It is now possible to describe—imperfectly, but in more than outline—the types of neurons by which the retina carries out its computations. This is the rough draft of a “neurome,” a conscious effort to list all the tissue's neurons. Identifying the cell populations has led directly to a revised conception of the retina's input–output functions and of the fundamental elements from which visual perception is built.
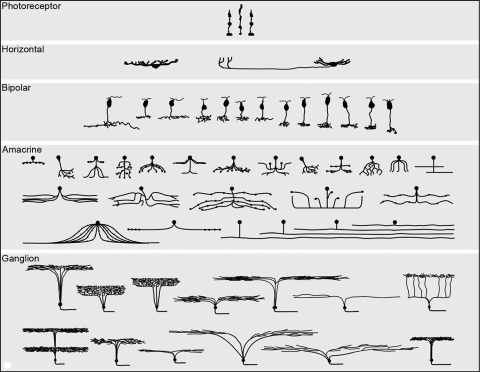
The retina is the first substantially complex structure of the central nervous system for which such a more or less complete parts list is available (Fig. 1). The resulting picture, the work of a community of investigators worldwide, resulted in a paradigm shift. A retina once imagined as based around a 6- to 12-cell module was replaced by a retina of multiple parallel arrays, containing at least 60 functionally different cell types and decomposing the visual input into more than a dozen different representations. The multiplex structure raises fundamental questions about the events that must occur later, in the central visual system.
Figure 1.
The major cell populations of a generic mammalian retina, shown semi-schematically in section view. Mammalian retinas contain in excess of 60 distinct cell types, each carrying out a distinct, dedicated function. The populations of photoreceptor, horizontal, and bipolar cells are represented quite completely. Amacrine cells are fairly complete, except that more types of wide-field amacrine cells exist than could be represented here.1 The ganglion cells shown are from a survey carried out in the retina of the rabbit. Ganglion cells vary more from species to species than other retinal cell types and there is reason to believe that, in most species, the number of ganglion cell types is at least 20. The illustration is reproduced with permission from Masland RH. The fundamental plan of the retina. Nature Neurosci. 2001;4:877–886, © Elsevier, where it is discussed in more detail.
Here, I will give a global view of the retina's cell populations, aimed, like the Proctor Lecture itself, at an audience of nonspecialist vision scientists. I will review how the cell populations were revealed and how they participate in the retina's functions. Along the way, I will point out some of the places where our concepts of the retina were overturned and comment on the next generation of questions.
Assembling a Neurome: Many Types of Retinal Neurons
We knew from the work of Cajal2 and from modern surveys by Boycott,3 Kolb4 and their colleagues that retinal neurons present themselves in a bewildering collection of shapes and sizes. Because those studies relied entirely on the fickle Golgi stain, however, there was no way to create a systematic program 5,6 and bring it to closure (Fig. 2). That was changed by technical advances—in vitro technique, immunostaining, molecular probes, and digital microscopy—that enabled unprecedented resolution and the effortless collection of large image samples.
Figure 2.
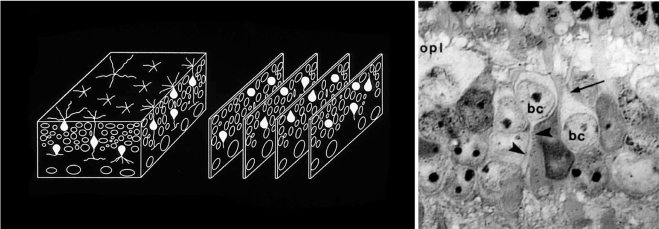
Defining the major classes of interneurons. Horizontal, Müller, bipolar, and amacrine cells are not readily distinguishable in ordinary histologic stains. To inventory the specific types of retinal neurons, it was first necessary to establish a baseline—to quantitatively describe the major classes of retinal interneurons. We devised a way to count the cells independent of any staining methodology. The inner plexiform layer was treated as a three-dimensional solid. Samples (blocks of tissue) from locations around the retina were serially sectioned, and every cell within each block was identified. The identification was made by visualizing their major processes at their exit from the cell bodies (right). Contrary to earlier belief, the retinas of different mammalian species had very similar cell populations, as shown in Figure 3. This quantitative inventory of the major cell classes could be used as a foundation on which the inventories of individual bipolar and amacrine cell populations could be built. OPL, outer plexiform layer; BC, bipolar cell; arrow, bipolar cell dendrite; arrowhead, bipolar cell axon. Left: Adapted from Masland RH. Neuronal diversity in the retina. Current Opin Neurobiol. 2001;11:431–436. © Elsevier. Right: Adapted from Strettoi E, Masland RH. The organization of the inner nuclear layer of the rabbit retina. J Neurosci. 1995;15:875–888. ©Society for Neuroscience.
Photoreceptor Cells
The first cells to be well described were the rod and cone photoreceptors, easy to distinguish as broad classes by morphology and as functional types by immunostaining or in situ hybridization, using the proteins associated with their light-sensing functions as a primary guide. A generic mammalian retina contains a single type of rod photoreceptor and two types of cone photoreceptor: one sensitive to short wavelengths and one sensitive to long ones. By comparing the outputs of these two cones, the animal can locate the wavelength of a stimulus along the spectral continuum between them. This is the basic form of mammalian color vision.
Mammalian retinas follow a single biological plan, even to the proportions of horizontal, amacrine, and bipolar cells (Fig. 3). For obvious reasons, much attention is given to the human retina, but in the big picture, primate retinas are odd: They contain a highly specialized fovea, and very late in evolution they evolved a third cone opsin, improving their discrimination between long wavelengths. Otherwise, however, they follow the generic plan, and in this article I will focus on that plan.
Figure 3.
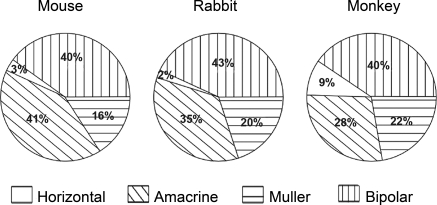
The populations of retinal interneurons in the mouse, rabbit, and monkey.7 Data for the mouse and the rabbit were obtained using the serial reconstruction method shown in Figure 2. Data from the monkey are from Martin and Grünert.8 The comparison is made for regions of retina in the three species where the density of cones is similar—from the central retina in the rabbit and mouse and the midperiphery in the monkey. Horizontal cells in monkeys are somewhat more numerous (they are also smaller in lateral extent) than are horizontal cells in mice and rabbits. What is most striking about the comparison among the three species, however, is their overall similarity.
