Abstract
Rho-family GTPases play regulatory roles in many fundamental cellular processes. Caenorhabditis elegans UNC-73 RhoGEF isoforms function in axon guidance, cell migration, muscle arm extension, phagocytosis, and neurotransmission by activating either Rac or Rho GTPase subfamilies. Multiple differentially expressed UNC-73 isoforms contain a Rac-specific RhoGEF-1 domain, a Rho-specific RhoGEF-2 domain, or both domains. The UNC-73E RhoGEF-2 isoform is activated by the G-protein subunit Gαq and is required for normal rates of locomotion; however, mechanisms of UNC-73 and Rho pathway regulation of locomotion are not clear. To better define UNC-73 function in the regulation of motility we used cell-specific and inducible promoters to examine the temporal and spatial requirements of UNC-73 RhoGEF-2 isoform function in mutant rescue experiments. We found that UNC-73E acts within peptidergic neurons of mature animals to regulate locomotion rate. Although unc-73 RhoGEF-2 mutants have grossly normal synaptic morphology and weak resistance to the acetylcholinesterase inhibitor aldicarb, they are significantly hypersensitive to the acetylcholine receptor agonist levamisole, indicating alterations in acetylcholine neurotransmitter signaling. Consistent with peptidergic neuron function, unc-73 RhoGEF-2 mutants exhibit a decreased level of neuropeptide release from motor neuron dense core vesicles (DCVs). The unc-73 locomotory phenotype is similar to those of rab-2 and unc-31, genes with distinct roles in the DCV-mediated secretory pathway. We observed that constitutively active Gαs pathway mutations, which compensate for DCV-mediated signaling defects, rescue unc-73 RhoGEF-2 and rab-2 lethargic movement phenotypes. Together, these data suggest UNC-73 RhoGEF-2 isoforms are required for proper neurotransmitter signaling and may function in the DCV-mediated neuromodulatory regulation of locomotion rate.
THE Rho GTPase signaling pathway is involved in multiple cellular processes such as gene transcription, cytokinesis, cell-cycle progression, and regulation of the actin cytoskeleton and more recently was found to play a role in neurotransmission (Jaffe and Hall 2005; Steven et al. 2005; McMullan et al. 2006; Williams et al. 2007). In Caenorhabditis elegans Rho upregulates neurotransmission in at least two ways. GTP-bound Rho, activated by the Rho guanine nucleotide exchange factor (RhoGEF) RHGF-1, binds to and inhibits diacylglycerol kinase (DGK), which results in increased levels of its substrate diacylglycerol (DAG) at the synapse (Hiley et al. 2006; McMullan et al. 2006). Increased DAG facilitates the release of neurotransmitter likely due to an accumulation of UNC-13, a component of the synaptic vesicle release machinery, via UNC-13 binding to DAG in the synaptic membrane (Lackner et al. 1999). Increased acetylcholine release through the Rho pathway ultimately leads to an increased rate of locomotion (McMullan et al. 2006).
Rho also affects neurotransmission in a DGK– and UNC-13–independent manner since inactivation of Rho in a dgk-1 mutant background still decreases locomotion rate and an UNC-13 DAG-binding mutant only partially blocks constitutive RHO-1 activity (McMullan et al. 2006). UNC-73, the C. elegans homolog of mammalian Trio and Kalirin, is a candidate RhoGEF acting in the DGK-independent pathway (Debant et al. 1996; Alam et al. 1997; Steven et al. 2005; Hiley et al. 2006). The unc-73 gene encodes multiple isoforms, all of which contain at least one RhoGEF domain, indicating they are potential activators of Rho family GTPases. The smaller UNC-73 isoforms contain either a RhoGEF-1 domain, which specifically activates Rac family GTPases in biochemical assays (Steven et al. 1998; Wu et al. 2002; Kubiseski et al. 2003), or a RhoGEF-2 domain, which is specific to Rho (Spencer et al. 2001). The UNC-73B RhoGEF-1 isoform and the Rac pathways have well-characterized roles in axon guidance, cell migration, muscle arm extension, and the phagocytosis of apoptotic cells, while the functions of the UNC-73 RhoGEF-2 isoforms are the focus of this study (Hedgecock et al. 1987; Steven et al. 1998; Honigberg and Kenyon 2000; Kishore and Sundaram 2002; Lundquist 2003; Debakker et al. 2004; Morita et al. 2005; Levy-Strumpf and Culotti 2007; Watari-Goshima et al. 2007; Axang et al. 2008; Alexander et al. 2009). Isoform-specific rescue experiments previously revealed the RhoGEF-2 domain-containing UNC-73C1, C2/F, and E isoforms act redundantly to regulate the speed of locomotion (Steven et al. 2005). Transgenic animals lacking these specific isoforms have a lethargic movement phenotype that is rescued by the expression of any individual isoform from the group. Point mutations that reduce UNC-73 RhoGEF-2 activity also result in a lethargic phenotype (Steven et al. 2005; Williams et al. 2007), but deletion of the entire RhoGEF-2 domain causes early larval arrest as a result of pharynx pumping defects (Steven et al. 2005).
Analysis of C. elegans heterotrimeric G-protein signaling revealed a network of Gαq, Gαo, and Gαs pathways regulating acetylcholine release from motor neurons (Perez-Mansilla and Nurrish 2009). Gαq (C. elegans EGL-30) and Gαs (GSA-1) positively influence neurotransmitter release while Gαo (GOA-1) activity reduces neurotransmitter release through inhibition of the Gαq/EGL-30 pathway (Hajdu-Cronin et al. 1999; Lackner et al. 1999; Miller et al. 1999; Reynolds et al. 2005; Schade et al. 2005; Charlie et al. 2006). Our previous genetic analysis placed unc-73 RhoGEF-2 activity downstream of Gαo/GOA-1 and Williams et al. (2007) more specifically identified the UNC-73E RhoGEF-2 isoform as a Gαq/EGL-30 effector acting in parallel to phospholipase Cβ (EGL-8) (Steven et al. 2005). In mammals, Gαq similarly interacts with the Rho-specific activators p63RhoGEF and Trio, an UNC-73 homolog (Lutz et al. 2005; Rojas et al. 2007). C. elegans egl-30 and unc-73 RhoGEF-2 loss-of-function mutations have lethargic or slow movement phenotypes that are opposite to the hyperactive movement phenotype of transgenic animals carrying a Rho gain-of-function mutation (Brundage et al. 1996; Steven et al. 2005; McMullan et al. 2006; Williams et al. 2007). However, the precise role of UNC-73 RhoGEF-2–stimulated Rho activity in the regulation of locomotion is not known.
Neurotransmission involves two separate mechanisms of neurotransmitter release (Sudhof 2008). Classical small molecule neurotransmitters such as acetylcholine and glutamate are stored in small, clear vesicles that are filled and released at presynaptic sites. In response to presynaptic depolarization the vesicles fuse to the plasma membrane and neurotransmitters are released into the synapse where they have immediate effects to initiate depolarization in the postsynaptic neuron. Neuromodulatory neurotransmitters such as neuropeptides, on the other hand, are held in large, dense core vesicles (DCVs) produced by the Golgi apparatus in the cell body. They are transported down the axon for exocytosis at the synapse, as well as other sites, where they tend to have slower and longer-lasting effects on the nervous system compared to classical neurotransmitters (Katz and Frost 1996; Husson et al. 2007).
The results of this study suggest UNC-73 RhoGEF-2 isoforms affect C. elegans motility through the modulation of neurotransmission signaling mechanisms. unc-73 RhoGEF-2 mutants are weakly resistant to the acetylcholinesterase inhibitor aldicarb and are hypersensitive to the acetylcholine agonist levamisole, indicating alterations in cholinergic signaling. DCV-mediated neuropeptide signaling is also altered in unc-73 RhoGEF-2 mutants and the unc-73 RhoGEF-2 lethargic movement phenotype is very similar to the locomotory phenotypes of rab-2 and unc-31, two genes with established roles in DCV-mediated signaling (Avery et al. 1993; Speese et al. 2007; Chun et al. 2008; Edwards et al. 2009; Sumakovic et al. 2009). Consistent with these results we observe that activating mutations in the Gαs/GSA-1 pathway, which increase DCV exocytosis and compensate for neuropeptide release defects in C. elegans (Charlie et al. 2006; Zhou et al. 2007), rescue the unc-73 and rab-2 lethargic movement phenotypes. Our data suggest that UNC-73 RhoGEF-2 isoforms and the Rho GTPase pathway alter DCV-mediated signaling involving neuropeptides or other neuromodulatory molecules that stimulate locomotion in C. elegans.
