Abstract
Maintenance of redox homeostasis is critical for the survival of all aerobic organisms. In the budding yeast Saccharomyces cerevisiae, as in other eukaryotes, reactive oxygen species (ROS) are generated during metabolism and upon exposure to environmental stresses. The abnormal production of ROS triggers defense mechanisms to avoid the deleterious consequence of ROS accumulation. Here, we show that the Rho1 GTPase is necessary to confer resistance to oxidants in budding yeast. Temperature-sensitive rho1 mutants (rho1ts) are hypersensitive to oxidants and exhibit high accumulation of ROS even at a semipermissive temperature. Rho1 associates with Ycf1, a vacuolar glutathione S-conjugate transporter, which is important for heavy metal detoxification in yeast. Rho1 and Ycf1 exhibit a two-hybrid interaction with each other and form a bimolecular fluorescent complex on the vacuolar membrane. A fluorescent-based complementation assay suggests that the GTP-bound Rho1 associates with Ycf1 and that their interaction is enhanced upon exposure to hydrogen peroxide. The rho1ts mutants also exhibit hypersensitivity to cadmium, while cells carrying a deletion of YCF1 or mutations in a component of the Pkc1–MAP kinase pathway exhibit little or minor sensitivity to oxidants. We thus propose that Rho1 protects yeast cells from oxidative stress by regulating multiple downstream targets including Ycf1. Since both Rho1 and Ycf1 belong to highly conserved families of proteins, similar mechanisms may exist in other eukaryotes.
CELLS growing aerobically are constantly exposed to ROS, which are generated during normal cellular metabolism and upon exposure to oxidants or metals. Although ROS can regulate several intracellular signaling pathways, these molecules can damage DNAs, proteins, and lipids. Thus maintenance of the intracellular redox state is critical for cellular integrity (Finkel 2003). The abnormal production of ROS leads to the induction of defense mechanisms to avoid the deleterious consequence of ROS accumulation, and oxidative stress occurs when cells cannot efficiently neutralize or eliminate ROS. Several studies in the budding yeast Saccharomyces cerevisiae, including genome-wide expression profiling, have identified many genes whose transcripts or protein levels are elevated or repressed in response to oxidants (Morgan et al. 1997; Godon et al. 1998; Lee et al. 1999; Gasch et al. 2000; Cohen et al. 2002; He and Fassler 2005). These studies have provided insight into the regulatory responses and the oxidative stress response regulons including the two transcription factors Yap1 and Skn7. However, it is not clear how these gene products function to protect cells from oxidative stress. It is also noteworthy that most genes required for resistance to oxidative stress are not induced in response to oxidative stress (Thorpe et al. 2004). How cells respond to and recover from oxidative stress is thus largely unknown.
The Rho1 GTPase in budding yeast is involved in a number of different signaling events including the cell wall integrity (CWI) pathway, which is activated by various stresses such as heat shock, hypo-osmotic shock, and nutritional stress (Levin 2005; Park and Bi 2007). Rho1 activates Pkc1, a yeast homolog of mammalian protein kinase C, which participates in activating a MAP kinase (MAPK)-activation cascade composed of a MEKK (Bck1), a redundant pair of MEKs (Mkk1/2), and a MAPK (Mpk1/Slt2) in response to cell wall stresses (Lee and Levin 1992; Kamada et al. 1995; Harrison et al. 2004). Rho1 regulates actin organization via the CWI pathway (Delley and Hall 1999; Harrison et al. 2001) and by activating the formin Bni1 (Kohno et al. 1996; Evangelista et al. 1997; Dong et al. 2003). Rho1 also regulates 1,3-β-glucan synthesis as a direct regulatory subunit of glucan synthase (encoded by FKS1 and GSC2/FKS2) (Drgonova et al. 1996; Qadota et al. 1996). A systematic analysis of several high-temperature–sensitive (ts) mutations of RHO1 led to identification of the distinct functional domains of Rho1—one group of rho1ts mutants including rho1-2 and rho1-5 is defective in activation of Pkc1, while another group including rho1-3 is defective in activation of glucan synthase (Saka et al. 2001). Rho1 exhibits a two-hybrid interaction with Skn7 (Alberts et al. 1998), which regulates the osmotic or oxidative stress response genes (He et al. 2009). It is not clear, however, whether Rho1 or the cell integrity MAPK cascade is activated by oxidative stress.
Cells lacking Rom2, a guanine nucleotide exchange factor (GEF) for Rho GTPases, are hypersensitive to oxidants, suggesting possible involvement of Rho1 or other GTPases in the oxidative stress response (Park et al. 2005; Vilella et al. 2005). Interestingly, another Rho1 GEF, Tus1, was shown to interact with Ycf1 (yeast cadmium factor) by a membrane two-hybrid analysis and co-immunoprecipitation (Paumi et al. 2007). Ycf1 is a vacuolar glutathione S-conjugate transporter of the ATP-binding cassette family, and plays an important role in detoxifying metals such as cadmium and arsenite (Li et al. 1997). Tus1 stimulates Ycf1 transporter activity in a Rho1-dependent manner (Paumi et al. 2007). Numerous studies suggest that metals induce oxidative stress in a variety of cell types (Ercal et al. 2001; Valko et al. 2005). For example, cadmium is a nonredox metal that has been shown to induce oxidative stress by increasing ROS indirectly in S. cerevisiae and neurons (Brennan and Schiestl 1996; López et al. 2006; Cuypers et al. 2010). These previous studies provided a potential link between Rho1 and Ycf1, but also raised some important questions. Does Ycf1 act upstream of Rho1 or as a downstream effector of Rho1? Does Tus1 activate Rho1 on the vacuolar membrane? Rho1 localizes to the plasma membrane and to other sites including bud tips, the mother-bud neck, and endomembranes (McCaffrey et al. 1991; Drgonova et al. 1996; Qadota et al. 1996; Yoshida et al. 2009), while Ycf1 localizes to the vacuolar membrane (Wemmie and Moye-Rowley 1997; Mason and Michaelis 2002). Tus1 localizes to the presumptive bud site in unbudded cells and to the mother-bud neck during cytokinesis (Yoshida et al. 2006; Kono et al. 2008), but has not been observed on the vacuolar membrane.
These remaining questions led us to investigate a possible role of Rho1 under oxidative stress and the potential interaction between Rho1 and Ycf1 in vivo. Here we report that Rho1 is necessary to confer resistance to oxidants and that Rho1 interacts with Ycf1 in a GTP-dependent manner. Our findings thus suggest that Rho1 is involved in reducing ROS in the cell by regulating Ycf1 and other downstream targets.
Materials and Methods
Plasmids and yeast strains
Standard methods of yeast genetics and recombinant DNA manipulation were used (Guthrie and Fink 1991; Ausubel et al. 1999). Yeast cells were grown under standard growth conditions at 30° unless otherwise indicated. Yeast strains used in this study are listed in Table 1. Details of plasmid constructions are described in supporting information, File S1, and plasmids used in this study are listed in Table S1.
