The restricted overexpression of Pax6 in the mouse cornea combined with the abnormal corneal, but not lens, phenotype suggests that Pax6 is essential and functions autonomously in the cornea.
Abstract
Purpose.
To assess whether Pax6 functions directly in the cornea, a corneal-preferred promoter was used to overexpress Pax6 specifically in the cornea.
Methods.
Transgenic mice harboring a construct containing mouse Pax6 coding sequences fused downstream of the aldehyde dehydrogenase 3a1 (Aldh3a1) promoter were generated (Pax6 Tg). Pax6 expression was analyzed by Western blot and immunohistochemistry. Eye sections were stained with hematoxylin and eosin, Schiff reagent, and fluorescein, to assess morphologic changes, the presence of goblet cells, and barrier integrity, respectively. Gene expression changes in mildly affected Pax6 Tg corneas were compared to age-matched, wild-type (WT) corneas by microarray analysis and quantitative PCR. Promoter regulation of several differentially expressed genes was examined by monitoring luciferase activity of reporter constructs after cotransfection with Pax6 in COS7 cells.
Results.
Corneal overexpression of Pax6 produces an abnormal cornea with altered epithelial cell morphology, neovascularization, immune cell invasion, and a compromised barrier; the lens appeared normal. Major changes in expression of genes involved in immune function, vascularization, and epithelial differentiation occurred in corneas from Pax6 Tg versus WT mice. The keratin (K) profile was dramatically altered in the Pax6 Tg corneas, as were several components of the Wnt signaling pathway. In severely affected Pax6 Tg corneas, K12 was reduced, and Pax6 was redistributed into the cytoplasm. Promoters from the chitinase 3-like 3, Wnt inhibitory factor 1, and fms-related tyrosine kinase 1/soluble VEGF receptor genes were upregulated five-, seven-, and threefold, respectively, by Pax6 in transfected COS7 cells.
Conclusions.
Pax6 functions directly to maintain normal, corneal epithelial cells.
Normal development and maintenance of the vertebrate and invertebrate eye depends on the proper amount (dosage) of wild-type Pax6 protein. When Pax6 protein is absent, so are the eyes,1 whereas misexpression of wild-type Pax6 can result in ectopic eye formation.2–4 More subtle alterations in the levels of Pax6 also produce eye abnormalities with incomplete penetrance and variable expressivity. Heterozygous PAX6+/− humans and Pax6+/− mice and rats expressing low levels of Pax6 exhibit defects in the cornea, lens, iris, ciliary body, and retina and a reduction in eye size (in mice only).5–14 Similarly, overexpression of Pax6 by gene duplication in humans15 or by introduction of a yeast artificial chromosome (YAC)-based transgene containing the human PAX6 locus into mice results in ocular problems.16 Interestingly, some of the ocular abnormalities in Pax6-overexpressing mice are similar to those reported in the Pax6+/− mice, but some are different.
Optical clarity of the cornea must be maintained to retain good vision. The cornea consists of three compartments: an outer, multilayered, nonkeratinized squamous epithelium; a central stroma with orderly collagen lamellae populated with keratocytes; and an inner, single-layered endothelium that abuts the anterior chamber of the eye. Together with the tear film, the cornea provides a barrier to the rest of the eye against trauma, invading microorganisms, and environmental toxins. The corneal phenotype is variably affected by perturbations in ocular Pax6 levels. Mice manifest both overlapping and distinctive features, depending on whether Pax6 dosage is increased or decreased.5,12,17,18 The corneas of Pax6+/− mice exhibit a thickened stroma with inflammatory cell infiltration and neovascularization, whereas goblet cells and erosions appear in the epithelium, eventually leading to corneal opacity and serve as an excellent model for aniridia-related keratopathy, a collection of corneal changes associated with aniridia in PAX6+/− individuals.5,12 In contrast, examination of a YAC line overexpressing Pax6, Pax77, revealed transparent microcorneas without the presence of goblet cells or blood vessels and a normal-looking stroma.18 There was, however, a reduced number of corneal epithelial cell layers, an increase in proliferative markers, a reduction in keratin (K)12 expression, and an altered wound-healing response, as was shown for the Pax6+/− cornea.5,11,13,19 Further, corneal (and other ocular) phenotypes differed when the Pax77 construct was analyzed on different genetic backgrounds, implying a role for modifier genes in Pax6 function.17
How Pax6 is involved at the molecular level, directly and/or indirectly, in orchestrating a normal corneal phenotype has been the subject of several recent studies. Using patterns of keratin expression as a means of studying the progression of epithelial differentiation,20 several groups have shown that the corneal epithelial cell-specific keratin K12, is reduced in Pax6 under- and overexpressing corneas.5,14,18,19 Similarly, a downregulation of Pax6 precedes the loss of K12 when mouse corneal epithelium transdifferentiates into epidermis after culturing on dermis, presumably the result of a dermally derived Wnt activation signal,21 or when the Wnt pathway is activated through a loss of either the Wnt inhibitor Dkk2 or of Notch 1.22–24 Conversely, Pax6 has been shown to regulate Wnt signaling components in both the lens25 and the central nervous system.26
The coupled development of the lens and corneal epithelium from Pax6-expressing surface ectoderm,27 the influence of the lens on anterior segment development28–30 and the continued Pax6 expression in the lens and cornea of adult eyes31 has made it difficult to discern whether Pax6 functions directly in each of these tissues, or action in one compartment indirectly affects the other one, or both. Current strategies to analyze the effects of Pax6 levels in ocular tissues have typically altered expression in both the lens and cornea. For example, the Pax77 mice contain a promoter that replicates the spatiotemporal pattern of endogenous Pax6 gene expression in the eye, including the lens and cornea,16–18,32 whereas the conditional knockout of Pax6 using the LE-Cre promoter reduces Pax6 expression in the lens as well as the cornea.33 To address the question of whether Pax6 functions directly in the cornea, we used a cornea-preferred promoter from the Aldh3a1 gene to drive expression of Pax6.34 Overexpression of Pax6 in an FVB N genetic background results in an abnormal cornea that shares some features of the Pax6+/− mouse and the Pax77 mouse, including defective epithelial differentiation, neovascularization, and immune cell invasion. Microarray analysis of corneas from Pax6 Tg versus WT sibling mice showed major changes in the expression of genes involved in immune function, vascularization, and epithelial differentiation. Further, we showed that promoters from several of these potential Pax6 target genes are regulated by Pax6 in vitro. Taken together, these results indicate that Pax6 directly contributes to the differentiation and maintenance of the mouse cornea.
N genetic background results in an abnormal cornea that shares some features of the Pax6+/− mouse and the Pax77 mouse, including defective epithelial differentiation, neovascularization, and immune cell invasion. Microarray analysis of corneas from Pax6 Tg versus WT sibling mice showed major changes in the expression of genes involved in immune function, vascularization, and epithelial differentiation. Further, we showed that promoters from several of these potential Pax6 target genes are regulated by Pax6 in vitro. Taken together, these results indicate that Pax6 directly contributes to the differentiation and maintenance of the mouse cornea.