Materials and Methods
C. elegans strains
Strains were maintained at 21° on plates containing standard nematode growth media unless otherwise noted. The following strains were used in this study: N2 Bristol (wild type), NL3231 acy-1(pk484) III; dpy-20(e1362) IV; pkIs296[hsp::gsa-1(QL); dpy-20(+)] X (Korswagen et al. 1997); KG518 acy-1(ce2), VC671 egl-3(ok979), RM2221 egl-8 (md1971), egl-30(tg26) (Doi and Iwasaki 2002); KG421 gsa-1(ce81), juIs1[unc-25p::snb-1::gfp] (Hallam and Jin 1998); KG532 kin-2(ce179), nuIs183[unc-129p::nlp-21::YFP; myo-2p::NLS::gfp] IV (Sieburth et al. 2007); QT47 nzIs1[HSp::rho-1(gf); ttx-3p::gfp] I (McMullan et al. 2006); MT1093 rab-2(n501) (rab-2 is also known as unc-108), XA7314 unc-73(ev802) I; qaIs7312[unc-73D1; F25B3.3p::gfp] V, XA7330 qaEx7327[unc-73D1; unc-73E; F25B3.3p::gfp]; unc-73(ev802), CB928 unc-31(e928), XA7300 unc-73(ev802)/unc-11(e47) dpy-5(e61), and KG1278 unc-73(ce362). Double-mutant strains were constructed using standard genetic methods without additional marker mutations. Mutations in the double mutants were confirmed by PCR, sequencing, or outcrossing to him-5 males and examination of the F2 progeny phenotypes.
Transgenic lines
Standard microinjection techniques were used to generate stable transgenic C. elegans lines carrying extrachromosomal DNA arrays. For the unc-73(ev802) rescue experiments, between 50 and 100 ng/μl of the gene-Xp::unc-73E::gfp constructs were mixed with 50–100 ng/μl of pXS2 encoding unc-73D1 and injected into unc-73(ev802)/unc-11dpy-5 without a cotransformation marker. pXS2 was added to rescue unc-73(ev802) lethality. Progeny were screened for stable expression of the extrachromosomal array and homozygous unc-73(ev802) lines containing the array were established. For unc-73(ce362) rescue experiments, transgenic lines were first established in a wild-type background, by injection of 100 ng/μl gene-Xp::unc-73E::gfp without a cotransformation marker, and then the lines were crossed into unc-73(ce362). Exceptions were the dat-1p and eat-4p constructs, which were injected into wild type at 50 ng/μl along with 50 ng/μl unc-122p::gfp (coelomocyte-specific promoter) as a cotransformation marker. PCR-based genotyping was used to confirm unc-73(ev802) and unc-73(ce362) rescued lines were homozygous.
Gain-of-function acy-1 cDNA constructs [KG#81 myo-3p::acy-1(gf) and KG#83 rab-3p::acy-1(gf)] (Reynolds et al. 2005) at 10 ng/μl with 50 ng/μl unc-122p::gfp and 90 ng/μl herring sperm DNA were injected into N2 to establish stable transgenic lines that were crossed into unc-73(ce362). The synapse marker KP#282 acr-2p::snb-1::cfp (Nurrish et al. 1999) at 50 ng/μl mixed with 50 ng/μl pRF4 rol-6(su1006dm) and 50 ng/μl herring sperm DNA were injected into N2 and unc-73(ce362) animals to establish stable lines. The plasmid KP#315 acr-2p::egl-30(Q209L) (Lackner et al. 1999) at 10 ng/μl mixed with 50 ng/μl F25B3.3p::gfp (a cotransformation marker expressed in neurons) and 70 ng/μl herring sperm DNA were injected into N2 and unc-73(ce362) to establish stable transgenic lines. At least three transgenic lines were isolated for each injected plasmid mix and it was confirmed that each set of lines had the same phenotype and/or GFP expression pattern.
Molecular biology
The gene-Xp::unc-73E::gfp cell-specific promoter constructs were made by directionally cloning promoter DNA into the unique NotI (5′) and EcoRV or EcoRI (3′) sites of the unc-73E::gfp plasmid pXS6. In this construct the unc-73E transcript is encoded by cDNA for the first two exons with the remainder encoded by a genomic DNA fragment extending ∼150 bp beyond the 3′ end of the coding region of the transcript. Promoters generated by PCR using wild-type genomic DNA or the plasmids pPD49.83 (heat-shock promoter; from Andy Fire, Stanford University, Palo Alto, CA), pPD136.64 (myo-3 promoter; from Andy Fire), or pRM621 (modified unc-17 promoter as described in Charlie et al. 2006; from Jim Rand, Oklahoma Medical Research Foundation, Oklahoma City, OK) as the template were cloned into pJET (Fermentas) or pGEM (Promega, Madison, WI) PCR cloning vectors and confirmed by sequencing. We used the same promoter regions previously defined in mutant rescue experiments (Lee et al. 1999; Nass et al. 2002; Carvelli et al. 2004; Carnell et al. 2005; Liu et al. 2007). Two promoters were not generated by PCR, but directly cloned from plasmids. pBY103 (from Dave Pilgrim, University of Alberta, AB, Canada) contains the 1.2-kb HindIII/EcoRI unc-119 promoter fragment and pJL35 (from Bruce Bamber, University of Toledo, Toledo, OH) contains the 1.2-kb unc-47 promoter fragment.
Phenotype analysis
The aldicarb sensitivity protocol was modified from Mahoney et al. (2006). Larval stage four (L4) worms were picked the day before the assay was performed and left to grow overnight on plates with nematode growth media (NGM) and bacteria. The next day the young adults were transferred to NGM plates containing 1.0 mM aldicarb (Chem Services). Plates were stored at 4° for 1 to 5 days and seeded with bacteria the day of use. Approximately 30 animals per strain were examined blind for paralysis every 10 min for 2 hr, using a Leica MZ6 stereomicroscope. Animals were defined as paralyzed if after a maximum of three worm pick taps on the head and three taps on the tail there was no body movement; however, animals exhibiting only small foraging movements of the head or small movements of the tail were still considered paralyzed. The assay was repeated three times for each strain and an average for each time point was calculated.
Levamisole sensitivity assays were performed as described above for aldicarb sensitivity. Staged young adult worms were examined on seeded NGM plates containing 0.5 mM levamisole (Sigma, St. Louis) over a period of 1 hr. Plates were prepared as described above for aldicarb and at least three trials were performed for each strain.
Rates of locomotion were determined at room temperature (∼21°) as described previously (Steven et al. 2005). Healthy and fed young adult animals were transferred to NGM assay plates without bacteria and left for at least 20 sec before counting. Locomotion rate is defined as the number of body bends exhibited in 20 sec of uninterrupted forward movement. If an animal stopped moving or reversed direction, the count was abandoned. One body bend was defined as a complete cycle of terminal bulb motion starting from the top position of the sinusoidal wave track through to the bottom and back to the top. Between 25 and 30 animals were examined for each strain.
NGM plates containing the phorbol ester phorbol 12-myristate 13 acetate (PMA) (Enzo Life Sciences) were made as described by Reynolds et al. (2005). Approximately 15–20 adult worms for each strain were placed on separate PMA or control (containing the ethanol carrier) plates at room temperature and observed 2.5–3 hr later. PMA treatment of the lethargic unc-73 and rab-2 mutants increased the locomotion rates of these mutants, but they tended to coil and reverse frequently, which made it impossible to measure their locomotion rates in our body bend assays. Video images were captured with a Dino-Lite AM413T digital microscope in a “Worm Tracker” rig and processed using Worm Tracker 2.0 software (Schafer Lab).
Coelomocyte endocytosis in C. elegans strains was examined by monitoring Texas Red-conjugated bovine serum albumin (TR-BSA) (Invitrogen) uptake by coelomocytes (Zhang et al. 2001). Synchronized young adults were injected with a short pulse of 1 mg/ml TR-BSA into the pseudocoelom in the region of the pharynx between the metacorpus and the terminal bulb. Animals were left to recover on seeded NGM plates for 1 hr and then collected in a drop of ice-cold 1% paraformaldehyde (Electron Microscopy Sciences) in M9 on a slide with a 2% agarose pad. They were maintained in the drop for at least 20 min and up to 2 hr, on ice, as all the injected worms were collected, and then covered with a coverslip and observed under the microscope.
