Abstract
A major pathological feature of chronic airway diseases is the elevated expression of gel-forming mucins. NF-κB activation in airway epithelial cells has been shown to play a proinflammatory role in chronic airway diseases; however, the specific role of NF-κB in mucin gene expression has not been characterized. In this study, we show that the proinflammatory cytokines, IL-1β and IL-17A, both of which use the NF-κB pathway, are potent inducers of MUC5B mRNA expression in both well differentiated primary normal human bronchial epithelial cells and the human bronchial epithelial cell line, HBE1. MUC5B induction by these cytokines was both time- and dose-dependent, and was attenuated by the small molecule inhibitor, NF-κB inhibitor III, as well as p65 small interfering RNA, suggesting that the regulation of MUC5B expression by these cytokines is via an NF-κB–based transcriptional mechanism. Deletion analysis of the MUC5B promoter demonstrated that IL-1β– and IL-17A–induced promoter activity resides within the −4.17-kb to −2.56-kb region relative to the transcriptional start site. This region contains three putative κB-binding sites (NF-κB-1, −3,786/−3,774; NF-κB-2, −3,173/−3,161; and NF-κB-3, −2,921/−2,909). Chromatin immunoprecipitation analysis confirmed enhanced binding of the p50 NF-κB subunit to the NF-κB-3 site after cytokine stimulation. We conclude that an NF-κB-based transcriptional mechanism is involved in MUC5B regulation by IL-1β and IL-17A in airway epithelium. This is the first demonstration of the participation of NF-κB and its specific binding site in cytokine-mediated airway MUC5B expression.
Keywords: cytokines, gene regulation, mucin, transcription factors, lung
CLINICAL RELEVANCE.
Mucin overproduction is a major clinical hallmark associated with airway inflammation. The elucidation of the molecular mechanism involved in cytokine-induced MUC5B expression will provide a therapeutic basis for the treatment to reduce the overproduction.
Homeostasis in mucus production is essential for proper mucociliary clearance and innate immune function in normal airways (1, 2); excessive production of mucin in inflamed airways can increase morbidity and mortality by obstructing mucociliary clearance and air flow (3, 4). To date, 11 mucin (MUC) genes (MUC1, 2, 3, 4, 5AC, 5B, 6, 7, 8, 13, and 19) have been described as being expressed in the lung (2). Among these, MUC5AC and MUC5B are the most prominent mucins in the airway. MUC5B is mainly expressed in mucous glands under normal conditions; however, the airway epithelium is also known to express MUC5B as well as MUC5AC, particularly in disease states, such as chronic obstructive pulmonary disease (COPD) and usual interstitial pneumonia (5, 6). In a mouse model of asthma, airway epithelial cells have also been shown to up-regulate Muc5b expression in association with mucous cell hyperplasia (7). Although extensive work has been done to elucidate the molecular mechanism of cytokine-induced MUC5AC expression (8–11), there are few studies describing the regulation of cytokine-mediated MUC5B gene expression. We have previously reported that IL-6 and IL-17A could stimulate MUC5B gene expression via a c-Jun kinase/extracellular signal–regulated kinase signaling pathway in normal human bronchial epithelial (NHBE) cells (12); however, the transcription factors involved in cytokine-induced MUC5B expression still remain to be determined.
NF-κB is a pleiotropic transcription factor with multiple critical roles in regulation of immune responses (13–15). NF-κB becomes activated in response to inflammatory cytokines, mitogens, physical and oxidative stress, infection, and microbial products (16). Before stimulation, NF-κB subunits are sequestered in the cytoplasm by IκB. After cell stimulation, IκB-α is phosphorylated by IκB kinase (IKK) 2. Phosphorylation of IκB-α results in the ubiquitination and degradation of IκB-α, leading to the nuclear localization of NF-κB, and transcriptional activation of target genes (14). The proinflammatory role of NF-κB in chronic airway diseases has been well documented. Enhanced activation of NF-κB has been implicated in both asthma and COPD (17, 18). Through the use of transgenic mice and conditional ablation strategies, activation of NF-κB within the airway epithelium has been shown to be necessary to induce airway inflammation and mucus overproduction (19, 20). However, little is known about the direct involvement of NF-κB in airway mucin gene regulation. The few studies published point to the involvement of NF-κB in MUC5B up-regulation by cigarette smoke (21) and MUC5AC up-regulation by lipoproteins of Haemophilus influenza or Mycoplasma pneumonia (22, 23). However, these NF-κB studies were not performed in a primary human cell system, nor did they address the role of NF-κB in proinflammatory cytokine–induced MUC5B expression.