Rods and cones can easily be recognized in wholemounts by differential interference contrast microscopy, by immunostaining of the short wavelength–sensitive opsin, by the cones' binding of a specific peanut lectin, and even by imaging in living human eyes.9,10 This advance has allowed analysis of their spatial distributions in the retinas of various species. The rods and cones are distributed across the retina in differing patterns. Animals with very mobile eyes, such as carnivores and primates, may have a central region of high cone density; crepuscular animals like rabbits often have a cone-rich region aligned to the horizon; and mice have very little regional specialization at all. It is worth noting that the density of ganglion cells usually mirrors the distribution of cones, which in turn reflects the amount of cortical space devoted to that region of the retina.11 Thus, the cone distribution reflects the importance to the brain—and to the visual life of the animal—of a particular region of the visual world. Whether or not the animal foveates images of interest (as do carnivores and primates), the principle is the same.
The two chromatic types of cones are poorly distinguishable by shape alone, but they can be distinguished by the short- and long-wavelength–sensitive opsins that impart their different spectral sensitivities. They thus can be mapped across the retina.12–14 Once again, there are variations in the spectral distributions of cone types that may depend on the animal's living habitat.15,16 Mice and rabbits, for example, have a blue-cone–rich area in the inferior retina, corresponding to the upper visual field. A popular speculation is that these animals, which make few eye movements, profit by devoting a greater fraction of their inferior retinal cones to the wavelengths dominant in the sky. Why this does not happen in other ground-dwelling mammals is not clear.17 These variations are fun to speculate about—but dangerous, too: Similar ecological thinking led to the widespread but false belief that the Rod was the evolutionarily primordial photoreceptor, when in fact the cone came first.
Horizontal Cells
As cell populations, horizontal cells are equally simple. Although much remains to be learned about their physiology, most mammals have only two types of horizontal cells.18 In one case (type A cell) a relatively simple, radial dendritic arbor reaches outward to the axonal terminals of the cones. The horizontal cell receives an excitatory synaptic input from the cones and makes an inhibitory output back onto the cones. The second type of horizontal cell (type B cell) is structurally more complex. It has two separate dendritic arbors, connected by a long, thin, connecting process. One dendritic arbor—the one associated with the cell body and nucleus—is associated, like the first type, with the synaptic terminals of the cone photoreceptor cells. The other, at the remote end of the long connecting process, is associated with the rods. For some reason, mice, rats and gerbils have only B type horizontal cells. Horizontal cells, like the rods and cones, are readily immunostained, and they have been quantified and mapped in several mammalian species. (The distributions of many retinal neurons can be found on an excellent web archive.19)
Much was once made of the possibility that horizontal cells contribute to edge enhancement—to sharpening the visual image at its edges. Their function can be imagined in this way, but other formulations are also possible, and it may be that “edge enhancement” is in fact an epiphenomenon of a different task. Indeed, the simplicity of the horizontal cell populations and their synaptic connectivity makes them attractive for formal analysis (see Refs. 20, 21), using other concepts based in signal processing. For example, a different formulation of the horizontal cells' role is that they adjust the gain of the retina's input: They measure the average brightness within a local region and then subtract a proportional value from the output of the rods and cones. This can be imagined as a local form of light adaptation. Its effect is to prevent the saturation of the retina's response range in regions where there is a locally bright object. Photographers will instantly recognize this as the difficulty encountered when photographing faces in a room that also contains windows to the outdoors. In such a situation the range of brightness exceeds the range of most cameras. If one turns down the camera's sensitivity, faces in the room are underexposed and cannot be recognized; if one adjusts the sensitivity to a level that allows interior detail to be visible, the image of the window is saturated, and it appears only as an undifferentiated white rectangle. In the retina, horizontal cell feedback locally turns down the sensitivity—but only locally—so that the window and the faces can be seen at the same time. It is like “flattening” the pixel values in a simple image-processing program, except that the bright pixels are compressed only in the visual region where it is needed, not throughout the whole scene.
Bipolar Cells
The earliest electrophysiological recordings from bipolar cells showed at least four functional responses to light: some cells responded at light onset, others at offset, some transiently, others more sustainedly.22,23 It was learned during the 1970s that ON bipolar cells arborize in the inner part of the inner plexiform layer and OFF bipolar cells in the outer,24,25 thus establishing right away that there is a correlation between the structural organization of the inner plexiform layer and its processing of information. Important studies in the cat retina showed that bipolar cells could be grouped into physically quantifiable types.26,27 Other early studies detailed the populations of bipolar cells in the rat and monkey retinas. It was learned that mammalian retinas contain a single type of rod bipolar cell, a finding that has been confirmed many times over. This work also established the fundamental principles of their connectivity with cones, reviewed in a previous Proctor Lecture.28 These studies classified cone bipolar cells into six to nine types.
A more advanced classification was undertaken in the rabbit retina.29 It was based on bipolar cell images from three independent sources. The first was a population of cells that had been injected with Lucifer yellow. These images were available from a study30 in which the cells were characterized electrophysiologically. Second was an archive of Golgi-stained material. The third was a sample of photofilled cells (see below). Image stacks were created of the Golgi-stained bipolar cells and the photofilled cells, thus generating three-dimensional representations that allowed systematic analysis of the depth of their stratification. The Golgi stain reveals cells with unparalleled contrast and detail, but has the variable sampling properties that plague that method. The photofilled cells yield images of lower contrast but a more reliably quantitative sample of the population. The physiological characterization gave an additional, entirely independent basis for classifying a neuron: the nature of its response to light. The bipolar cells of the rabbit were divided into 1 rod bipolar cell and 12 types of cone bipolar cells (Fig. 4).
Figure 4.
The types of bipolar cells in the rabbit retina. A more recent study in the mouse retina,31 using XFP-expressing transgenic animal strains and immunostaining to distinguish the bipolar cell types, also arrived at 12 types of cone bipolar cells. These are drawings of individual Golgi-stained cells. They are representative of a much larger sample. The 12 types of cone bipolars illustrated here (and one rod bipolar) were classified by study of a large population of cells stained by the photofilling method, by Golgi staining, and by injection with Lucifer yellow after recording.29 Most have narrow dendritic and axonal fields, but one (wide-field Cba2) contacts the sparse blue cones exclusively32 and has correspondingly wide arbors. The naming of the cells is not definitive; it is likely that the final nomenclature will use either the names of the cells' molecular markers or a simple numbering.31
In a tour de force of modern anatomic technique, Wässle and colleagues31 have recently studied the bipolar cells of the mouse, identifying cell types by a combination of guided microinjection, immunostaining, and transgenic strains with cell-type–specific expression of marker proteins. They found one type of rod-driven bipolar cell, and in their new categorization they also found 12 types that receive inputs primarily from cones. They added a final, critical step, which was to estimate the numbers of each of the bipolar cell types. They could do this because many of their cell types were stained as whole populations; the individual cell types could then be added up and compared to the total number of bipolar cells that had been counted in serial section reconstructions (Fig. 2) of the inner nuclear layer.4 The identified individual cell types correctly added up to the known total number of bipolar cells. This result confirms that the catalog of bipolar cell types in the mouse retina is essentially complete.