Microscopy and imaging analysis
Worms were immobilized with 30 mg/ml 2,3-butanedione monoxime (BDM) (Sigma) or 10 mM levamisole (Sigma) in M9. Images were captured with a Hamamatsu ORCA-ER camera mounted on a Leica DMRA2 microscope and processed using Openlab software (Improvision, Lexington, MA) or captured with a QICAM camera (QImaging) mounted on a Zeiss Axiophot microscope and processed with Q Capture Pro (QImaging). Confocal microscopy images were obtained with an Olympus Fluoview 300/IX70 confocal microscope. For NLP-21::YFP and TR-BSA analysis stacks of 0.4-μm-thick optical images were captured with Olympus Fluoview 5.0 software and fluorescence intensity was quantified using Volocity software (Improvision). Images were tightly cropped to contain the cells or axons of interest and fluorescent objects within the images were identified automatically by intensity. The threshold value for this process was kept constant for each experiment and was appropriately chosen to eliminate “background” fluorescence as observed in wild-type animals. Object intensities were quantified and summed for each image. Small objects containing <30 voxels (coelomocytes), 6 voxels (cell bodies), or 4 voxels (dorsal axons) were eliminated since these likely corresponded to nonspecific background specks. The arbitrary fluorescent unit of each measurement was standardized to the average fluorescent unit of wild type obtained for that day.
Synchronized young adults with dorsal cord axon bundles oriented toward the objective were imaged using a UPlanApo 40× objective. The fluorescent intensity was quantified as the arbitrary fluorescent unit per unit length. The DA6 and DB6 cell bodies in the region posterior to the vulva were imaged with a UPlanApo 100× objective in synchronized young adults with the ventral cord oriented toward the objective. Fluorescence intensity was quantified as the arbitrary fluorescent unit per two cell bodies. Single posterior coelomocytes oriented toward the objective were imaged in L4 larvae using the UPlanApo 100× objective. Fluorescence intensity was quantified as the arbitrary fluorescent unit per coelomocyte.
Results
UNC-73 RhoGEF-2 isoforms modulate acetylcholine signaling
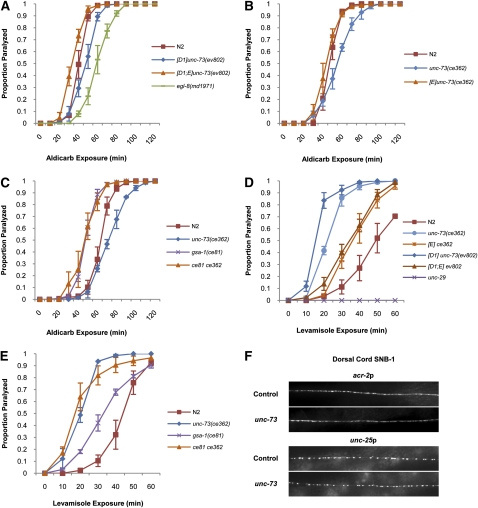
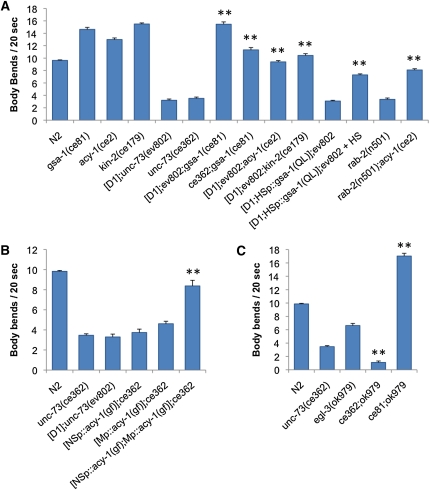
To better understand how UNC-73 RhoGEF-2 isoforms regulate locomotion we first checked for defects in classical synaptic vesicle neurotransmission. Measuring resistance to the acetylcholinesterase inhibitor aldicarb is an indirect method of examining alterations in C. elegans cholinergic signaling. Aldicarb exposure increases the amount of synaptic acetylcholine, which paralyzes worms (Nguyen et al. 1995). Control egl-8 mutants, with defective phospholipase C, release less acetylcholine into the synapse and were therefore moderately resistant to aldicarb in comparison to wild-type animals (Figure 1A) (Lackner et al. 1999). We tested two unc-73 mutants for aldicarb resistance. unc-73(ce362) animals have a point mutation in the RhoGEF-2 domain that severely reduces RhoGEF activity (Williams et al. 2007). Transgenic Is[D1]; unc-73(ev802) animals have a deletion eliminating the RhoGEF-2 domain but they express the UNC-73D1 RhoGEF-2 isoform in the pharynx under the control of the endogenous unc-73D1 promoter, which rescues the unc-73(ev802) larval arrest phenotype (Steven et al. 2005). The unc-73(ce362) and Is[D1]; unc-73(ev802) strains both exhibited a lethargic movement phenotype (Figure 2) (Steven et al. 2005; Williams et al. 2007). Wild-type (N2) and transgenic Ex[D1; E]; unc-73(ev802) and Ex[E]; unc-73(ce362) rescued animals, which have wild-type rates of movement, served as controls (Figure 2) (Steven et al. 2005; Williams et al. 2007). Both unc-73 mutants exhibited weak aldicarb resistance in comparison to the control animals (Figure 1, A and B), indicating unc-73 RhoGEF-2 mutants have altered acetylcholine signaling consistent with a minor or modulatory role for RhoGEF-2 activity in cholinergic neurotransmission.
Figure 1 .
unc-73 RhoGEF-2 mutants have altered neurotransmitter signaling. (A–C) Animals were examined for paralysis on NGM plates containing 1 mM aldicarb, an acetylcholinesterase inhibitor. unc-73 RhoGEF-2 mutants are weakly resistant to aldicarb compared to wild-type N2 animals and Ex[D1; E]; unc-73(ev802) and Ex[E]; unc-73(ce362) rescued strains. Control egl-8 mutants are moderately resistant to aldicarb. gsa-1(ce81) gain-of-function mutants and gsa-1(ce81) unc-73(ce362) double mutants are hypersensitive to aldicarb. (D and E) Animals were examined for paralysis on NGM plates containing 0.5 mM levamisole, an acetylcholine receptor agonist. unc-73 RhoGEF-2 mutants are hypersensitive to levamisole. Control unc-29 levamisole-sensitive acetylcholine receptor subunit mutants are completely resistant. Error bars indicate SEM. Square brackets denote transgenes present in a particular strain. For example, [D1; E] indicates the unc-73D1 and unc-73E isoform encoding transgenes. (F) Gross synapse morphology is unchanged in unc-73 RhoGEF-2 mutants. SNB-1::CFP (synaptobrevin) localization in dorsal cord cholinergic motor neuron axons [acr-2 promoter (Nurrish et al. 1999)] is similar in unc-73(ce362) mutants and control N2 animals (top). SNB-1::GFP localization in dorsal cord GABAergic motor neuron axons [unc-25 promoter (Hallam and Jin 1998)] is similar in Is[D1]; unc-73(ev802) mutants and control unc-73(ev802)/unc-11 dpy-5 animals (bottom).
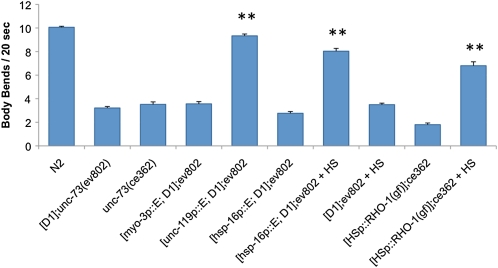
Figure 2 .
UNC-73E functions in the adult nervous system to regulate locomotion. Expression of UNC-73E in the nervous system (unc-119 promoter), but not body wall muscles (myo-3 promoter) rescues Is[D1]; unc-73(ev802) lethargy. Heat-shock (HS)-induced UNC-73E or RHO-1(gf) expression (using the hsp-16 promoter) in adult animals also rescues unc-73 RhoGEF-2 mutant locomotion defects. Error bars indicate SEM. **P < 0.001 in comparison to Is[D1]; unc-73(ev802) or unc-73(ce362) using Student’s t-test.
Aldicarb resistance may result from lower levels of acetylcholine release at synapses or a diminished postsynaptic response to acetylcholine. The postsynaptic response to acetylcholine was examined in unc-73 mutants using the acetylcholine receptor agonist levamisole (Lewis et al. 1980). unc-73(ce362) and Is[D1]; unc-73(ev802) animals did not have a reduced levamisole response and were actually hypersensitive to the drug (Figure 1D) (Williams et al. 2007). Levamisole hypersensitivity was reduced in control unc-73 rescued strains (Figure 1D). Therefore, unc-73 aldicarb resistance and lethargic movement phenotypes are not due to a reduced response to acetylcholine by body wall muscles and it is possible a more significant unc-73 aldicarb resistance phenotype is masked by an increased sensitivity to acetylcholine. Interestingly, other genes with roles in neurotransmission including snt-1, unc-18, unc-41, and unc-75 also display levamisole hypersensitivity phenotypes for unknown reasons, but possibly due to receptor upregulation to compensate for neurotransmission defects (Miller et al. 1996).
