Abstract
Rationale: The characterization of young adults who develop late-onset diseases may augment the detection of novel genes and promote new pathogenic insights.
Methods: We analyzed data from 2,500 individuals of African and European ancestry in the COPDGene Study. Subjects with severe, early-onset chronic obstructive pulmonary disease (COPD) (n = 70, age < 55 yr, FEV1 < 50% predicted) were compared with older subjects with COPD (n = 306, age > 64 yr, FEV1 < 50% predicted).
Measurements and Main Results: Subjects with severe, early-onset COPD were predominantly females (66%), P = 0.0004. Proportionally, early-onset COPD was seen in 42% (25 of 59) of African Americans versus 14% (45 of 317) of non-Hispanic whites, P < 0.0001. Other risk factors included current smoking (56 vs. 17%, P < 0.0001) and self-report of asthma (39 vs. 25%, P = 0.008). Maternal smoking (70 vs. 44%, P = 0.0001) and maternal COPD (23 vs. 12%, P = 0.03) were reported more commonly in subjects with early-onset COPD. Multivariable regression analysis found association with African American race, odds ratio (OR), 7.5 (95% confidence interval [CI], 2.3–24; P = 0.0007); maternal COPD, OR, 4.7 (95% CI, 1.3–17; P = 0.02); female sex, OR, 3.1 (95% CI, 1.1–8.7; P = 0.03); and each pack-year of smoking, OR, 0.98 (95% CI, 0.96–1.0; P = 0.03).
Conclusions: These observations support the hypothesis that severe, early-onset COPD is prevalent in females and is influenced by maternal factors. Future genetic studies should evaluate (1) gene-by-sex interactions to address sex-specific genetic contributions and (2) gene-by-race interactions.
Keywords: chronic obstructive pulmonary disease, female, African Americans
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) has evolved from a disorder primarily affecting males to one of increased prevalence and mortality in females. Females and African Americans may be more susceptible to the deleterious effects of smoking, as they have been shown to exhibit similar severity of COPD despite less intense smoking over shorter durations.
What This Study Adds to the Field
We provide evidence that female sex is significantly associated with early-onset COPD in participants from the Genetic Epidemiology of COPD (COPDGene) Study. Also, African American race appears to be a risk factor for severe early-onset COPD. Maternal history and maternal respiratory disease are significantly associated with risk for severe early-onset COPD.
Chronic obstructive pulmonary disease (COPD) is typically a disease that presents in late adulthood, often associated with cigarette smoking (1, 2). Although the prevalence of COPD has been changing, COPD is more prevalent in individuals older than 65 years of age (3). Previously considered a disease affecting primarily males, the prevalence of COPD in women and the number of women dying from COPD have increased (4). Maturity-onset diseases are complex; they may have multiple causes with contributions from environmental exposures, comorbidities, age-related degenerative changes, and genetic factors. Diseases occurring at an earlier age than traditionally expected may result from the interaction of inherited factors and environmental exposures.
In 1998, a family-based cohort composed of 44 probands with severe, early-onset COPD recruited from specialized programs in lung transplantation, lung volume reduction surgery, and local pulmonary clinics and 249 first- or second-degree relatives was reported (5). Eligibility criteria for inclusion as a proband included the following: (1) age not greater than 52 years, (2) the absence of severe α1-antitrypsin deficiency, and (3) FEV1 less than 40% predicted. This first report was notable for the high percentage of female probands (80%). However, whether early-onset COPD is a specific phenotype and whether it is a female predominant disease have been questioned. No reports have specifically focused on the characteristics of African American subjects with severe, early-onset COPD. However, two single-center analyses (6, 7), and a retrospective study of participants in the National Emphysema Treatment Trial (NETT) (8), noted that African American subjects with COPD, with equally severe COPD, tend to be younger than white subjects with COPD and smoke less intensely. We hypothesized that female sex and maternal factors would be associated with early-onset COPD in African American and white subjects in comparison with older subjects with equally severe COPD. To determine the effect of sex, maternal characteristics, and race on the risk for severe, early-onset COPD, we analyzed data from the first 2,500 subjects enrolled in the Genetic Epidemiology of COPD (COPDGene) Study (15APR2010 dataset), a multicenter investigation focused on identifying genes influencing COPD in non-Hispanic whites and African Americans (9).
Methods
Study Subject Eligibility
The study was approved by the institutional review boards of each clinical center. Participants in the COPDGene Study were between the ages of 45 and 80 years with at least 10 pack-years of cigarette smoking. Ethnicity was determined by self-report. Previous participants in the Boston Early-onset COPD Study were not eligible to participate in COPDGene.
