SYNOPSIS
Chronic, subacute decidual hemorrhage (i.e., abruptio placenta and retrochorionic hematoma formation) is an important contributor to preterm parturition. Such hemorrhage induces thrombin from decidual tissue factor, which play a pivotal role in the development of preterm premature rupture of membranes and preterm delivery by acting through protease-activated receptors to promote the production of pro-inflammatory cytokines, and matrix-degrading metalloproteinases. Severe, acute abruption can lead to maternal and fetal mortality. Current management of abruption is individualized based on severity of disease, underlying etiology, and gestational age.
Keywords: Decidua, hemorrhage, prematurity, abruption, inflammation, cytokines
Introduction
Despite intense investigations into its pathogenesis and prevention, preterm delivery (PTD) remains a major public health concern. In 2008, the preterm birth rate in United States was 12.3% with PTD a major world-wide contributor of perinatal morbidity and mortality.1 Long-term sequelae of prematurity include pulmonary dysfunction, blindness, hearing loss, gastrointestinal malfunction, intraventricular hemorrhage, mental retardation, and cerebral palsy. Healthcare costs associated with these acute and chronic complications of prematurity exceed $26 billion per year.2
An increasing body of evidence now links decidual hemorrhage (DH) to PTD, with approximately 10% of all PTD attributed to bleeding-related events.3 While vaginal bleeding can rarely arise from cervical polyps, most cases originate from the decidua basalis (placental abruption) or the decidua parietalis (retrochorionic hematoma). The occurrence of vaginal bleeding in the first trimester and in more than one trimester is respectively associated with two- and seven-fold increased risks of PTD compared to no bleeding.4–8 The occurrence of a clinically evident abruption, together with occult DH and retrochorionic hematoma formation, complicates almost 40% of PTDs due to preterm labor with intact membrane and preterm premature rupture of the fetal membranes (PPROM) between 22–38 weeks, versus 0.8% after term delivery (p<0.01).7
Beyond inducing prematurity, pregnancies complicated by placental abruption display an increase in perinatal mortality beginning at about 28 weeks of gestation (Fig. 1). The resulting neonates exhibit increased rates of perinatal asphyxia, intraventricular hemorrhage, periventricular leukomalacia, cerebral palsy and mortality, compared with age-matched controls.9,10 The demographic profile of DH-associated PTD differs from infection-associated PTD, with the former occurring more frequently in patients who are married, older, white, parous, college educated and tobacco users.11 Other risk factors for DH include PPROM, thus DH is both a cause and consequence of this condition, as well as trauma, substance use, hypertensive disorders, renal disorders, multiple gestations, rapid uterine decompression, and history of abruption in a prior pregnancy.12–15 (Table 1)
Figure 1.
Perinatal mortality in pregnancies with and without abruption across gestation, 2000–2002.9
Circles, pregnancies with abruption. Diamonds, pregnancies without abruption.
(From Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol 2006;108(4):1005-16, with permission.)
Table 1.
| Risk factor | OR or RR |
|---|---|
| Trauma | 4.3–15.0 |
|
| |
| Cocaine use | 5.0–10.0 |
|
| |
| Oligohydramnios | 2.5–10 |
|
| |
| Chorioamnionitis | 2.0–2.5 |
|
| |
| Maternal age and parity | 1.1–3.7 |
|
| |
| Hypertensive disorders | |
| Eclampsia | 3.3–3.9 |
| Chronic hypertension with preeclampsia | 7.8 |
| Chronic hypertension | 1.8–5.1 |
| Mild and severe preeclampsia | 0.4–4.5 |
|
| |
| PPROM | 1.8–5.1 |
|
| |
| Multiple gestations | 1.5–3.0 |
|
| |
| Alcohol use | 1.5–1.7 |
|
| |
| Renal disorders | 1.4–1.8 |
|
| |
| Tobacco use | 1.4–2.5 |
|
| |
| Dietary or nutritional deficiency | 0.9–2.0 |
|
| |
| Male fetus | 0.9–1.3 |
Decidualization
Each menstrual cycle begins with hypoxia-induced angiogenesis and estradiol (E2)-induced proliferation of epithelium and stromal cells, which had been sloughed off at the end of the previous infertile cycle. Coincident with the occurrence of ovulation is an elevation in plasma progesterone (P4) levels which blocks further mitotic activity and initiates differentiation of the E2-primed cells. In the luteal phase of the human menstrual cycle, P4 stimulates E2-primed human endometrial stromal cells to decidualize.16,17