Defects in neurotransmission and locomotory behavior may result from structural defects within the nervous system. Synapse structure was examined on a gross level in Is[D1]; unc-73(ev802) and unc-73(ce362) mutants using fluorophore-tagged SNB-1, which localizes to presynaptic vesicle clusters in neurons (Nonet 1999). Tagged SNB-1 was examined in unc-73 mutant GABAergic and cholinergic motor neurons using unc-25 and acr-2 promoters, respectively (Figure 1F). The size and spacing of the vesicle clusters in dorsal cord axons was similar in unc-73 mutants and control animals, suggesting unc-73 RhoGEF-2 isoforms are not required for normal presynaptic structure.
unc-73 RhoGEF-2 isoforms function in adult neurons to regulate locomotion
UNC-73 RhoGEF-2 isoforms that rescue the Is[D1]; unc-73(ev802) lethargic phenotype have differential expression patterns, but they are all expressed to some extent in the nervous system (Steven et al. 2005). We used UNC-73E as a representative RhoGEF-2 isoform in tissue-specific rescue experiments to better define where UNC-73 RhoGEF-2 activity is required for wild-type rates of locomotion. UNC-73E expression in the nervous system (unc-119 promoter), but not in the body wall muscles (myo-3 promoter) rescued the Is[D1]; unc-73(ev802) lethargic movement phenotype (Figure 2). Ex[unc-119p::unc-73E::gfp; D1]; unc-73(ev802) animals moved at a rate comparable to that of wild-type N2, while the movement of Ex[myo-3p::unc-73E::gfp; D1]; unc-73(ev802) animals was not significantly different from that of lethargic Is[D1]; unc-73(ev802) animals. The unc-73D1 transgene was included in these transgenic strains to rescue the lethality of the unc-73(ev802) allele. UNC-73E::GFP fusion protein expression in the expected tissues was confirmed in all of our transgenic strains by fluorescence microscopy (Supporting Information, Figure S2 and Figure S3).
To assess the temporal requirements of UNC-73E expression for normal locomotion we used a heat-shock promoter (hsp-16.41) to control UNC-73E expression. If Is[D1]; unc-73(ev802) lethargy is a result of defects in neurotransmission and not due to developmental defects, such as axon pathfinding errors, then expressing one of the C1, C2, or E isoforms postdevelopment in adult animals should rescue the lethargy. Indeed, a 40-min heat shock of adult Ex[hsp-16p::unc-73E::gfp; D1]; unc-73(ev802) animals induced UNC-73E expression and restored movement close to wild-type rates, while animals that did not receive the heat shock remained lethargic (Figure 2 and Figure S2D). This indicated UNC-73E, and by implication also the C1 and C2 isoforms, plays a nondevelopmental role in the regulation of locomotion, consistent with a previous report of UNC-73E function in adult neurons observed with a different allele (Williams et al. 2007).
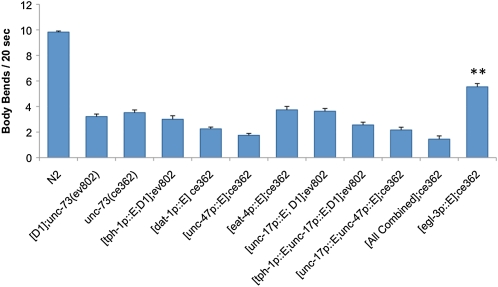
Additional promoters were used to examine the cellular requirements of UNC-73E expression for wild-type locomotion. UNC-73E expression in different classes of neurons including dopaminergic, GABAergic, glutamatergic, serotonergic, and cholinergic neurons did not rescue the Is[D1]; unc-73(ev802) or unc-73(ce362) lethargic phenotypes when the constructs were injected individually or together in combination (Figure 3 and Figure S3). In contrast, UNC-73E expression in peptidergic neurons using the egl-3 promoter (Kass et al. 2001) did rescue unc-73(ce362) and Is[D1]; unc-73(ev802) slow movement phenotypes (Figure 3 and data not shown). egl-3 encodes a proprotein convertase that is copackaged in dense core vesicles with neuropeptides and is expressed in peptidergic neurons, a large subset of neurons, which includes the cholinergic motor neurons (Kass et al. 2001). Although UNC-73E expression driven by the egl-3 promoter was widespread in the nervous system (Figure S3E), these results suggested that UNC-73 RhoGEF-2 isoforms might play a role in peptidergic neurotransmission to influence locomotion rates.
Figure 3 .
UNC-73E expression in peptidergic neurons, but not other neuronal subtypes, rescues unc-73 RhoGEF-2 mutant lethargy. UNC-73E expression in ventral cord cholinergic (unc-17 promoter), dopaminergic (dat-1 promoter), GABAergic (unc-47 promoter), glutamatergic (eat-4 promoter), or serotonergic neurons (tph-1 promoter) alone or in the indicated combinations, including all constructs combined (“all combined”), failed to rescue Is[D1]; unc-73(ev802) or unc-73(ce362) locomotory defects. However, partial rescue was obtained using the egl-3 promoter, which drives expression in peptidergic neurons. Error bars indicate SEM. **P < 0.001 in comparison to Is[D1]; unc-73(ev802) or unc-73(ce362) using Student’s t-test.
The UNC-73 RhoGEF-2 domain specifically activates the GTPase Rho in vitro (Spencer et al. 2001; Williams et al. 2007). We used a rho-1 gain-of-function cDNA driven by a heat-shock promoter to examine whether rho-1 acts downstream of UNC-73 to regulate the rate of locomotion in vivo. Heat-shocked adult Ex[hsp-16p::rho-1(gf)]; unc-73(ce362) animals moved approximately three times faster than animals that were not heat-shocked (Figure 2). This partial rescue might be expected since constitutive activation of Rho GTPases can disrupt many cell behaviors and cycling between the active and inactive forms is likely required for normal function (Luo et al. 1994; Morita et al. 2005). However, our results are consistent with the in vitro results and indicate RHO-1 functions downstream of UNC-73 RhoGEF-2 activity to regulate locomotion rate in adult animals in vivo.
unc-73 RhoGEF-2 isoforms modulate neuropeptide levels
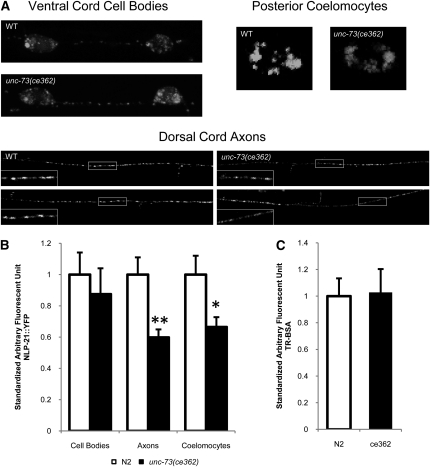
Our cell-specific rescue experiments revealed that UNC-73E expression in peptidergic neurons rescued unc-73 mutant lethargy. We used a neuropeptide release assay, recently developed independently by the Kaplan and Jorgensen laboratories, to more directly assess the role of UNC-73 RhoGEF-2 isoforms in peptidergic neurotransmission (Sieburth et al. 2007; Speese et al. 2007). Fluorescently labeled neuropeptide, NLP-21::YFP, was expressed in DA and DB motor neurons [using the neural unc-129 promoter (Colavita et al. 1998)], which also express UNC-73 RhoGEF-2 isoforms (Steven et al. 2005). Neuropeptide release from these neurons was examined indirectly by measuring YFP fluorescence in scavenger coelomocyte cells, which collect secreted proteins from the pseudocoelom. Coelomocyte YFP fluorescence in unc-73(ce362) mutants was ∼33% lower (P = 0.01) than the fluorescence from wild-type animals, suggesting less neuropeptide was released from UNC-73 RhoGEF-2 mutant neurons in this assay (Figure 4). Coelomocyte fluorescence after microinjection of fluorescently labeled BSA into the pseudocoelom revealed unc-73(ce362) mutants did not have coelomocyte uptake defects that would interfere with the interpretation of the neuropeptide release assay (Figure 4C).
Figure 4 .
Neuropeptide level is reduced in unc-73 RhoGEF-2 mutants. NLP-21::YFP expressed in cholinergic motor neurons was used to examine neuropeptide levels and neuropeptide release. (A) Representative images of DA6 and DB6 cholinergic motor neuron cell bodies in the ventral cord, axons in the dorsal cord, and posterior coelomocytes in wild-type and unc-73(ce362) animals containing unc-129p::nlp-21::yfp. Insets reveal the punctate localization of NLP-21::YFP in axons. (B) Quantification of the arbitrary fluorescence intensity standardized to wild type. No significant difference between mutant and wild-type DA6 and DB6 cell body fluorescence was observed (P = 0.58). YFP fluorescence levels were decreased in unc-73(ce362) dorsal cord axons (**P < 0.001) and posterior coelomocyte cells (*P = 0.01) in comparison to wild type. (C) Coelomocyte uptake of Texas Red-conjugated bovine serum albumin injected into the pseudocoelom is similar in N2 and unc-73(ce362) animals (P = 0.86). Error bars show SEM. P-values were calculated using Wilcoxon’s two-sample test.