Table 1. Yeast strains used in this study.
| Strain | Relevant genotypea | Source/comments | |
|---|---|---|---|
| NY2284* | α | ura3 leu2 trp1 his3 ade2 lys2 rho1Δ::HIS3 ade3::RHO1::LEU2 | Guo et al. (2001) |
| NY2285* | a | rho1Δ::HIS3 ade3::rho1-2E45V::LEU2 | Guo et al. (2001) |
| NY2286* | α | rho1Δ::HIS3 ade3::rho1-3L60P::LEU2 | Guo et al. (2001) |
| NY2287* | a | rho1Δ::HIS3 ade3::rho1-5G121C::LEU2 | Guo et al. (2001) |
| HPY1710* | α | YCF1-VN::kanMX6 | See text |
| HPY1737* | α | tus1Δ::TRP1 YCF1-VN::kanMX6 | See text |
| HPY1738* | α | ycf1Δ::kanMX4 | See text |
| HPY1739* | a | rho1Δ::HIS3 ade3::rho1-5G121C::LEU2 ycf1Δ::kanMX4 | See text |
| HPY1574* | α | RHO1::GFP-RHO1-URA3 | Derived from NY2284b |
| HPY1730* | a | rho1-2::GFP-RHO1-URA3 | Derived from NY2285b |
| HPY1731* | α | rho1-3::GFP-RHO1-URA3 | Derived from NY2286b |
| HPY1732* | a | rho1-5::GFP-RHO1-URA3 | Derived from NY2287b |
| HPY1955* | α | YCF1-GFP::TRP1 | See text |
| EG123# | α | ura3-52 leu2-3,112 trp1-1 his4 can1 | Same as HPY11, Park et al. (1993) |
| DL106# | α | pkc1∆::LEU2 YCp50-PKC1 | Levin and Bartlett-Heubusch (1992) |
| DL511# | α | pkc1∆::LEU2 YCp50-pkc1-1 | Levin and Bartlett-Heubusch (1992) |
| DL506# | α | pkc1∆::LEU2 YCp50-pkc1-2 | Levin and Bartlett-Heubusch (1992) |
| DL504# | α | pkc1∆::LEU2 YCp50-pkc1-3 | Levin and Bartlett-Heubusch (1992) |
| DL253# | α | bck1Δ::URA3 | Lee and Levin (1992) |
| JVG216# | a | mpk1Δ::TRP1 | Krause and Gray (2002) |
| BY4741@ | a | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| HPY1904@ | a | ycf1Δ::kanMX4 | Open Biosystems |
| HPY1905@ | a | ybt1Δ::kanMX4 | Open Biosystems |
| HPY1906@ | a | bpt1Δ::kanMX4 | Open Biosystems |
| THY AP4^ | a | leu2, ura3, trp1::(lexAop)-lacZ (lexAop)-HIS3 (lexAop)-ADE2 | Obrdlik et al. (2004) |
| YCF1–CT^ | a | YCF1-Cub-LexA-VP16 KanMX | Derived from THY AP4 |
| ArBT–CT^ | a | SHO1::MFαSS-CD4tm-Cub-LexA-VP16 KanMX | Derived from THY AP4 |
| YCF1–CT ΔT^ | a | YCF1-Cub-LexA-VP16 KanMX tus1Δ::NatR | Derived from THY AP4 |
| ArBT–CT ΔT^ | a | SHO1::MFαSS-CD4tm-Cub-LexA-VP16 KanMX tus1Δ::NatR | Derived from THY AP4 |
Strains marked with * are isogenic to NY2284, except as indicated; strains marked with @ are isogenic to BY4741, except as indicated; strains marked with # are isogenic to EG123 (Park et al. 1993), except as indicated; strains marked with ^ are isogenic to THY AP4 except as indicated; and the background of the strains marked with * and @ is S288C.
pRS306–GFP–RHO1 (pHP1699) was integrated into the RHO1 locus after digestion with BglII.
Plate assays
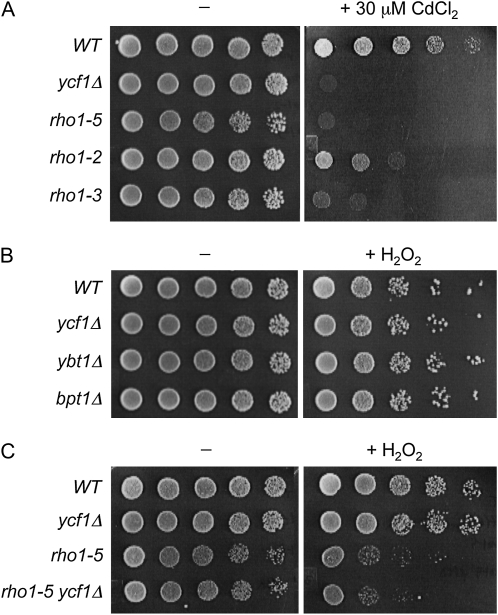
The sensitivity of the rho1ts mutants to paraquat (Sigma-Aldrich) and diethyl maleate (DEM) (Sigma-Aldrich) was determined at 30°, as previously described (Singh et al. 2008) with slight modification. The wild-type and rho1ts strains were diluted to OD600 = 0.4 from mid-to-late logarithmic-phase cultures in YPD and then serially diluted as indicated. These cells were spotted on YPD plates containing 400 µg/ml paraquat, 1 mM DEM, or no oxidant. The plates were incubated at 30° for 2–5 days. To test the sensitivity to H2O2, cells were diluted to OD600 = 0.8 and then treated with 2 or 3 mM H2O2 for 200 min before plating on YPD or SC plates as indicated. The sensitivity to various concentrations of H2O2 was tested by halo assays. First, cells from a mid logarithmic-phase culture were diluted to OD600 = 0.2. To make a lawn of cells, 200 µl of the diluted culture was spread on YPD or SC plates as indicated. Sterilized filter disks (Whatman filter paper) were placed on the plate and then soaked with 5 µl of H2O2 (concentrations ranging from 0.1 to 4 M). The plates were then incubated at 30° for 1–2 days to monitor zones of growth inhibition around the filter disks.
The sensitivity of the pkc1 mutants (gifts from D. Levin, Johns Hopkins University, Baltimore, MD) to H2O2 was tested similarly except that fivefold serial dilutions were made starting from OD600 = 1, and cells were plated on SC−Ura containing 1 M sorbitol after treatment with 2 mM H2O2 for 200 min or after mock treatment. The bck1Δ and mpk1Δ mutants (gifts from J. Gray, University of Glasgow, Glasgow, UK) were tested similarly, except plated on the YPD plates. The laboratory wild-type strains exhibited varying degrees of sensitivity to H2O2 depending on the background: BY4741 was more sensitive to H2O2 compared with other wild-type strains tested (Figure 9, B and C and Figure S1), as previously reported (Higgins et al. 2002; Singh et al. 2008). The sensitivity of the ycf1Δ and rho1-5 mutants to cadmium was tested by making fivefold serial dilutions starting from OD600 = 2, followed by plating on SC containing 30 μM CdCl2. The plates were then incubated at room temperature for 3–7 days.
Figure 9 .
rho1ts mutants are hypersensitive to cadmium while ycf1Δ mutant are slightly sensitive to H2O2. (A) Fivefold serial dilutions (from left to right, starting from OD600 = 2) of wild-type (NY2284), ycf1Δ (HPY1738), rho1-5 (NY2287), rho1-2 (NY2285), and rho1-3 (NY2286) strains, all of which are in the isogenic background, were grown on SC or SC plate containing 30 μM CdCl2 at room temperature for 4 days (−) or 7 days (+30 μM CdCl2). (B) Fivefold serial dilutions (from left to right, starting from OD600 = 1) of wild type (BY4741) and isogenic deletion mutants of vacuolar transporters (ycf1Δ, ybt1Δ, and bpt1Δ) were treated with 2 mM H2O2 or mock treated, spotted on SC plates, and incubated at 30° for 2 days. (C) Fivefold serial dilutions (from left to right, starting from OD600 = 1) of wild type (NY2284), ycf1Δ (HPY1738), rho1-5 (NY2287), and rho1-5 ycf1Δ (HPY1739) were treated as in Figure 9B.
Determination of ROS accumulation
ROS accumulation was monitored indirectly by fluorescence microscopy and flow cytometry, as previously described (Singh et al. 2008) with slight modifications. The rho1ts mutant cells, grown overnight in YPD at room temperature, were diluted threefold and grown for 3 additional hours. These cells were incubated with dihydrorhodamine 123 (DHR) (Sigma Chemical) for 2 hr at 30°, along with or without 1 mM H2O2, and then analyzed by fluorescence microscopy with the FITC filter. For flow cytometry analysis, cells were grown similarly, except that the cultures were diluted to OD600 = 0.6 before adding DHR at 30°. Half of the cells were shifted to 37° for 2 hr, while the remaining cells were maintained at 30°. Both cultures were then analyzed with the FACSCalibur (Becton Dickinson) with λex = 488 nm excitation and FL1 (530/30 BP) filter.