The process of decidualization begins around blood vessels and beneath the glands. It involves changes in differentiation that transform the interstitial extracellular matrix (ECM) of the follicular phase endometrium to an ECM enriched with basal laminar-type proteins and proteins that promote hemostasis. Under continued P4 stimulation, decidualization spreads wave-like throughout the late luteal phase endometrium.18
After implantation, blastocyst-derived extravillous trophoblasts (EVTs) traverse the decidua and enter the inner third of the myometrium. This stepwise process begins with attachment of EVT-expressed adhesion molecules to specific basal laminar proteins in the decidual ECM.19 Trophoblasts then breach decidual capillaries to provide the embryo with a rich source of oxygen and nutrients, and eventually remodel maternal spiral arteries to form high-capacitance, low-resistance vessels that increase blood flow to the fetal-placental unit.20 Invasion of decidual capillaries and arteries occurs within a matrix of decidual cells that express tissue factor (TF) under continued stimulation by P4. Elevated decidual cell expression of TF is temporally and spatially positioned to promote hemostasis during this invasive process.21
Role of Tissue Factor
TF is a transmembrane 45-kDa glycoprotein member of the class 2 cytokine receptor family.21–23 Unlike endothelial cells, which do not normally express TF, tissues that are highly vulnerable to bleeding such as the brain and placental villi constitutively express high levels of TF.24,25 Expression of TF may provide an evolutionary advantage in prevention of fatal hemorrhage. In human endometrial stromal cells, P4 uniquely induces high levels of TF to prevent hemorrhage during placentation, but in the absence of a conception, P4 withdrawal promotes inhibition of TF expression to facilitate menstruation.22
In response to vascular injury, the clotting cascade is initiated by binding of extravasated circulating factor VII to the hydrophilic extracellular domain of perivascular cell membrane-bound TF. Factor VII auto-activates upon binding to TF to form the TF/VIIa complex, and triggers a greater than 100-fold increase in catalytic activity.26 The TF/VIIa complex cleaves pro-thrombin to thrombin, which then ultimately converts fibrinogen to fibrin. The final hemostatic plug is formed when fibrin monomers self-polymerize and are cross-linked by thrombin-activated factor XIIIa.27–28 Simultaneously, thrombin induces platelet plug formation. The clotting cascade (Fig. 2) meets maternal hemostatic demands, beginning at the initial breach of decidual capillaries by syncytiotrophoblast, through EVT remodeling of spiral arteries,20,29 and finally during physiologic placental detachment at the third stage of labor.29
Figure 2. Hemostatic, thrombotic, and fibrinolytic pathways60.
Ø, inhibitory effect; (+), stimulatory effect.
(From Han CS, Paidas MJ, Lockwood CJ. Clotting Disorders. In: High Risk Pregnancy: Management Options. Eds: James DK, Steer PJ, Weiner CP et al. New York, NY: Saunders; 2010, with permission.)
The maternal-fetal interface at term displays prominent immunostaining for TF in decidual cells while cytotrophoblasts are virtually devoid of TF immunostaining.30 That abruption-induced thrombin formation is promoted by decidual cell-expressed TF and linked to PTD is indicated by: a) the standard use of fibrinogen consumption to measure abruption severity; b) enhanced fibrin deposition in the decidua; and c) the high sensitivity and specificity with which elevated circulating thrombin-antithrombin (TAT) complex levels predict the subsequent occurrence of PTD associated with PTL and/or PPROM.31–34
Direct and Indirect Roles for Thrombin in PTD and PPROM
Through this protective cascade, abruption results in an intense local generation of thrombin which paradoxically predisposes to PPROM and PTD. In addition to promoting hemostasis via its extracellular actions, thrombin induces several biological effects via decidual cell membrane-bound protease-activated receptors (PARs), a family of four distinct “seven membrane” G protein-coupled receptors. As is the case for thrombin, each PAR ligand is a serine protease that binds to and cleaves its receptor to expose an N-terminal tethered ligand domain that autoactivates the cleaved receptor.34