To distinguish whether the reduced neuropeptide release in the unc-73 mutant was due to defects in the production, transport, or release of the neuropeptide we also examined YFP fluorescence in the axons and cell bodies of NLP-21::YFP-expressing neurons. YFP fluorescence in wild-type and mutant dorsal cords was punctate as expected from dense core vesicle cotransport of proprotein convertase cleaved neuropeptide and YFP down the axons (Figure 4A) (Kass et al. 2001; Sieburth et al. 2007). The unc-73(ce362) dorsal cord axon fluorescence was 40% lower compared to wild type (P = 0.001), but the cell bodies of mutant and wild-type animals had similar fluorescence levels (P = 0.58) (Figure 4). We conclude that the UNC-73 RhoGEF-2 isoforms are required for the production or maintenance of wild-type neuropeptide levels in axons, which ultimately affects the amount of neuropeptide released from neurons.
The Gαs pathway functions downstream of UNC-73 RhoGEF-2 activity
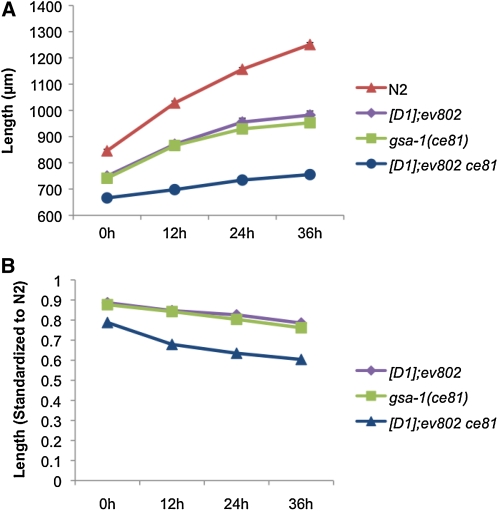
To better define where UNC-73 and Rho GTPase signaling may fit in the pathways regulating locomotion we performed an epistasis analysis with unc-73 lethargic mutants and genes that are known to regulate locomotion in C. elegans and display a hyperactive movement phenotype. Double mutants were constructed with either unc-73(ce362) or Is[D1]; unc-73(ev802), which have similar lethargic movement phenotypes. unc-73 double mutants made with hyperactive dgk-1 or goa-1 loss-of-function mutants were lethargic, suggesting UNC-73 RhoGEF-2 isoforms function downstream of or in parallel to dgk-1 and goa-1 (Steven et al. 2005). Is[D1]; unc-73(ev802) lethargy also suppressed the hyperactivity of the egl-30(tg26) gain-of-function allele and the hyperactivity of animals expressing a constitutively active egl-30 cDNA in cholinergic motor neurons, which is consistent with a study identifying UNC-73E as a downstream effector of Gαq/EGL-30 (Williams et al. 2007) (data not shown). In contrast, mutations that constitutively activate the Gαs/GSA-1 pathway, including gsa-1(ce81), acy-1(ce2) (adenylyl cyclase-1), and kin-2(ce179) (protein kinase-2; PKA regulatory subunit) completely suppressed the lethargy of Is[D1]; unc-73(ev802) and unc-73(ce362) animals (Figure 5A) and for unknown reasons also caused a reduction in animal length (Figure 6). A gsa-1 gain-of-function cDNA under the control of a heat-shock promoter also suppressed Is[D1]; unc-73(ev802) lethargic movement in heat-shocked adult animals (Figure 5A). These results indicate the Gαs/GSA-1 pathway functions downstream of or in parallel to the UNC-73 RhoGEF-2 isoforms and GSA-1 activity, like that of the UNC-73 RhoGEF-2 isoforms, is not required during development, but instead functions to regulate locomotion in the mature animal.
Figure 5 .
Constitutive Gαs/GSA-1 pathway activity rescues unc-73 RhoGEF-2 mutant lethargic locomotion. (A) acy-1(ce2) and gsa-1(ce81) gain-of-function mutations and the kin-2(ce179) loss-of-function mutation suppress Is[D1]; unc-73(ev802), unc-73(ce362), and rab-2(n501) locomotion defects. gsa-1(gf) cDNA expression in heat-shocked (HS) adult mutant animals also rescues unc-73 RhoGEF-2 mutant lethargy. (B) Expression of an acy-1(gf) cDNA exclusively in muscles (M) or the nervous system (NS) fails to rescue the unc-73 RhoGEF-2 lethargic phenotype, although expression of the cDNAs in combination does rescue the lethargy. (C) The unc-73(ce362); egl-3(ok979) locomotion rate is significantly lower than that of either single mutant alone. gsa-1(ce81) rescues the egl-3(ok979) lethargic movement phenotype. Error bars show SEM. **P < 0.001 in comparison to the corresponding unc-73, rab-2, or egl-3 mutant using Student’s t-test.
Figure 6 .
unc-73 RhoGEF-2 and gsa-1(gf) mutants are shorter than wild type. (A) Is[D1]; unc-73(ev802) and gsa-1(ce81) mutants are shorter than wild type. The Is[D1]; gsa-1(ce81) unc-73(ev802) double mutants are shorter than the single-mutant animals (P = 0.0002; ANOVA two-factor without replication). Worm lengths were measured starting at the L4 “crescent vulva” stage and three more times every 12 hr. n ≥ 25 for each strain. Error bars show SEM. (B) Worm lengths shown as a percentage standardized to wild type.
Constitutively active Gαs/GSA-1 pathway mutants are hypersensitive to aldicarb, indicating they release more acetylcholine into the synapse or they are more sensitive to acetylcholine (Figure 1C) (Schade et al. 2005). Increased acetylcholine release and sensitivity are likely factors involved in the activated Gαs/GSA-1 pathway rescue of unc-73 RhoGEF-2 lethargy as gsa-1(ce81) unc-73(ce362) and Is[D1]; gsa-1(ce81) unc-73(ev802) double mutants were hypersensitive to both aldicarb and 0.5 mM levamisole (Figure 1, C and E, and Figure S1A; data not shown). Increased acetylcholine release may be the more significant factor in aldicarb hypersensitivity since gsa-1(ce81) mutants are actually resistant to a lower concentration of 0.1 mM levamisole (Schade et al. 2005). Interestingly, levamisole sensitivity does not correlate with the locomotory phenotypes of these mutants. For example, rescued gsa-1(ce81) unc-73(ce362) double mutants with wild-type locomotion rates and lethargic unc-73(ce362) single mutants are both strongly hypersensitive to levamisole (Figures 1E and 5A). Similarly, transgenic animals that are lethargic due to reduced RHO-1 activity or hyperactive as a result of rho-1(gf) expression are hypersensitive to levamisole (McMullan et al. 2006).
unc-73 lethargic movement is similar to that of rab-2 and unc-31
The lethargic yet coordinated locomotion phenotype of unc-73 RhoGEF-2 mutants is separate from the severe uncoordinated movement phenotypes of genes such as snb-1 (synaptobrevin), which are fundamental to the mechanisms of synapse function involving synaptic vesicles, and is perhaps more suggestive of a role for RhoGEF-2 activity in the modulatory mechanisms controlling locomotion rate (Figure 2) (Hall and Hedgecock 1991; Maruyama and Brenner 1991; Nonet et al. 1998; Steven et al. 2005). For example, C. elegans RAB2 (RAB-2) function is critical for the modulatory regulation of locomotion through its role in DCV maturation (Edwards et al. 2009; Sumakovic et al. 2009). Importantly, the rab-2 locomotion phenotype (Park and Horvitz 1986; Chun et al. 2008) is very similar to Is[D1]; unc-73(ev802) and unc-73(ce362) slow movement phenotypes; animals are extremely slow on food, but increase their speed off food to a rate about one-third that of wild type (Figure 5A; see File S1, File S3, and File S5). We also observed that rab-2(n501) lethargy was suppressed by either a Gαs/GSA-1 pathway gain-of-function mutation or treatment with phorbol ester (Figure 5A; see File S1, File S2, File S3, and File S4). Phorbol esters are stable analogs of DAG, which activate PKC and UNC-13 and can stimulate both synaptic vesicle and DCV-mediated neurotransmission (Gillis et al. 1996; Stevens and Sullivan 1998; Lackner et al. 1999; Rhee et al. 2002; Sieburth et al. 2007). The lethargy of unc-73 RhoGEF-2 mutants was rescued by phorbol esters as well (Williams et al. 2007) (see File S5 and File S6). Also consistent is the fact that unc-73 RhoGEF-2 mutants and rab-2 mutants both were weakly resistant to aldicarb and were hypersensitive to the acetylcholine receptor agonist levamisole, suggesting similar alterations to cholinergic signaling may occur in these mutants, although Sumakovic et al. (2009) reported that rab-2 mutants have wild-type levamisole sensitivity using a different method (Figure 1, A and B, and Figure S1, B and C) (Edwards et al. 2009). Our observations place the UNC-73 RhoGEF-2 isoforms in a category of proteins including RAB-2 and UNC-31 [C. elegans calcium-dependent activator protein for secretion (CAPS)], which play distinct roles in the DCV-mediated modulation of locomotion and whose very similar slow movement phenotypes are rescued by constitutive activation of the Gαs/GSA-1 pathway (Avery et al. 1993; Charlie et al. 2006).