Material and Methods
Generation of Pax6 Tg Mice
A transgenic DNA construct was made by fusing a 4.5-kb mouse Aldh3a1 promoter to the coding region of the mouse Pax6 gene. A plasmid DNA containing the entire Pax6 coding region was used as a template in a semiquantitative, reverse-transcriptase (RT) polymerase chain reaction (PCR) with top-strand (TS) 9421Pax6 (5′GGCCGTCGACATGCAGAACAGTCACAGCGGAG3′) and bottom-strand (BS) 9474Pax6 (5′GGCCTCTAGATTACTGTAATCGAGGCCAGTAC3′) primers for 35 cycles in a thermocycler. The amplified, 1319-bp product was gel purified, restricted with SalI and XbaI, and incubated in a three-way ligation reaction with a 4.5-kb HindIII/SalI Aldh3a1 promoter fragment,34 and the pCAT basic vector restricted with HindIII and XbaI. The points of insertion and the entire Pax6 coding region were sequenced. A 7592-bp HindIII/PvuI linear DNA fragment containing the Aldh3a1 promoter linked to the Pax6 coding region was purified and injected into FVB N two-cell blastula to generate the transgenic mouse MD91. Transgene-containing Fo mice were identified by PCR with genomic DNA from tail preparations (Qiagen Blood and Tissue Kit; Qiagen, Gaithersburg, MD) with TS 9435Aldh3a1 (5′GTTCACTGCAGAATAGTGTC3′) primer complementary to the Aldh3a1 promoter within the first intron and a BS 9839Pax6 (5′GCTGTGAGCTAGCTCTACGAT3′) primer complementary to the Pax6 coding region. Lines from three founders, MD91-10, MD91-21, and MD91-32 were established and bred for further analysis. The mice were maintained in accordance with the guidelines set forth by the Animal Care and Use Committee of the National Eye Institute, National Institutes of Health, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
N two-cell blastula to generate the transgenic mouse MD91. Transgene-containing Fo mice were identified by PCR with genomic DNA from tail preparations (Qiagen Blood and Tissue Kit; Qiagen, Gaithersburg, MD) with TS 9435Aldh3a1 (5′GTTCACTGCAGAATAGTGTC3′) primer complementary to the Aldh3a1 promoter within the first intron and a BS 9839Pax6 (5′GCTGTGAGCTAGCTCTACGAT3′) primer complementary to the Pax6 coding region. Lines from three founders, MD91-10, MD91-21, and MD91-32 were established and bred for further analysis. The mice were maintained in accordance with the guidelines set forth by the Animal Care and Use Committee of the National Eye Institute, National Institutes of Health, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Histology and Immunohistochemistry
Whole eyes were immersion fixed in 4% paraformaldehyde or 10% buffered formalin, washed in phosphate-buffered saline (PBS), cleared in xylene, and embedded in paraffin. Before staining, 10-μm sections were deparaffinized, rehydrated using a graded series of ethanol washes and rinsed in PBS. Eye sections were stained in Gill's hematoxylin and eosin or periodic acid-Schiff reagent,5 as described previously. Images shown are representative of the corneal phenotype that occurred in all three transgenic lines.
Ocular surface integrity was examined using fluorescein imaging. Age-matched Pax6 Tg and WT mice were euthanatized, and 1 drop of fluorescein in 2% in PBS (Sigma-Aldrich, St. Louis, MO) was applied to both eyes for 1 minute followed by repeated washing with PBS. The eyes were examined immediately, to avoid diffusion of the fluorescein and photographed with a camera (Stemi SV11; Carl Zeiss Meditec, Inc., Dublin, CA), fitted with a green fluorescent protein (GFP) filter (485/20 excitation).
Immunostaining for Pax6 was achieved by incubating dewaxed sections with a primary anti-Pax6 antibody (1:200; Covance, Princeton, NJ) overnight at 4°, and processed as described previously.5 The slides were coverslipped with aqueous mounting medium (Aqua-Poly/Mount; Polysciences, Warrington, PA) and photographed (Axioplan 2; Carl Zeiss Meditec, Inc.).
RNA and Protein Analysis
Total mRNA was extracted from the corneas of adult Pax6 Tg and WT siblings (RNA-B; Tel-Test, Friendswood, TX). The mRNA was converted into cDNA according to the manufacturer's instructions (SuperScript First-Strand Synthesis System; Invitrogen, Carlsbad, CA) and used as template in RT and/or quantitative (q)PCR assays. The amount of cDNA template was normalized with primers specific for GAPDH (Mm99999915_g1) (Taqman; Applied Biosystems, Inc. [ABI] Foster City, CA). qPCR was performed in a thermocycler (model 7900HT; ABI), and the results were analyzed with commercial software (SDS Software 2.1; ABI).
Gene expression at the mRNA level was analyzed by qPCR with cDNA synthesized from pooled Pax6 Tg corneas displaying mild vascularization versus age-matched WT corneas with gene-specific primers purchased from ABI (Taqman) for Chi3l3 (Mm00657889_mH), Chi3l4 (Mn00840870_m1), Cxcl7 (Mm00470163_m1), Plod2 (Mm00478767_m1), Sox18 (Mm00656049_gH), Wif1 (Mn00442355_m1), Wnt11 (Mm00437328_m1), Wnt4 (Mm00437341_m1), Wnt5a (Mm00437347_m1) and keratin-4 (Mm00492996_g1), -12 (Mm00839769_m1), -13 (Mm00495194_m1), -14 (Mm00516876_m1), -16 (Mm00492979_g1), and -17 (Mm00495207_m1). In addition, Pax6 and K12 levels were assessed in Pax6 Tg corneas that exhibited severe vascularization and surface erosion, by using cDNA prepared as stated above and the primers for endogenous Pax6 (Mm00443072_m1) and K12 (Mm00839769) (Taqman; ABI). qPCR assays were normalized for cDNA by using GAPDH as described above.
Pax6 protein expression was analyzed with corneas and lenses solubilized in 1× lysis buffer (150 mM NaCl, 50 mM Tris [pH 7.4], 0.5% NP-40, 0.5% sodium deoxycholate, 5 mM EDTA, 0.25% SDS, pepstatin, leupeptin, PMSF, and aprotinin). Protein concentration was determined with the Bradford assay (Bio-Rad, Hercules, CA). PAGE was performed with 10% Bis-Tris precast gels (NuPAGE; Invitrogen), buffers, and 2× SDS sample buffer containing 50 mM dithiothreitol, followed by transfer to a PVDF membrane in 1× transfer buffer according to the manufacturer's directions (NuPAGE; Invitrogen). The membrane was incubated with a rabbit anti-Pax6 antibody or a goat anti-Pax6 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and the immunoreactive complex was visualized (Supersignal West Femto Maximum Sensitivity Substrate; ThermoScientific, Rockford, IL). The blot was stripped (Restore Plus Western Blot Stripping Buffer; ThermoScientific) and reprobed with β-actin antibody (AC-74; Sigma-Aldrich) to control for sample loading. Semiquantitation of signals was performed with Image J software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Transfection and Promoter Activity Analysis
COS-7 cells (10-cm dish) were transfected transiently with Aldh3a1 promoter/Pax6 transgenic DNA (5 μg) plus pou2f1 (0.28 μg), shown to activate this promoter,35 or the pCMV Pax6 plasmid using a transfection reagent according to the manufacturer's instructions (Fugene; Roche, Indianapolis, IN). The cells were harvested 48 hours later, and lysates were examined for Pax6 expression by Western blot as described above.
Reporter DNA constructs containing fms-related tyrosine kinase 1/soluble VEGF receptor (Flt1), chitinase 3-like 4 (Chi3l4), or Wnt inhibitory factor 1 (Wif1) promoter sequences driving firefly luciferase activity were generated by RT-PCR amplification of FVB N genomic DNA, using primers complementary to the 5′ noncoding regions of these genes: TS Flt1 (5′GGCCGAGCTCGTTTTGCTTCTAGGAAGC3′) and BS Flt1 (5′GGCCCTCGAGCACCGCGCTCCGAGCCT3′) primers created a 745-bp Flt1 promoter product; TS Chi3l4 (5′GGCCGGTACCAACAGTATTTGAATATAGAC3′) and BS Chi3l4 (5′GGCCAAGCTTGTGTCTTCAGGATTGCTTC3′) produced a 1058-bp Chi3l4 promoter product; and TS Wif1 (5′GGCCGAGCTCGTAATGCTGCAAGCCGCCGC3′) and BS Wif1 (5′GGCCAAGCTTTCCGAGCCATGGTGCTCAGGAC3′) produced a 1112-bp Wif1 promoter product. The PCR conditions were as described above except for the Flt1 promoter synthesis where DNA polymerase was used as recommended (TaqPCRx; Invitrogen), with 0.5× used as the final enhancer concentration. The resulting promoter products were processed as described above and ligated into the pGL3 basic luciferase vector to create the pChi3l4Luc, pFlt1Luc, and pWif1Luc reporter plasmids.