IL-1β is a proinflammatory cytokine that has been shown to play an important role in airway diseases characterized by increased mucus production (24–26), and has been shown to be capable of activating the classical NF-κB signaling pathway (27). IL-17A is a member of a novel family of proinflammatory cytokines that is composed of six members: IL-17A, -B, -C, -D, -E, and -F (28). IL-17A has been shown to play important roles in a variety of inflammatory lung conditions, including asthma, COPD, and Gram-negative bacterial pneumonia infection (29–31). IL-17A stimulates the production of inflammatory cytokines and chemokines, and mediates pulmonary neutrophil migration (32, 33). Our recent studies have demonstrated that IL-17A stimulates the degradation of IκB-α, followed by the nuclear translocation of p50 and p65 subunits of NF-κB (34, 35), and induces mucin gene expression (11, 12) and production of human β-defensin-2 (36), CCL-20 (37), CXCL-1, -2, -3, -5, -6, and IL-19 (34) production by primary NHBE cells. Although NF-κB plays a central role in both IL-1β and IL-17A signaling cascades, no evidence is available about an NF-κB–based transcriptional mechanism in IL-1β– and IL-17A–induced MUC5B expression.
The purpose of this current study was to elucidate the molecular events associated with IL-1β– and IL-17A–induced MUC5B expression. Here, we demonstrate that IL-1β and IL-17A induced MUC5B expression in an NF-κB–dependent manner. Using promoter analysis and chromatin immunoprecipitation (ChIP) studies, we have identified an NF-κB–binding element in the promoter region of the MUC5B gene.
MATERIALS AND METHODS
Culture Conditions
Human bronchial tissues were obtained with informed consent from patients of the University of California–Davis Medical Center (Sacramento, CA) and the National Disease Research Interchange (Philadelphia, PA). The University Human Subjects Review Committee approved and periodically reviewed the protocol. Primary NHBE cells were isolated and cultured under an air–liquid interface condition, as described previously (11) The immortalized NHBE cell line, HBE1 (38), was used for most of the transfection experiments that were performed with a Lipofectamine 2000–mediated protocol (Invitrogen, Carlsbad, CA). Culture conditions for the HBE1 cell line have been described previously (11).
Cytokine and Inhibitor Treatments
Recombinant human cytokines, IL-1β and IL-17A or IL-17F, were obtained from Invitrogen and R&D systems (Minneapolis, MN), respectively. NF-κB activation inhibitor III (20 μM; Calbiochem, San Diego, CA), a thiazoloamide, was dissolved in DMSO before use. We observed no cytotoxic effects of the inhibitor (determined by trypan blue exclusion) at the dose used in this study (data not shown).
Real-Time RT-PCR
Isolated DNA-free RNA was used for quantitative real-time RT-PCR to obtain relative mRNA amounts of each gene after normalizing to the β-actin or GAPDH message abundance, as described previously (33–36). The primer sequences were as follows: GAPDH forward, TGGGCTACACTGAGCACCAG; GAPDH reverse, GGGTGTCGCTGTTGAAGTCA; β-actin forward, AGTCGGTTGGAGCGAGCAT; β-actin reverse, AAAGTCCTCGGCCACATTGT; MUC5B forward, GTGAGGAGGACTCCTGTCAAGT; MUC5B reverse, CCTCGCAGAAGGTGATGTTG; p65 forward, AGCTCAAGATCTGCCGAGTG; p65 reverse, ACATCAGCTTGCGAAAAGGA.
Mucin ELISA and Western Blot Analysis
Mucin secreted by primary NHBE cells was measured by a double-sandwich ELISA method using a monoclonal antibody specific to airway sputum mucin, 17B1, as described previously (39). The amount of mucin secreted in the culture was expressed as nanogram protein per million cells per day.
For Western blot analysis of MUC5B expression in NHBE cells, a deglycosylation step was performed on the blotted membrane before immunoreaction with monoclonal antibody 5B19-2E (Santa Cruz Biotechnology, Santa Cruz, CA), as described previously (40, 41).