Among its protean cellular effects, thrombin induces proliferation and chemotaxis of pro-inflammatory immune and mesenchymal cells and activates endothelial cells via PAR-1, 3 and 4. In contrast, PAR-2 is primarily a receptor for trypsin and trypsin-like enzymes, which includes the TF/VIIa complex.35–37 Binding to PARs can directly induce production of matrix metalloproteinases (MMPs) which degrade various ECM components.38–40 Bioengineering measurements taken together with comprehensive histological and biochemical observations indicate that the fibrillar collagen-rich amnion and choriodecidua ECM provide greater than additive tensile strength and structural integrity that withstand disruptive forces derived from the human fetus and myometrium.41 The weakest membrane component, the choriodecidua, was observed to rupture first, followed by its amnion support structure. Proteases secreted by neighboring cell types degrade collagens I and IV in the ECM of choriodecidua and amnion, thereby impairing the integrity and strength of these ECMs.42
The MMPs are the primary mediators of ECM degradation. Specifically, 1) the collagenases, exemplified by MMP-1, effectively degrade fibrillar collagens; 2) the stromelysins, exemplified by MMP-3, degrade a broad array of ECM components, as well as activate the secreted zymogenic forms of pro-MMP-1 and pro-MMP-9 to promote an ECM-degrading cascade; and 3) the gelatinases, exemplified by MMP-9 and MMP-2, preferentially degrade basal laminar proteins such as collagen IV.38–43, In cultured human term decidual cell s, thrombin markedly augments MMP-1 and MMP-3.46,47 Third trimester human decidua contains abundant, constitutively expressed MMP-2, as well as the key regulators of MMP activity, tissue inhibitor of MMPs (TIMP-1 and TIMP-2).19 A rich decidual infiltrate of neutrophils, which express high levels of such ECM-degrading proteases as elastase, collagenase and MMP-9, accompanies abruption-induced PPROM even in the absence of infection.48–50
The association between PPROM and intra-amniotic infections is well-established and strong evidence now links PPROM with placental abruption in the absence of infection.5 In the amniotic fluid of women with PTD and coexisting chorioamnionitis, levels of interleukin-8 (IL-8), the primary neutrophil chemoattractant and activator51–52, and IL-6, a decisive mediator of chronic inflammation, are elevated.53–54 Similarly, we observed that thrombin elicited a dose-dependent elevation in IL-8 secretion by decidual cells compared to untreated cultures (P<0.05), with 2.5 U/mL of thrombin increasing IL-8 levels by greater than 14-fold.55 The decidua of placental sections of abruption-induced PPROM in the absence of infection showed co-localization of the immunoreactive IL-8 with fibrin deposits consistent with thrombin-induction of IL-8 production by decidual cells. Furthermore, immunostaining for the neutrophil marker CD15 in placentas after overt abruption, with or without PPROM, showed marked decidual neutrophil infiltration that peaked after PPROM, whereas decidua from gestational age-matched controls were virtually devoid of neutrophils. Neutrophil infiltrates also co-localized with fibrin deposition. As a rich source of proteases, neutrophils contribute to degradation of ECM and abruption-mediated PPROM.52
Additionally, thrombin has been shown to be a potent direct uterotonic agent both in vitro and in vivo56 Using a rat model, thrombin was shown to increase frequency, intensity and tone of myometrial contractions in a dose-dependent manner. Pre-treatment of thrombin with hirudin, a thrombin inactivator, or heparin suppressed the uterotonic effects of thrombin.
Therefore, during DH, thrombin generated from TF localized on the cell membranes of decidual cells is positioned to induce PPROM and PTD directly via enhanced decidual cell expressed MMP expression, and indirectly by promoting neutrophil trafficking and uterine contractility, as summarized in Fig. 3. These pathologic mechanisms together contribute to a 7-fold increase in risk of PPROM when vaginal bleeding is seen in more than one trimester.6–8
Figure 3. Pathway to prematurity from Decidual Hemorrhage.
FVIIa: activated factor VII; TF: tissue factor; PAR: protease-activated receptors; MMP: matrix metalloproteinases; ECM: extracellular matrix.
(Courtesy of Charles J. Lockwood, MD, New Haven, Connecticut)
Clinical manifestations of Decidual Hemorrhage
Abruptions associated with preterm labor and PPROM usually reflect sub-acute, chronic processes. However, acute, clinically evident abruptions can lead to both maternal and fetal death. Abruptions are defined as the partial or complete separation of a normally implanted placenta from the placental implantation site prior to delivery. Abruption occurs in 0.48% to 1.8% of pregnancies, and its incidence may be increasing.57,58 In recent decades, a disproportionate increase has been seen in African-American women.59 The incidence of abruption is highest between 24–28 weeks of gestation and decreases as gestation advances.9,10 (Fig. 4)
Figure 4.