We defined severe, early-onset COPD as FEV1/FVC less than 0.70 and FEV1 percent predicted less than 50% on postbronchodilator spirometry in subjects less than 55 years of age. Modification of the original criteria used by Silverman and colleagues (5) resulted in a more robust sample size. Individuals who met the previously published criteria of Silverman and colleagues (FEV1 percent predicted < 40% and age < 53 yr) are reported in the online supplement (5, 10). The comparison group for the primary analysis was composed of subjects with COPD with a postbronchodilator FEV1 percent predicted value less than 50%, FEV1/FVC less than 0.70, and age greater than 64 years.
Questionnaires
All subjects completed standardized questionnaires to assess respiratory history and symptoms, respiratory medications, smoking history, family history, and other medical history. The Respiratory Disease Questionnaire is a modified version of the American Thoracic Society/Division of Lung Diseases Respiratory Epidemiology Questionnaire (11). The specific family history questions used to generate the data presented in Table 2 are included in Table E2 in the online supplement. Pack-years were calculated as the average number of cigarettes smoked per day, divided by 20, and multiplied by the number of years of smoking. Subjects were queried about their age at initiation of smoking, current smoking, history of pneumonia, age at first episode of pneumonia, presence of a usual cough, number of years with a cough, number of years with phlegm production, history of asthma, history of maternal COPD, history of maternal smoking, history of paternal COPD, and history of paternal smoking. In lieu of extensive socioeconomic data in our cohort, we dichotomized education achieved as a high school degree or equivalent versus more advanced education. In addition, we constructed a composite COPD variable: a single variable composed of reports of maternal (or paternal) chronic obstructive pulmonary disease, chronic bronchitis, and emphysema.
TABLE 2.
MULTIVARIABLE LOGISTIC REGRESSION MODELS FOR PREDICTION OF EARLY-ONSET COPD IN THE COPDGENE STUDY
| Univariate Analyses | Multivariable Model | |||
| Characteristic (%) | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Female sex (47) | 2.6 (1.5–4.5) | 0.0006 | 3.1 (1.1–8.7) | 0.03 |
| AA race (16) | 4.4 (2.4–8.1) | <0.0001 | 7.5 (2.3–24) | 0.0007 |
| Pack-years of smoking | 0.97 (0.96–0.98) | <0.0001 | 0.98 (0.96–1.0) | 0.03 |
| Maternal smoking (49) | 2.9 (1.7–5.1) | 0.0002 | 1.7 (0.6–4.7) | 0.3 |
| Maternal COPD (14) | 2.2 (1.1–4.5) | 0.03 | 4.7 (1.3–17) | 0.02 |
Definition of abbreviations: AA = African American; CI = confidence interval; COPD = chronic obstructive pulmonary disease; OR = odds ratio.
Note: Clinical center, nonsignificant in the stepwise selection, is not reported here.
Dependent variable: severe COPD < age 55 years versus severe COPD > 64 years.
Hosmer-Lemeshow goodness of fit test = 0.8.
Spirometry
Spirometry was performed in a standardized manner with an Easy-One spirometer (ndd Medical Technologies, Andover, MA) with the subjects seated and wearing nose clips. A central automated review of spirometry was performed with manual review of cases not meeting American Thoracic Society/European Respiratory Society criteria (12). After prebronchodilator spirometry, two inhalations of albuterol were administered via spacer, and postbronchodilator spirometry was performed 15–40 minutes later. Spirometric reference values were based on National Health and Nutrition Survey (NHANES) predicted spirometry values (13).
Chest computed tomography (CT) scanning was performed on all subjects using Siemens, Phillips, or General Electric Medical Systems scanners. Image reconstruction slice thickness was standardized. Image reconstruction kernels varied according to the scanner manufacturer. An inspiratory chest CT scan was performed to assess airway wall thickness and emphysema. An expiratory chest CT scan was performed to assess air trapping. The lungs were segmented as previously described (14, 15). Total percent emphysema and total percent air trapping were determined by CT using SLICER software (www.slicer.org) (16, 17). Emphysema was defined as the percentage of lung less than –950 Hounsfield units (HU) on the inspiratory CT scan. Air trapping was defined as the percentage of lung less than –856 HU on the expiratory scan. We used the following thresholds to distinguish scans with and without lung pathology: at least 5% CT emphysema and at least 15% air trapping on CT. Airway analysis was performed using VIDA Pulmonary Workstation 2 (VIDA Diagnostics, Coralville, IA; www.vidadiagnostics.com). CT-based metrics of airway disease included mean bronchial wall thickness calculated as an average of the six segmental values for each subject and wall area percent (100 × [wall area/total bronchial area]).
Body mass index was calculated as [weight (kg)/height (cm2)] × 10,000. Subjects completed a 6-minute walk test to assess exercise capacity in accordance with American Thoracic Society guidelines (18). The BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) Index score was calculated for each subject (19). The modified Medical Research Council (MMRC) dyspnea score was based on responses to questions about perceived dyspnea at specific levels of exertion (20).