Promoter–Reporter Constructs and Small Interfering RNA
Two MUC5B 4.17 kb and 2.56 kb promoter-luciferase reporter constructs previously constructed in our laboratory (7, 40) were used for the MUC5B promoter study. Cells were cotransfected with pRL-TK (Promega, Madison, WI) to control for transfection efficiency. NF-κB p65 small interfering RNA (siRNA) and random oligomer negative control were purchased from Ambion Biotech (Austin, TX).
ChIP Assay
ChIP assays were performed according to the ChIP protocol from Millipore (Billerica, MA), with minor modifications as described previously (34, 40, 41). Primers used for putative NF-κB sites of MUC5B were: NF-κB-1 (from −3,861 to −3,712): forward primer, 5′-GTGCGTCTGGCCTGGTAAG-3′; reverse primer, 5′-CCCAGGATGTGTACTCAGAGC-3′; NF-κB-2 (from −3,195 to −3,070): forward primer, 5′-GCAAGTTCCTGGCACGTC-3′; reverse primer, 5′-AAGGCGCTGAAAACAGAAGA-3′; NF-κB-3 (from −3,006 to −2,851): forward primer, 5′-CCGGGATGTCTCAATAGCTG-3′; reverse primer, 5′-GGCACACAGTGACACCAAAC-3′.
Statistical Analysis
Data are expressed as means (±SE). Experiments were performed in triplicate, and performed in at least two independent cultures. Group differences were calculated using the Student's t test. Differences were considered significant for P values less than or equal to 0.05.
RESULTS
IL-1β and IL-17A Stimulate MUC5B Gene Expression in NHBE Cells
We examined the potency of IL-1β and IL-17A stimulation of MUC5B expression in well differentiated NHBE cells cultured under air–liquid interface conditions. As shown in Figure 1, both IL-1β and IL-17A induced MUC5B mRNA expression in primary NHBE cells in both a dose- and time-dependent manner. For IL-1β, a significant stimulation of MUC5B was observed at concentrations as low as 0.2 ng/ml (Figure 1A). A time-course analysis indicated that maximum stimulation of MUC5B expression occurred at 24 hours (Figure 1B) after addition of 10 ng/ml IL-1β. A similar dose–response curve was seen for IL-17A, except that a decrease in MUC5B gene expression occurred with concentrations higher than 10 ng/ml (Figure 1C). Maximum stimulation was seen 24 hours after treatment with 10 ng/ml of IL-17A (Figure 1D). These results confirm that both IL-1β and IL-17A are potent stimulators of MUC5B gene expression in well differentiated NHBE cells. Similar time- and dose-dependent results were seen in studies with the HBE1 cell line (data not shown).
Figure 1.
Stimulation of MUC5B mRNA expression by IL-1β and IL-17A. (A and C) Dose–response effects of IL-1β and IL-17A on MUC5B mRNA expression. Normal human bronchial epithelial (NHBE) cells grown in air–liquid interface (ALI) conditions for 1 week were starved for 16 hours (without growth factors) before being treated with various concentrations of IL-1β (0–20 ng/ml) and IL-17A (0–50 ng/ml). At 24 hours after cytokine treatment, total RNA was harvested, and MUC5B mRNA was analyzed using SYBR Green quantitative real-time PCR and normalized to a housekeeping gene, GAPDH, as described in Materials and Methods. (B and D) Time course effects of IL-1β and IL-17A treatment on MUC5B mRNA expression. NHBE cells were treated with 10 ng/ml IL-1β (B) or 20 ng/ml IL-17A (D). RNA samples were harvested from these cultures at different time points (0, 12, 24, 48 h) after treatment. MUC5B and GAPDH mRNA levels were quantified using real-time PCR. Triplicate dishes were used for each experiment, and experiments were repeated with cultures derived from two different human donors. Statistically significant: *P < 0.05, **P < 0.01, compared with unstimulated control.