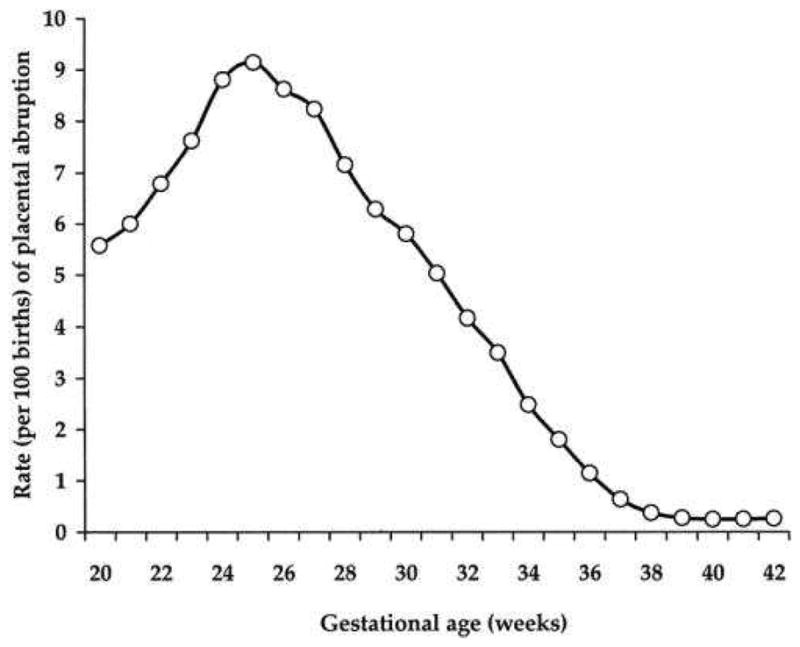
Rates of abruption across gestation, United States, 2000–20029
(From Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol 2006;108(4):1005-16, with permission.)
Diagnosis of placental abruption is based on classical clinical findings of vaginal bleeding, abdominal pain and increased uterine contractions. Differential diagnoses include placenta previa, vasa previa, cervical lesions, infection (cervicitis), trauma, urinary source, and others.60 Approximately, 10–35% of abruptions may be concealed within the uterus, and therefore result in clinical underestimation of blood loss.61–62
Physical examination may reveal a rigid abdomen secondary to tetanic contractions or Couvelaire uterus, vaginal bleeding, and evidence of maternal hypoperfusion or fetal distress. Cardiotocography may display uterine tachysystole with or without a NICHD Category III fetal heart rate tracing. Although ultrasound evaluation of the placenta can sometimes show presence of a retroplacental or retrochorionic hematoma, approximately 50% of placental abruptions produce no distinct ultrasound findings.10 Clinical sequelae include 1) fetal manifestations of uteroplacental insufficiency, 2) maternal complications of hemorrhage, or 3) as previously described, premature birth.
The severity of DH ranges from subclinical separation of the placenta, incidentally detected on ultrasound or on gross examination of placenta after delivery, to major abruption with associated clinical sequelae. The grading of placental abruptions is seen in Table 2. Grade 3 placental abruption occurs in approximately 0.2% of pregnancies.61 Formation of a hematoma in the decidua may result in compression of the overlying intervillous space, local destruction of the placental parenchyma, and fetal hypoxia. Therefore, chronic placental abruption often manifest as uteroplacental insufficiency, fetal growth restriction, or oligohydramnios. Most pregnancies complicated by abruption result in the delivery of an infant weighing less than the 10th percentile for gestational age.62
Table 2.
Grading of placental abruption70
| Grade | Vaginal bleeding | Uterine tetany | Fetal distress | Maternal shock | Ultrasound finding |
|---|---|---|---|---|---|
| 0 | − | − | − | − | Small retroplacental clot |
| 1 | + | +/− | − | − | +/− |
| 2 | +/− | +/− | + | − | +/− |
| 3 | +/− | + | Fetal demise | + with possible coagulopathy | +/− |
In severe case, placental abruption involving greater than 50% separation can result in intrauterine fetal demise.10 With a perinatal mortality rate of approximately 12%, placental abruption carries a 15-fold increase in risk of intrauterine and neonatal demise compared to controls. At the optimal gestational age of 40 weeks (when overall perinatal mortality normally nadirs), perinatal mortality was 19-fold greater for abruption complicated than for non-abruption associated births (34.6 vs. 1.8 per 1,000 births, respectively). Mortality with abruption was more likely to occur antepartum than after delivery (relative risk for stillbirth was 18 compared with 10 for neonatal death).63
Maternal sequelae of placental abruption include complications of severe hemorrhage, such as hypotension, hysterectomy, disseminated intravascular coagulation (DIC), massive transfusions, intensive care unit admission, and multisystem organ failure. Maternal mortality rate ranges from 0.04% to 1%.64–65 Approximately 10% of patients with placental abruption show significant coagulopathy, with the incidence increasing to 20–30% in cases of massive abruption. The robust thrombin production in severe placental abruption is the pathophysiology behind DIC with consumptive hypofibrinogenemia.10
Women who have experienced an abruption have increased long term health risks. Placental abruption with fetal compromise has been associated with maternal cardiovascular sequelae later in life in a large population-based study of over a million women, with an adjusted hazard ratio of 2.0 (95% CI: 1.7–2.2).66 These future cardiovascular events, such as ischemic heart disease, cerebrovascular accident, and peripheral artery disease, may be a delayed manifestation of the underlying maternal vascular pathology that predisposed to adverse pregnancy outcomes.