Statistical Methods
Continuous variables were compared by Student t test and are displayed as means ± standard deviation. Categorical values were compared by chi square testing and are displayed as percentages of the total. We used the amount of education attained as a proxy for socioeconomic status in some of our multivariable models. To determine the maternal contribution to the development of severe, early-onset COPD, we constructed a logistic regression model using a stepwise selection algorithm with severe, early-onset COPD as the dependent variable and older severe COPD as the comparison group, P = 0.1 as the threshold for inclusion, with race, sex, pack-years of smoking, maternal COPD, and maternal smoking as the independent variables. In addition to our main analysis, we employed an alternative study design. We divided the cohort into tertiles based on age less than 55 years, 55–64 years, and 64 years or older. Covariates that were statistically significant in models contrasting early-onset COPD versus older COPD were analyzed in each age group with severe COPD, defined as FEV1 percent predicted less than 50%, as the dependent variable. Logistic regression models were also constructed for evaluation of paternal factors. As paternal characteristics were not significant in any of our multivariable regression models, no paternal characteristics were included. Two interaction terms, described as follows: (1) smoking-by-sex, in which pack-years were used to represent smoking history, and (2) race-by-sex, were nonsignificant and deleted from the model. Logistic regression analysis was performed with SAS, version 9.2 (SAS Institute, Cary, NC).
Results
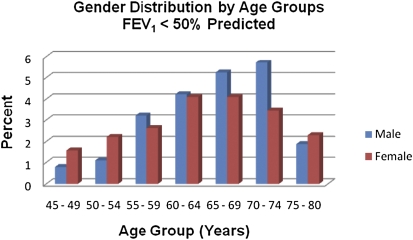
Of the first 2,500 subjects enrolled in the COPDGene Study, 49% (1,221 of 2,500) were female and 26% (640 of 2,500) were African American. We excluded 227 individuals who could not be classified as control subjects or COPD cases (FEV1 < 80% predicted and FEV1/FVC ratio ≥ 0.70). On the basis of the actual age at study enrollment, there were 70 subjects with severe, early-onset COPD. There were 306 subjects with COPD who met our criteria as severe COPD at age greater than 64 years. The sex distribution of all subjects with FEV1 less than 50% predicted, arrayed by incremental age groups, is displayed in Figure 1.
Figure 1.
In the first 2,500 subjects from the Genetic Epidemiology of COPD (COPDGene) Study with severe chronic obstructive pulmonary disease (COPD) (n = 532), females predominated at both ends of the age spectrum: ages 45–54 and 75–80 years. Calculations: [(females with FEV1 < 50% predicted/total females) × 100] and [(males with FEV1 < 50% predicted/total males) × 100].
Characteristics of Subjects with Severe, Early-Onset COPD
Of the 70 subjects with severe, early-onset COPD (2.8% of the total 2,500 COPDGene subjects), the mean age was 51 years, 46 subjects were female (66%), and 24 were male (Table 1). Twenty-five of the 70 subjects (36%) were African American and 16 of the African American subjects with early-onset COPD were female. By comparison, African Americans represented 19% (198 of 1,061) of subjects who were classified as GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage 2–4 in the entire 2,500-subject study population. Among subjects with early-onset COPD, African Americans were significantly less likely to have advanced education after high school, 10 versus 90%, P = 0.0008. Although individuals with advanced education smoked an average of 55 versus 60 pack-years for those with a high school degree or equivalent, the difference was not statistically significant, P = 0.09.
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF SUBJECTS WITH SEVERE, EARLY-ONSET CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) AND OLDER SUBJECTS WITH SEVERE COPD: THE COPDGENE STUDY
| Subjects with Early-onset COPD | Older Subjects with COPD | ||
| (n = 70) | (n = 306) | P Value | |
| Age, yr* | 51 ± 3 | 71 ± 4 | <0.0001 |
| Age at smoking initiation, yr* | 16 ± 4 | 17 ± 5 | 0.003 |
| Sex, % female | 66 | 43 | 0.0004 |
| Race | |||
| White, % | 64 | 89 | |
| African American, % | 36 | 11 | <0.0001 |
| Maternal COPD, % | 23 | 12 | 0.03 |
| Maternal smoking, % | 70 | 44 | 0.0001 |
| Maternal lung cancer, % | 13 | 4 | 0.02 |
| Paternal COPD, % | 21 | 13 | 0.2 |
| Paternal smoking, % | 78 | 77 | 0.9 |
| Pack-years of smoking* | 42 ± 24 | 61 ± 30 | <0.0001 |
| FEV1 percent predicted* | 34 ± 11 | 34 ± 10 | 0.9 |
| BMI* | 28 ± 9 | 27 ± 6 | 0.2 |
| BODE Index score* | 4.7 ± 1.5 | 4.6 ± 1.6 | 0.5 |
| CT scan percent emphysema* | 17 ± 14 | 23 ± 14 | 0.001 |
| CT scan percent gas trapping* | 48 ± 20 | 56 ± 16 | 0.003 |
| Bronchodilator responsiveness, % FEV1* | 9.0 ± 15 | 10 ± 14 | 0.7 |
| Current smoking, % | 56 | 17 | <0.0001 |
Definition of abbreviations: BMI = body mass index; BODE = BMI, airflow obstruction, dyspnea, and exercise capacity; COPD = chronic obstructive pulmonary disease; CT = computed tomography.