Each of these types of bipolar cells appears to report a different facet of the cone's output to the inner retina. Some of these are known: ON versus OFF, sustained versus transient, and the blue-ON cell, which selectively contacts short-wavelength cones.33,34 Other types of bipolar cell remain less well understood, although interesting studies show hints of diversity and classifications have been made.30,35 There are now techniques for recording from an identified bipolar cell and a specific individual rod or cone that drives it. These powerful methods show great promise for sorting out the functional types of bipolar cells.36
Why is it biologically necessary for the output of a cone to be decomposed into a dozen separate bipolar cell channels? The occasional response is that this is because of the informational load: The bandwidth of a cone's output may be too great for a single bipolar cell to handle, so that the signal must be split among several of them. However, the combined information can evidently be transmitted along the length of a cone photoreceptor cell, so why not along a bipolar cell? Both are short, fat neurons, and both use ribbon synapses at their outputs. A more appealing possibility is that the segregation is needed for establishing connectivity in the inner retina. The different channels (bipolar cell axon terminals) are routed to different strata of the inner plexiform layer, where they make contact with precise sets of postsynaptic partners. Perhaps there is an economy of wiring in creating a physical separation—a rigorous laminar organization—of the different types of information that ultimately will be used to create different physiologies in the different types of retinal ganglion cells.
Amacrine Cells
Amacrine cells (Fig. 5) are the most diverse population of interneurons in the retina. In the rabbit, so far the best studied species, they range from the tiny “narrow S1” cell, with a lateral spread of only 40 μm, to wide-field amacrine cells that cover many millimeters—a substantial fraction of the whole retina.37–39 Partly because they pose such an obvious challenge, they were the first retinal interneuron for which a systematic definition of the whole cell population was attempted.
Figure 5.
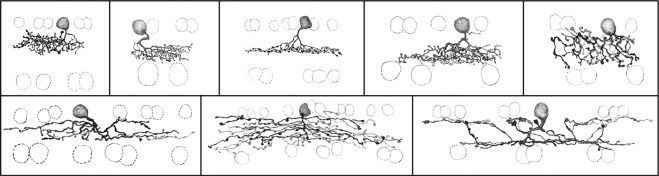
Some of the types of amacrine cell present in the rabbit retina. This is a subset of the total population of amacrine cells distinguishable in photofilling and Golgi stains. In the initial study, 29 types of amacrine cell were identified.37,38 Later work62 indicated that the number of types of wide-field amacrine cells had been underestimated, because a small number of wide-field cells of any one type can still blanket the retinal surface completely, and they are thus rarely encountered by any sampling method. Note that many of the amacrine cells shown here violate the division of the inner plexiform layer into ON (inner) and OFF (outer) halves: They carry ON information to the OFF cells, and OFF information to the ON cells.
The puzzle came into focus in the mid-1990s. At that point the implementation of immunocytochemistry and other ways of creating cell-type–specific markers had led to the descriptions of a half dozen types of amacrine cells.40 Even then the cells were flagrant in their diversity, from the small and numerous AII, stained for the Ca2+-binding protein parvalbumin, to the enormous but starkly sparse dopaminergic cells, stained for tyrosine hydroxylase, which can stretch most of the way across the retina. These cells were uncovered mainly by chance—by fortuitous stumbling on cell types that uniquely express candidate molecules (usually neurotransmitter-related proteins) that allow them to be selectively stained. At the time, this was a big advance, because once markers were available, the cells could be described with unprecedented precision.
But it was also frustrating, because there was no way to know how many amacrine cells there were for which no marker existed—how many were simply invisible to the available panel of staining methods. It was clear that unstained amacrine cells existed, but there was no way to know if they were many or few, if the known cells represented a large fraction of the total or a small one. The reason was that it is not obvious in ordinary nuclear stains what is an amacrine cell and what is a bipolar or Müller cell. To know how many members of the amacrine cell population had been identified, we needed first to learn the exact number of the retina's amacrine, bipolar, Müller, and horizontal cells.
This accounting was accomplished by a brute-force method in which the retina was approached as a three-dimensional solid, sectioned serially, and the class of every neuron in blocks of the inner nuclear layer was identified.5,6 Our collaborator Enrica Strettoi devised a staining methodology that allowed very-high-resolution light microscopic images of the cells in semithin sections—enough so that the processes of individual cells could be visualized as they passed from the somata into the plexiform layers. These images allowed identification of the major cell classes from their root definitions, independent of any type of selective staining (Fig. 2). The answer was shocking: We and others had believed that major progress had been made, yet the amacrine cells thus far identified represented only 22% of the total amacrine cells that exist.41
How to identify the remaining amacrine cells? While one possibility would have been to wait for cell-type–specific amacrine cell markers, it was not clear when they would become available. (As it happens, it would have been a long wait—they are only now beginning to appear, 15 years later.) Instead, we used pure morphology to define the cells. This is not as “soft” a method as some might think. The shapes of neuronal arbors are not just a matter of esthetics. The cells have the shapes that they have because of the connections that they have: each pattern of neural connectivity creates a distinct neuronal arbor in the connected cells. In Raviola's phrase,42 the shapes are the visible expression of the synaptic connections.
To sample the population of amacrine cells, we sought a way to collect an unbiased sample of amacrine cell images. The method finally devised was a photochemical one in which optically irradiating a single neuron caused a diffusible marker to be generated within the cell.34,35 Collecting an image stack through the cell allowed a three-dimensional view, including, critically, the level at which its dendrites arborize. In our initial survey, we found 29 distinguishable types of amacrine cell. Surprisingly, the cells were distributed rather evenly among the 29 types. We had expected a few major types surrounded by a cast of minor players. Instead, we found no predominant types at all. To be sure, wide-field cells are less numerous than narrow-field, but the most numerous amacrine cell made up 12% of the total population and the second only 4%.
The functions of amacrine cells, as much as they are known, are as diverse as their structures. Amacrine AII, the most numerous, is a narrow-field cell that serves as a link between the rod system and the rest of the retina. It allows the late-evolving rods to piggyback on the specialized circuitry that had already evolved for the cones, cone bipolars, and ganglion cells.43,44 At the opposite extreme, the sparse dopaminergic cells are neuromodulators, releasing dopamine in a paracrine fashion to control some of the activities across the retina in light and darkness. (Interestingly, they also modulate neurotransmission by more conventional GABA-ergic synapses on amacrine AII45). In contrast, the starburst amacrine cells do a precise job: They create direction selectivity in one type of retinal ganglion cell.46,47 As will be discussed later, other wide-field amacrine cells create contextual effects on the retina's output. But the functions of most of the amacrine cells are unknown. What is clear from the known examples is that no generalization will encompass them all. They carry out unique functions in microcircuits that are built for specific tasks.