Management of Placental Abruption
Management of suspected placental abruption should include prompt assessment of maternal and fetal status, and a subsequent individualized management algorithm based on severity of disease, underlying etiology, and gestational age. In severe cases of placental abruption with life-threatening hemorrhage, maternal hemodynamic stabilization is the priority. As previously mentioned, the majority of maternal complications result from hypovolemic shock secondary to massive blood loss, whether concealed or clinically visible. Maternal vital signs, including blood pressure, heart rate, and urine output should be closely monitored. Hypovolemia may be masked in cases of abruption in which severe hypertension is the etiology.
Crystalloid, colloid, and blood product resuscitation may be initiated through two wide-bore intravenous lines, if necessary. Identification and correction of underlying risk factors, such as trauma, or a hypertensive crisis also play an important role in the management of placental abruption. A multidisciplinary approach should be undertaken, if available, with appropriate consultations with perinatologists, neonatologists, anesthesiologists, operating room staff, blood bank, and intensive care specialists.
Laboratory evaluation should include blood type and Rhesus-D status, complete blood counts, coagulation studies, and cross-matching of blood products. Hypofibrinogenemia is a result of the intense thrombin response and is the most sensitive indicator of coagulopathy. Prothrombin time and partial thromboplastin time may be elevated in cases of severe abruption. Kleihauer-Betke, an acid elution test employed to assess the presence of fetal hemoglobin in maternal circulation, may be useful in calculation of Rhesus-D immunoglobulin dosage in patients who are Rhesus-D negative.
If the fetus is at a gestational age deemed to be potentially viable, continuous fetal cardiotocography should be initiated to determine fetal well-being. Antenatal corticosteroids may be administered between 24 + 0/7 to 33 + 6/7 weeks of gestation for promotion of fetal lung maturity and prevention of prematurity-associated complications. If fetal decompensation unresponsive to intrauterine resuscitation occurs or the fetus is above 34 + 0/7 weeks of gestation, delivery should be expeditiously effected. Vaginal delivery can be attempted if the fetal heart rate tracing remains reassuring and the maternal status remains stable. Given the intense uterotonic effects of thrombin, patients may often undergo spontaneous labor without need for induction or augmentation. Cesarean delivery should be performed if the patient is remote from delivery with evidence of fetal distress, intolerance of labor, or for routine obstetrical indications.
In the case of an intrauterine fetal demise secondary to a massive abruption, the mode of delivery is dependent on maternal status, severity of hemorrhage, and other obstetrical complicating factors, such as prior classical hysterotomy incision. Vaginal delivery is the preferred modality in most cases, unless urgent delivery is necessary for maternal stabilization.
If the mother and fetus are both stable, such as in a mild, subacute, or chronic abruption, ultrasound evaluation of the intrauterine environment may be undertaken, including assessment of the placental location, placental appearance, amniotic fluid index, and fetal growth. Placenta previa may be present in 10% of placental abruptions.
Ultrasonographic appearance of a hematoma evolves over time. In the acute phase, the blood is hyperechoic to isoechoic when compared to the placenta. (Figs. 5 and 6) As the hematoma organizes and resolves, the insult appears hypoechoic within a week and may be anechoic within two weeks.67 (Figs. 7 and 8) Exclusion of a retroplacental hematoma, however, does not exclude the presence of placental abruption.
Figures 5 and 6.
Retroplacental hematoma
Figure 7 and 8.
Resolving hematoma with hypoechoic appearance
The use of tocolytics in placental abruption has classically been discouraged, however, a few small non-randomized studies have shown possible benefit to administration of tocolytic agents to prolong gestation.68–69 Further studies are needed to elucidate the risks and benefits of tocolysis.
Careful management of a patient with placental abruption should continue into the postpartum period, given the risks of DIC and postpartum uterine atony. Uterotonic agents, such as oxytocin, carboprost, misoprostol, and methyl-ergonovine, should be readily available.