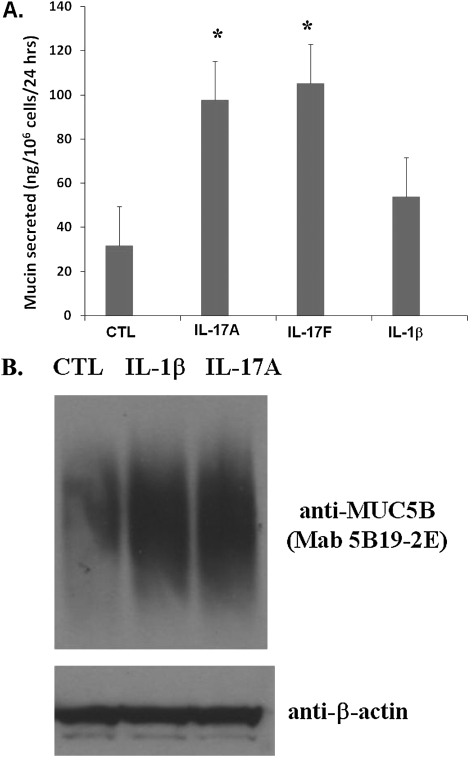
We performed both mucin ELISA and Western blot analysis to examine effects on protein expression. As shown in Figure 2A, both IL-17A and IL-17F were able to stimulate mucin production significantly, approximately threefold, over untreated cells, from 30 to nearly 100 ng/106 cells/day, whereas IL-1β provoked a twofold increase in mucin production. Western blot analysis using a MUC5B N-terminal–specific antibody confirmed the enhanced accumulation of high–molecular weight MUC5B protein after cytokine stimulation (Figure 2B).
Figure 2.
Effects of cytokines on mucin production and MUC5B glycoprotein expression in NHBE cells. NHBE cells were treated with 10 ng/ml IL-1β and 20 ng/ml IL-17A/F. (A) Medium was collected 24 hours after stimulation for mucin ELISA quantification, as described in the text and Ref. 39. (B) Cells were harvested 24 hours after stimulation and lysed for Western blot analysis with monoclonal antibody (Mab) 5B19-2E specific for MUC5B N-terminal peptide (40) and anti–β-actin antibody, which was used to monitor the protein input. *P < 0.05. CTL, unstimulated control.
NF-κB Is Required for Both IL-1β– and IL-17A–Induced MUC5B Expression
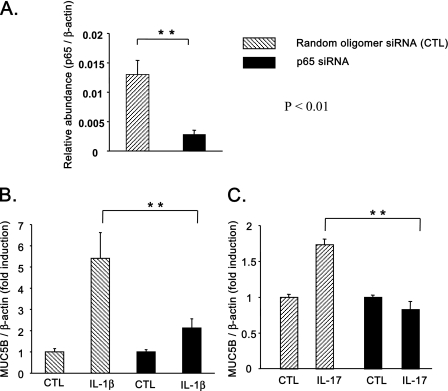
To evaluate the involvement of NF-κB in cytokine-induced MUC5B expression, an NF-κB activation inhibitor and NF-κB siRNA were used. As shown in Figures 3A and 3B, both IL-1β– and IL-17A–induced MUC5B expression was sensitive to the NF-κB activation inhibitor in NHBE cells. To further these results confirm, HBE1 cells were transfected with p65 NF-κB siRNA. As shown in Figure 4A, transfection with p65 NF-κB siRNA significantly attenuated p65 message in HBE1 cells. A similar inhibition at the protein level was seen with p65 siRNA treatment (11). p65 siRNA also attenuated IL-1β–induced MUC5B expression, compared with the negative control (Figure 4B). A similar attenuation was seen for IL-17A–induced MUC5B expression (Figure 4C). These results demonstrate that NF-κB is involved in IL-1β– and IL-17A–induced MUC5B expression.
Figure 3.
Effects of NF-κB inhibitor III on cytokine-induced MUC5B expression. NF-κB inhibitor III (20 μM) or an equal amount of vehicle (DMSO) was added 1 hour before 10 ng/ml IL-1β (A) or 20 ng/ml IL17A (B) treatment on primary NHBE cells, as described in Materials and Methods. At 12 and 24 hours after treatment, RNA samples were collected from these cultures. SYBR Green quantitative RT-PCR was used to quantify the message levels of MUC5B and GAPDH (or β-actin) in these RNA samples. Triplicate dishes were used for each time point, and experiments were repeated three times for different cultures derived from different donors. Statistically significant: *P < 0.05, **P < 0.01.
Figure 4.