Integrated membrane yeast two-hybrid analysis
Integrated membrane yeast two-hybrid (iMYTH) assays and construct generation were performed as previously described (Paumi et al. 2007; Snider et al. 2010). Construction details of the NubG fusions of Rho1 and the TUS1 deletion strains are provided in File S1. THY AP4 MYTH reporter strains, which harbor chromosomally encoded Ycf1 or unrelated control bait fused to the Cub–LexA–VP16 tag at the C terminus, were transformed with plasmids encoding NubG-tagged Rho1 or control constructs. Cells were plated on SC−Trp as a control to show the presence of prey plasmid and comparative growth between strains. The bait–prey interaction was monitored on SC−Trp−Ade−His containing X-Gal.
Fluorescence microscopy and bimolecular fluorescence complementation
Image acquisition of GFP–Rho1 was carried out essentially as previously described (Kang et al. 2001) using a Nikon E800 microscope (Nikon, Tokyo, Japan) fitted with a 100× oil-immersion objective (N.A. = 1.30), a Uniblitz electronic shutter, a Prior Z-axis drive, and a Hamamatsu Orca ER cooled charge-coupled device. A series of optical sections was captured at 0.3-µm intervals using Slidebook software (Intelligent Imaging Innovations, Denver, CO) by exposing for 1 sec. Cells were treated with 1–2 mM H2O2 for 2–4 hr or mock treated, where indicated, before imaging.
Bimolecular fluorescence complementation (BiFC) assays were performed as previously described (Singh et al. 2008; Kang et al. 2010) with slight modifications. Rho1 was fused to the C-terminal fragment of YFP (YFPC) at its N terminus and was expressed from a CEN or 2μ plasmid (where indicated). Ycf1 was fused to the N-terminal fragment of Venus (VN), a variant YFP (Nagai et al. 2002), at its C terminus, and was expressed from its chromosomal locus (see File S1 for details of the plasmid and strain construction). To monitor BiFC signals, a single optical section was captured using the YFP filter by exposing cells to UV for 8 sec. Imaging and image processing were performed under identical conditions for all BiFC assays. Where indicated, the vacuolar membrane was visualized by staining cells with FM4-64 for 30 min at room temperature as previously described (Vida and Emr 1995). Localization pattern and pixel intensity of the bimolecular fluorescent complex and Ycf1–GFP were analyzed by counting at least 100 cells per experiment from three independent experiments. Image analysis and processing were performed with ImageJ software, and the data are presented as mean ± SD. Statistical significance was determined using Student's t-test.
Results
rho1ts mutants are hypersensitive to various oxidants
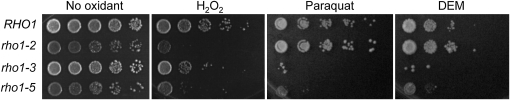
To determine whether RHO1 regulates the cellular response to oxidative stress, we examined sensitivity of the rho1ts mutants, rho1-2, rho1-3, and rho1-5, to oxidants including paraquat, diethyl maleate (DEM), and hydrogen peroxide (H2O2). Paraquat is a superoxide-generating agent (Cochemé and Murphy 2008), and DEM is a thiol-specific oxidant that depletes glutathione in the cell (Nguyên-Nhu and Knoops 2002). Both drugs increase intracellular ROS levels. Hydrogen peroxide is in itself poorly reactive but can be readily converted to the highly reactive hydroxyl radical upon exposure to UV or by interaction with metal ions (Valko et al. 2005). When serial dilutions of these rho1 mutants were spotted on rich plates containing paraquat or DEM at 30°, rho1-3 and rho1-5 were hypersensitive to these oxidants compared to wild type, while rho1-2 exhibited little sensitivity to these drugs (Figure 1). These rho1 mutants also exhibited sensitivity to H2O2 to different extents, with rho1-2 and rho1-5 being particularly hypersensitive to H2O2 (Figure 1 and Figure S1A). Taken together, these results suggest that Rho1 is necessary to confer resistance to oxidants.
Figure 1.
The rho1ts mutants are hypersensitive to oxidants. Fivefold serial dilutions (from left to right) of wild-type (NY2284), rho1-2 (NY2285), rho1-3 (NY2286), and rho1-5 (NY2287) cells were grown at 30° for 2–4 days on YPD after treating with 3 mM H2O2 for 200 min or mock treatment, and on YPD containing 400 μg/ml of paraquat or 1 mM DEM.
Cells of the rho1ts mutants exhibit high ROS accumulation
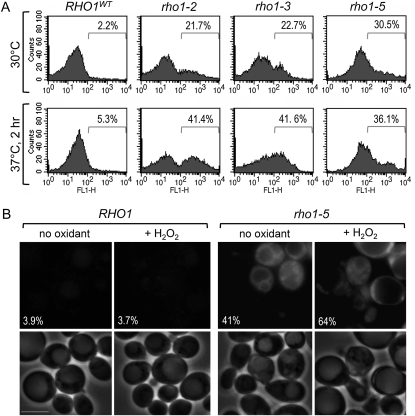
To test whether the hypersensitivity of the rho1ts mutants to oxidants resulted from its specific defect in maintaining cellular redox balance rather than general sickness, we indirectly monitored the intracellular ROS level using DHR, which becomes fluorescent rhodamine 123 upon oxidation (Herker et al. 2004). When these cells were examined by flow cytometry after adding DHR, we found that a high level of ROS was present in the rho1 mutants even when they were grown at the semipermissive temperature of 30°, but not in wild-type cells (Figure 2A). A higher percentage of the rho1 mutant cells exhibited increased fluorescence upon shifting the cultures to 37° (Figure 2A). When the rho1-5 mutant was examined under the fluorescence microscope, high fluorescence was observed in the cytoplasm at 30° and in an even higher percentage of the cells after exposure to H2O2 (Figure 2B). These results thus suggest that ROS were not efficiently removed in the cytoplasm of the rho1ts mutants.
Figure 2 .
The rho1ts mutants exhibit a high level of ROS accumulation. (A) FACS analysis of wild-type (NY2284) and rho1 mutant cells (NY2285–NY2287) grown at 30° or shifted to 37° for 2 hr and stained with DHR. Histograms of single representative experiment are shown from three independent experiments. n = 10,000 for each sample. (B) Cells of wild type and rho1-5 were grown at 30° and visualized by fluorescence microscopy after staining with DHR during a 2-hr incubation with or without 1 mM H2O2. Fluorescence (top) and phase contrast (bottom) images of representative cells are shown from three independent experiments (n = 450–650 cells for each sample), and the average percentages of the cells with detectable fluorescence are shown. Bars, 5 μm.