Ganglion Cells
The congruence of ganglion cell morphology and functional type was established long ago, in classic studies of the morphologically distinctive α- and β-cells in the cat. These are associated with distinctive physiological codings of the visual input, establishing a core principle that has seen no exception to this day: distinct dendritic structure means distinct physiological function.48–50 All early investigators noted the presence of many other types of retinal ganglion cell in the cat (which at the time was the most popular species for electrophysiological study.) Although they make up ∼50% of the total ganglion cell population, these other types have tended to be played down in later accounts—especially physiological studies concentrating on the grating responses of the cells—and it remained for a series of precisely detailed studies by Berson and his colleagues51 to convincingly document the presence of at least 12 other cell types in the cat retina. Remarkably, the physiological characteristics of these “nonclassic” cell types are only beginning to be understood. Similarly, the monkey retina was once thought to be populated almost entirely by one small (midget) and one larger (parasol) cell type. Even in the central fovea, this is unlikely to be correct, and for most of the retina, these are now known to be accompanied by at least a dozen other types of retinal ganglion cells,52 with most researchers estimating the total as closer to 20.
Ganglion cells are the least distinctive retinal neurons in terms of biochemical markers, and their dendritic architectures are dramatically variable from species to species—a variation not seen in the outer retinal neurons. This has made definitive taxonomies of ganglion cell types a challenge. In the mouse, the availability of transgenic, cell-type-specific animals is now accelerating progress.53–55 A combined anatomic and physiological study in the rabbit estimated about a dozen types and showed directly that the different morphologies correspond to different physiologies.56 A multitechnique anatomic survey of ganglion cell types in the rabbit found 12 morphologic types plus a group that could not be classified. As will be discussed later, several laboratories find comparable numbers of cell types in the mouse and there is now reason to think that this estimate—in the mouse and probably in the other mammals—is too low. Despite these difficulties in arriving at the exact number, the overall design is unambiguous: Mammalian retinas contain at least a dozen functional types of retinal ganglion cell, each telling the brain something different about any given point in the visual scene.
Discovery Science: Answers to Questions You Did Not Know to Ask
In the 1980s, Sydney Brenner58 and his colleagues painstakingly reconstructed from several thousand serial electron micrographs the entire nervous system of Caenorhabditis elegans, expecting the hard-won results to help explain the behavior of this simple creature. As it turned out, the results did not lead to revelations about worm behavior, and critics expecting breakthroughs considered this a piece of very bad luck. Later, though, its more fundamental value became clear. As one of the participants remarked, “Ask the less flashy question: imagine what it would be like to study the neurobiology of C. elegans today without having the map of its nervous system.” Similarly, the retinal neurome informs every experiment on how the retina works.
I have already alluded to the first and biggest surprise, which was the enormous diversity of neuronal types and the decomposition of the visual image that they subserve. But structure has also taught very specific lessons, some of which reform the textbook view of the retina in precise ways. An example is the unimportance of horizontal cells for the processing of image shape. Despite their prominent place in the canonical diagram of the retina's circuitry—an example is shown in Figure 6 (left)—horizontal cells are numerically rare. They are vastly outnumbered by bipolar and amacrine cells (Fig. 6, right). As mentioned earlier, the textbook view attributes to horizontal cells the generation of edge enhancement, a phenomenon easily demonstrated perceptually and long ago recognized as the “lateral inhibition” seen in classic studies of Limulus. Since horizontal cells have a wider lateral spread than do photoreceptor or bipolar cells, it is likely that they do have that net effect. However, the mechanism of horizontal cell feedback remains unclear59–61 and it is likely that, when scenes less impoverished than laboratory gratings or dots are concerned, horizontal cells are minor players compared with those of the inner retina, where massive laterally directed circuits and dozens of laterally directed cell types are available.
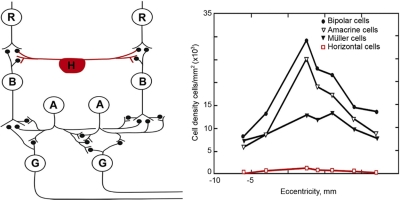
Figure 6.
Despite their prominent place in the textbook representation of the retina, horizontal cells are a numerically insignificant fraction of the retina's total interneurons. Left: this classic drawing (courtesy of Dr. John Dowling) accurately conveys the main types of retinal neuron and their synaptic interrelations. What this type of drawing cannot show is the relative number of the cells, which adds an additional dimension to our understanding. The counts are shown at right, from serial reconstructions as shown in Figure 2 and 3. The lateral connections of the retina are dominated quantitatively by amacrine cells. R, photoreceptor cell; H, horizontal cell; B, bipolar cell; A, amacrine cell; G, ganglion cell.
Indeed, another surprise was the plethora of wide-field amacrine cells—not only their numbers but their breathtaking span across the retina.39,45,62 These cells reach far beyond the boundaries of any ganglion cell's sampling region and carry out functions far more sophisticated than the simple “shift” effects seen with primitive stimuli.63 In the mouse retina, 16 distinct types of wide-field amacrine cell have been identified—“types” here defined as cells having different levels of stratification, patterns of dendrites and axons, or both.62 Their functions remain to be studied in detail, but it is already clear from physiological experiments.64–66 that the response of a ganglion cell to a local stimulus is heavily influenced by the surrounding visual context.
Another dogma destroyed was that the inner plexiform layer is rigorously divided into separate ON and OFF halves: many amacrine cell arbors cross that previously sacred border (see Fig. 5). Because the distances are electronically short, it is unavoidable that they carry ON information into the OFF layer and OFF information into the ON layer. This mixing of the channels—recently termed “crossover inhibition”—could in principle generate a variety of useful computations,67,68 although concrete examples are still few.