Gαs/GSA-1 pathway function
Our results indicate UNC-73 RhoGEF-2 isoforms function specifically in the nervous system where they may regulate DCV-mediated transport of neuromodulators affecting locomotion. Increased GSA-1 signaling elevates DCV exocytosis from neurons, suggesting a possible explanation for Gαs/GSA-1 pathway rescue of the unc-73 RhoGEF-2 slow movement phenotype (Zhou et al. 2007). To test whether the Gαs/GSA-1 pathway functions in neurons for rescue we expressed an ACY-1(P280S) gain-of-function protein (Schade et al. 2005) specifically in the nervous system of unc-73(ce362) mutants. Surprisingly, these animals and ce362 animals with ACY-1(P280S) expressed specifically in the muscles remained lethargic; however, ce362 animals containing ACY-1(P280S) in both the nervous system and in muscles were no longer lethargic (Figure 5B). Therefore, it appears Gαs/GSA-1 pathway activation in both neurons and muscles is required to compensate for unc-73 RhoGEF-2 neuronal defects.
UNC-73 RhoGEF-2 isoforms have functions in addition to neuropeptide signaling
EGL-3 is a proprotein convertase, which cleaves propeptides and is required for the production of functional neuropeptides (Kass et al. 2001). Like unc-73 RhoGEF-2 mutants, egl-3 mutants are resistant to aldicarb and hypersensitive to levamisole (Jacob and Kaplan 2003). Since our results indicate UNC-73 RhoGEF-2 isoforms affect the level of neuromodulatory protein in the DCVs of peptidergic neurons, we looked for evidence of a genetic interaction between unc-73(ce362) and a putative egl-3(ok979) null mutant. The unc-73(ce362); egl-3(ok979) double mutants move very slowly, at a rate ∼95% slower than wild type, which is slower than the rate for either single mutant alone, suggesting EGL-3 and the UNC-73 RhoGEF-2 isoforms act in parallel pathways (Figure 5C). It is important to consider, however, that it is difficult to predict the effect of EGL-3 inactivation in double mutants since neuropeptides can have either positive or negative effects on locomotion and egl-3 mutations may not affect all neuropeptides or completely inactivate them since neuropeptide staining with an FMRFamide antibody is only moderately reduced in egl-3 mutants (Nelson et al. 1998; Kass et al. 2001; Jacob and Kaplan 2003; Husson et al. 2006). This makes the unc-73; egl-3 phenotype difficult to interpret and may also explain why egl-3 null mutants have such a mild locomotion phenotype with rates just 30% lower than wild type (Figure 5C). The severe lethargy of unc-73; egl-3 double mutants at least suggests UNC-73 RhoGEF-2 isoforms may affect locomotion through molecules in addition to neuropeptides. Indeed, we observed that unc-73 RhoGEF-2 mutations, particularly in a gsa-1 mutant background, can influence animal length, which may result from DCV signaling defects affecting the function of proteins or growth factors in addition to neuropeptides (Figure 6).
Finally, we examined the relationship between the Gαs/GSA-1 pathway and neuropeptide pathways affected by EGL-3 processing. In a gsa-1(ce81) gain-of-function background egl-3(ok979) lethargic movement was rescued to speeds even faster than wild type, indicating constitutive Gαs/GSA-1 pathway activation can compensate for neuropeptide processing defects affecting locomotion (Figure 5C).
Discussion
This study examines the role of Rho GTPase pathway signaling in the regulation of C. elegans locomotory behavior. Mutations affecting the Rho GTPase-specific UNC-73 RhoGEF-2 isoforms result in a decreased rate of locomotion while animals continue to move in a coordinated sinusoidal manner. Our results suggest UNC-73 RhoGEF-2 isoforms affect C. elegans motility through changes in neurotransmission signaling involving acetylcholine and the regulation of DCV neuromodulatory protein levels in mature neurons. Cholinergic signaling alterations observed in unc-73 RhoGEF-2 mutants treated with pharmacological agents may be due to UNC-73 RhoGEF-2 isoforms playing a direct role in cholinergic signaling mechanisms. However, phenotypic similarities between unc-73 and DCV signaling genes and our observation of DCV signaling defects in unc-73 mutants instead suggest a role for UNC-73 RhoGEF-2 isoforms in the DCV-mediated modulatory mechanisms regulating locomotion with observed changes in cholinergic signaling possibly a secondary effect of DCV-mediated signaling. Importantly, we observed that unc-73 RhoGEF-2 mutant lethargy is rescued by constitutive activation of the Gαs/GSA-1 pathway. This is consistent with the proposed role for UNC-73 RhoGEF-2 isoforms in DCV secretory pathway regulation since Gαs/GSA-1 pathway activation increases DCV exocytosis and can bypass defects in DCV signaling (Charlie et al. 2006; Zhou et al. 2007).
unc-73, rab-2, and unc-31 have similar phenotypes
In addition to unc-73 the genes rab-2 and unc-31 have very similar lethargic, yet coordinated movement phenotypes that are also rescued by Gαs/GSA-1 pathway activation (Figure 5) (Charlie et al. 2006). UNC-31 is the C. elegans homolog of mammalian CAPS, a component of the DCV release machinery required for hormone and neuropeptide release from neuroendocrine cells (Walent et al. 1992; Rupnik et al. 2000). Although CAPS and its paralog CAPS-2 may have additional functions, including synaptic vesicle priming and catecholamine uptake into DCVs, the role of the only C. elegans CAPS homolog, UNC-31, specifically involves DCV docking in the process of exocytosis from neurons (Speidel et al. 2005; Gracheva et al. 2007; Jockusch et al. 2007; Speese et al. 2007; Zhou et al. 2007; Hammarlund et al. 2008). Drosophila CAPS function at the neuromuscular junction is also restricted to DCV exocytosis with additional cell nonautonomous effects on synaptic vesicle release believed to result from the lack of neuromodulators released from DCVs (Renden et al. 2001). After DCVs bud from the Golgi, the C. elegans RAB2 GTPase, RAB-2/UNC-108, is required for DCV maturation in a process that occurs in neuronal cell bodies upstream of DCV release mechanisms (Edwards et al. 2009; Sumakovic et al. 2009).
Thus, unc-31, rab-2, and unc-73 RhoGEF-2 mutants not only have very similar phenotypes, including weak or no resistance to aldicarb, hypersensitivity to levamisole, and almost identical lethargic movement phenotypes that can be rescued by phorbol esters or Gαs/GSA-1 pathway activation, but also each of the proteins encoded by these genes functions in the DCV signaling pathway (Figures 1, A and B, and 5A and Figure S1, B and C) (Avery et al. 1993; Miller et al. 1996; Daniels et al. 2000; Steven et al. 2005; Charlie et al. 2006; Williams et al. 2007; Chun et al. 2008; Sumakovic et al. 2009). The similar lethargic phenotypes of these genes are interesting in that the mutants are extremely slow on food, but they increase their speed to a rate about one-third that of wild type when removed from bacteria. It is also consistent that cholinergic motor neuron-specific unc-73 or rab-2 expression is not sufficient to rescue their respective locomotion phenotypes, although unc-31 cholinergic motor neuron expression rescues unc-31 locomotion to 80% of the wild-type rate (Figure 3) (Charlie et al. 2006; Chun et al. 2008). On the basis of these similarities we describe the unc-73, rab-2, and unc-31 genes as having an Lrg phenotype (lethargic, but coordinated movement rescued by Gαs activation) and predict that other genes in the neuromodulatory pathways controlling locomotion will also have a Lrg phenotype.
UNC-73 RhoGEF-2 isoforms and neuromodulatory protein signaling
Decreased NLP-21::YFP neuropeptide fluorescence in axons and coelomocytes, but not the cell bodies of unc-73 RhoGEF-2 mutants suggests at least two possible functions for UNC-73 RhoGEF-2 isoforms in the secretory pathway: (1) producing, trafficking, or maintaining the correct number of DCVs traveling down the axon and/or (2) loading or maintaining the correct number of neuromodulators in DCVs. UNC-73 RhoGEF-2 isoforms are not required for the initial production of neuropeptides since there is no difference in NLP-21::YFP cell body fluorescence in mutants compared to wild type.