The Pkc1–MAPK pathway may play a minor role under oxidative stress
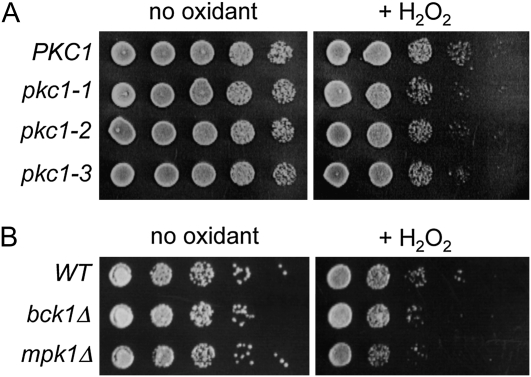
What is the downstream target of Rho1 that is involved in the oxidative stress response? Since rho1-2 and rho1-5, which are defective in activating Pkc1 (Saka et al. 2001), were hypersensitive to H2O2, we wondered whether Rho1 regulates the Pkc1–MAPK pathway under oxidative stress. We thus examined the sensitivity of pkc1ts mutants to H2O2. A pkc1Δ mutant with a plasmid carrying the pkc1 allele, pkc1-1, pkc1-2, or pkc1-3, exhibited slight sensitivity to H2O2 at 25–33° on the plate containing sorbitol as an osmotic stabilizer and cell wall protective agent (Figure 3A). Similarly, we found that cells lacking the downstream components of Pkc1, bck1Δ and mpk1/slt2Δ, were also slightly more sensitive to H2O2 than wild type (Figure 3B), suggesting that the Pkc1–MAPK pathway may play a minor role in recovery from oxidative stress.
Figure 3 .
The pkc1 mutants and cells lacking downstream components of Pkc1 are mildly sensitive to H2O2. (A) Fivefold serial dilutions (from left to right starting from OD600 = 1) of pkc1Δ mutant carrying YCp–PKC1 (DL106), YCp–pkc1-1 (DL511), YCp–pkc1-2 (DL506), and YCp–pkc1-3 (DL504) were grown at 25, 30, and 33° for 2–4 days on SC−Ura plates containing 1 M sorbitol after treatment with 2 mM H2O2 for 200 min or mock treatment. The results were about the same at all temperatures tested, and only the plate at 30° is shown. (B) Cells of bck1Δ (DL253) and mpk1Δ (JVG216) mutants were treated similarly, except plated on YPD plates at 30°.
Rho1 interacts with Ycf1 in vivo
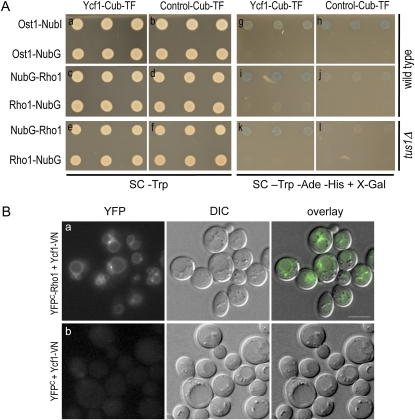
Since the phenotype of the pkc1 or mpk1 mutant upon exposure to H2O2 was much milder than that of rho1 mutants, Rho1 might regulate another downstream target involved in the oxidative stress response. Because Tus1 interacts with Ycf1 (Paumi et al. 2007), we wondered whether Ycf1 might be such a Rho1 target. We hypothesized that dysfunction of Ycf1 in the rho1ts mutants might lead to increased ROS accumulation in the cytoplasm. To test whether Rho1 interacts with Ycf1 in vivo, first we performed an integrated split-ubiquitin membrane yeast two-hybrid (iMYTH) analysis (Snider et al. 2010). The reporter strains, which harbored chromosomally encoded Ycf1 or unrelated control bait fused to Cub–LexA–VP16, were transformed with a plasmid encoding NubG-tagged Rho1 or a control construct (see Materials and Methods and File S1) and then plated onto SC−Trp (Figure 4A, a–f). The bait–prey interactions were then determined by monitoring growth and β-galactosidase expression on SC plates lacking Trp, Ade, and His but containing X-Gal (Figure 4A, g–l). Both Ycf1–Cub–LexA–VP16 and the control bait strains grew and exhibited blue color in the presence of the positive control prey Ost1–NubI (Figure 4A, g and h, top row) but not in the presence of the noninteracting control prey Ost1–NubG (Figure 4A, g and h, bottom row). The Ycf1 strain, but not the control bait, exhibited robust growth and blue color in the presence of NubG–Rho1 (Figure 4A, i and j, top row). In contrast, the reporter strain expressing Rho1 with the NubG tag at its C terminus (Rho1–NubG) or the control bait strain did not show such growth and blue coloration (Figure 4A, i and j, bottom row). This absence of interaction is likely due to the C-terminal NubG tag preventing proper membrane targeting of Rho1. Taken together, these data indicate that Rho1 interacts specifically with Ycf1 in vivo.
Figure 4 .
Rho1 associates with Ycf1 in vivo. (A) iMYTH assays to determine the Rho1–Ycf1 interaction. THY AP4 MYTH reporter strains, expressing either C-terminally Cub–LexA–VP16 tagged Ycf1 or unrelated control bait, carry NubG-tagged Rho1 or other control plasmid as indicated. These cells were plated on SC−Trp to show the presence of prey plasmid and comparative growth among strains (a–f) and on SC−Trp−Ade−His containing X-Gal to monitor bait–prey interactions (g–l). Strains used for each panel are: YCF1–CT (a, c, g, and i); ArBT–CT (b, d, h, and j); YCF1–CT ΔT (e and k); and ArBT–CT ΔT (f and l). (B) BiFC assays were performed in wild-type cells (HPY1710), which express Ycf1–VN from its chromosomal locus and carry pRS316–YFPC–RHO1 (a) or YCp50–YFPC (b). Cells were grown in SC−Ura at 30°. Images were captured with the YFP filter for 8-sec exposures. Fluorescent images (YFP), DIC images (DIC), and fluorescent images overlaid with the DIC images (overlay) are shown for the representative cells. Bars, 5 μm. See Figure 5B for quantitation of the localization pattern of the Rho1–Ycf1 bimolecular fluorescent complex.
Next, we performed a BiFC assay to monitor the Rho1–Ycf1 interaction in vivo. This assay allows visualization of protein–protein associations in live cells by monitoring YFP fluorescence, which appears when truncated YFP fragments (YFPN and YFPC) are brought together by association of the two proteins fused to them (Hu et al. 2002). We expressed YFPC–Rho1 from a low-copy plasmid in a strain expressing Ycf1–VN from the chromosome (Materials and Methods). Ycf1–VN and YFPC–Rho1 were partially functional on the basis of complementation of cadmium sensitivity of ycf1Δ and H2O2 sensitivity of rho1–5, respectively (Figure S2, A and B). When these fusion proteins were coexpressed, the majority of cells exhibited a strong YFP signal on the vacuolar membrane (Figure 4B, a). Some of these cells also showed one or two fluorescent puncta at the sites where two vacuolar lobes overlapped (see Figure 5B for quantitation). In contrast, no cells exhibited detectable fluorescence in a control strain that coexpressed Ycf1–VN and YFPC (without a Rho1 fusion) (Figure 4B, b). These results thus indicate that Rho1 interacts directly or closely associates with Ycf1 in vivo.
Figure 5 .
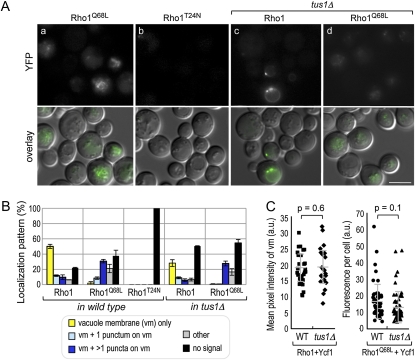
The Rho1–Ycf1 bimolecular fluorescent complex formation is dependent on the GTP-bound state of Rho1 in vivo. (A) BiFC assays were performed in the YCF1–VN strain (HPY1710), which carries (a) pRS316–YFPC–RHO1Q68L or (b) pRS316–YFPC–RHO1T24N, and the YCF1–VN tus1Δ strain (HPY1737), which carries (c) pRS316–YFPC–RHO1 or (d) pRS316–YFPC–RHO1Q68L. Cells were grown in SC−Ura at 30°. Images were captured, processed, and presented as in Figure 4B. Bar, 5 μm. (B) Localization pattern of the Rho1–Ycf1 bimolecular fluorescent complex: the vacuolar membrane (vm) only; the vm and a punctum on the vm; the vm and a few puncta on the vm; and others, which are mixed patterns with diffuse signal often enriched in the vacuole. Localization pattern of bimolecular fluorescent complex was quantitated from three independent experiments (n = 300–400), and mean (%) ± SD are shown. (C, left) Mean pixel intensity of the vacuolar membrane of each individual cell was plotted and quantified using ImageJ software: WT (HPY1710 with pRS316–YFPC–RHO1), 18.7 ± 5.0 (in arbitrary units, a.u.) and tus1Δ (HPY1737 with pRS316–YFPC–RHO1), 19.5 ± 6.3 (in a.u.) (P = 0.6). (Right) Fluorescence intensity of each individual cell was analyzed: WT (HPY1710 with pRS316–YFPC–RHO1Q68L), 16.9 ± 10.9 (in a.u.) and tus1Δ (HPY1737 with pRS316–YFPC–RHO1Q68L), 13.5 ± 9.4 (in a.u.) (P = 0.1).