Mean ± SD.
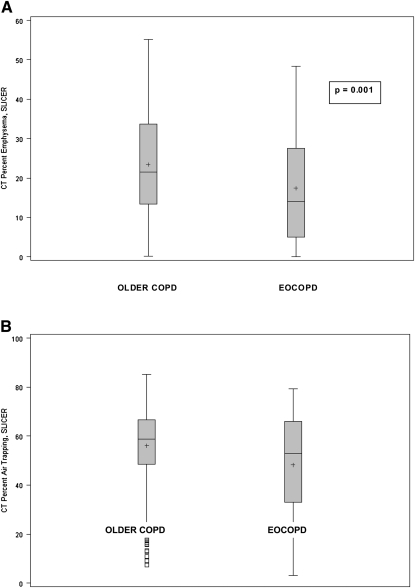
Subjects with severe, early-onset COPD did not significantly differ in severity of lung function in comparison with older subjects with COPD (FEV1 percent predicted, 34 ± 11 vs. 34 ± 10, P = 0.9). However, the subjects with early-onset COPD started smoking earlier, 16 versus 17 years (P = 0.003), smoked significantly less intensely, 42 versus 61 pack-years (P < 0.0001), and were more likely to currently smoke, 56 versus 17% (P < 0.0001). These subjects with COPD also experienced their first episode of pneumonia earlier, 35 versus 46 years (mean), P = 0.0001. The subjects with early-onset COPD displayed less percent emphysema, 17 versus 23% (P = 0.001), and less air trapping, 48 versus 56% (P = 0.003), determined radiographically by computed tomography (Figures 2A and 2B, respectively). Wall area percentage measurements were available for a subset of our cohort: 58 subjects with early-onset COPD and 242 older subjects with COPD. Segmental wall area percentage in subjects with early-onset COPD was 64 ± 3 versus 63 ± 3% in older subjects with COPD, P = 0.04. Subsegmental wall area percentage in subjects with early-onset COPD was 67 ± 2.3 versus 66 ± 2.1%, P = 0.01.
Figure 2.
(A) Percent emphysema from computed tomography, calculated from the histogram percentage of voxels at –950 Hounsfield units or less, using SLICER software. Mean percent emphysema in the subjects with early-onset chronic obstructive pulmonary disease (EOCOPD) was 17% compared with 23% in older subjects with COPD. (B) Air trapping determined as the percentage of lung less than –856 Hounsfield units on the expiratory computed tomography (CT) scan using SLICER software. Mean percent air trapping in the subjects with EOCOPD was 48 versus 56% in the older subjects with COPD.
Maternal respiratory history factored prominently in these subjects with early-onset COPD (Table 1). Maternal smoking (70 vs. 44%, P = 0.0001) and maternal COPD (23 vs. 12%, P = 0.03) were reported more frequently in subjects with early-onset COPD. In addition, our data suggested that subjects with early-onset COPD were more likely to report a history of maternal lung cancer, 10 versus 4% (P = 0.045). No paternal factors were associated with risk for severe, early-onset COPD (P > 0.1 for all tests).
Regression Models
Female characteristics were significantly associated with early-onset COPD and included female sex (odds ratio [OR], 2.6; 95% confidence interval [CI], 1.5–4.5; P = 0.0006), maternal smoking (OR, 2.9; 95% CI, 1.7–5.1; P = 0.0002), and maternal COPD (OR, 2.2; 95% CI, 1.1–4.5; P = 0.03). In a multivariable logistic regression model analyzing maternal respiratory factors, including sex, race, pack-years of smoking, maternal COPD, maternal smoking, and clinical center, only female sex (OR, 3.1; 95% CI, 1.2–8.7; P = 0.03), maternal COPD (OR, 4.7; 95% CI, 1.3–17; P = 0.02), African American race (OR, 7.5; 95% CI, 2.3–24; P = 0.0007), and pack-years of smoking (OR, 0.98; 95% CI, 0.96–1.0; P = 0.03) retained significance (Hosmer-Lemeshow P = 0.8) (Table 2). Additional logistic regression models for severe, early-onset COPD are displayed in the online supplement, analyzing smoking intensity (Table E3, part A) or a composite COPD variable for maternal COPD, emphysema, and chronic bronchitis (Table E3, part B).