N genomic DNA, using primers complementary to the 5′ noncoding regions of these genes: TS Flt1 (5′GGCCGAGCTCGTTTTGCTTCTAGGAAGC3′) and BS Flt1 (5′GGCCCTCGAGCACCGCGCTCCGAGCCT3′) primers created a 745-bp Flt1 promoter product; TS Chi3l4 (5′GGCCGGTACCAACAGTATTTGAATATAGAC3′) and BS Chi3l4 (5′GGCCAAGCTTGTGTCTTCAGGATTGCTTC3′) produced a 1058-bp Chi3l4 promoter product; and TS Wif1 (5′GGCCGAGCTCGTAATGCTGCAAGCCGCCGC3′) and BS Wif1 (5′GGCCAAGCTTTCCGAGCCATGGTGCTCAGGAC3′) produced a 1112-bp Wif1 promoter product. The PCR conditions were as described above except for the Flt1 promoter synthesis where DNA polymerase was used as recommended (TaqPCRx; Invitrogen), with 0.5× used as the final enhancer concentration. The resulting promoter products were processed as described above and ligated into the pGL3 basic luciferase vector to create the pChi3l4Luc, pFlt1Luc, and pWif1Luc reporter plasmids.
COS-7 cells were transfected transiently with 0.5 μg reporter plasmid DNA per well and 2 ng pSV40RL per well (to normalize for transfection efficiency) of a 12-well plate in triplicate. Various amounts of pKW10 (vector only) or pKW10Pax6 plasmid DNA were cotransfected per well. The cells were harvested 48 hours after transfection, and luciferase activity was determined according to the manufacturer's directions (Dual Luciferase Assay; Promega, Madison, WI). Transfections were repeated on at least three separate occasions. The changes in luciferase activity were calculated by normalizing for transfection efficiency.
Microarray Analysis
Whole eyes were enucleated and examined under the microscope. Corneas harboring one to two blood vessels were dissected from six Pax6 Tg eyes, pooled, and flash frozen. Six corneas from age-matched, WT eyes were prepared in a similar fashion. The Pax6 Tg and WT corneal samples were sent to Expression Analysis (Durham, NC) for microarray analysis, to enable comparison of corneal gene expression (Genechip Analysis Software; Affymetrix). RNA was extracted from each pooled sample, labeled, and hybridized to mouse gene arrays (Mouse 430 2.0; Affymetrix, Santa Clara, CA) containing 22,000 transcripts identified by Entrez Gene numbers.
Results
Generation of Transgenic Mice Overexpressing Pax6 in the Cornea but not in the Lens
To assess the effects of overexpressing Pax6 in the cornea, transgenic mice were generated by using a cornea-preferred promoter from the mouse Aldh3a1 gene identified previously.34 The Aldh3a1 promoter contains 1050 bp upstream of exon 1, exon 1 (wherein transcription initiation occurs), intron 1, and a part of exon 2. The transgenic construct was created by fusing the complete mouse Pax6 coding sequences downstream of the Aldh3a1 promoter (Fig. 1A). Corneal expression is preferred over lens expression using this promoter: five independent transgenic mouse lines expressing luciferase fused to this promoter showed 10- to 1957-fold higher luciferase activity in the cornea than in the lens (Davis J and Piatigorsky J, unpublished results, 2008).
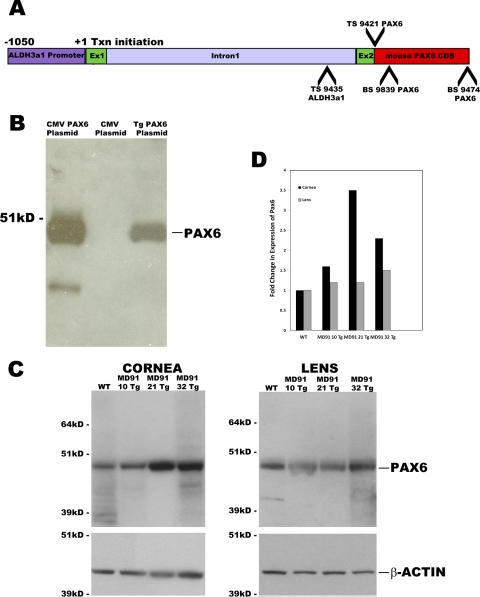
Figure 1.
(A) Transgenic mice were generated using a corneal-preferred promoter from the mouse Aldh3a1 gene. The Aldh3a1 promoter contains 1050-bp upstream of exon 1, exon 1, intron 1, and a part of exon 2. The transgenic construct was created by fusing the complete mouse Pax6 CDS of the Aldh3a1 promoter. TS9421 and BS9474 were used to clone the mouse Pax6 CDS, and TS9435 and BS9839 primers were used for genotyping. (B) Western blot analysis shows the production of bona fide Pax6 protein from the transgenic construct after transfection into COS7 cells (lane 3). The Pax6-immunoreactive product is comparable in size to that produced after transfection of COS7 cells with a positive control CMVPax6 plasmid (lane 1). (C) Three Pax6 Tg founders were identified—MD91-10, MD91-21, and MD91-32—by genotype analysis. Increased amounts of Pax6 protein were observed in total corneal extracts from 6-week-old Tg mice compared with age-matched, WT siblings by Western blot analysis, but not in lens samples from the same mice, indicating that Pax6 is selectively overexpressed in the cornea. (D) Semiquantitative ratio of change in Pax6 expression from corneal and lens extracts using β-actin as a loading control shows an increase in Pax6 protein levels in the transgenic corneas. CDS, coding sequences; Ex, exon.
To confirm that the transgenic construct produced bona fide Pax6 protein, we transfected COS-7 cells with Aldh3a1 Pax6 transgenic plasmid DNA plus a transcription factor, pou2f, shown to activate the Aldh3a1 promoter in COS-7 cells.35 Western blot analysis showed the production of a Pax6 immunoreactive product of the predicted molecular weight that migrates to the same position as the immunoreactive product in COS-7 cells transfected with the positive control pKW10Pax6 plasmid that harbors a CMV promoter driving Pax6 synthesis, indicating that Pax6 protein is synthesized from the transgenic construct (Fig. 1B).
Three Pax6 Tg founders were identified—MD91-10, MD91-21, and MD91-32—by genotype analysis (see Methods; data not shown). To determine whether Pax6 protein is overexpressed, Western blots were performed on cell lysates from corneas and lenses. Pax6 levels were increased 1.6-, 3.5-, and 2.3-fold in the corneas of the MD91-10, MD91-21, and MD91-32 mice, respectively, relative to the WT corneas, whereas the level of Pax6 protein did not increase appreciably in the Pax6 Tg lens (Figs. 1C, 1D). β-Actin levels were used as a loading control and are shown in the bottom panel (Fig. 1C).