Association of Rho1 with Ycf1 is likely to depend on its GTP-bound state
Next, to determine whether Rho1 interacts with Ycf1 in a GTP-dependent manner, we expressed YFPC–Rho1Q68L and YFPC–Rho1T24N, which are expected to be in the GTP- and GDP-locked state in vivo, respectively (Nonaka et al. 1995), in the YCF1–VN strain. Cells coexpressing YFPC–Rho1Q68L and Ycf1–VN exhibited BiFC signals (Figures 5A, a), although the percentage of cells with little signal was increased (see Discussion). In contrast, cells coexpressing YFPC–Rho1T24N and Ycf1–VN showed little fluorescence (Figure 5A, b). The Rho1Q68L–Ycf1 BiFC signal was often observed on the vacuolar membrane and in several puncta on the vacuolar membrane (see Figure 5B for quantitation). The YFP signals in these cells appeared less discrete than those observed in the cells coexpressing YFPC–Rho1 and Ycf1–VN. This is likely due to the vacuolar shape in cells expressing YFPC–Rho1Q68L, as visualized by differential interference contrast (DIC) microscopy. Staining with FM4-64 also revealed different morphology of the vacuolar membrane in these cells (Figure S3). Despite these differences, these data thus suggest that Rho1–GTP interacts with Ycf1.
We hypothesized that the formation of the Rho1–Ycf1 complex would depend on Tus1, which converts Rho1 to the GTP-bound state. To test this idea, we performed BiFC assays in a strain deleted for TUS1. When the interaction between YFPC–Rho1 and Ycf1–VN was examined in the tus1Δ mutant, fewer cells indeed show a detectable BiFC signal (compare Figure 5A, c to Figure 4B, a). However, a significant percentage of tus1Δ cells still showed the Rho1–Ycf1 bimolecular fluorescent complex (Figure 5B). When fluorescence of these cells with positive BiFC signals was compared, the mean pixel intensity of the vacuolar membrane was about the same in wild-type and tus1Δ cells (Figure 5C). Consistent with the BiFC results, iMYTH analysis in a tus1Δ reporter strain indicated that Rho1 interacts with Ycf1 specifically even in the absence of TUS1 (Figure 4A, k and l). It is thus likely that another GEF compensates for the loss of Tus1 in tus1Δ cells. The Rho1Q68L–Ycf1 bimolecular fluorescent complex was also observed in tus1Δ cells (Figure 5A, d and 5B), and the total fluorescence intensity in individual cells was not statistically different between wild type and the tus1Δ mutant.
The Rho1–Ycf1 interaction may increase upon exposure to H2O2
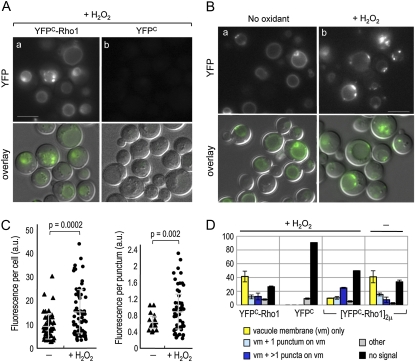
Since Ycf1 formed a bimolecular fluorescent complex with the GTP-locked Rho1 but not with the GDP-locked Rho1, Rho1 might be activated upon exposure to oxidants and thus form more Rho1–Ycf1 bimolecular fluorescent complex. To test the idea, we performed BiFC assays in cells coexpressing Ycf1–VN and YFPC–Rho1 after treatment with H2O2. While the BiFC signals appeared on the vacuolar membrane similar to those in untreated cells, more cells showed several puncta with stronger fluorescence on the vacuolar membrane (Figure 6A, a). This is particularly evident in cells expressing YFPC–Rho1 from a multicopy plasmid after exposure to H2O2 (Figure 6B, b and 6D). Quantification of fluorescence intensity of these cells indeed indicated that the pixel intensity of individual cells and in each punctum increased from 9.57 ± 5.4 to 15.13 ± 9.9 (in arbitrary units, a.u.; P = 0.0002) and from 0.64 ± 0.1 to 0.94 ± 0.5 (P = 0.002), respectively, after H2O2 treatment (Figure 6C). Despite the cell-to-cell variation, these differences are statistically significant. Cells expressing Ycf1–VN and YFPC (without the Rho1 fusion), however, did not show such signal after H2O2 treatment (Figure 6A, b), suggesting that these dots represent the Rho1–Ycf1 bimolecular fluorescent complex rather than any other endogenous proteins that became fluorescent after H2O2 treatment. The fluorescence signal was occasionally observed in the vacuolar lumen in some cells upon exposure to H2O2, which might result from mistargeting of the bimolecular fluorescent complex under oxidative stress. Taken together, these results suggest that the interaction between Rho1 and Ycf1 increased after H2O2 exposure (see Discussion).
Figure 6 .
The Rho1–Ycf1 bimolecular fluorescent complex formation after exposure to H2O2. (A) BiFC assays were performed in the YCF1–VN strain (HPY1710), carrying (a) pRS316–YFPC–RHO1 or (b) YCp–YFPC (pHP1730). Cells were grown in SC−Ura at 30° and incubated with 2 mM H2O2 for 2 hr before imaging. Images were captured, analyzed, and presented as in Figure 4B. Bar, 5 μm. (B) BiFC assays were performed in HPY1710, carrying pRS426–YFPC–RHO1. Cells were grown in SC−Ura at 30° and incubated with 2 mM H2O2 for 4 hr (+H2O2) or mock treated (no oxidant) before imaging. Images were captured, analyzed, and presented as in Figure 4B. Bar, 5 μm. (C, left) Fluorescence intensity of individual cells of HPY1710 with pRS426–YFPC–RHO1 was plotted and quantified using ImageJ software: pixel intensity in untreated cells, 9.57 ± 5.4 (in a.u.) and in cells treated with 2 mM H2O2 for 4 hr, 15.1 ± 9.9 (in a.u.) (P = 0.0002). (C, right) Fluorescence intensity of each punctum in HPY1710 with pRS426–YFPC–RHO1 was analyzed similarly: pixel intensity in untreated cells, 0.64 ± 0.1 (in a.u.) and in cells treated with 2 mM H2O2 for 4 hr, 0.94 ± 0.5 (in a.u.) (P = 0.002). (D) Localization pattern of the Rho1–Ycf1 bimolecular fluorescent complex was analyzed as in Figure 5B from strains HPY1710 with pRS316–YFPC–RHO1, YCp–YFPC, or pRS426–YFPC–RHO1 after treatment with H2O2 for 4 hr and HPY1710 with pRS426–YFPC–RHO1 after mock treatment. Data are from three independent experiments (n = 300–400), and mean (%) ± SD are shown.