Effects of p65 small interfering RNA (siRNA) on cytokine-induced MUC5B expression. HBE1 cells were treated with p65 siRNA or random oligomer (RO) siRNA as the control treatment (CTL), as described in the text. Two days after siRNA treatment, cells were starved for 6 hours, then treated with IL-1β (B) or IL-17A (C) for 16 hours. The efficiency of siRNA-p65 in reducing endogenous p65 mRNA was confirmed by performing real-time PCR (A). Relative MUC5AC message levels were averaged from triplicate dishes, and the experiment was repeated three times with HBE1 cells from different passage numbers. Statistically significant: **P < 0.01.
Deletion Analysis of Cytokine-Induced MUC5B Promoter Activity
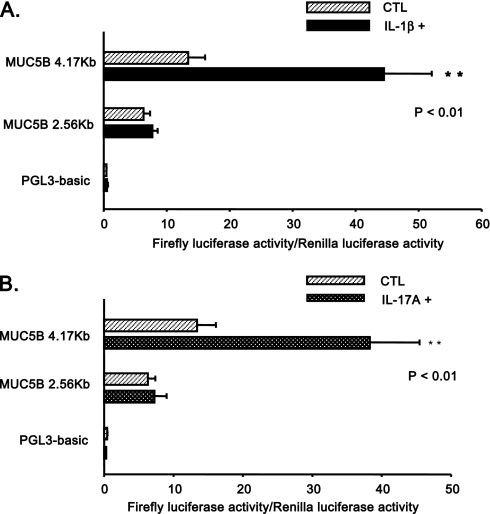
As shown in Figure 5A, IL-1β significantly increased luciferase activity of the MUC5B 4.17-kb promoter construct; however, there was no significant induction of the MUC5B 2.56-kb promoter construct. Similar results were observed for IL-17A (Figure 5B). Together, these results indicate that the region of the MUC5B promoter spanning −4.17 kb to −2.56 kb contains cis-acting element(s) that are required for both IL-1β– and IL-17A–stimulated gene expression.
Figure 5.
Deletion analysis of MUC5B promoter–reporter gene activities in response to cytokines. HBE1 cells were cotransfected with various MUC5B promoter luciferase reporter constructs and the control pRL-TK plasmid, as described in Materials and Methods. At 2 days after transfection, cells were left unstimulated (CTL) or simulated with IL-1β (A) or IL-17A (B) at the 10-ng/ml level for 16 hours. Luciferase activities in these cells were measured and normalized as described in Materials and Methods. Data from triplicate dishes were averaged and experiments performed in three independent cultures. Statistically significant: **P < 0.01 compared with the CTL case without cytokine treatment.
Demonstration of NF-κB Binding to MUC5B Promoter by ChIP Assay
To identify the putative enhancer element(s) in the MUC5B promoter, sequence analysis using MatInspector (Genomatix Software GmbH, Ann Arbor, MI) revealed three putative κB-binding sites between −4.17 kb and −2.56 kB of the MUC5B promoter (Figure 6A). These are as follows: NF-κB-1, antisense (−) 5′ gtGGGAccctcca 3′ (−3,786/−3,774); NF-κB-2, sense (+) 5′ gtGGGAggctcct 3′ (−3,173/−3,161); and NF-κB-3, sense (+) 5′ cgGGGAggtgcct 3′ (−2,921/−2,909). To determine if these putative NF-κB sites are involved in cytokine-induced MUC5B expression, a ChIP assay was performed. Because of the difficulty in obtaining consistent results with the anti-p65 antibody during ChIP analyses, an antibody to the p50 subunit of NF-κB was used for these experiments. As shown in Figure 6B, IL-1β enhanced the binding of the p50 subunit of NF-κB to the promoter region containing the NF-κB-3 site, which was detected by real-time PCR quantification. On the other hand, the binding of p50 to either the NF-κB-1 or the NF-κB-2 site was not enhanced by cytokine treatment (Figure 6B). IL-17A treatment resulted in similar p50 binding patterns (data not shown). These results confirm the presence of an NF-κB–binding element in the MUC5B promoter region, and show that cytokine treatment enhances binding of the p50 subunit of NF-κB to this region.
Figure 6.