In the end, the lesson is that the retina more resembles an image processor—Adobe Photoshop or the like—than it does a camera (Fig. 7). We have barely scratched the surface of the computations carried out in the inner plexiform layer. Because these are local, spatially restricted, microcircuits, they are especially amenable to electrophysiological attack.1,7,68,69 In this, powerful aid is coming from genetically modified mice, which allow the experimenter repeatably to target the same cell or pairs of cells, use viruses to trace their connections, and alter the cells' functions in controlled ways. Because of these new tools, the microcircuits of the inner retina are now solvable problems.70–74
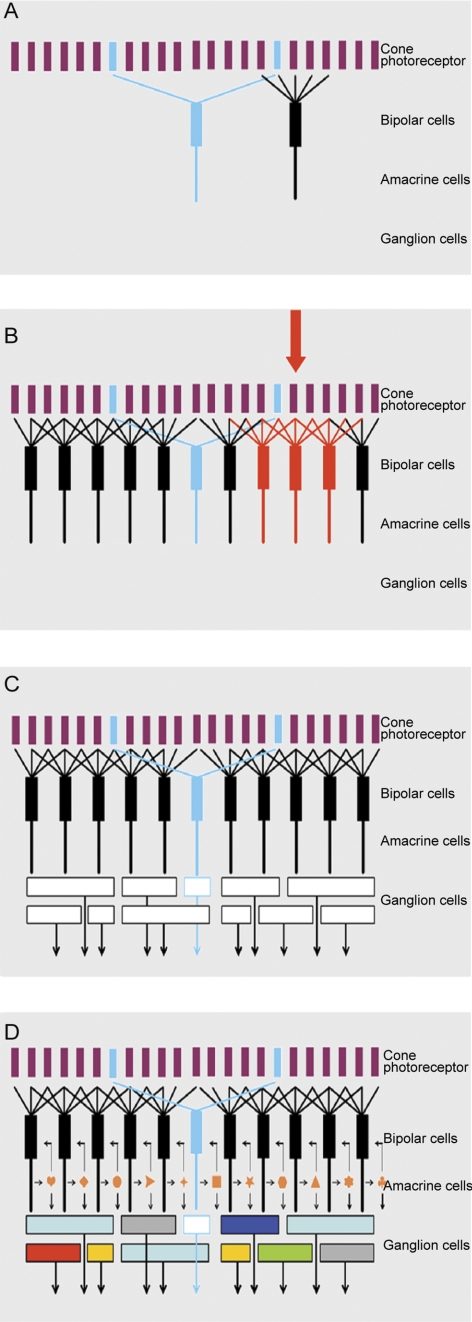
Figure 7A.
The four panels of this figure show exploded views of the retina's circuitry. Here, the mosaic of cone photoreceptors is shown at the top. (The late-evolving rod system is not shown, as it is a minor part of the retina's circuitry, and is used only in deep darkness.) The mosaic of cone photoreceptors is sampled by the “diffuse” bipolar cells (black), which receive input from all the cones within their reach, and by a small group of “blue bipolars,” which selectively contact the infrequent blue cones. This selectivity preserves the chromatic purity of the responses of the blue bipolar cells. Later retinal circuitry (not shown) compares the output of the blue (short wavelength) cones to that of the long wavelength cones. This comparison is the fundamental basis of mammalian color vision.
Figure 7B. The diffuse bipolar cells are far more numerous than the blue bipolars and come in 11 functional types, each transmitting a different component of the cone's output. Stimulation of an individual cone (red arrow) transmits information to all the bipolar cells that contact it (red bipolar cells in the illustration). In this case the red bipolar cells would all be of one functional type; for example, all of them would be ON-transient bipolars. But that same (arrow) cone would also be contacted by bipolar cells of the other 10 types, carrying different components of the cone's output. The whole array cannot be shown in a planar diagram like this one—there is not enough space for all the bipolar cells in a single plane, and 12 different colors of bipolar cell would be required.
Figure 7C. The different types of bipolar cells synapse on different types of ganglion cells. Since each type of bipolar cell conveys a different type of information to the inner retina, each conveys its own particular version of the visual input to the ganglion cell(s) on which it synapses. This is the initial step in the creation of ganglion cell diversity. In the example, the blue bipolar cell synapses on a specific type of ganglion cell, which then becomes a blue-sensitive ganglion cell. The same is true for each of the other bipolar cell types. If this were the whole story, the organization of the inner retina would be relatively simple. There might still be 12 (or more) functional types of ganglion cells, but the bipolar cells would provide the only drive to the ganglion cells, so that the ganglion cell response would be determined purely by the response tuning of the bipolar cell, the physiology of the bipolar cell synapse and the ganglion cell's ion channels.
Figure 7D. The final responses of the ganglion cells are highly selective: Some respond only to a particular direction of stimulus movement, some specifically to low or to high contrast, some specifically to rapid movement, and so on. This final diversity is created by the action of amacrine cells. Amacrine cells receive a direct excitatory input from bipolar cells, and they make inhibitory synaptic inputs back onto the axon terminals of the bipolar cells. This is a classic feedback system, creating a control point at the bottleneck where information enters the inner retina from the outer retina. However, amacrine cells also synapse on each other; and they feed forward on retinal ganglion cells. These feed-forward synapses are responsible for some of the more interesting properties of the ganglion cell response. A famous case is the starburst amacrine cell, which creates direction selectivity in one type of retinal ganglion cell.46,47 Not shown are the wide-field amacrine cells, which can span the whole width of the retinal surface.
Questions for the Future
There is no doubt that the overall shape of the “retina neurome”—more than 60 types of neurons, arrayed into at least a dozen functional modules—is correct, but the list of cell types shown in Figure 1 is, like the human genome, a first draft. Refinements will occur and imprecise places will be rendered exact. Where is the job of structural description essentially done, and where is it more of a work in progress? In one domain, the job of structural description is of course far from done. The emerging connectome project aspires to describe not only all the cell types but all of their connections.75–78 Even for the connectome, however, a medium-scale description—classification at the level of whole cells—is still critical, and this is the scale that finds the greatest usefulness for physiological and genetic analyses. What pressing questions remain?
As cell populations, the photoreceptors, horizontal cells, and bipolar cells are now well defined.19 There are a great many unresolved questions about their functions. A few examples: Are there mixed rod and cone inputs to bipolar cells in some species, and if so, why? What is the interneuronal circuitry of the red and green cones in primates? What is the synaptic mechanism of horizontal cell feedback? For bipolar cells, the great issue is not to define the cell populations structurally, but to understand their physiology. What are the molecular signatures of the bipolar cells, and what information does each type convey from the outer retina to the inner? But the identities of the players are known.
Amacrine cells are more complex and the description of the amacrine cell population less complete. For rabbit amacrine cells (Figs. 1, 8) the photofilling technique is quantitative, in the sense that the recovery rate for targeted cells was 94%. When compared with known amacrine cell populations, the sample turned out to be accurate: Starburst cells are known to comprise 3% of the total amacrine population and were 5% of the photofilled sample; AII cells 11% and 13%; IAC 2% and 4%; DAPI-3 cells 3% and 3%. These fiducial cells span the range of morphologies and stratification of rabbit amacrine cells, yielding some confidence that the method accurately samples the existing population of small and medium field amacrine cells.