Abnormal Corneas in Pax6 Tg Eyes
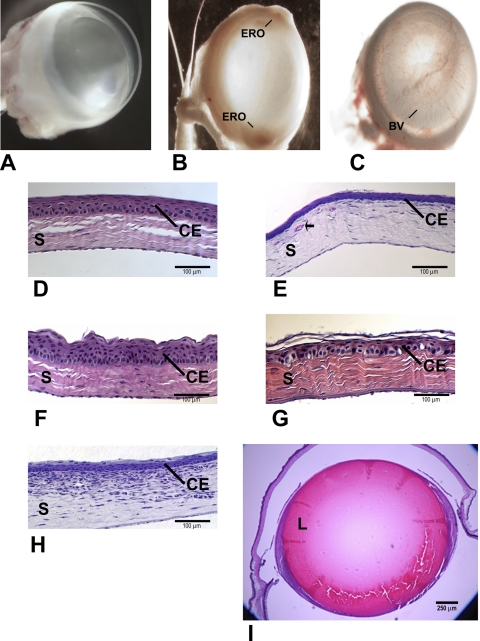
Microscopic examination of whole eyes revealed opacity, vascularization, and/or surface erosion in the corneas from all three Pax6 Tg mouse lines compared with WT siblings (Figs. 2A–C). Histologic preparations stained with hematoxylin and eosin confirmed the presence of blood vessels in the stroma of Pax6 Tg mice (Figs. 2D, 2E). In addition, an immune infiltrate was present in some of the corneas (Figs. 2H); otherwise, the corneal stroma and endothelium appeared normal. The corneal epithelium was variable from animal to animal (and from eye to eye in the same animal) and showed changes in thickness and organization, swollen basal cells, and sloughing of the superficial layers. The corneal epithelium was noticeably thinner or thicker compared with WT (Figs. 2D–F). In some corneas, the neatly ordered architecture of the WT epithelium was absent, the basal cell layer was prominently enlarged and/or the surface cells were loose, and gaps appeared in the most anterior layers of the epithelium (Fig. 2G). The epithelium did not harbor carbohydrate-laden cells (mucin-containing goblet cells), as determined by periodic acid-Schiff staining (data not shown). There was no temporal pattern in the appearance of individual corneal defects, and not all the Pax6 Tg mouse corneas examined looked abnormal. The percentage of transgenic mice exhibiting one or more of the atypical phenotypes ranged from 56% to 69%, depending on the founder, compared with an incidence of 6.6% corneal defects in WT, age-matched siblings (Table 1). The appearance of corneal aberrations did not correlate with increasing age: Six-week-old mice were as likely to have defects as were mice 1 year of age or older. In contrast to the corneal defects noted in the Pax6 Tg mice, the lens looked normal (Fig. 2I). These results indicate that abnormal corneal defects are independent of the lens phenotype.
Figure 2.
Microscopic examination of whole eyes (A–C) revealed opacity, vascularization, and/or surface erosion in the corneas of Pax6 Tg mice (B, C) compared to WT (A). Histologically, Pax6 Tg corneal epithelium was variable from mouse to mouse (E–H), showing changes in thickness, swollen basal cells, and sloughing of the superficial layers compared with WT (D). (E) Blood vessels, indicated by an arrow and/or (H) immune infiltrate were present in the stroma (S) of some corneas. In contrast to the Pax6 Tg corneas, the Pax6 Tg lens (L) appeared normal (I). BV, blood vessel; ERO, erosion; CE, corneal epithelium; S, stroma; L, lens.
Table 1.
Frequency of Abnormal Corneas from Different Pax6 Tg Founders
| Founder | Abnormal Corneas/Corneas Examined | % Abnormal Corneas |
|---|---|---|
| WT | 8/122 | 6.6 |
| MD91-10 | 47/84 | 56 |
| MD91-21 | 32/50 | 64 |
| MD91-32 | 44/64 | 69 |
Differential Expression of Immune-, Vascular-, and Differentiation-Related Genes in the Pax6 Tg Corneas
To investigate the underlying mechanism(s) for the observed phenotypes, gene expression of Pax6 Tg versus age-matched, WT corneas was examined by microarray analysis. Pax6 Tg corneas with one to two blood vessel arborizations, but otherwise normal-appearing at a gross level, were selected to maximize analysis of early, potentially causative, changes in gene expression rather than those due to late-stage deterioration of the cornea. Despite the selection of corneas with a mild phenotype, more than 1000 genes were differentially expressed with a twofold change or greater (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6726/-/DCSupplemental). The top 46 genes with altered expression in the Pax6 Tg corneas are shown in Table 2. The microarray data were validated with qPCR (with a second pool of corneas containing blood vessels). The changes in expression for chitinase 3-like 3 (Chi3l3), Chi3-like 4 (Chi3l4), chemokine (C-X-C motif) ligand 7 (CxCl7), SRY-containing gene 18 (Sox18), and Wnt inhibitory factor 1 (Wif1) mRNA by qPCR are consistent with changes observed in the microarray analysis (Table 3). The discrepancy in the fold change value between microarrays and qPCR can be attributed to the limitations of microarrays including a more narrow dynamic range than qPCR, signal saturations, and cross-hybridizations.36 Finally, microarrays have a significant decrease in overall accuracy of differential expression detection at low expression levels,36 a situation relevant in the present study, as indicated by the qualitative “absent” call in the WT cornea for most of the genes that were analyzed by these two methods.
Table 2.
Highest Differences in Gene Expression in Pax6 Tg Corneas Relative to WT
| Unigene | Gene Description | Fold Change |
|---|---|---|
| Mm_387173 | Chitinase 3-like 3 | 1260 |
| Mm_425140 | Chitinase 3-like 4 | 1260 |
| Mm_6853 | Small proline-rich protein 2A | 512 |
| Mm_293614 | Chemokine ligand 7 | 181 |
| Mm_153688 | Ribonuclease A family, member 11 | 158 |
| Mm_290390 | Membrane-spanning 4-domains, 6D | 128 |
| Mm_196110 | Hemoglobin alpha, adult chain 1 | 91 |
| Mm_37426 | CD163 antigen | 91 |
| Mm_348025 | Leucine-rich alpha-2-glycoprotein 1 | 79 |
| Mm_209715 | Procollagen, type XI, alpha 1 | 79 |
| Mm_198803 | UDP-glucose ceramide glucosyltransferase | 69 |
| Mm_383216 | Asporin | 64 |
| Mm_249555 | Procollagen, type III, alpha 1 | 56 |
| Mm_195788 | Keratin 76 | 56 |
| Mm_235324 | Colony stimulating factor 2 receptor, beta 1 | 56 |
| Mm_100348 | Reg of G-protein signaling 7 binding protein | 56 |
| Mm_2570 | Complement component 1, Q | 49 |
| Mm_137 | Chemokine ligand 6 | 49 |
| Mm_21767 | Cadherin 5 | 49 |
| Mm_3468 | Suppressor of cytokine signaling 3 | 45 |
| Mm_79983 | Procollagen, 2-oxoglutarate 5-dioxygenase | −45 |
| Mm_42029 | Chemokine ligand 8 | 42 |
| Mm_30138 | Dermokine | 42 |
| Mm_264904 | SRY-box containing gene 18 | 42 |
| Mm_582 | Fatty acid binding protein 4 | 37 |
| Mm_260768 | Plasmalemma vesicle associated protein | 37 |
| Mm_292100 | Fibrino gen-like protein 2 | 37 |
| Mm_143831 | Guanylate cyclase 1, soluble, alpha 3 | 37 |
| Mm_24097 | Activated macrophage WAP domain pro | 37 |
| Mm_416125 | Chemokine ligand 9 | 37 |
| Mm_200916 | Glutathione peroxidase 3 | 34 |
| Mm_2055 | Matrix metalopeptidase 12 | 34 |
| Mm_31748 | NADPH oxidase 4 | 34 |
| Mm_268521 | Insulin-like growth factor 1 | 34 |
| Mm_360747 | Carbohydrate sulfotransferase 1 | 34 |
| Mm_439747 | Serotonin receptor 2B | 32 |
| Mm_340090 | Myosin, heavy polypeptide 3 | 32 |
| Mm_22768 | Claudin 5 | 30 |
| Mm_220853 | Chemokine ligand 21b | 30 |
| Mm_20954 | Regulator of G-protein signaling 5 | 29 |
| Mm_27343 | Endomucin | 29 |
| Mm_38274 | Chitinase 3-like 1 | 29 |
| Mm_212333 | Pancreatic lipase-related protein 2 | −29 |
| Mm_241205 | Protein tyrosine phosphatase-like | 29 |
| Mm_440388 | Phosphatidylinositol glycan anchor | 29 |
| Mm_30805 | NCK associated protein 1 like | 26 |
Table 3.
Fold Change by qPCR for Select Genes
| Gene Name | Fold Change |
|---|---|
| Chi3l3 | 12003 ± 640 |
| Chi3l4 | 438734 ± 17433 |
| Cxcl7 | 3537 ± 468 |
| Sox18 | 774 ± 8.1 |
| Wif1 | 22.67 ± 1.1 |
| Plod | 2.63 ± 0.18 |
Data are expressed as the mean ratio of change ± SD.