Placental evaluation
Gross examination of a placenta may reveal a fresh clot attached to the maternal surface of the placenta in cases of recent placental abruption. Chronic or remote abruption may be seen as fibrin deposition at the site of abruption with infarcts or depression of the overlying placental parenchyma. Microscopic examination reveals hemosiderin-laden macrophages and evidence of villous hemorrhage.70
Histological etiologies for DH include ischemic placental diseases, poor spiral artery remodeling, spiral artery thrombosis and sclerotic lesions in myometrial arteries.6,8,57 It is important to note, however, that evidence of abruption can be seen in up to 4.5% of placentas examined routinely, suggesting that small episodes of placental abruption are more common than those diagnosed clinically.71
Recurrence, Prediction, and Prevention
The recurrence rate of placental abruption ranges from 6% to 17% after a first episode, similar to the recurrence risk of PTD and increases to 25% after two episodes. Approximately 7–15% of massive abruptions resulting in intrauterine fetal demise have the same outcome in a subsequent pregnancy.10,72 Thirty percent of all future pregnancies in women who have had a prior placental abruption do not carry to viability.72
Although multiple trials exist for various treatments in the prevention of preeclampsia, no randomized-controlled trials exist for prevention of placental abruption. Prevention of recurrent abruption begins with correction of underlying modifiable risk factors. Consideration should be given to interval resection of a uterine septum or submucous myoma. Other established risk factors for placental abruption, such as cigarette smoking, drug use, chronic hypertension, pregnancy-induced hypertension, and preeclampsia, should be addressed. Preconceptional and prenatal counseling on the harmful effects of smoking and drug abuse during pregnancy can help to reduce the incidence of placental abruption and other adverse outcomes of pregnancy.3
Summary
Decidual hemorrhage is an important contributor to PTD and associated healthcare costs. Management of abruption should be individualized, and the patient should be counseled on risks of recurrence. Recent translational investigations on the role of TF and thrombin have helped to shed light on the complex disease process. Additional future research is imperative to development of targeted therapies in the treatment and prevention of placental abruption and PTD.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Osterman MJ, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS Data Brief. 2010;39(39):1–8. [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS. Societal Costs of Prematurity. In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academies Press; 2007. pp. 398–429. [PubMed] [Google Scholar]
- 3.Ananth CV, Berkowitz GS, Savitz DA, et al. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282(17):1646–51. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Mittendorf R, Lieberman E, et al. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet Gynecol. 1991;78(1):14–8. [PubMed] [Google Scholar]
- 5.Harger JH, Hsing AW, Tuomala RE, et al. Risk factors for preterm premature rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol. 1990;163(1 Pt 1):130–7. doi: 10.1016/s0002-9378(11)90686-3. [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Peltier MR, Chavez MR, et al. Recurrence of ischemic placental disease. Obstet Gynecol. 2007;110(1):128–33. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]
- 7.Salafia CM, Lopez-Zeno JA, Sherer DM, et al. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am J Obstet Gynecol. 1995;173(4):1065–70. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- 8.Naeye RL. Maternal age, obstetric complications, and the outcome of pregnancy. Obstet Gynecol. 1983;61(2):210–6. [PubMed] [Google Scholar]
- 9.Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108(4):1005–16. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 10.Creasy RK, Resnik R, Iams JD, et al. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 11.Strobino B, Pantel-Silverman J. Gestational vaginal bleeding and pregnancy outcome. Am J Epidemiol. 1989;129(4):806–15. doi: 10.1093/oxfordjournals.aje.a115195. [DOI] [PubMed] [Google Scholar]
- 12.Yeo L, Ananth CV, Vintzileos AM. In: Placental Abruption. Sciarra J, editor. Hagerstown, MD: Lippincott, Williams & Wilkins; 2003. [Google Scholar]
- 13.Schiff MA. Pregnancy outcomes following hospitalisation for a fall in Washington State from 1987 to 2004. BJOG. 2008;115(13):1648–54. doi: 10.1111/j.1471-0528.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 14.Ananth CV, Smulian JC, Demissie K, et al. Placental abruption among singleton and twin births in the United States: risk factor profiles. Am J Epidemiol. 2001;153(8):771–8. doi: 10.1093/aje/153.8.771. [DOI] [PubMed] [Google Scholar]