UNC-73 RhoGEF-2 domain activity is specific to the GTPase Rho (Spencer et al. 2001). Consistent with UNC-73 RhoGEF-2 function in the secretory pathway Rho localizes to secretory granule membranes in chromaffin cells and modulates the secretory pathway in MAST cells (Price et al. 1995; Gasman et al. 1998; Ory and Gasman 2011). Another interesting finding is that ARAP1, which has both RhoGAP (GTPase activating protein) and ArfGAP domains, localizes to the Golgi apparatus and can change Golgi morphology when overexpressed (Miura et al. 2002).
UNC-73 mammalian homologs Trio and Kalirin also function in the cellular secretory pathway. Kalirin was originally identified through its association with peptidylglycine-amidating monooxygenase (PAM), a neuropeptide processing enzyme that functions in DCVs (Alam et al. 1997; Mains et al. 1999). Kalirin and Trio modulate DCV maturation in pituitary derived AtT-20 neuroendocrine cells and their isoforms are differentially associated with Golgi, immature DCVs, and endosomes (Ferraro et al. 2007). Increasing Kalirin or Trio activity depletes immature DCVs of their hormone cargo, while Kalirin or Trio inhibition increases the amount of mature hormone product in mature DCVs. This role for Kalirin and Trio in the secretory pathway is mediated by their RhoGEF-1 domains, suggesting the UNC-73 RhoGEF-1 domain may have a role in the secretory pathway. Our study, on the other hand, indicates Trio and Kalirin RhoGEF-2 activity may also function in the secretory pathway.
Which secreted proteins are influenced by UNC-73 RhoGEF-2 function?
Many neuropeptides and neuropeptide receptors are known to influence muscle and neuronal activity and ultimately locomotion in C. elegans and other nematodes (Marks et al. 2001; Rogers et al. 2001; Keating et al. 2003; Husson et al. 2007). It is possible that one or more neuromodulators required to regulate locomotion are released from neuronal DCVs in wild-type animals but are not transported properly through the neuronal secretory pathway in unc-73 RhoGEF-2 or rab-2 mutants and not properly released from unc-31 mutant neurons. The identity of this neuromodulator(s) is not known and although we use NLP-21::YFP neuropeptide to monitor movement of neuromodulatory proteins through the secretory pathway in our assays, NLP-21 is not a likely candidate since nlp-21 RNAi does not cause locomotory defects (Kamath et al. 2003). In fact, a neuropeptide may not be the neuromodulator affected in unc-73 RhoGEF-2 mutants since the severity of the locomotion defect in these mutants increases in an egl-3 neuropeptide-processing mutant background (Figure 5C). Also, unc-31 mutants, with compromised DCV exocytosis, are much more lethargic than egl-3 and egl-21 neuropeptide-processing mutants, suggesting other neuromodulatory proteins in addition to neuropeptides are regulating locomotion (Kass et al. 2001; Jacob and Kaplan 2003; Speese et al. 2007).
For example, TGFβ signaling may be affected in unc-73 RhoGEF-2 mutants. The C. elegans TGFβ family member UNC-129 has a locomotory phenotype, but it is thought to result from axon guidance defects during development (Colavita et al. 1998). Mutations in other TGFβ signaling molecules can suppress unc-2 mutant calcium channel lethargy, but the mechanisms are not defined (Estevez et al. 2004). C. elegans TGFβ pathways are more well known for the control of dauer formation and importantly, animal size, a trait affected in unc-73 RhoGEF-2 mutants (Figure 6). Also relevant are observations that vertebrate TGFβ affects the activity of mature synapses at the neuromuscular junction (Chin et al. 2002; Fong et al. 2010). Although no vertebrate neurotrophin family homologs exist in C. elegans, it is possible other proteins regulated by UNC-73 RhoGEF-2 isoforms have neurotrophin-like functions in synaptic activity modulation (Lessmann and Brigadski 2009). Finally, biogenic amines such as serotonin and dopamine can be released from DCVs, but on the basis of phenotype analysis, our cell-specific rescue experiments, and previous double-mutant characterization these molecules do not appear to be neuromodulators affected in unc-73 RhoGEF-2 mutants (Steven et al. 2005; Sudhof 2008).
Where is UNC-73 RhoGEF-2 function required to regulate locomotion?
UNC-73 RhoGEF-2 isoforms function in the nervous system to modulate C. elegans locomotion rate (Figure 2). Body wall muscles, which facilitate C. elegans locomotion, are innervated by cholinergic motor neurons, possible candidates for the specific site of UNC-73 RhoGEF-2 function within the nervous system. Indeed, we observe that neuropeptide signaling is reduced in unc-73 RhoGEF-2 mutant cholinergic motor neurons (Figure 4) and C3-mediated Rho inactivation in these same neurons results in a slow movement phenotype, indicating UNC-73 RhoGEF-2 isoforms and the Rho pathway have roles in cholinergic motor neuron function (McMullan et al. 2006). However, UNC-73 RhoGEF-2 isoforms likely function in additional neurons since unc-73 lethargy is not rescued with cholinergic motor neuron-specific expression of the UNC-73E isoform (Figure 3). Interestingly, the aldicarb resistance phenotypes of the neuropeptide processing genes egl-3 and egl-21 and, as mentioned previously, rab-2 lethargy, also are not rescued by cholinergic motor neuron-specific expression (Jacob and Kaplan 2003; Chun et al. 2008). These observations and the rescue of unc-73 lethargy with peptidergic unc-73E expression (Figure 3) are consistent with UNC-73 RhoGEF-2 isoforms, RAB-2, and the neuropeptide processing enzymes EGL-3 and EGL-21 performing a neuromodulatory function in unidentified control neurons upstream of the cholinergic motor neurons.
Gαs/GSA-1 pathway activation rescues UNC-73 RhoGEF-2 mutant locomotory defects
cAMP and PKA regulate DCV and synaptic vesicle release in multiple cell types and are required for the modulation of synaptic plasticity in mammals through the modification of ion channels, the synaptic release machinery, or transcription factors. (Brandon et al. 1997; Seino and Shibasaki 2005). In C. elegans, defects in UNC-31/CAPS-mediated neuromodulator release from DCVs are suppressed by cAMP production and PKA activation downstream of Gαs/GSA-1. Specifically, unc-31 DCV docking and exocytosis defects, observed by total internal reflection fluorescence microscopy and membrane capacitance measurements, are ameliorated by both forskolin application and genetic Gαs/GSA-1 pathway activation (Zhou et al. 2007), while unc-31 locomotion defects are also suppressed by Gαs/GSA-1 pathway activation (Charlie et al. 2006).
Our analysis and rab-2 reports (Edwards et al. 2009; Sumakovic et al. 2009) suggest UNC-73 RhoGEF-2 isoforms and RAB-2 function upstream of the UNC-31–mediated mechanisms of exocytosis at the plasma membrane; therefore, one possibility is that increased Gαs/GSA-1 pathway activity enlarges the readily releasable pool of DCVs at the plasma membrane to compensate for the reduced level of neuromodulator proteins sent for exocytosis in unc-73 RhoGEF-2 and rab-2 mutants (Zhou et al. 2007). Our observations with egl-3 neuropeptide processing mutants, however, suggest the Gαs/GSA-1 pathway may instead act downstream of neuromodulatory protein release from DCVs. It seems unlikely that locomotory defects resulting from unprocessed neuropeptides would be compensated for by an increased release of the unprocessed neuropeptides from DCVs, yet we observe that egl-3 locomotion defects are rescued by Gαs/GSA-1 pathway activation (Figure 5C). Another possibility is that the proposed neuromodulatory protein(s), whose secretion is affected by UNC-73, RAB-2, and UNC-31, activates a Gαs/GSA-1–coupled receptor-modulating locomotion. GSA-1 activation would therefore increase acetylcholine release and increase locomotion rates in unc-73, rab-2, and unc-31 mutants downstream of their respective DCV signaling defects. Our results further show that Gαs/GSA-1 pathway activity is required in neurons and body wall muscle for complete rescue of unc-73 RhoGEF-2 mutant lethargy (Figure 5B). This is consistent with the role of the Gαs/GSA-1 pathway in C. elegans, which functions in both the nervous system and body wall muscles to modulate the rate of locomotion (Reynolds et al. 2005).