Many of the genes whose mRNA levels changed in the Pax6 Tg corneas (at least twofold) are involved in immune function, blood vessel formation, and epithelial cell differentiation. Table 4 lists a selection of these genes with known functions that fall within one of those classes.
Table 4.
Functional Categories of Genes That are Differentially Expressed in Corneas from WT versus Pax6 Tg Mice
| Immune-Related |
| Chitinase 3-like 1, 3, & 4 |
| Chemokine ligands 6, 7, 8, 9, 11, & 21b |
| Complement component 1, 2, 3, & 4 |
| Toll-like receptor 12 |
| CXC12 |
| CXCR4 |
| CSF2-R1 |
| Fc receptor |
| T cell receptor |
| Ig light and heavy chain receptors |
| Il receptor-1 |
| CD163 antigen |
| TNF receptor 1b |
| CD209E |
| CD45 |
| CD300 |
| CD34 on mast cells |
| CD53 |
| SOCS3 |
| Fibrinogen-like protein 2 |
| Fatty acid binding protein 4 |
| Matrix metallopeptidase 2, 3, 12, & 13 |
| Differentiation-Related |
| Sprp 1A, 2A, & 2F |
| EMP3 |
| Dermokine |
| SPARC |
| K4, K13, 15, 16, 17, & 76 |
| Wnt inhibitory factor 1 |
| Wnt 5a, 11 |
| Wnt inducible signaling protein |
| Secreted frizzled-related protein 2 |
| Procollagen Ia1, Ia2, IIIa1, Va2, Va3, XIa1, XIVa1 |
| Blood Vessel-Related |
| Angiotensin receptor like-1 |
| Angiomotin |
| Vegfc |
| Endothelin receptor A |
| VCAM |
| Hemoglobin alpha, adult chain 1 |
| Cadherin 5 |
| Claudin 5 |
| Angiopoietin-like 2 |
| IGF-1 |
| IGFBP 3, 4, & 6 |
| SRY-box containing gene 18 |
| Endosialin |
| Fatty acid binding protein 4 |
| NADPH oxidase 4 |
| Regulator of G-protein signaling 5 |
Genes representing components of both the innate and adaptive branches of the immune system underwent the most dramatic changes in the Pax6 Tg corneas. mRNA levels from several Chi3-like genes, the chemokine ligands 6, 7, 8, 9, 11, 12, and 21b and chemokine receptor 4, complement factors 1 to 4, as well as T-, Fc-, and Ig heavy/light chain immune cell receptors and toll-like receptor 12 were among the many in this category to be upregulated (Table 4). It is not clear whether the changes in immune-related genes are a consequence of epithelial, resident immune, and/or immune infiltrate cell gene expression.
Not surprisingly, a second category of genes that showed differential expression was those involved in blood vessel formation. This was anticipated considering the deliberate selection of corneas with small blood vessels versus the transparent corneas of WT mice. Genes whose mRNA levels were increased in the Pax6 Tg mouse corneas included angiopoietin-like 2, angiomotin, Vegfc, endothelin receptor A, and Sox18 (Table 4).
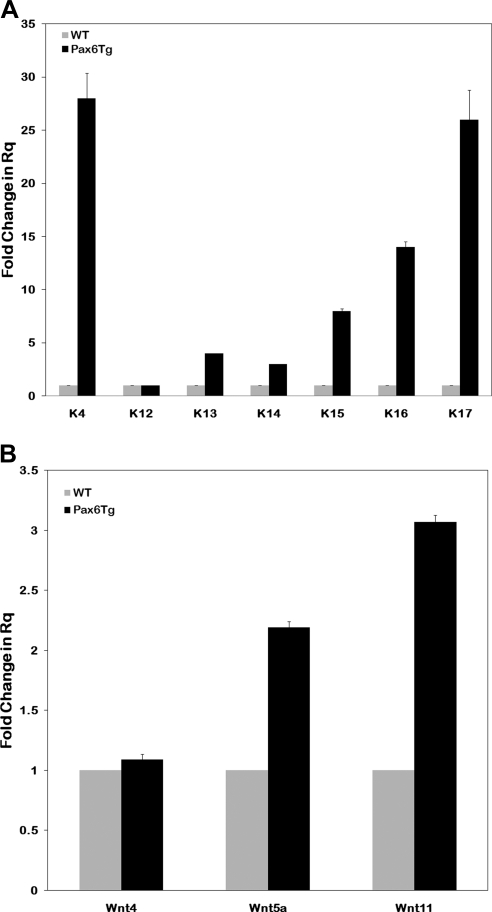
Genes involved in differentiation constitute the last functional category with altered expression in the Pax6 Tg corneas. The mRNA levels of several keratin genes were strongly affected (Table 4). Microarray results were corroborated by qPCR where levels of K4, K13, K14, K15, K16, and K17 mRNA increased 28-, 4-, 3-, 8-, 14-, and 26-fold, respectively (Fig. 3A). In contrast, the corneal-specific keratin, K12, a classic marker of corneal epithelial cell identity, remained unchanged in these mildly affected corneas. Other genes whose mRNA levels increased included epithelial membrane protein (EMP) 3, the small proline-rich proteins (Sprp) Sprp2A, 2F, and 1A, many procollagen genes, SPARC, Wif1, secreted frizzled-related protein 2 (Sfrp2), and Wnt inducible signaling protein (Table 4). To determine whether other components of the Wnt pathway changed, the levels of several Wnt ligands were examined by qPCR. Wnt5a and Wnt11 increased two- and threefold, respectively, whereas levels of Wnt4 remained unchanged (Fig. 3B).
Figure 3.
(A) The mRNA levels of several keratin genes were strongly affected (A). Microarray results were corroborated by qPCR, which showed levels of K4, K13, K14, K15, K16, and K17 mRNA to increase 28-, 4-, 3-, 8-, 4-, and 26-fold, respectively. In contrast, K12, a corneal epithelial cell-specific marker, remained unchanged in these mildly affected corneas. (B) Several components of the Wnt pathway were altered in the microarray analysis. qPCR confirmed the microarray results, showing that Wnt5a and -11 mRNAs were increased two and threefold, respectively, in the Pax6 Tg mice corneas relative to the WT.
Changes in the Ocular Surface, a Reduction in Endogenous Pax6 and K12 mRNA, and Loss of Nuclear Pax6 Accompany an Abnormal Corneal Phenotype
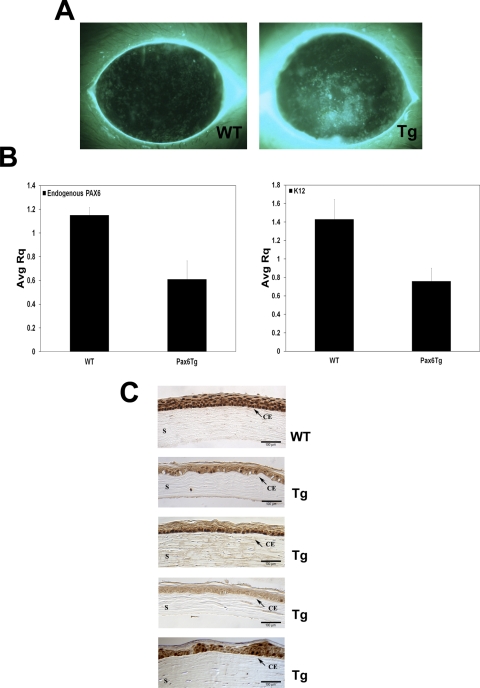
The possibility that the enhanced immune system response in Pax6 Tg corneas reflects an alteration at the corneal surface was explored by fluorescein staining of age-matched, WT and transgenic mice eyes from between 6 and 12 weeks of age. Eyes that appeared normal at a gross level—no blood vessels, erosions, or opacity—were selected for treatment with fluorescein. The surface of a representative Pax6 Tg cornea stained intensely and diffusely with fluorescein compared with a WT cornea, photographed for the same amount of time, which showed residual retention of the fluorescein dye (Fig. 4A). The uptake of fluorescein by the Pax6 Tg corneas indicates that the integrity of the ocular surface has been breached and may account for the activation of an immune response.