Chromatin immunoprecipitation (ChIP) assays on p50 protein binding on MUC5B promoter region in cells after IL-1β treatment. (A) Identification of putative κB-binding sites in the 5′-flanking region of MUC5B promoter. (B) Quantitative PCR analysis of anti-p50 antibody–precipitated and control IgG antibody–precipitated chromatin from HBE1 cells treated with 10 ng/ml of IL-1β for 1 hour. Coprecipitated DNA and the corresponding input DNA before precipitation were quantified by real-time PCR analysis using primers to amplify the MUC5B promoter DNA, which contain different κB-binding sites (top, NF-κB-1; middle, NF-κB-2; bottom, NF-κB-3). Results from the quantitative real-time PCR after normalization with input control DNA template were averaged from three independent experiments. **P < 0.01 compared with non–cytokine-treated control.
DISCUSSION
In the present study, we demonstrate that NF-κB plays a role in both IL-1β– and IL-17A–induced MUC5B expression in both well differentiated primary NHBE cells and the HBE1 cell line. Both IL-1β and IL-17A stimulated MUC5B gene expression in a time- and dose-dependent manner. Attenuation of NF-κB activity by use of a specific inhibitor or treatment of the cells with a p65 siRNA both suppressed MUC5B induction by either cytokine. Using a reporter-based promoter study, we showed that enhancer elements located in the MUC5B promoter between −4.17 kb and −2.56 kb of the transcriptional start site play a critical role in IL-1β– and IL-17A–induced promoter activation. Importantly, we also provide an in vivo ChIP evidence to demonstrate an enhanced physical interaction between the NF-κB p50 subunit and the MUC5B promoter after cytokine treatment. This is the first report describing a critical role for NF-κB in transcriptional regulation of airway MUC5B expression by IL-1β and IL-17A, as well as the identification of an NF-κB–binding element in the promoter of the MUC5B gene. A similar demonstration has been recently reported for induced MUC5AC gene expression (11). For IL-1β, this is the first demonstration that IL-1β is capable of stimulating MUC5B expression in addition to MUC5AC. This is in contrast to a previous report (42), which could only demonstrate the stimulation of MUC5AC, but not MUC5B message by IL-1β. This difference may be difficult to explain due to the variation in different culture conditions among different laboratories.
We previously reported that IL-17A could stimulate MUC5B expression in primary NHBE cultures (12), and that this stimulation could be partially blocked by an anti–IL-6 receptor neutralizing antibody. This result suggests that an IL-17A–mediated IL-6 autocrine/paracrine loop could be involved in regulation of mucin gene expression. Further studies have shown that this mechanism reached a maximum stimulation at 48–72 hours (Y.C., unpublished data). In contrast, the IL-1β and IL-17A induction of MUC5B in this study is an early event that occurs within 24 hours after treatment.
NF-κB activation in airway epithelial cells plays a central role in airway inflammation (20, 43–45); however, no report has demonstrated the involvement of an NF-κB–based mechanism in cytokine-stimulated MUC5B up-regulation. Here, we show that NF-κB activation is indispensable for IL-1β– and IL-17A–induced MUC5B gene expression. Our findings indicate that NF-κB activation in airway epithelium results in airway inflammation and mucus overproduction, which are two major features of chronic airway disease, and highlight the potential clinical benefit of focused targeting on the NF-κB pathway in inflamed airways.
Few published studies have addressed involvement of NF-κB in MUC5B induction. Preciado and colleagues (21) reported that cigarette smoke could activate NF-κB and induce Muc5b expression in mouse middle ear cells, but they did not address whether or not NF-κB is directly responsible for Muc5b induction. In the present study, attenuation of NF-κB activity using both inhibitor and siRNA approaches significantly decreased IL-1β– and IL-17A–induced MUC5B gene expression in NHBE and HBE1 cells. In addition, we recently demonstrated that both IL-1β and IL-17A stimulation of HBE1 cells induced the degradation of IκB-α, and led to nuclear localization of NF-κB subunits, p50 and p65 (35). These findings provide evidence that NF-κB activation is involved in IL-1β– and IL-17A–stimulated MUC5B induction. Furthermore, using MUC5B promoter luciferase constructs, we also demonstrate that cytokine-mediated transcriptions act on cis element(s) located within the −4.17 kb to −2.56 kb region of the MUC5B promoter. Importantly, sequence analysis revealed three potential κB-binding sites located within this region. Using ChIP analysis, we demonstrated that IL-1β stimulation enhanced the binding of p50 to the region of MUC5B promoter from −3,006 to −2,851, which contains the κB-binding site (NF-κB-3 site, −2,921/−2,909). Further experiments, such as site-directed mutagenesis, should been done to verify the functionality of the NF-κB–binding site in MUC5B promoter activation.