Of the 2,500 study subjects in the COPDGene Study, 532 (21%) exhibited severe COPD defined as postbronchodilator FEV1 percent predicted less than 50%. Using the alternative study design with data parsed by age tertiles (Table E4), maternal COPD and pack-years of smoking were associated with severe COPD in all three age groups. Support for the association of female sex with severe COPD was seen with the younger age group and suggested for male sex with the older age group (Table E4). The importance of family history is similar to the findings reported by Hersh and colleagues (21). These results for characteristics associated with severe COPD, parsed by age group, add substantiation to the contribution of female sex for history of early-onset COPD and history of maternal COPD for severe COPD. Race, however, was not significant in these models.
Discussion
Severe, early-onset COPD is rare and only a small percentage of subjects with COPD from the COPDGene Study met our criteria for severe, early-onset COPD at presentation for study enrollment. Nonetheless, early-onset COPD has the potential to provide important insight into COPD risk factors. Our findings from the first 2,500 subjects enrolled in the COPDGene Study are consistent with and support the hypothesis that female sex is important in the development of severe, early-onset COPD as female sex predominated with our case definition, the case definition used by Silverman and colleagues, and others (5, 10, 22). In comparison with older subjects with COPD, subjects with early-onset COPD had significantly less tobacco exposure in terms of pack-years of smoking. This implies that the magnitude of tobacco exposure is not the primary factor in the development of early-onset COPD, suggesting that additional factors, such as genetic determinants, maternal factors, and their interactions, may be more important. Although we strongly believe that further elucidation of these additional factors is of paramount importance, an alternative explanation for the reduced risk for pack-years of smoking in the early-onset COPDGene subjects [OR, 0.98; 95% CI, 0.96–1.0; P = 0.03] is that subjects with early-onset COPD are significantly younger than the older subjects with severe COPD and have had less time to accumulate additional pack-years of smoking. The presence of maternal respiratory disease may be among these additional factors that promote acceleration of lung function loss due to smoking in these susceptible individuals. These findings support the importance of investigating specific contributions from mitochondrial and X chromosome genes as well as gene-by-sex interactions.
In the initial, family-based report of early-onset COPD by Silverman and colleagues (5), first-degree relatives who actively smoked had significantly lower lung function compared with currently smoking or ex-smoking control subjects, suggesting that susceptibility to lung impairment was not explained solely by tobacco smoking. Relatives of probands were 58% more likely to report parental COPD (OR, 4.4; 95% CI, 1.5–12.4) and chronic bronchitis. A subsequent report from this cohort has determined that current or formerly smoking first-degree relatives have more bronchodilator responsiveness (23). Further data suggesting a genetic predisposition to early-onset COPD have been supported by the finding of familial aggregation in nonsmokers for a spirometric phenotype, FEF25–75 (forced expiratory flow, midexpiratory phase) (24). This cohort was also the subject of the first and subsequent linkage analyses for COPD-related phenotypes (25–28). These findings and our data suggest the excellent potential for investigating early-onset COPD to provide clues to COPD pathogenesis.
Lung growth and development differ by sex. Airway and parenchymal growth are proportional in females in contrast to the dysynapsis that occurs in males, where parenchymal growth exceeds that of the airways. Female lung function growth peaks before that of males. Studies have demonstrated that growth in FEV1 peaks at age 13 in girls (300 ml/yr) and plateaus at age 16, whereas growth in FEV1 peaks at age 14 in boys (400 ml/yr) and plateaus after age 18 (29). Other factors that affect maximal lung function include height and thoracic width, which are smaller in females. Current or former smoking female first-degree relatives of subjects in the original Boston Early-onset COPD Study were demonstrated to have significantly more bronchodilator responsiveness compared with current or formerly smoking control subjects, defined as the percentage increase from baseline FEV1 (5.8 ± 8.1 vs. 2.9 ± 5.1%, P < 0.01) (10). In addition, women with mild COPD in the Lung Health Study were demonstrated to have significantly more bronchial hyperreactivity in response to methacholine challenge (30), a factor that predicted greater loss in lung function over time (31). It has been articulated that COPD should be considered a pediatric disease as prenatal and early life exposures in concert with maternal smoking, maternal atopy, and maternal genotype result in alterations in childhood lung health leading to susceptibility to develop COPD in adulthood (32). Our results are similar to those of Svanes and colleagues, where maternal asthma and maternal smoking were associated with reduced lung function in a population-based study of young adults (33). Exposure to maternal smoking in utero has also been demonstrated to affect maximally attainable lung function and airway development (29, 34–37), with greatest effect among children with asthma (38). Although we did not fully evaluate the impact of maternal smoking during pregnancy in our cohort, the problem is not trivial. It has been reported that 10% of pregnant African American women and 16% of pregnant white women report smoking during pregnancy (39). Early life exposure to tobacco smoke has also been postulated to affect alterations in immune function with increased susceptibility to respiratory disease (40). Therefore, severe, early-onset COPD may potentially develop in susceptible females because of the intricate interaction of early or decreased attainment of maximal lung function or reduction in lung growth potentiated by maternal behaviors and maternal respiratory history, tobacco smoking, asthma, genetic factors, or hormonal influences.