Figure 4.
(A) Diffuse, punctate staining of the corneal surface using fluorescein reveals a breach in barrier function in the Pax6 Tg mice compared with WT. (B) Gene expression in severely affected corneas with total corneal erosions and vascularization was analyzed by qPCR. K12 and endogenous Pax6 mRNA levels were reduced ∼50% in Tg corneas relative to age-matched, WT corneas. (C) The amount of Pax6 protein was reduced and localized to the cytoplasm in Pax6 Tg corneal epithelium (four different mouse corneas are shown) by immunohistochemistry with a Pax6 antibody. CE, corneal epithelium; S, stroma.
To examine gene expression in more severely affected corneas, individual mice with total corneal erosions and/or widespread corneal vascularization were collected and compared to age-matched, WT corneas by qPCR. K12 levels were reduced from an average, WT RQ (relative quantity) of 1.4 ± 0.215 to 0.76 ± 0.14 in the Pax6 Tg corneas (Fig. 4B). Surprisingly, like K12 mRNA levels, endogenous Pax6 levels were reduced in the Pax6 Tg corneas ∼50%, from an average WT RQ of 1.1 ± 0.065 to 0.615 ± 0.155 in the transgenic corneas (Fig. 4B). The amount of cDNA template was normalized using primers specific for GAPDH (Taqman; ABI). Examination of Pax6 protein by immunohistochemistry revealed that the staining intensity was also reduced in the Pax6 Tg corneal epithelium versus the WT mouse, consistent with the observed reduction in Pax6 mRNA levels (Fig. 4C). Interestingly, there was also dramatically reduced nuclear staining in the transgenic corneal epithelium compared with that in the WT (Fig. 4C), indicating that both Pax6 levels and cellular distribution were abnormal in the severely affected transgenic corneas.
Pax6 Regulates the Chi3l4, Flt1, and Wif1 Promoters in Vitro
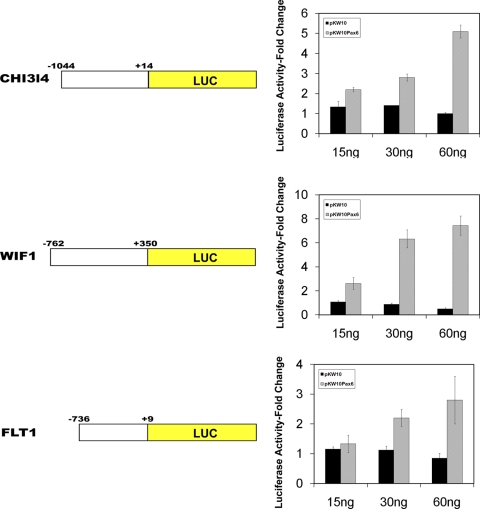
To confirm that Pax6 can regulate expression of those genes shown by microarray to change, a promoter region from a gene representing each of the functional categories was fused to a reporter gene and analyzed by transfection. The pChi3l4Luc plasmid containing a 1044-bp promoter sequence of Chi3l4, an immune-related gene, located upstream of the luciferase gene, was cotransfected with 0, 15, 30, or 60 ng of pKW10 or pKW10Pax6 plasmid in COS-7 cells. Increasing amounts of pKW10Pax6 activated the promoter in a dose–response fashion, such that the maximum amount (60 ng) resulted in a 4.8-fold activation of luciferase activity relative to luciferase activity after pChi3l4Luc transfection only (Fig. 5). As a control, increasing amounts of pKW10, a plasmid containing vector sequences only, did not appreciably alter promoter activity (Fig. 5). A similar strategy was used to investigate the Pax6-responsiveness of a pFlt1Luc plasmid, a construct containing 736 bp of the Flt1 promoter, a gene encoding a vascular endothelial growth factor receptor, ligated to the luciferase gene. Although the microaarray evidence for Flt1 gene expression is inconclusive due to primer site selection (Davis J and Piatigorsky J, personal communication, 2011), the gene was selected for transfection analysis because of its importance in regulating corneal neovascularization.37 At the highest concentrations of Pax6, the Flt1 promoter underwent a 3.1-fold increase in activity (Fig. 5). Finally, a gene representing the third functional category of differentiation, Wif1, was analyzed. The pWif1Luc plasmid, containing 762 bp of Wif1 promoter sequences, was activated 2.6-, 6.3-, and 7.4-fold by 15, 30, and 60 ng of pKW10Pax6, respectively, but not by equivalent amounts of pKW10 plasmid DNA (Fig. 5). In summary, the transfection experiments confirm the findings by microarray analysis that Pax6 regulates, directly or indirectly, the Chi3l3, Flt1, and Wif1 genes.
Figure 5.
Promoter sequences of a representative gene from each of the functional categories were fused to the luciferase reporter gene and analyzed by COS7 cell cotransfection. Maximum amounts of Pax6 plasmid (60 ng) resulted in a 4.8-, 7.4-, and 3.1- fold activation of Chi3l4, Wif1, and Flt1 promoter/reporter constructs, respectively, relative to vector plasmid only. The transfection experiments confirmed the microarray findings that Pax6 regulates the Chi3l4 and Wif1 genes.
Discussion
The restricted overexpression of Pax6 in the cornea combined with the abnormal corneal phenotype suggests that Pax6 is essential and functions autonomously in the cornea. Moreover, the lenses of these mice appear normal, indicating that corneal defects can occur in the absence of a lens defects. While these findings, as well as those of others,16,17,38 suggest a direct role for Pax6 in the cornea, they do not negate the role of the lens in corneal development.28–30 The status of the Pax6 Tg cornea can be characterized by (at least) two phases: an early phase where the apparent phenotype is mild—a few blood vessels and/or a compromised barrier—but otherwise normal-appearing corneal surface. During this early phase, Pax6 is overexpressed, and the expression of many genes involved in inflammation, vascularization, and epithelial differentiation is altered. A second phase ensues in which the cornea becomes opaque, ulcerated, and eroded. In these severely affected corneas, Pax6 and K12 levels have decreased, presumably because of a severe disruption of normal corneal epithelial differentiation in the Pax6 Tg mice.
Pax6 Tg and Pax77 mice exhibited similar and different phenotypic changes. Only the cornea was altered in the Pax6 Tg mice, whereas Pax77 mice manifested defects in the cornea, lens, retina, ciliary body, and iris,16 probably a reflection of Pax6 overexpression in the cornea only versus all tissues where Pax6 is normally expressed.16–18,39 Corneal features found in both Pax6-overexpressing models included changes in the number, organization, and shape of the corneal epithelial cells, and a reduction in K12 (see below). Differences in the size and clarity of the corneas as well as in the presence or absence of blood vessels and immune infiltrate may be attributable to different genetic backgrounds.17,40,41 Different corneal phenotypes may also be due to promoter differences. The overexpression of Pax6 in the lens29,30,42 or ciliary body43 in the Pax77 mice may have indirect effects on the development of the cornea. Further, the YAC construct would likely be subject to the same regulation as the endogenous promoter/gene with respect to onset and strength of Pax6 expression, including positive and negative autoregulation by Pax6 itself.39,44 However, the amount of Pax6 in mature corneas is comparable in the two mouse models (at least in the milder phenotype) and probably does not account for the difference in corneal phenotypes. Finally, different promoter usage may result in changes in the relative levels of Pax6 isoforms, and hence, regulation of different downstream target genes.38,45–47 Although the Pax6:Pax6(5a) ratio was nearly the same as that of the WT siblings in the Pax77 YAC eyes,17 the Aldh3a1 promoter driving Pax6 in the present study increased the amount of Pax6 relative to Pax6(5a).