Although a significant role for NF-κB in the transcriptional regulation of IL-1β– and IL-17A–stimulated MUC5B expression has been shown in this study, the possibility that other transcription factors are involved cannot be ruled out. Recently, we reported that specificity protein 1 (SP1) activation plays an important role in both basal MUC5B promoter activity and phorbol 12-myristate 13-acetate–induced MUC5B gene expression in NHBE cells (41). Choi and coworkers (46) reported that the cAMP response element–binding protein and a cAMP-response element site on the −956 region of the MUC5B promoter is required for 17β-estradiol–induced MUC5B expression in normal human nasal epithelial cells and NCI-H292 cells. It is possible that an intricate network of transcriptional factors is involved in the regulation of MUC5B expression under various conditions. Given the broad nature of NF-κB activation, it seems likely that other factors might be involved in the regulation of IL-1β– and IL-17A–induced MUC5B expression, which will be a topic of exploration in future studies.
In conclusion, we have found that both IL-1β and IL-17A, two prominent proinflammatory cytokines associated with chronic airway inflammation, can mediate MUC5B induction in airway epithelial cells. We further show that NF-κB activation is an essential mechanism for both IL-1β– and IL-17A–induced MUC5B expression, and we have identified the functional region (from −4.17 kb to −2.56 kb) of the MUC5B promoter that contains the NF-κB–binding site (−2,921/−2,909). As IL-1β and IL-17A have both been demonstrated as positively promoting airway inflammation in various disease states, our results are consistent with these findings, and further suggest one manner in which these cytokines contribute to the pathogenesis of airway inflammatory diseases. This study highlights the importance of NF-κB as a transcriptional regulator of mucin gene expression in airway epithelium, and may provide new strategies for controlling mucus overproduction in chronic airway diseases.
This work was supported by in part by National Institutes of Health (NIH) grants HL-77902, HL77315, and ES00628, by California Tobacco-Related Disease Research Program grant 16RT-0127), and by NIH training grant T32 HL07013 (S.V.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0313OC on October 22, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002;109:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol 2008;70:405–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WD. Lung mucus: a clinician's view. Eur Respir J 1997;10:1914–1917. [DOI] [PubMed] [Google Scholar]
- 4.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zhao YH, Di YP, Wu R. Characterization of human mucin 5b gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region. Am J Respir Cell Mol Biol 2001;25:542–553. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. Muc5b is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;178:1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Zhao YH, Wu R. In silico cloning of mouse Muc5b gene and upregulation of its expression in mouse asthma model. Am J Respir Crit Care Med 2001;164:1059–1066. [DOI] [PubMed] [Google Scholar]
- 8.Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1 beta and tumor necrosis factor–alpha induce Muc5ac overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases–MSK1-CREB activation in human airway epithelial cells. J Biol Chem 2003;278:23243–23250. [DOI] [PubMed] [Google Scholar]
- 9.Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, Suarez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol 2007;37:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujisawa T, Ide K, Holtzman MJ, Suda T, Suzuki K, Kuroishi S, Chida K, Nakamura H. Involvement of the p38 MAPK pathway in IL-13–induced mucous cell metaplasia in mouse tracheal epithelial cells. Respirology 2008;13:191–202. [DOI] [PubMed] [Google Scholar]
- 11.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1β and IL-17A: the NF-κB paradigm. J Immunol 2009;183:6236–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 2003;278:17036–17043. [DOI] [PubMed] [Google Scholar]
- 13.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999;18:6853–6866. [DOI] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell 2008;132:344–362. [DOI] [PubMed] [Google Scholar]
- 15.Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol 2008;29:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–260. [DOI] [PubMed] [Google Scholar]
- 17.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor–kappaB, in asthma. Am J Respir Crit Care Med 1998;158:1585–1592. [DOI] [PubMed] [Google Scholar]
- 18.Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, et al. Increased expression of nuclear factor–kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J 2002;20:556–563. [DOI] [PubMed] [Google Scholar]