- 15.Roque H, Paidas MJ, Funai EF, et al. Maternal thrombophilias are not associated with early pregnancy loss. Thromb Haemost. 2004;91(2):290–5. doi: 10.1160/TH03-09-0596. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood CJ, Krikun G, Hickey M, et al. Decidualized human endometrial stromal cells mediate hemostasis, angiogenesis, and abnormal uterine bleeding. Reprod Sci. 2009;16(2):162–70. doi: 10.1177/1933719108325758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabanelli S, Tang B, Gurpide E. In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol. 1992;42(3–4):337–44. doi: 10.1016/0960-0760(92)90137-8. [DOI] [PubMed] [Google Scholar]
- 18.Kearns M, Lala PK. Life history of decidual cells: a review. Am J Reprod Immunol. 1983;3(2):78–82. doi: 10.1111/j.1600-0897.1983.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 19.Lockwood CJ, Oner C, Uz YH, et al. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78(6):1064–72. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KL. The Developing Human. Philadelphia, PA: W. B. Saunders Company; 1998. [Google Scholar]
- 21.Lockwood CJ, Nemerson Y, Guller S, et al. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab. 1993;76(1):231–6. doi: 10.1210/jcem.76.1.8421090. [DOI] [PubMed] [Google Scholar]
- 22.Lockwood CJ, Krikun G, Papp C, et al. The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann N Y Acad Sci. 1994;734:57–79. doi: 10.1111/j.1749-6632.1994.tb21736.x. [DOI] [PubMed] [Google Scholar]
- 23.Runic R, Schatz F, Krey L, et al. Alterations in endometrial stromal cell tissue factor protein and messenger ribonucleic acid expression in patients experiencing abnormal uterine bleeding while using Norplant-2 contraception. J Clin Endocrinol Metab. 1997;82(6):1983–8. doi: 10.1210/jcem.82.6.3992. [DOI] [PubMed] [Google Scholar]
- 24.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–97. [PMC free article] [PubMed] [Google Scholar]
- 25.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24(6):1015–22. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 26.Neuenschwander PF, Fiore MM, Morrissey JH. Factor VII autoactivation proceeds via interaction of distinct protease-cofactor and zymogen-cofactor complexes. Implications of a two-dimensional enzyme kinetic mechanism. J Biol Chem. 1993;268(29):21489–92. [PubMed] [Google Scholar]
- 27.Nemerson Y. Tissue factor and hemostasis. Blood. 1988;71(1):1–8. [PubMed] [Google Scholar]
- 28.Bach RR. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23(4):339–68. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 29.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Lockwood CJ, Murk W, Kayisli UA, et al. Progestin and thrombin regulate tissue factor expression in human term decidual cells. J Clin Endocrinol Metab. 2009;94(6):2164–70. doi: 10.1210/jc.2009-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaiworapongsa T, Espinoza J, Yoshimatsu J, et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11(6):368–73. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 32.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185(5):1059–63. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 33.Rosen T, Kuczynski E, O’Neill LM, et al. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001;10(5):297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- 34.Macfarlane SR, Seatter MJ, Kanke T, et al. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–82. [PubMed] [Google Scholar]
- 35.Gabazza EC, Taguchi O, Kamada H, et al. Progress in the understanding of protease-activated receptors. Int J Hematol. 2004;79(2):117–22. doi: 10.1532/ijh97.03165. [DOI] [PubMed] [Google Scholar]
- 36.Cottrell GS, Amadesi S, Schmidlin F, et al. Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans. 2003;31(Pt 6):1191–7. doi: 10.1042/bst0311191. [DOI] [PubMed] [Google Scholar]
- 37.Riewald M, Ruf W. Orchestration of coagulation protease signaling by tissue factor. Trends Cardiovasc Med. 2002;12(4):149–54. doi: 10.1016/s1050-1738(02)00153-6. [DOI] [PubMed] [Google Scholar]
- 38.Bohm SK, McConalogue K, Kong W, et al. Proteinase-Activated Receptors: New Functions for Old Enzymes. News Physiol Sci. 1998;13:231–40. doi: 10.1152/physiologyonline.1998.13.5.231. [DOI] [PubMed] [Google Scholar]
- 39.Grand RJ, Turnell AS, Grabham PW. Cellular consequences of thrombin-receptor activation. Biochem J. 1996;313(Pt 2):353–68. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lockwood CJ, Paidas M, Murk WK, et al. Involvement of human decidual cell-expressed tissue factor in uterine hemostasis and abruption. Thromb Res. 2009;124(5):516–20. doi: 10.1016/j.thromres.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13(8):781–92. [PubMed] [Google Scholar]