Our data are unique in the inclusion and evaluation of African American subjects with COPD for the clinical characteristics associated with severe, early-onset COPD. The reason why COPD develops in African Americans at younger ages and with less intense smoking, in comparison with white subjects with COPD, is unknown. There may be multiple potential mechanisms influencing this varied susceptibility to the adverse consequences of cigarette smoking. There are known differences in the pharmacokinetics of nicotine; African Americans have higher serum cotinine levels per cigarette smoked, lower renal clearance of cotinine, higher intake of nicotine per cigarette smoked (41), and more loss of lung function per cigarette smoked (7). There is a high prevalence of asthma in African Americans and differences in airway reactivity that when compounded by tobacco smoking could result in disproportionately increased risk for airflow obstruction (42). Significant differences in occupational and environmental exposures may also potentially exist. The explanation for the early development of COPD in African Americans is likely complex and multifactorial with interactions between social, environmental, and genetic risk factors. The airway wall findings and the lesser degree of CT emphysema in our subjects with severe, early-onset COPD are suggestive that early-onset COPD may be an airway wall–predominant disorder.
Limitations to our study include that the COPDGene Study is not population based and differences in recruitment could have contributed to our findings. Our cross-sectional study design and study questionnaires do not allow us to exclude the possibility that a proportion of the older subjects in the cohort may have initially had early-onset COPD. In fact, 38 (12%) of the older COPD group self-reported a diagnosis of COPD at an age less than 55 years. Seventeen were male (6%) and 21 were female (7%). As a result, our effect estimates are likely underestimations. Thus, the definition of severe early-onset COPD in subjects less than 55 years of age can be definitively applied only to the younger portion of our cohort. Although our sample size of subjects with severe, early-onset COPD is small, we nonetheless found differences by race and sex. Our finding that African American race may also be associated with the development of severe, early-onset COPD is plausible as it has been reported that this group may develop COPD at earlier ages and with shorter durations of smoking (6, 8, 43). In contrast to the report by Chatila and colleagues (8), where 42 (3.4%) African American subjects out of the 1,218 total subjects enrolled in the NETT Study were analyzed, our study enrolled 640 (26%) African Americans. We do recognize that overrepresentation of younger African Americans could also impact our findings; however, the prevalence of COPD in African Americans may be influenced by early mortality from other smoking-related disorders (44). We acknowledge that recall bias for maternal factors such as smoking and lung disease might differ in early-onset compared with later-onset COPD. Importantly, maternal COPD history was associated across all age strata, suggesting maternal history may be a susceptibility marker for severe COPD. In addition, our study does not thoroughly address the role of socioeconomic factors in explaining the occurrence of severe early-onset COPD in African American or white subjects. Last, although known α1-antitrypsin (AAT)–deficient subjects were not eligible for COPDGene, it is possible that some undiagnosed subjects with AAT deficiency have been included.
Changes in the epidemiology of COPD have shifted the focus from a male-predominant disease to one in which the prevalence and adverse consequences are increasingly affecting females. Our findings in the COPDGene Study support the recommendation that future genetic and molecular studies of COPD should include particular attention to the contribution of maternally inherited factors such as mitochondrial and X chromosome genes, as well as gene-by-sex interactions and gene-by-race interactions.
Supplementary Material
Footnotes
Supported by U01HL089897, U01HL089856, K01 HL092601 (M.G.F.), an Alpha-1 Foundation grant (M.G.F.), R01 HL089438 (D.L.D.) from the National Heart, Lung, and Blood Institute, and a Doris Duke Clinical Scientist Development Award (D.L.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The COPDGene project is also supported by the COPD Foundation through contributions made to an industry advisory board composed of AstraZeneca, Boehringer Ingelheim, Novartis, and Sepracor.