Localization of GFP–Rho1 remains similar, while the Ycf1–GFP level is elevated after exposure to H2O2
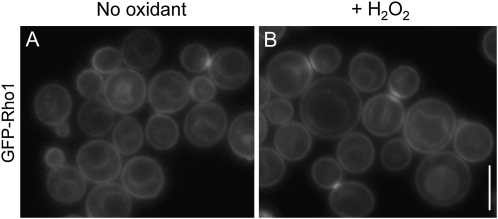
We questioned whether localization of the Rho1–Ycf1 bimolecular fluorescent complex indeed indicates the sites at which these two proteins interact with each other in vivo and how localization of Rho1 and Ycf1 is affected upon exposure to H2O2. We thus examined localization of Rho1 before and after exposure to H2O2 using a strain, which expressed GFP–Rho1 under its native promoter from the chromosome. Expression of GFP–Rho1 in rho1ts mutants restored the resistance to H2O2, although less efficiently than wild type (compare Figure S2C to Figure 1), indicating that GFP–Rho1 was partially functional. GFP–Rho1 localized to the plasma membrane and to the sites of polarized growth as well as to the vacuolar membrane as expected (Figure 7a). This localization pattern of GFP–Rho1 remained similar after exposure to H2O2, although diffuse signals were also occasionally seen in the vacuolar lumen in some cells (Figure 7b). Thus, Rho1 is likely to interact with Ycf1 on the vacuolar membrane where the two proteins colocalize, and localization of GFP–Rho1 is mostly unaffected by H2O2.
Figure 7 .
Localization of GFP–Rho1, expressed from the chromosome, was examined in wild-type cells (HPY1574), grown in SC−Ura at 30°, (A) before and (B) after exposure to 2 mM H2O2 for 2 hr. A series of Z sections was captured with the GFP filter and a single, representative Z section is shown. Bar, 5 μm.
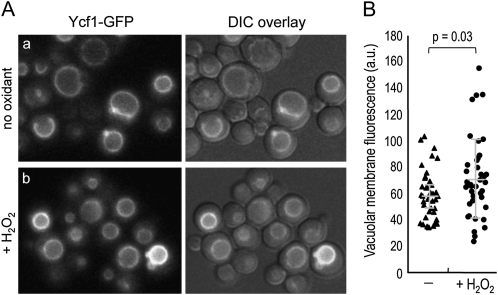
We next examined localization of Ycf1–GFP, which was expressed from the YCF1 chromosomal locus. While Ycf1–GFP localized to the vacuolar membrane similarly before and after exposure to H2O2 (Figure 8A), the mean pixel intensity of the vacuolar membrane increased from 59.2 ± 18.8 to 71.6 ± 30.1 (in a.u.) after H2O2 treatment (Figure 8B). This increase is statistically significant (P = 0.03), albeit rather heterogeneous among individual cells, suggesting that the Ycf1 level is elevated under oxidative stress.
Figure 8 .
(A) Localization of Ycf1–GFP was examined in the YCF1–GFP strain (HPY1955), grown in SC−Trp at 30°, before and after exposure to 2 mM H2O2 for 2 hr. A series of Z sections was captured with the GFP filter and a single, representative Z section is shown. (B) Fluorescence intensity of the vacuolar membrane was plotted and quantified using ImageJ software: pixel intensity in untreated cells, 59.2 ± 18.8 (in a.u.); and in cells treated with H2O2, 71.6 ± 30.1 (in a.u.) (P = 0.03).
rho1ts mutants are hypersensitive to cadmium, while an ycf1Δ mutant exhibits slight sensitivity to H2O2
On the basis of our data described above, together with previous observations (Paumi et al. 2007), we hypothesized that Rho1 activates Ycf1. If this were the case, we would expect a rho1ts mutant to be hypersensitive to cadmium and an ycf1Δ mutant to be sensitive to H2O2. To test these predictions, we examined the sensitivity of the rho1-2, rho1-3, and rho1-5 mutants to cadmium. Indeed, these rho1 mutants were sensitive to CdCl2 to different extents (Figure 9A), and the pattern of the differential sensitivity was similar to those seen for paraquat and DEM (Figure 1).
Next, we examined the H2O2 sensitivity of an ycf1Δ mutant in two strain backgrounds. A ycf1Δ mutant exhibited similar sensitivity to H2O2 compared to each isogenic wild-type strain (Figure 9, B and C) and the mutants lacking other vacuolar ABC transporters, ybt1Δ and bpt1Δ (Figure 9B). At relatively higher H2O2 concentrations, however, ycf1Δ was slightly more sensitive to H2O2 than wild type (Figure S1B). In addition, when the rho1-5 and ycf1Δ mutations were combined, the double mutant was slightly more sensitive to H2O2 than rho1-5 (Figure 9C). Taken together, these observations thus suggest that Ycf1 contributes to resistance to both metals and oxidants, although loss of YCF1 alone does not result in hypersensitivity to H2O2. These results also indicate that other targets of Rho1 as well as Ycf1 are likely to modulate cytoplasmic ROS level, since rho1ts was much more sensitive to H2O2 than ycf1Δ (see Discussion).
Discussion
Rho1 activates the “cell integrity” MAPK pathway in response to various stresses (Levin 2005), but it has not been clear whether Rho1 or any other component of the MAPK pathway is also involved in the oxidative stress response. Although the Rho1 GEF, Tus1, interacts with Ycf1 (Paumi et al. 2007), it remained unclear whether Ycf1 functions upstream or as a target of Rho1. The studies reported here now clarify some of these outstanding issues and uncover a heterogeneous and complex cellular response to oxidative stress.
Temperature-sensitive rho1 mutants were hypersensitive to oxidants and exhibited an elevated level of ROS accumulation in the cytoplasm. A membrane two-hybrid analysis and a fluorescence-based complementation assay demonstrate that Rho1 interacts with Ycf1 in vivo, likely in its GTP-bound state (see below). Together with the previous finding that Ycf1 activity depends on Rho1 (Paumi et al. 2007), our findings thus suggest that Rho1 activates Ycf1 to regulate the redox balance in the cell. Neither the ycf1Δ nor the pkc1ts mutants, however, exhibited such hypersensitivity to H2O2, suggesting that Rho1 regulates the oxidative stress response probably through multiple downstream targets. We observed high cell-to-cell variation in cellular response to oxidative stress, including the levels of Ycf1, the Rho1–Ycf1 bimolecular fluorescent complex, and ROS accumulation upon exposure to H2O2. This is likely due to a different age and physiological state of individual cells. In fact, cellular age in eukaryotes is a particularly well-known determinant of heterogeneous resistance to oxidative burden (Avery 2006).
Cells expressing the GTP-locked Rho1Q68L showed a positive BiFC signal, whereas cells expressing the GDP-locked Rho1T24N did not, suggesting that Rho1–GTP interacts with Ycf1. It is thus likely that Ycf1 is a downstream target of Rho1. The localization pattern of the Rho1Q68L–Ycf1 bimolecular fluorescent complex appeared different from that of Rho1, reflecting the different vacuole morphology in cells expressing Rho1Q68L (Figure S3). Indeed, Rho1 is also involved in vacuole membrane fusion (Eitzen et al. 2001; Logan et al. 2010). It might also correspond to the intrinsic difference between the GTP-locked Rho1 and the GTP-bound Rho1, which can cycle back to the GDP-bound state, with respect to their association with Ycf1. Although fewer cells exhibited BiFC signals with Rho1Q68L than with the wild type, this is likely due to the sickness of cells expressing Rho1Q68L (Nonaka et al. 1995), which might have caused loss of the YFPC–Rho1Q68L plasmid in some cells. Since Tus1 also interacts with Ycf1 (Paumi et al. 2007), Tus1 may facilitate the interaction between Rho1 and Ycf1 on the vacuolar membrane as well as the GDP–GTP exchange on Rho1. We were, however, unable to observe convincing Tus1 localization to the vacuolar membrane before or after exposure to H2O2, likely due to transient localization or a very weak signal of Tus1–GFP. Rho1 still interacted with Ycf1 in tus1Δ cells, albeit less efficiently, suggesting that another Rho1 GEF substitutes Tus1 function in a tus1Δ mutant.