Figure 8.
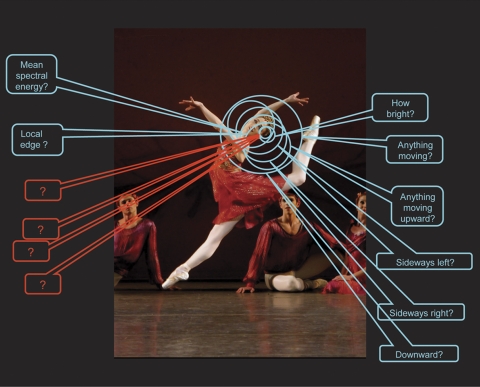
The retina interrogates any point in visual space about a number of distinct qualities. Retinal ganglion cells tile the retinal surface: Each of the ∼20 distinct types of retinal ganglion cell covers the retinal surface uniformly, and this mosaic is independent of the mosaics of the other 19 types.57 Thus, at every point on the retina at least one example of each of the 20 ganglion cell types is present. Correspondingly, a point in visual space is surveyed by at least one of each of the types of retinal ganglion cell, each reporting to the brain a different feature of the visual world. In this example, we consider a small region on the trailing side of the dancer's head. Different retinal ganglion cells report on whether there is, within that area of space, movement, movement in a particular direction, an object of a particular spectral composition, among other reportings. The nature of eight of these reportings are known (there are eight well-characterized types of ganglion cell in the rabbit retina, on which the illustration is based). Many more are known to exist, from the presence of structurally distinct ganglion cell types, but their physiology remains to be characterized. From structural evidence, the number of encodings appears to be similar in all mammalian retinas. Image: New York City Ballet's Ashley Bouder in Jerome Robbins' “The Four Seasons.” Photo credit: Paul Kolnik.
But that study had two notable weaknesses. First, because amacrine cells are distributed among at least 29 types, the number of examples of any one type was very small. When dividing types based on small numbers of examples, the lines that separate the types may occasionally have been incorrectly drawn. And even when the major features are clearly correct—as usually turned out to be the case when the cells could be visualized more clearly in the Golgi material—the details will surely be refined as methods for marking particular cell types become available. An example is the “fountain” cell, so named because of the distinctive recurving pattern of its dendrites.37,38 This cell type was recognized and its main features correctly described from a sample of only five photofilled examples. Fortunately, the basic description was confirmed in a later study74 in which a large number of the cells were injected, providing higher quality micrographs and a more precise description of the course of the dendrites. As molecular markers become available for more amacrine cells, this level of detail should be achieved for most cell types and for species other than the rabbit.
Second, even a large sample will encounter few of the retina's wide-field amacrine cells. The cells spread so widely that the retinas's surface can be effectively blanketed using small absolute numbers of cells; any sampling technique will encounter them rarely. They are seen by a few specialized methods,45,49 and in the transgenic mouse GFP-M,62,80 whose Thy-1 promoter favors expression of GFP in wide-field amacrine cells (as well as the ganglion cells for which this mouse strain is better known.) They are present in most or all levels of the inner plexiform layer and thus affect many kinds of retinal computation. As already noted, electrophysiological studies have borne this out, revealing a variety of response modulations from outside the classic receptive field. It is clear that the retina's response to any object will be affected by the visual context on which that object appears—but there is at present no way systematically to ensure that these infrequent amacrine cells have been adequately surveyed.
Retinal ganglion cells present even greater difficulties, not the least of which is that there are major variations from species to species. This contrasts with the other retinal cell classes: Some retinas have cone-rich regions centrally, but a cone is generally recognizable as a cone no matter where it occurs. Horizontal cells in monkeys have a narrower spatial extent than horizontal cells in rabbits or cats, but they are plainly the same functional element. As far as is known, the same goes for variations in the shapes of bipolar cells. Remarkably, the four classic, easy-to-stain amacrine cells (AII, starburst, A17, and TH) could hardly be more different in morphology, and each has a distinctive connectivity; yet these are conserved in the retinas of mice, rats, rabbits, cats, monkeys, and humans.
At the level of the ganglion cells, this pattern of conservation breaks down, and significant species differences appear. The central principle remains the same: Retinal ganglion cells in any mammalian species come in more than a dozen anatomic and physiological types, and thus send more than a dozen different representations of the visual input to the brain. But the shapes, and apparently the functions, of retinal ganglion cells vary widely. Decades of argument have failed to resolve even the apparently simple question of homology (or not) between the small (β/midget) and large (α/parasol) ganglion cells of cats and monkeys. The monkey, previously touted as a paragon of ganglion cell simplicity, possesses at least a dozen easily distinguishable ganglion cell types and probably more.52 In the mouse, much studied for its genetic advantages, the number appears close to 20; there is agreement on many of the cell types, but an agreed-on taxonomy remains elusive.81–84
Why is this a critical problem? The answer, of course, is that the outputs of the retinal ganglion cells are the building blocks of visual perception (Fig. 8). If each point in the visual scene is reported to the higher brain centers by 20 different representations, the brain must use that information in some way—yet textbook concepts of central visual function give no acknowledgment to their existence. A canonical statement of the problem is given by the “smooth cell,” a type of retinal ganglion cell in the macaque monkey meticulously studied by Crook and her colleagues.85,86 This cell has a physiology indistinguishable (to standard testing) from the classic α/Y/parasol cell, being particularly sensitive to stimuli that flash or move. And yet it is clearly a different cell: (1) The smooth cell is instantly distinguishable from parasol cells in dendritic morphology, (2) it has twice the dendritic field diameter of a parasol cell, and (3) it tiles the retina with a uniform mosaic independent of the mosaic of parasol cells. Thus, the smooth cells send to the brain a coding of the visual input similar to that of the parasol cells, but each smooth cell reports on a region of visual space approximately four times as big as that sampled by a parasol cell. The smooth cells project to the lateral geniculate body, the way station to the cortex. Why does the cortex need to view the world through two different-sized samplings of the same features? Is there some other difference in the encoding transmitted by the smooth cell—something not revealed by testing with standard grating stimuli? And how do these separate representations combine to create visual perception?
To understand what kind of information is carried by each of the diverse ganglion cell types is a very hard problem,87 a challenge for even the most thoughtful contemporary students of sensory coding.88–91 Anatomic structure cannot answer these questions. But it can, together with molecular tools, make a contribution, which is to limit the universe of possibilities: it can specify, in a concrete and unchangeable way, how many channels electrophysiologists should seek, and a definitive structural classification of retinal ganglion cells is therefore a pressing goal.
Acknowledgments
I am grateful to my collaborators and co-workers, too many to name here but recognized as co-authors on the publications of our laboratory. It is these friendships that have made the work possible and the life of science enjoyable.