Contributing authors: M.G.F., L.Z., J.M., N.N.H., B.M., J.E.H., G.W., E.A.R., J.D.C., E.K.S., D.L.D. Funding: J.D.C., E.K.S.; concept and design: M.G.F., E.K.S., D.L.D.; data collection: M.G.F., B.M., J.E.H., E.A.R., J.D.C., E.K.S.; data analysis: M.G.F., L.Z., J.M., N.N.H., B.M., J.E.H., G.W., E.A.R., J.D.C., E.K.S., D.L.D.; statistical support: L.Z., J.M., M.G.F., E.K.S., D.L.D.; writing/editing: M.G.F., L.Z., J.M., N.N.H., B.M., J.E.H., G.W., E.A.R., J.D.C., E.K.S., D.L.D.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201011-1928OC on May 11, 2011
Author Disclosure: M.G.F. received institutional grant support from the Alpha-1 Foundation. L.Z. received institutional grant and travel support from National Jewish Health. N.N.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.M. received support for travel from the COPD Foundation. He was on the Advisory Board for Forest, AstraZeneca (AZ), Novartis, Dey, Nycomed, Respironics, Schering, Johnson & Johnson, Sequal, Embryon, Respironics, Boehringer Ingelheim (BI), Pfizer, and MedImmune. He was a consultant for Astellas, Talecris, and Chiesi. He received institutional grant support from AZ, GlaxoSmithKline (GSK), Pfizer, NABI Biopharmaceuticals, BI, Sunovion, and MedImmune. He received lecture fees from GSK, BI, and Pfizer. He receives royalties from UpToDate and received institutional compensation from Spiration for the review of documents related to a clinical trial. He received compensation from BI and Pfizer for a video presentation and from the University of British Columbia for questionnaire development. He received honoraria from the American Thoracic Society, the American College of Chest Physicians, the American Academy of Family Practice, the Cleveland Clinic, Breathe LA, Abbott, and the Veterans Health Administration. He received compensation from WebMD for an audio for an educational program and from the France Foundation for the development of educational programs. J.E.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.W. was a consultant for MedImmune and Spiration. E.A.R. and J.D.C. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.K.S. received institutional grant support and support for travel from the COPD Foundation. He received grant support from GSK and was a consultant for GSK and AZ. He received lecture fees from GSK and AZ. D.L.D. received grant support from the Doris Duke Charitable Foundation.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–773 [DOI] [PubMed] [Google Scholar]
- 2.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J 2007;30:993–1013 [DOI] [PubMed] [Google Scholar]
- 3.Soriano JB, Maier WC, Egger P, Visick G, Thakrar B, Sykes J, Pride NB. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax 2000;55:789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Deaths from chronic obstructive pulmonary disease–United States, 2000–2005. MMWR Morb Mortal Wkly Rep 2008;57:1229–1232 [PubMed] [Google Scholar]
- 5.Silverman EK, Chapman HA, Drazen JM, Weiss ST, Rosner B, Campbell EJ, O'Donnell WJ, Reilly JJ, Ginns L, Mentzer S, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease: risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 1998;157:1770–1778 [DOI] [PubMed] [Google Scholar]
- 6.Chatila WM, Wynkoop WA, Vance G, Criner GJ. Smoking patterns in African Americans and whites with advanced COPD. Chest 2004;125:15–21 [DOI] [PubMed] [Google Scholar]
- 7.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med 2006;100:1110–1116 [DOI] [PubMed] [Google Scholar]
- 8.Chatila WM, Hoffman EA, Gaughan J, Robinswood GB, Criner GJ. Advanced emphysema in African-American and white patients: do differences exist? Chest 2006;130:108–118 [DOI] [PubMed] [Google Scholar]
- 9.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) Study design. COPD 2010;7:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ, Denish P, Silverman RA, Celedon JC, Reilly JJ, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:2152–2158 [DOI] [PubMed] [Google Scholar]
- 11.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978;118:1–120 [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338 [DOI] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–187 [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging 2001;20:490–498 [DOI] [PubMed] [Google Scholar]
- 15.Ross J, Estepar R, Diaz A, Westin C, Kikinis R, Silverman E, Washko G. Lung extraction, lobe segmentation and hierarchical region assessment for quantitative analysis on high resolution computed tomography images. Med Image Comput Assist Interv 2009;12:690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washko GR, Dransfield MT, Estepar RS, Diaz A, Matsuoka S, Yamashiro T, Hatabu H, Silverman EK, Bailey WC, Reilly JJ. Airway wall attenuation: a biomarker of airway disease in subjects with COPD. J Appl Physiol 2009;107:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest 2007;132:464–470 [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 19.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012 [DOI] [PubMed] [Google Scholar]