The Rho1–Ycf1 bimolecular fluorescent complex was observed on the vacuolar membrane and occasionally as one or two dots on the vacuolar membrane. Although the exact nature of these puncta remains unclear, both patterns of the BiFC signals were dependent on Rho1 and Ycf1. Interestingly, the number of these puncta on the vacuolar membrane and their pixel intensity increased after exposure to H2O2, suggesting an increased interaction between Rho1 and Ycf1 upon exposure to H2O2. This might be due to the activation of Rho1 as well as elevation of the Ycf1 protein level upon exposure to H2O2 (Figure 8), consistent with the fact that Yap1 regulates the expression of YCF1 (Sharma et al. 2002). It is also possible that these puncta reflect the coalescence of the Rho1–Ycf1 bimolecular fluorescent complexes after exposure to H2O2. Ycf1–GFP also appeared as one or two dots on the vacuolar membrane, which are thought to be multivesicular bodies (MVBs) (C. M. Paumi, unpublished observation), in addition to the vacuolar membrane, but these puncta did not particularly increase upon exposure to H2O2 (Figure 8).
While the interaction between Rho1 and Ycf1 is clear from this study, Ycf1 is unlikely to be the only Rho1 effector involved in the oxidative stress response. Cells lacking YCF1 exhibited little (or slight) hypersensitivity to hydrogen peroxide depending on H2O2 concentration. This could be due to the functional redundancy of other vacuolar membrane-residing transporters such as Ybt1 and Bpt1. However, none of the double or triple mutants of the vacuolar transporters was as sensitive as the rho1ts mutants to H2O2 (M.-E. Lee, C. M. Paumi, and H.-O. Park, unpublished observation). Despite the lack of clear sensitivity of ycf1Δ to oxidants, a couple of observations suggest that the Rho1–Ycf1 interaction is significant to confer resistance to both metals and oxidants. The differential sensitivity of the rho1ts mutants to paraquat and DEM is correlated with their sensitivity to cadmium (Figures 1 and 9A), which is well established as an inducer of oxidative stress in various cell types including yeast (Brennan and Schiestl 1996). A ycf1 deletion confers an increased sensitivity of a rho1ts mutant to H2O2 (Figure 9C).
The unique response of each rho1ts mutant to various oxidants also suggests that the hypersensitivity to oxidants is unlikely due to the general sickness of the rho1 mutants. This observation is consistent with the idea that different oxidants may trigger cellular responses by distinct mechanisms, as previously suggested (Thorpe et al. 2004). Hydrogen peroxide is an uncharged species (unlike superoxide, O2−) that penetrates membranes freely (Imlay 2008). While other oxidants such as diamide may affect the cell wall, H2O2 seems to affect the intracellular function (Vilella et al. 2005). We found that the rho1-2 and rho1-5 mutants, which are specifically defective in Pkc1 activation (Saka et al. 2001), were particularly hypersensitive to H2O2, but their sensitivities to other oxidants were opposite. Thus their hypersensitivity to H2O2 could be due in part to the defect in Pkc1 activation, but the role of the Pkc1–MAPK pathway in response to other oxidants seems less clear.
The bck1Δ and mpk1/slt2Δ mutants as examined here were mildly sensitive to H2O2. This is consistent with a previous report (Staleva et al. 2004), but differs from another study, which indicated that the bck1 and mpk1 mutants were not sensitive to H2O2 and diamide (Vilella et al. 2005). None of the pkc1 mutants that we tested exhibited such severe sensitivity to H2O2, unlike the report by Vilella et al. (2005). This discrepancy might be due to the different PKC1 alleles and the strain background. It is thus not certain whether the Pkc1–MAPK cascade plays a role under oxidative stress. The bifunctional transcription factor Skn7 might also be involved in the Rho1-mediated oxidative stress response (Alberts et al. 1998). Further investigation will be required to fully understand the mechanism by which Rho1 regulates the oxidative stress response.
In this study, we found that Rho1 is necessary for survival under oxidative stress. In contrast, Rho5 is necessary for cell death under excessive oxidative stress (Singh et al. 2008). Thus, despite the similar structure of these Rho GTPases, Rho1 and Rho5 seem to play opposite roles under oxidative stress. Cells may use an alternative program to promote either survival or death depending on the level of stress or cellular damage. It remains uncertain how cell fate is determined under different levels of oxidative stress. Although the details of the mechanism remain unknown, our findings suggest that Rho1 may regulate Ycf1 to get rid of heavy metals or other xenobiotics from the cytoplasm, and thus help yeast cells recover from oxidative stress. Because both Rho1 and Ycf1 belong to highly conserved families of proteins, Rho GTPases might also be involved in regulation of an ABC transporter in mammals.
Acknowledgments
We thank D. Levin, J. Gray, Y. Ohya, W. Guo, E. Bi, and W-K. Huh for providing strains and plasmids; K. Pan for help with image analysis; and P. J. Kang, L. Huang, and A. Simcox for discussion and comments on the manuscript. We are also grateful to M. Rose and the anonymous reviewers for insightful comments. This work was supported in part by research grants from the National Institutes of Health (NIH)/National Institute of General Medical Sciences (GM076375) and the American Heart Association to H.-O. P., and NIH/National Center for Research Resources (P20 RR020171) to C.M.P. The Stagljar lab is supported by grants from the Canadian Foundation for Innovation, the Canadian Institutes of Health Research, the Canadian Cancer Society Research Institute, the Heart and Stroke Foundation, the Cystic Fibrosis Foundation, the Ontario Genomics Institute, and Novartis.