- 42.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27(8):783–93. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Moore RM, Mansour JM, Redline RW, et al. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11–12):1037–51. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663–70. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 46.Rosen T, Schatz F, Kuczynski E, et al. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med. 2002;11(1):11–7. doi: 10.1080/jmf.11.1.11.17. [DOI] [PubMed] [Google Scholar]
- 47.Mackenzie AP, Schatz F, Krikun G, et al. Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol. 2004;191(6):1996–2001. doi: 10.1016/j.ajog.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12(4):237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 49.Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183(1):94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 50.Van den Steen PE, Proost P, Wuyts A, et al. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96(8):2673–81. [PubMed] [Google Scholar]
- 51.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 52.Zeilhofer HU, Schorr W. Role of interleukin-8 in neutrophil signaling. Curr Opin Hematol. 2000;7(3):178–82. doi: 10.1097/00062752-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63(5):321–9. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 54.Saji F, Samejima Y, Kamiura S, et al. Cytokine production in chorioamnionitis. J Reprod Immunol. 2000;47(2):185–96. doi: 10.1016/s0165-0378(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 55.Lockwood CJ, Toti P, Arcuri F, et al. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol. 2005;167(5):1443–9. doi: 10.1016/S0002-9440(10)61230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elovitz MA, Saunders T, Ascher-Landsberg J, et al. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol. 2000;183(4):799–804. doi: 10.1067/mob.2000.108897. [DOI] [PubMed] [Google Scholar]
- 57.Hall DR. Abruptio placentae and disseminated intravascular coagulopathy. Semin Perinatol. 2009;33(3):189–95. doi: 10.1053/j.semperi.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Rasmussen S, Irgens LM, Bergsjo P, et al. The occurrence of placental abruption in Norway 1967–1991. Acta Obstet Gynecol Scand. 1996;75(3):222–8. doi: 10.3109/00016349609047091. [DOI] [PubMed] [Google Scholar]
- 59.Ananth CV, Oyelese Y, Yeo L, et al. Placental abruption in the United States, 1979 through 2001: temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192(1):191–8. doi: 10.1016/j.ajog.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 60.Han CS, Paidas MJ, Lockwood CJ. Clotting Disorders. In: James DK, Steer PJ, Weiner CP, et al., editors. High Risk Pregnancy: Management Options. New York, NY: Saunders; 2010. [Google Scholar]
- 61.Knuppel AR, Drukker JE. Bleeding in Late Pregnancy: Antepartum Bleeding. In: Hayashi RH, Castillo MS, editors. High Risk Pregnancy: A Team Approach. Philadelphia, PA: Saunders; 1986. p. 547. [Google Scholar]
- 62.Fraser R, Watson R. Bleeding during the Latter Half of Pregnancy. In: Chalmers I, editor. High Risk Pregnancy: A Team Approach. London: Oxford University Press; 1989. p. 89. [Google Scholar]
- 63.Ananth CV, Wilcox AJ. Placental abruption and perinatal mortality in the United States. Am J Epidemiol. 2001;153(4):332–7. doi: 10.1093/aje/153.4.332. [DOI] [PubMed] [Google Scholar]
- 64.Tikkanen M, Gissler M, Metsaranta M, et al. Maternal deaths in Finland: focus on placental abruption. Acta Obstet Gynecol Scand. 2009;88(10):1124–7. doi: 10.1080/00016340903214940. [DOI] [PubMed] [Google Scholar]
- 65.Egley C, Cefalo R. In: Abruptio Placenta. Studd J, editor. London: Churchill Livingstone; 1985. p. 108. [Google Scholar]
- 66.Ray JG, Vermeulen MJ, Schull MJ, et al. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 67.Nyberg DA, Cyr DR, Mack LA, et al. Sonographic spectrum of placental abruption. AJR Am J Roentgenol. 1987;148(1):161–4. doi: 10.2214/ajr.148.1.161. [DOI] [PubMed] [Google Scholar]
- 68.Saller DN, Jr, Nagey DA, Pupkin MJ, et al. Tocolysis in the management of third trimester bleeding. J Perinatol. 1990;10(2):125–8. [PubMed] [Google Scholar]
- 69.Towers CV, Pircon RA, Heppard M. Is tocolysis safe in the management of third-trimester bleeding? Am J Obstet Gynecol. 1999;180(6 Pt 1):1572–8. doi: 10.1016/s0002-9378(99)70053-0. [DOI] [PubMed] [Google Scholar]
- 70.Bernischke K, Kaufmann P. Pathology of the Human Placenta. New York, NY: Springer; 2000. [Google Scholar]
- 71.Fox H, Sebire N. Pathology of the Placenta (Major Problems in Pathology) London: Saunders; 2007. [Google Scholar]
- 72.Hibbard BM, Jeffcoate TN. Abruptio placentae. Obstet Gynecol. 1966;27(2):155–67. [PubMed] [Google Scholar]
- 73.Sher G, Statland BE. Abruptio placentae with coagulopathy: a rational basis for management. Clin Obstet Gynecol. 1985;28(1):15–23. doi: 10.1097/00003081-198528010-00003. [DOI] [PubMed] [Google Scholar]