- 20.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–586 [DOI] [PubMed] [Google Scholar]
- 21.Hersh CP, Hokanson JE, Lynch DA, Washko GR, Make BJ, Crapo JD, Silverman EK. Family history is a risk factor for chronic obstructive pulmonary disease. Chest (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, Demeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65:480–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celedon JC, Speizer FE, Drazen JM, Weiss ST, Campbell EJ, Carey VJ, Reilly JJ, Ginns L, Silverman EK. Bronchodilator responsiveness and serum total IgE levels in families of probands with severe early-onset COPD. Eur Respir J 1999;14:1009–1014 [DOI] [PubMed] [Google Scholar]
- 24.DeMeo DL, Carey VJ, Chapman HA, Reilly JJ, Ginns LC, Speizer FE, Weiss ST, Silverman EK. Familial aggregation of FEF25–75 and FEF25–75/FVC in families with severe, early onset COPD. Thorax 2004;59:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman EK, Mosley JD, Palmer LJ, Barth M, Senter JM, Brown A, Drazen JM, Kwiatkowski DJ, Chapman HA, Campbell EJ, et al. Genome-wide linkage analysis of severe, early-onset chronic obstructive pulmonary disease: airflow obstruction and chronic bronchitis phenotypes. Hum Mol Genet 2002;11:623–632 [DOI] [PubMed] [Google Scholar]
- 26.Silverman EK, Palmer LJ, Mosley JD, Barth M, Senter JM, Brown A, Drazen JM, Kwiatkowski DJ, Chapman HA, Campbell EJ, et al. Genomewide linkage analysis of quantitative spirometric phenotypes in severe early-onset chronic obstructive pulmonary disease. Am J Hum Genet 2002;70:1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer LJ, Celedon JC, Chapman HA, Speizer FE, Weiss ST, Silverman EK. Genome-wide linkage analysis of bronchodilator responsiveness and post-bronchodilator spirometric phenotypes in chronic obstructive pulmonary disease. Hum Mol Genet 2003;12:1199–1210 [DOI] [PubMed] [Google Scholar]
- 28.DeMeo DL, Celedon JC, Lange C, Reilly JJ, Chapman HA, Sylvia JS, Speizer FE, Weiss ST, Silverman EK. Genome-wide linkage of forced mid-expiratory flow in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:1294–1301 [DOI] [PubMed] [Google Scholar]
- 29.Boezen HM, Jansen DF, Postma DS. Sex and gender differences in lung development and their clinical significance. Clin Chest Med 2004;25:237–245 [DOI] [PubMed] [Google Scholar]
- 30.Kanner RE, Connett JE, Altose MD, Buist AS, Lee WW, Tashkin DP, Wise RA. Gender difference in airway hyperresponsiveness in smokers with mild COPD: the Lung Health Study. Am J Respir Crit Care Med 1994;150:956–961 [DOI] [PubMed] [Google Scholar]
- 31.Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA; Lung Health Study Research Group Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:1802–1811 [DOI] [PubMed] [Google Scholar]
- 32.Bush A. COPD: a pediatric disease. COPD 2008;5:53–67 [DOI] [PubMed] [Google Scholar]
- 33.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, de Marco R, Norback D, Raherison C, Villani S, et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010;65:14–20 [DOI] [PubMed] [Google Scholar]
- 34.Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy: effects on lung function during the first 18 months of life. Am J Respir Crit Care Med 1995;152:977–983 [DOI] [PubMed] [Google Scholar]
- 35.Boezen HM, Vonk JM, van Aalderen WM, Brand PL, Gerritsen J, Schouten JP, Boersma ER. Perinatal predictors of respiratory symptoms and lung function at a young adult age. Eur Respir J 2002;20:383–390 [DOI] [PubMed] [Google Scholar]
- 36.Milner AD, Rao H, Greenough A. The effects of antenatal smoking on lung function and respiratory symptoms in infants and children. Early Hum Dev 2007;83:707–711 [DOI] [PubMed] [Google Scholar]
- 37.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O'Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax 2009;64:810–814 [DOI] [PubMed] [Google Scholar]
- 38.Li YF, Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Rappaport EB, Peters JM. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med 2000;162:2097–2104 [DOI] [PubMed] [Google Scholar]
- 39.Department of Health and Human Services (HHS) Maternal and child health. : Healthy people 2010, Vol. 2, Chapter 16. Washington DC, HHS, 2000 [Google Scholar]
- 40.Prescott SL. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatr Respir Rev Mar 2008;9:3–9, quiz 10 [DOI] [PubMed] [Google Scholar]
- 41.Perez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA 1998;280:152–156 [DOI] [PubMed] [Google Scholar]
- 42.Dransfield MT, Bailey WC. COPD: racial disparities in susceptibility, treatment, and outcomes. Clin Chest Med 2006;27:463–471 [DOI] [PubMed] [Google Scholar]
- 43.Kirkpatrick P, Dransfield MT. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Curr Opin Pulm Med 2009;15:100–104 [DOI] [PubMed] [Google Scholar]
- 44.Wise RA. Changing smoking patterns and mortality from chronic obstructive pulmonary disease. Prev Med 1997;26:418–421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.