The change in expression of many immune- and vascular-related genes, possibly a consequence of a compromised barrier,48,49 suggests a primary role for Pax6 in mediating a cellular response to stress or injury. Previously, Sivak et al.50 showed that Pax6 was activated during corneal epithelial wound healing. Although we find the significant upregulation of metalloproteinase (MMP)-2, -3, -12, and -13 in the early phase of the Pax6 Tg phenotype intriguing (Supplementary Table S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6726/-/DCSupplemental), a change in MMP-9 expression as observed in the wounded corneas, may not occur until the phenotype becomes more advanced. Expression of three members of a family of nonenzymatic, mammalian chitinases—Chi3l1, Chi3l3, and Chi3l4—are significantly increased in the Pax6 Tg corneas. Induction of Chi3l1 has been associated with chronic inflammation,51,52 stress,53 and tissue remodeling.51,54,55 Further, our cotransfection results showed a fivefold promoter activation of Chi3l4, suggesting that Chi3l4 is a target, directly or indirectly, of Pax6. Similarly, we showed that Pax6 can activate the anti-/proangiogenic gene Flt1 in vitro.
Several members of the keratin superfamily are upregulated when a mild phenotype is evident including K4, K13- to -17, and K76. Induction of K16 and 17 is associated with stress, injury, inflammation, and hyperproliferative disorders, such as squamous metaplasia and at the wound edge during healing.56,57 It is possible that increases in K4 and K13 represent encroachment by the conjunctival epithelium; however, the absence of conjunctival-derived goblet cells in the Pax6 transgenic corneas suggests otherwise. Further studies are needed to determine whether these keratin genes are targets of Pax6.
Our results coincide with findings from studies examining ocular surface diseases,58 as well as mutant5,12,14,18 and knockout mouse models,22–24,59–61 that have shown an association between a change in levels of both Pax6 and K12 (generally a reduction in both) and differentiation abnormalities in the cornea epithelium. In the cornea-to-epidermis mouse model,21 corneal epithelial cells first become dedifferentiated. Several features of the dedifferentiated phenotype can be observed in the Pax6 Tg cornea including a reduction and redistribution of Pax6 to the cytoplasm,11,21 loss of K12 expression, and the induction of the limbal basal cell markers K14 and -17.21,62,63 In addition, our study shows that K15, a marker of limbal stem cells,63,64 is increased in the Pax6 Tg corneas, corroborating the notion that the corneal epithelium has dedifferentiated. The upregulation of Sprp-1A, -2A, and -2F,65 an early indicator of abnormal keratinization in squamous metaplasia and many other ocular diseases,58,66–68 is also consistent with a defect in differentiation.
The change in expression of several Wnt signaling components may contribute to the abnormal differentiation phenotype in the Pax6 Tg corneas. Suppression of Wnt signaling is critical in maintaining corneal epithelial cell fate.22,61,69,70 Depending on how and where the Wnt suppression is relieved, corneal epithelial cells can dedifferentiate,21,61 grow in an unregulated fashion,61 transdifferentiate to an epidermal cell fate,21,22,59,69 and/or exhibit an impaired wound-healing response.24 The transfection data suggest that Pax6 may play a role in suppressing the Wnt pathway via transcriptional activation of the Wif1 gene. Supporting this notion, the microarray analysis of the Pax6 Tg corneas showed an increase in Wif1 mRNA levels as well as those of a second Wnt inhibitor, Sfrp2. Pax6 is known to regulate Wnt inhibitors in the lens25 and central nervous system.26
Despite the upregulation of Wnt inhibitors that may act to suppress Wnt signaling, the corneas of the Pax6 Tg mice fail to differentiate normally. An explanation may lie in the simultaneous increase in the Wnt ligands (activators) Wnt5a and -11. As mentioned earlier, an activating Wnt signal is thought to downregulate Pax6 expression leading to a loss of corneal epithelial identity.21,22,61 The increase in levels of several downstream targets of Wnt signaling, K1720 and Wnt inducible signaling protein,71 also suggests that Wnt signaling is activated in the Pax6 Tg corneas; however, definitive proof necessitates additional analysis. The coincident induction of Wnt activators and inhibitors by Pax6 has been observed in the central nervous system.26 Although our data do not allow us to tell whether Pax6 directly activates the Wnt5a and -11 promoters, nor do they reveal whether Wnt ligands and inhibitors are localized to overlapping or distinct regions of the cornea, they do suggest that Pax6 contributes to the regulation of the Wnt pathway while being regulated itself by this pathway.
In summary, Pax6 acts autonomously in the cornea to maintain normal corneal function. We propose that the initial stimulus in the Pax6 Tg mice leading to abnormal phenotypes is the alteration of genes involved in epithelial differentiation by the overexpression of Pax6, possibly resulting in changes in the barrier, followed by a change in immune- and vascular-related genes. Eventually, Pax6 and K12 are reduced, resulting in the loss of corneal epithelial identity.
Supplementary Material
Footnotes
Disclosure: J. Davis, None; J. Piatigorsky, None
References
- 1. Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110 [PubMed] [Google Scholar]
- 2. Kozmik Z, Daube M, Frei E, et al. Role of Pax genes in eye evolution: a cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev Cell. November 5(5):773–785, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267(5205):1788–1792 [DOI] [PubMed] [Google Scholar]
- 4. Tomarev SI, Callaerts P, Kos L, et al. Squid Pax-6 and eye development. Proc Natl Acad Sci U S A. 1997;94(6):2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis J, Duncan MK, Robison WG, Jr., Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003;116:2157–2167 [DOI] [PubMed] [Google Scholar]
- 6. Glaser T, Lane J, Housman D. A mouse model of the aniridia-Wilms tumor deletion syndrome. Science. 1990;250(4982):823–827 [DOI] [PubMed] [Google Scholar]
- 7. Hanson IM, Fletcher JM, Jordan T, et al. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet. 1994;6(2):168–173 [DOI] [PubMed] [Google Scholar]
- 8. Hill RE, Favor J, Hogan BL, et al. Mouse small eye results from mutations in a paired-like homeobox- containing gene. Nature. 1991;354(6354):522–525 [DOI] [PubMed] [Google Scholar]
- 9. Callaerts P, Halder G, Gehring WJ. PAX-6 in development and evolution. Annu Rev Neurosci. 1997;20:483–532 [DOI] [PubMed] [Google Scholar]
- 10. Matsuo T, Osumi-Yamashita N, Noji S, et al. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993;3(4):299–304 [DOI] [PubMed] [Google Scholar]
- 11. Ou J, Walczysko P, Kucerova R, et al. Chronic wound state exacerbated by oxidative stress in Pax6+/− aniridia-related keratopathy. J Pathol. August 2008;215(4):421–430 [DOI] [PubMed] [Google Scholar]
- 12. Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003;44(5):1871–1878 [DOI] [PubMed] [Google Scholar]
- 13. Ramaesh T, Ramaesh K, Leask R, et al. Increased apoptosis and abnormal wound-healing responses in the heterozygous Pax6+/− mouse cornea. Invest Ophthalmol Vis Sci. 2006;47(5):1911–1917 [DOI] [PubMed] [Google Scholar]
- 14. Ramaesh T, Ramaesh K, Martin Collinson J, Chanas SA, Dhillon B, West JD. Developmental and cellular factors underlying corneal epithelial dysgenesis in the Pax6+/− mouse model of aniridia. Exp Eye Res. 2005;81(2):224–235 [DOI] [PubMed] [Google Scholar]
- 15. Aalfs CM, Fantes JA, Wenniger-Prick LJ, et al. Tandem duplication of 11p12–p13 in a child with borderline development delay and eye abnormalities: dose effect of the PAX6 gene product? Am J Med Genet. 1997;73(3):267–271 [DOI] [PubMed] [Google Scholar]
- 16. Schedl A, Ross A, Lee M, et al. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86(1):71–82 [DOI] [PubMed] [Google Scholar]
- 17. Chanas SA, Collinson JM, Ramaesh T, et al. Effects of elevated Pax6 expression and genetic background on mouse eye development. Invest Ophthalmol Vis Sci. 2009;50(9):4045–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dora N, Ou J, Kucerova R, Parisi I, West JD, Collinson JM. PAX6 dosage effects on corneal development, growth, and wound healing. Dev Dyn. 2008;237(5):1295–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramaesh K, Ramaesh T, Dutton GN, Dhillon B. Evolving concepts on the pathogenic mechanisms of aniridia related keratopathy. Int J Biochem Cell Biol. 2005;37(3):547–557 [DOI] [PubMed] [Google Scholar]