I owe the greatest debt to my teacher, mentor, and friend, Adelbert Ames, III, who was for more than 30 years Professor at Harvard Medical School and the Massachusetts General Hospital. Reading Del's papers inspired me to enter retina research. As a fellow in his laboratory, I learned about the cell biology of the nervous system, but also about personal conduct and the values of science. Del made important discoveries: He was the first to show, long ago, that mammalian central nervous tissue could in fact survive and function outside its ordinary protective coverings, opening the way to the multitude of reduced preparations that are the mainstay of modern neuroscience. He did important work on the formation of the cerebrospinal fluid and on the mechanism of tissue damage in stroke, work that had direct consequences for the treatment of patients. Along the way, he developed Ames Medium, widely used today for in vitro studies of mammalian CNS tissue. (Characteristically, he never expected compensation for this invention.) He is a modest person who avoids the spotlight, but he has served as a beacon of clear thinking and scientific values to all who come into contact with him at Harvard and in the wider scientific community. I owe him a great debt, and dedicate this article to him.
Footnotes
Supported over the years by the National Institutes of Health Grant EY017169, the Howard Hughes Medical Institute, and Research to Prevent Blindness.
Disclosure: R.H. Masland, None
References
- 1. Manookin MB, Weick M, Stafford BK, Demb JB. NMDA receptor contributions to visual contrast coding. Neuron. 2010;67:280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cajal SR. The Structure of the Retina. 1892 Translated by Thorpe SA, Glickstein M. Springfield, IL: Thomas; 1972 [Google Scholar]
- 3. Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Phil Trans R Soc Lond Biol B. 1969;255:109–184 [DOI] [PubMed] [Google Scholar]
- 4. Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114 [DOI] [PubMed] [Google Scholar]
- 5. Strettoi E, Masland RH. The organization of the inner nuclear layer in the rabbit retina. J Neurosci. 1995;15:875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jeon C-J, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chávez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010;30:2330–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287 [DOI] [PubMed] [Google Scholar]
- 9. Johnson LV, Hageman GS, Blanks JC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1986;27:129–135 [PubMed] [Google Scholar]
- 10. Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. J Neurosci. 2005;25:9669–9679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wässle H, Grünert U, Röhrenbeck J, Boycott BB. Cortical magnification factor and the ganglion cell density of the primate retina. Nature. 1989;341:643–646 [DOI] [PubMed] [Google Scholar]
- 12. Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci. 1990;10:3390–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roorda A, Williams DR. The arrangement of the threecone classes in the living human eye. Nature. 1999;397:520–526 [DOI] [PubMed] [Google Scholar]
- 14. Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523 [DOI] [PubMed] [Google Scholar]
- 15. Schleich CE, Vielma A, Glösmann M, Palacios AG, Peichl L. Retinal photoreceptors of two subterranean tuco-tuco species (Rodentia, Ctenomys):morphology, topography, and spectral sensitivity. J Comp Neurol. 2010;518:4001–4015 [DOI] [PubMed] [Google Scholar]
- 16. Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc Ophthalmol. 1971;30:33–159 [DOI] [PubMed] [Google Scholar]
- 17. Peichl L. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1001–1012 [DOI] [PubMed] [Google Scholar]
- 18. Boycott BB. Horizontal cells of mammalian retinae. Neurosci Res Suppl. 1988;8:S97–111 [DOI] [PubMed] [Google Scholar]
- 19. Collin SP. A database of retinal topography maps. Database at URL http://www.retinalmaps.com.au Clin Exp Optom. 2008;91:85–95 [DOI] [PubMed] [Google Scholar]
- 20. Lipin MY, Smith RG, Taylor WR. Maximizing contrast resolution in the outer retina of mammals. Biol Cybern. 2010;103:57–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Hateren H. A cellular and molecular model of response kinetics and adaptation in primate cones and horizontal cells. J Vis. 2005;5:331–347 [DOI] [PubMed] [Google Scholar]
- 22. Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, necturus maculosus. II Intracellular recording. J Neurophysiol. 1969;32:339–355 No abstract available [DOI] [PubMed] [Google Scholar]
- 23. Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970;207:623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Famiglietti EV, Jr., Kaneko A, Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198:1267–1269 [DOI] [PubMed] [Google Scholar]
- 25. Nelson R, Famiglietti EV, Kolb H. Intracellular staining reveals different levels of stratification for on-center and off-center ganglion cells in the cat retina. J Neurophysiol. 1978;4:427–483 [DOI] [PubMed] [Google Scholar]
- 26. Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Philos Trans R Soc Lond B Biol Sci. 1990;330:305–321 [DOI] [PubMed] [Google Scholar]
- 27. Cohen E, Sterling P. Convergence and divergence of cones onto bipolar cells in the central area of cat retina. Philos Trans R Soc Lond B Biol Sci. 1990;330:323–328 [DOI] [PubMed] [Google Scholar]
- 28. Boycott B, Wässle H. Parallel processing in the mammalian retina: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1999;40:1313–1327 [PubMed] [Google Scholar]
- 29. MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The population of bipolar cells in the rabbit retina. J Comp Neurol. 2004;472:73–86 [DOI] [PubMed] [Google Scholar]
- 30. McGillem GS, Dacheux RF. Rabbit cone bipolar cells: correlation of their morphologies with whole-cell recordings. Vis Neurosci. 2001;18:675–685 [DOI] [PubMed] [Google Scholar]
- 31. Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacNeil MA, Gaul PA. Biocytin wide-field bipolar cells in rabbit retina selectively contact blue ones. J Comp Neurol. 2008;506:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186 [DOI] [PubMed] [Google Scholar]
- 34. Ghosh KK, Martin PR, Grünert U. Morphological analysis of the blue cone pathway in the retina of a New World monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1997;379:211–225 [PubMed] [Google Scholar]
- 35. Wu SM. Synaptic organization of the vertebrate retina: general principles and species-specific variations: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2010;51:1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li W, Chen S, DeVries SH. A fast rod photoreceptor signaling pathway in the mammalian retina. Nat Neurosci. 2010;13:414–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982 [DOI] [PubMed] [Google Scholar]
- 38. MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326 [PubMed] [Google Scholar]
- 39. Famiglietti EV. Polyaxonal amacrine cells of rabbit retina: PA2, PA3, and PA4 cells: light and electron microscopic studies with a functional interpretation. J Comp Neurol. 1992;316:422–446 [DOI] [PubMed] [Google Scholar]
- 40. Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retinal Res. 1991;9:49–100 [Google Scholar]