Literature Cited
- Alberts A. S., Bouquin N., Johnston L. H., Treisman R., 1998. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273: 8616–8622 [DOI] [PubMed] [Google Scholar]
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., et al. , 1999. Current Protocols in Molecular Biology. John Wiley & Sons, New York. [Google Scholar]
- Avery S. V., 2006. Microbial cell individuality and the underlying sources of heterogeneity. Nat. Rev. Microbiol. 4: 577–587 [DOI] [PubMed] [Google Scholar]
- Brennan R. J., Schiestl R. H., 1996. Cadmium is an inducer of oxidative stress in yeast. Mutat. Res. 356: 171–178 [DOI] [PubMed] [Google Scholar]
- Cochemé H. M., Murphy M. P., 2008. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 283: 1786–1798 [DOI] [PubMed] [Google Scholar]
- Cohen B. A., Pilpel Y., Mitra R. D., Church G. M., 2002. Discrimination between paralogs using microarray analysis: application to the Yap1p and Yap2p transcriptional networks. Mol. Biol. Cell 13: 1608–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A., Plusquin M., Remans T., Jozefczak M., Keunen E., et al. , 2010. Cadmium stress: an oxidative challenge. Biometals 23: 927–940 [DOI] [PubMed] [Google Scholar]
- Delley P. A., Hall M. N., 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147: 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Pruyne D., Bretscher A., 2003. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161: 1081–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova J., Drgon T., Tanaka K., Kollar R., Chen G. C., et al. , 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272: 277–279 [DOI] [PubMed] [Google Scholar]
- Eitzen G., Thorngren N., Wickner W., 2001. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J. 20: 5650–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal N., Gurer-Orhan H., Aykin-Burns N., 2001. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 1: 529–539 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Blundell K., Longtine M. S., Chow C. J., Adames N., et al. , 1997. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276: 118–122 [DOI] [PubMed] [Google Scholar]
- Finkel T., 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15: 247–254 [DOI] [PubMed] [Google Scholar]
- Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., et al. , 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11: 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon C., Lagniel G., Lee J., Buhler J.-M., Kieffer S., et al. , 1998. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 273: 22480–22489 [DOI] [PubMed] [Google Scholar]
- Guo W., Tamanoi F., Novick P., 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3: 353–360 [DOI] [PubMed] [Google Scholar]
- Guthrie C., Fink G. R., 1991. Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego [Google Scholar]
- Harrison J. C., Bardes E. S., Ohya Y., Lew D. J., 2001. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 3: 417–420 [DOI] [PubMed] [Google Scholar]
- Harrison J. C., Zyla T. R., Bardes E. S. G., Lew D. J., 2004. Stress-specific activation mechanisms for the “cell integrity” MAPK pathway. J. Biol. Chem. 279: 2616–2622 [DOI] [PubMed] [Google Scholar]
- He X.-J., Mulford K. E., Fassler J. S., 2009. Oxidative stress function of the Saccharomyces cerevisiae Skn7 receiver domain. Eukaryot. Cell 8: 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X. J., Fassler J. S., 2005. Identification of novel Yap1p and Skn7p binding sites involved in the oxidative stress response of Saccharomyces cerevisiae. Mol. Microbiol. 58: 1454–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herker E., Jungwirth H., Lehmann K. A., Maldener C., Frohlich K.-U., et al. , 2004. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164: 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins V. J., Alic N., Thorpe G. W., Breitenbach M., Larsson V., et al. , 2002. Phenotypic analysis of gene deletant strains for sensitivity to oxidative stress. Yeast 19: 203–214 [DOI] [PubMed] [Google Scholar]
- Hu C. D., Chinenov Y., Kerppola T. K., 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9: 789–798 [DOI] [PubMed] [Google Scholar]
- Imlay J. A., 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77: 755–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Jung U. S., Piotrowski J., Levin D. E., 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9: 1559–1571 [DOI] [PubMed] [Google Scholar]
- Kang P. J., Béven L., Hariharan S., Park H.-O., 2010. The Rsr1/Bud1 GTPase interacts with itself and the Cdc42 GTPase during bud-site selection and polarity establishment in budding yeast. Mol. Biol. Cell 21: 3007–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Sanson A., Lee B., Park H.-O., 2001. A GDP/GTP exchange factor involved in linking a spatial landmark to cell polarity. Science 292: 1376–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H., Tanaka K., Mino A., Umikawa M., Imamura H., et al. , 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15: 6060–6068 [PMC free article] [PubMed] [Google Scholar]
- Kono K., Nogami S., Abe M., Nishizawa M., Morishita S., et al. , 2008. G1/S cyclin-dependent kinase regulates small GTPase Rho1p through phosphorylation of RhoGEF Tus1p in Saccharomyces cerevisiae. Mol. Biol. Cell 19: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S. A., Gray J. V., 2002. The protein kinase C pathway is required for viability in quiescence in Saccharomyces cerevisiae. Curr. Biol. 12: 588–593 [DOI] [PubMed] [Google Scholar]
- Lee J., Godon C., Lagniel G., Spector D., Garin J., et al. , 1999. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 274: 16040–16046 [DOI] [PubMed] [Google Scholar]
- Lee K. S., Levin D. E., 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Bartlett-Heubusch E., 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116: 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Lu Y. P., Zhen R. G., Szczypka M., Thiele D. J., et al. , 1997. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA 94: 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M. R., Jones L., Eitzen G., 2010. Cdc42p and Rho1p are sequentially activated and mechanistically linked to vacuole membrane fusion. Biochem. Biophys. Res. Commun. 394: 64–69 [DOI] [PubMed] [Google Scholar]
- López E., Arce C., Oset-Gasque M. J., Cañadas S., González M. P., 2006. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Biol. Med. 40: 940–951 [DOI] [PubMed] [Google Scholar]
- Mason D. L., Michaelis S., 2002. Requirement of the N-terminal extension for vacuolar trafficking and transport activity of yeast Ycf1p, an ATP-binding cassette transporter. Mol. Biol. Cell 13: 4443–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey M., Johnson J. S., Goud B., Myers A. M., Rossier J., et al. , 1991. The small GTP-binding protein Rho1p is localized on the Golgi apparatus and post-Golgi vesicles in Saccharomyces cerevisiae. J. Cell Biol. 115: 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. A., Banks G. R., Toone W. M., Raitt D., Kuge S., et al. , 1997. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16: 1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., et al. , 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20: 87–90 [DOI] [PubMed] [Google Scholar]
- Nguyên-nhu N. T., Knoops B., 2002. Alkyl hydroperoxide reductase 1 protects Saccharomyces cerevisiae against metal ion toxicity and glutathione depletion. Toxicol. Lett. 135: 219–228 [DOI] [PubMed] [Google Scholar]
- Nonaka H., Tanaka K., Hirano H., Fujiwara T., Kohno H., et al. , 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14: 5931–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrdlik P., El-Bakkoury M., Hamacher T., Cappellaro C., Vilarino C., et al. , 2004. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 101: 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-O., Bi E., 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71: 48–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-O., Chant J., Herskowitz I., 1993. BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365: 269–274 [DOI] [PubMed] [Google Scholar]
- Park J.-I., Collinson E. J., Grant C. M., Dawes I. W., 2005. Rom2p, the Rho1 GTP/GDP exchange factor of Saccharomyces cerevisiae, can mediate stress responses via the Ras-cAMP pathway. J. Biol. Chem. 280: 2529–2535 [DOI] [PubMed] [Google Scholar]
- Paumi C. M., Menendez J., Arnoldo A., Engels K., Iyer K. R., et al. , 2007. Mapping protein-protein interactions for the yeast ABC transporter Ycf1p by integrated split-ubiquitin membrane yeast two-hybrid analysis. Mol. Cell 26: 15–25 [DOI] [PubMed] [Google Scholar]
- Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., et al. , 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272: 279–281 [DOI] [PubMed] [Google Scholar]
- Saka A., Abe M., Okano H., Minemura M., Qadota H., et al. , 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276: 46165–46171 [DOI] [PubMed] [Google Scholar]
- Sharma K. G., Mason D. L., Liu G., Rea P. A., Bachhawat A. K., et al. , 2002. Localization, regulation, and substrate transport properties of Bpt1p, a Saccharomyces cerevisiae MRP-type ABC transporter. Eukaryot. Cell 1: 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Kang P. J., Park H.-O., 2008. The Rho5 GTPase is necessary for oxidant-induced cell death in budding yeast. Proc. Natl. Acad. Sci. USA 105: 1522–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J., Kittanakom S., Damjanovic D., Curak J., Wong V., et al. , 2010. Detecting interactions with membrane proteins using a membrane two-hybrid assay in yeast. Nat. Protoc. 5: 1281–1293 [DOI] [PubMed] [Google Scholar]
- Staleva L., Hall A., Orlow S. J., 2004. Oxidative stress activates FUS1 and RLM1 transcription in the yeast Saccharomyces cerevisiae in an oxidant-dependent manner. Mol. Biol. Cell 15: 5574–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe G. W., Fong C. S., Alic N., Higgins V. J., Dawes I. W., 2004. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 101: 6564–6569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Morris H., Cronin M. T., 2005. Metals, toxicity and oxidative stress. Curr. Med. Chem. 12: 1161–1208 [DOI] [PubMed] [Google Scholar]
- Vida T. A., Emr S. D., 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128: 779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella F., Herrero E., Torres J., de la Torre-Ruiz M. A., 2005. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280: 9149–9159 [DOI] [PubMed] [Google Scholar]
- Wemmie J. A., Moye-Rowley W. S., 1997. Mutational analysis of the Saccharomyces cerevisiae ATP-binding cassette transporter protein Ycf1p. Mol. Microbiol. 25: 683–694 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kono K., Lowery D. M., Bartolini S., Yaffe M. B., et al. , 2006. Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science 313: 108–111 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Bartolini S., Pellman D., 2009. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 23: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]