- 20. McGowan KM, Coulombe PA. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998;143(2):469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pearton DJ, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc Natl Acad Sci U S A. 2005;102(10):3714–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukhopadhyay M, Gorivodsky M, Shtrom S, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133(11):2149–2154 [DOI] [PubMed] [Google Scholar]
- 23. Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33(3):416–421 [DOI] [PubMed] [Google Scholar]
- 24. Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13(2):242–253 [DOI] [PubMed] [Google Scholar]
- 25. Machon O, Kreslova J, Ruzickova J, et al. Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/beta-catenin signaling in the lens surface ectoderm. Genesis. 2010;48(2):86–95 [DOI] [PubMed] [Google Scholar]
- 26. Kim AS, Anderson SA, Rubenstein JL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci. 2001;21(5):RC132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pei YF, Rhodin JA. The prenatal development of the mouse eye. Anat Rec. 1970;168(1):105–125 [DOI] [PubMed] [Google Scholar]
- 28. Collinson JM, Quinn JC, Buchanan MA, et al. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc Natl Acad Sci U S A. 2001;98(17):9688–9693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Genis-Galvez JM. Role of the lens in the morphogenesis of the iris and cornea. Nature. 1966;210(32):209–210 [DOI] [PubMed] [Google Scholar]
- 30. Genis-Galvez JM, Santos-Gutierrez L, Rios-Gonzalez A. Causal factors in corneal development: an experimental analysis in the chick embryo. Exp Eye Res. 1967;6(1):48–56 [DOI] [PubMed] [Google Scholar]
- 31. Koroma BM, Yang JM, Sundin OH. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997;38(1):108–120 [PubMed] [Google Scholar]
- 32. Manuel M, Pratt T, Liu M, Jeffery G, Price DJ. Overexpression of Pax6 results in microphthalmia, retinal dysplasia and defective retinal ganglion cell axon guidance. BMC Dev Biol. 2008;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14(21):2701–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kays WT, Piatigorsky J. Aldehyde dehydrogenase class 3 expression: identification of a cornea-preferred gene promoter in transgenic mice. Proc Natl Acad Sci U S A. 1997;94(25):13594–13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis J, Davis D, Norman B, Piatigorsky J. Gene expression of the mouse corneal crystallin Aldh3a1: activation by Pax6, Oct1, and p300. Invest Ophthalmol Vis Sci. 2008;49(5):1814–1826 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Barbacioru C, Hyland F, et al. Large scale real-time PCR validation on gene expression measurements from two commercial long-oligonucleotide microarrays. BMC Genomics. 2006;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;26 443(7114):993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A. Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression. J Cell Sci. 2000;113:3173–3185 [DOI] [PubMed] [Google Scholar]
- 39. Kleinjan DA, Seawright A, Mella S, et al. Long-range downstream enhancers are essential for Pax6 expression. Dev Biol. 2006;15 299(2):563–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quinn JC, West JD, Kaufman MH. Genetic background effects on dental and other craniofacial abnormalities in homozygous small eye (Pax6Sey/Pax6Sey) mice. Anat Embryol (Berl). 1997;196(4):311–321 [DOI] [PubMed] [Google Scholar]
- 41. Ramaesh T, Williams SE, Paul C, Ramaesh K, Dhillon B, West JD. Histopathological characterisation of effects of the mouse Pax6(Leca4) missense mutation on eye development. Exp Eye Res. 2009;89(2):263–273 [DOI] [PubMed] [Google Scholar]
- 42. Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220(2):424–431 [DOI] [PubMed] [Google Scholar]
- 43. Beebe DC. Development of the ciliary body: a brief review. Trans Ophthalmol Soc U K. 1986;105:123–130 [PubMed] [Google Scholar]
- 44. Manuel M, Georgala PA, Carr CB, et al. Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effect on cortical arealization. Development. 2007;134(3):545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim J, Lauderdale JD. Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Dev Biol. 2006;292(2):486–505 [DOI] [PubMed] [Google Scholar]
- 46. Kozmik Z, Czerny T, Busslinger M. Alternatively spliced insertions in the paired domain restrict the DNA sequence specificity of Pax6 and Pax8. EMBO J. 1997;16(22):6793–6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pinson J, Mason JO, Simpson TI, Price DJ. Regulation of the Pax6: Pax6(5a) mRNA ratio in the developing mammalian brain. BMC Dev Biol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knop E, Knop N. Anatomy and immunology of the ocular surface. Chem Immunol Allergy. 2007;92:36–49 [DOI] [PubMed] [Google Scholar]
- 49. Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Maintaining corneal integrity how the “window” stays clear. Prog Histochem Cytochem. 2001;36(3):185–259 [PubMed] [Google Scholar]
- 50. Sivak JM, Mohan R, Rinehart WB, Xu PX, Maas RL, Fini ME. Pax-6 expression and activity are induced in the reepithelializing cornea and control activity of the transcriptional promoter for matrix metalloproteinase gelatinase B. Dev Biol. 2000;222(1):41–54 [DOI] [PubMed] [Google Scholar]
- 51. Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol. 2009;15(42):5249–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang L, Wang M, Kang X, et al. Oxidative stress and asthma: proteome analysis of chitinase-like proteins and FIZZ1 in lung tissue and bronchoalveolar lavage fluid. J Proteome Res. 2009;8(4):1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen L, Wu W, Dentchev T, et al. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004;79(2):239–247 [DOI] [PubMed] [Google Scholar]
- 54. Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53(2):172–209 [PubMed] [Google Scholar]
- 55. Nishikawa KC, Millis AJ. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 2003;287(1):79–87 [DOI] [PubMed] [Google Scholar]
- 56. Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441(7091):362–365 [DOI] [PubMed] [Google Scholar]
- 57. Mazzalupo S, Wong P, Martin P, Coulombe PA. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev Dyn. 2003;226(2):356–365 [DOI] [PubMed] [Google Scholar]
- 58. Li W, Chen YT, Hayashida Y, et al. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol. 2008;214(1):114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joo JH, Kim YH, Dunn NW, Sugrue SP. Disruption of mouse corneal epithelial differentiation by conditional inactivation of pnn. Invest Ophthalmol Vis Sci. 2010;51(4):1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27(1):182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Y, Call MK, Yeh LK, et al. Aberrant expression of a beta-catenin gain-of-function mutant induces hyperplastic transformation in the mouse cornea. J Cell Sci. 2010;123:1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–4786 [DOI] [PubMed] [Google Scholar]
- 64. Lyngholm M, Hoyer PE, Vorum H, Nielsen K, Ehlers N, Mollgard K. Immunohistochemical markers for corneal stem cells in the early developing human eye. Exp Eye Res. 2008;87(2):115–121 [DOI] [PubMed] [Google Scholar]
- 65. Li S, Nikulina K, DeVoss J, et al. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49(1):34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beitch I. The induction of keratinization in the corneal epithelium: a comparison of the “dry” and vitamin A-deficient eyes. Invest Ophthalmol. 1970;9(11):827–843 [PubMed] [Google Scholar]
- 67. Jones DT, Monroy D, Ji Z, Pflugfelder SC. Alterations of ocular surface gene expression in Sjogren's syndrome. Adv Exp Med Biol. 1998;438:533–536 [DOI] [PubMed] [Google Scholar]
- 68. Li S, Gallup M, Chen YT, McNamara NA. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(5):2466–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gage PJ, Qian M, Wu D, Rosenberg KI. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317(1):310–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar S, Duester G. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol. 2010;340(1):67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.