- 41. Strettoi E, Masland RH. The number of unidentified amacrine cells in the mammalian retina. Proc Natl Acad Sci USA. 1996;93:14906–14911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Masland RH, Raviola E. Confronting complexity: strategies for understanding the microcircuitry of the retina. Ann Rev Neurosci. 2000;23:249–284 [DOI] [PubMed] [Google Scholar]
- 43. Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol. 1994;347:139–149 [DOI] [PubMed] [Google Scholar]
- 44. Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol. 2005;93:1476–1485 [DOI] [PubMed] [Google Scholar]
- 45. Gustincich S, Feigenspan A, Wu DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: a transgenic approach to neural networks. Neuron. 1997;18:723–736 [DOI] [PubMed] [Google Scholar]
- 46. Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852 [DOI] [PubMed] [Google Scholar]
- 47. Fried SI, Münch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron. 2005;46(1):117–127 [DOI] [PubMed] [Google Scholar]
- 48. Cleland BG, Levick WR, Wässle H. Physiological identification of a morphological class of cat retinal ganglion cells. J Physiol. 1975;248:151–171.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wässle H, Boycott BB, Illing RB. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B Biol Sci. 1981;212:177–195 [DOI] [PubMed] [Google Scholar]
- 50. Masland RH. Neuronal diversity in the retina. Current Opin Neurobiol. 2001;11:431–436 [DOI] [PubMed] [Google Scholar]
- 51. O'Brien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. J Physiol. 2002;538:787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dacey D. Origins of perception: retinal ganglion cell diversity and the creation of parallel visual pathways. In: Gazzaniga MS. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 2004 [Google Scholar]
- 53. Huberman AD, Manu M, Koch SM, et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482 [DOI] [PubMed] [Google Scholar]
- 55. Siegert S, Scherf BG, Del Punta K, Didkovsky N, Heintz N, Roska B. Genetic address book for retinal cell types. Nat Neurosci. 2009;12:1197–1204 [DOI] [PubMed] [Google Scholar]
- 56. Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587 [DOI] [PubMed] [Google Scholar]
- 57. Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proc Natl Acad Sci U S A. 2000;97:2303–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of Caenorhabditis elegans. Philos Trans R Soc Lond (Biol). 1986;14:l–340 [DOI] [PubMed] [Google Scholar]
- 59. Fahrenfort I, Klooster J, Sjoerdsma T, Kamermans M. The involvement of glutamate-gated channels in negative feedback from horizontal cells to cones. Prog Brain Res. 2005;147:219–229 [DOI] [PubMed] [Google Scholar]
- 60. Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol. 2010;518:1647–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008;28:456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin B, Masland RH. Populations of wide-field amacrine cells in the mouse retina. J Comp Neurol. 2006;499:797–809 [DOI] [PubMed] [Google Scholar]
- 63. Hamasaki DI, Maguire GW. A neural pathway for the shift response in the cat. Brain Res. 1985;337:51–58 [DOI] [PubMed] [Google Scholar]
- 64. Gollisch T, Meister M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron. 2010;65:150–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci. 2003;6:600–608 [DOI] [PubMed] [Google Scholar]
- 66. Chiao CC, Masland RH. Contextual tuning of direction-selective retinal ganglion cells. Nat Neurosci. 2003;6:1251–1252 [DOI] [PubMed] [Google Scholar]
- 67. Werblin FS. Six different roles for crossover inhibition in the retina:correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen X, Hsueh HA, Greenberg K, Werblin FS. Three forms of spatial temporal feedforward inhibition are common to different ganglion cell types in rabbit retina. J Neurophysiol. 2010;103:2618–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582:569–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boldogkoi Z, Balint K, Awatramani GB, et al. Genetically timed, activity-sensor and rainbow transsynaptic viral tools. Nat Methods. 2009;6:127–130 [DOI] [PubMed] [Google Scholar]
- 71. Margolis DJ, Gartland AJ, Euler T, Detwiler PB. Dendritic calcium signaling in ON and OFF mouse retinal ganglion cells. J Neurosci. 2010;30:7127–7138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hausselt SE, Euler T, Detwiler PB, Denk W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 2007. July;5(7):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Field GD, Gauthier JL, Sher A, et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010;467:673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lichtman JW, Sanes JR. Ome sweet ome: what can the genome tell us about the connectome? Curr Opin Neurobiol. 2008;18:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Seung HS. Reading the book of memory: sparse sampling versus dense mapping of connectomes. Neuron. 2009;62:17–29 [DOI] [PubMed] [Google Scholar]
- 78. Chklovskii DB, Vitaladevuni S, Scheffer LK. Semi-automated reconstruction of neural circuits using electron microscopy. Curr Opin Neurobiol. 2010;20:667–675 [DOI] [PubMed] [Google Scholar]
- 79. Wright LL, Vaney DI. The fountain amacrine cells of the rabbit retina. Vis Neurosci. 1999;16:1145–1156 [DOI] [PubMed] [Google Scholar]
- 80. Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51 [DOI] [PubMed] [Google Scholar]
- 81. Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351 [DOI] [PubMed] [Google Scholar]
- 82. Sun W, Li N, He S. Large-scale morphological survey of rat retinal ganglion cells. Vis Neurosci. 2002;19:483–493 [DOI] [PubMed] [Google Scholar]
- 83. Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136 [DOI] [PubMed] [Google Scholar]
- 84. Kong J-H, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol. 2005;489:293–310 [DOI] [PubMed] [Google Scholar]
- 85. Crook JD, Peterson BB, Packer OS, Robinson FR, Troy JB, Dacey DM. Y-cell receptive field and collicular projection of parasol ganglion cells in macaque monkey retina. J Neurosci. 2008;28:11277–11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Crook JD, Peterson BB, Packer OS, et al. The smooth monostratified ganglion cell: evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey. J Neurosci. 2008;28:12654–12671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Farrow K, Masland RH. Physiological Clustering of Visual Channels in the Mouse Retina. J Neurophysiol. 2011;105:1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meister M, Berry MJ., 2nd The neural code of the retina. Neuron. 1999;22:435–450 [DOI] [PubMed] [Google Scholar]
- 89. Fairhall AL, Burlingame CA, Narasimhan R, Harris RA, Puchalla JL, Berry MJ., 2nd Selectivity for multiple stimulus features in retinal ganglion cells. J Neurophysiol. 2006;96:2724–2738 [DOI] [PubMed] [Google Scholar]
- 90. Shapley R. Linear and nonlinear systems analysis of the visual system: why does it seem so linear?—a review dedicated to the memory of Henk Spekreijse. Vision Res. 2009;49:907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schwartz G, Taylor S, Fisher C, Harris R, Berry MJ., 2nd Synchronized firing among retinal ganglion cells signals motion reversal. Neuron. 2007;55:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]