- 19.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta–dependent genes in airway epithelium. Proc Natl Acad Sci USA 2005;102:17723–17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preciado D, Lin J, Wuertz B, Rose M. Cigarette smoke activates NF kappa B and induces Muc5b expression in mouse middle ear cells. Laryngoscope 2008;118:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R, Lim JH, Jono H, Gu XX, Kim YS, Basbaum CB, Murphy TF, Li JD. Nontypeable Haemophilus influenzae lipoprotein P6 induces Muc5ac mucin transcription via TLR2–TAK1–dependent p38 MAPK-AP1 and Ikkbeta–IkappaBalpha–NF-kappaB signaling pathways. Biochem Biophys Res Commun 2004;324:1087–1094. [DOI] [PubMed] [Google Scholar]
- 23.Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, Krause DC, Chu HW. Mycoplasma pneumoniae induces airway epithelial cell expression of Muc5ac in asthma. Eur Respir J 2008;31:43–46. [DOI] [PubMed] [Google Scholar]
- 24.Kadota J, Matsubara Y, Ishimatsu Y, Ashida M, Abe K, Shirai R, Iida K, Kawakami K, Taniguchi H, Fujii T, et al. Significance of IL-1beta and IL-1 receptor antagonist (IL-1ra) in bronchoalveolar lavage fluid (BALF) in patients with diffuse panbronchiolitis (DPB). Clin Exp Immunol 1996;103:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gessner C, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Engelmann L, Hoheisel G, Gillissen A, Sack U, Wirtz H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir Med 2005;99:1229–1240. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Tuder RM. Pathobiology of cigarette smoke–induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082. [DOI] [PubMed] [Google Scholar]
- 27.Subramaniam S, Stansberg C, Cunningham C. The interleukin 1 receptor family. Dev Comp Immunol 2004;28:415–428. [DOI] [PubMed] [Google Scholar]
- 28.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821–852. [DOI] [PubMed] [Google Scholar]
- 29.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 2001;25:335–340. [DOI] [PubMed] [Google Scholar]
- 30.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony–stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001;194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 2003;14:155–174. [DOI] [PubMed] [Google Scholar]
- 32.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001;108:430–438. [DOI] [PubMed] [Google Scholar]
- 33.Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med 2008;8:416–426. [DOI] [PubMed] [Google Scholar]
- 34.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1–dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol 2007;179:6504–6513. [DOI] [PubMed] [Google Scholar]
- 35.Kao CY, Kim C, Huang F, Wu R. Requirements for two proximal NF-kappaB binding sites and IkappaB-zeta in IL-17A–induced human beta-defensin 2 expression by conducting airway epithelium. J Biol Chem 2008;283:15309–15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 2004;173:3482–3491. [DOI] [PubMed] [Google Scholar]
- 37.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB–dependent signaling pathway. J Immunol 2005;175:6676–6685. [DOI] [PubMed] [Google Scholar]
- 38.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA Jr, Sarkadi B, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol 1993;264:C1219–C1230. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Carlson DM, St George JA, Plopper CG, Wu R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am J Respir Cell Mol Biol 1989;1:41–48. [DOI] [PubMed] [Google Scholar]
- 40.Wu YCD, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase–independent and –dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12–myristate 13–acetate. Am J Pathol 2007;170:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu DY, Wu R, Chen Y, Tarasova N, Chang MM. PMA stimulates MUC5B gene expression through an SP1-based mechanism in airway epithelial cells. Am J Respir Cell Mol Biol 2007;37:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 2004;286:320–330. [DOI] [PubMed] [Google Scholar]
- 43.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 2007;178:6504–6513. [DOI] [PubMed] [Google Scholar]
- 44.Haegens A, Barrett TF, Gell J, Shukla A, Macpherson M, Vacek P, Poynter ME, Butnor KJ, Janssen-Heininger YM, Steele C, et al. Airway epithelial NF-kappaB activation modulates asbestos-induced inflammation and mucin production in vivo. J Immunol 2007;178:1800–1808. [DOI] [PubMed] [Google Scholar]
- 45.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, et al. Nuclear factor–kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med 2008;177:959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi HJ, Chung YS, Kim HJ, Moon UY, Choi YH, Van Seuningen I, Baek SJ, Yoon HG, Yoon JH. Signal pathway of 17beta-estradiol–induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol 2009;40:168–178. [DOI] [PubMed] [Google Scholar]