Although our data are consistent with the above hypothesis, there is no definitive proof the Gαs/GSA-1 pathway functions downstream of DCV signaling to regulate C. elegans locomotion. Gαs/GSA-1 pathway activation increases acetylcholine release, which could rescue unc-73 RhoGEF-2 mutant lethargy whether UNC-73 functions in either a DCV or a synaptic vesicle signaling pathway (Figure 1C and Figure S1A) (Schade et al. 2005). However, aldicarb-hypersensitive dgk-1 mutants do not rescue the unc-73 RhoGEF-2 lethargic movement phenotype, indicating not all mutants that increase acetylcholine release can rescue unc-73 lethargy (Miller et al. 1999; Steven et al. 2005).
UNC-73 RhoGEF-2 isoforms have Gαq/EGL-30–independent functions
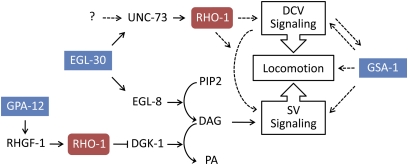
Screens for mutants that suppress the hyperactive and slow growth phenotypes of an overactive Gαq/EGL-30 pathway identified UNC-73E as a direct downstream effector of Gαq/EGL-30 acting in parallel to its well-known target phospholipase Cβ/EGL-8 (Figure 7) (Williams et al. 2007). Several observations lead us to believe UNC-73 RhoGEF-2 isoforms also have Gαq/EGL-30–independent functions in the regulation of locomotion: (1) unc-73 RhoGEF-2 mutants have weak resistance to the acetylcholinesterase inhibitor aldicarb and are hypersensitive to levamisole, while egl-30 mutants display strong aldicarb resistance and are no different from wild type on levamisole (Figure 1) (Lackner et al. 1999; Williams et al. 2007); (2) EGL-30 modulates locomotion through its activity in cholinergic motor neurons (Lackner et al. 1999), but UNC-73 RhoGEF-2 isoform effects on locomotion involve neurons in addition to cholinergic motor neurons (Figure 3); and (3) genetic activation of the Gαs/GSA-1 pathway completely rescues unc-73 RhoGEF-2 mutant locomotory defects (Figure 5A), but the same Gαs/GSA-1 pathway activation does not even partially rescue egl-30 locomotory defects (Reynolds et al. 2005). UNC-73E is likely a direct downstream Gαq/EGL-30 effector with regard to egg laying and growth (Williams et al. 2007); however, the relationship between EGL-30 and UNC-73E in the modulation of locomotion is not as clear and may involve one or more additional factors upstream of UNC-73 (“?” in Figure 7).
Figure 7 .
Rho GTPase and heterotrimeric G-protein pathway interactions regulating neurotransmission and locomotion. G-protein pathways (blue boxes) interact with Rho GTPase pathways (red boxes) directly (solid arrows) or through indirect or unknown mechanisms (dashed arrows). Rho exists in two separate populations, only one of which interacts with DGK-1 (Hiley et al. 2006; McMullan et al. 2006). The Gαo (GOA-1) pathway negatively regulates Gαq (EGL-30) and synaptic vesicle signaling, but is omitted for clarity (Hajdu-Cronin et al. 1999; Miller et al. 1999; Nurrish et al. 1999). UNC-73 RhoGEF-2 activity (UNC-73) functions downstream of EGL-30 and likely another factor(s) to modulate locomotion through dense core vesicle (DCV) and/or synaptic vesicle (SV) signaling. Constitutive Gαs (GSA-1) pathway activation can compensate for unc-73 RhoGEF-2 mutant locomotion defects, but the mechanism is not known. See the Discussion and references for details (Brundage et al. 1996; Kozasa et al. 1998; Lackner et al. 1999; Reynolds et al. 2005; Schade et al. 2005; Charlie et al. 2006; Hiley et al. 2006; McMullan et al. 2006; Williams et al. 2007; Zhou et al. 2007).
In conclusion, our analysis suggests UNC-73 RhoGEF-2 isoforms regulate locomotion through changes in neurotransmitter signaling. We propose that the UNC-73 RhoGEF-2 isoforms are required for DCV-mediated neuromodulatory protein signaling that regulates the rate of locomotion upstream of the Gαs/GSA-1 pathway in C. elegans. Further experiments are required to identify the specific connections between the Rho GTPase and Gαs/GSA-1 pathways and DCV signaling.
Acknowledgments
We thank Josh Kaplan, Ken Miller, Queelim Ch’ng, and the Caenorhabditis Genetics Center [which is funded by the National Institutes of Health (NIH) National Center for Research Resources] for nematode strains; Bruce Bamber, Andy Fire, Josh Kaplan, Ken Miller, Stephen Nurrish, Dave Pilgrim, Jim Rand, Piali Sengupta, and Derek Sieburth for plasmids; Qing Zhao for assistance with statistical analysis; and Rick Komuniecki, Gareth Harris, Thuy Tran, and the reviewers for helpful comments that improved the manuscript. This work was supported by NIH grant 1R15NS062406 (to R.M.S.).
Literature Cited
- Alam M. R., Johnson R. C., Darlington D. N., Hand T. A., Mains R. E., et al. , 1997. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J. Biol. Chem. 272: 12667–12675 [DOI] [PubMed] [Google Scholar]
- Alexander M., Chan K. K., Byrne A. B., Selman G., Lee T., et al. , 2009. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development 136: 911–922 [DOI] [PubMed] [Google Scholar]
- Avery L., Bargmann C. I., Horvitz H. R., 1993. The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics 134: 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axang C., Rauthan M., Hall D. H., Pilon M., 2008. Developmental genetics of the C. elegans pharyngeal neurons NSML and NSMR. BMC Dev. Biol. 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon E. P., Idzerda R. L., McKnight G. S., 1997. PKA isoforms, neural pathways, and behaviour: making the connection. Curr. Opin. Neurobiol. 7: 397–403 [DOI] [PubMed] [Google Scholar]
- Brundage L., Avery L., Katz A., Kim U. J., Mendel J. E., et al. , 1996. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron 16: 999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell L., Illi J., Hong S. W., McIntire S. L., 2005. The G-protein-coupled serotonin receptor SER-1 regulates egg laying and male mating behaviors in Caenorhabditis elegans. J. Neurosci. 25: 10671–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L., McDonald P. W., Blakely R. D., Defelice L. J., 2004. Dopamine transporters depolarize neurons by a channel mechanism. Proc. Natl. Acad. Sci. USA 101: 16046–16051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlie N. K., Schade M. A., Thomure A. M., Miller K. G., 2006. Presynaptic UNC-31 (CAPS) is required to activate the G alpha(s) pathway of the Caenorhabditis elegans synaptic signaling network. Genetics 172: 943–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J., Angers A., Cleary L. J., Eskin A., Byrne J. H., 2002. Transforming growth factor beta1 alters synapsin distribution and modulates synaptic depression in Aplysia. J. Neurosci. 22: RC220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D. K., McEwen J. M., Burbea M., Kaplan J. M., 2008. UNC-108/Rab2 regulates postendocytic trafficking in Caenorhabditis elegans. Mol. Biol. Cell 19: 2682–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita A., Krishna S., Zheng H., Padgett R. W., Culotti J. G., 1998. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 281: 706–709 [DOI] [PubMed] [Google Scholar]
- Daniels S. A., Ailion M., Thomas J. H., Sengupta P., 2000. egl-4 acts through a transforming growth factor-beta/SMAD pathway in Caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics 156: 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBakker C. D., Haney L. B., Kinchen J. M., Grimsley C., Lu M., et al. , 2004. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 14: 2208–2216 [DOI] [PubMed] [Google Scholar]
- Debant A., Serra-Pages C., Seipel K., O’Brien S., Tang M., et al. , 1996. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl. Acad. Sci. USA 93: 5466–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M., Iwasaki K., 2002. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron 33: 249–259 [DOI] [PubMed] [Google Scholar]
- Edwards S. L., Charlie N. K., Richmond J. E., Hegermann J., Eimer S., et al. , 2009. Impaired dense core vesicle maturation in Caenorhabditis elegans mutants lacking Rab2. J. Cell Biol. 186: 881–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M., Estevez A. O., Cowie R. H., Gardner K. L., 2004. The voltage-gated calcium channel UNC-2 is involved in stress-mediated regulation of tryptophan hydroxylase. J. Neurochem. 88: 102–113 [DOI] [PubMed] [Google Scholar]
- Ferraro F., Ma X. M., Sobota J. A., Eipper B. A., Mains R. E., 2007. Kalirin/Trio Rho guanine nucleotide exchange factors regulate a novel step in secretory granule maturation. Mol. Biol. Cell 18: 4813–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong S. W., McLennan I. S., McIntyre A., Reid J., Shennan K. I., et al. , 2010. TGF-beta2 alters the characteristics of the neuromuscular junction by regulating presynaptic quantal size. Proc. Natl. Acad. Sci. USA 107: 13515–13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasman S., Chasserot-Golaz S., Hubert P., Aunis D., Bader M. F., 1998. Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J. Biol. Chem. 273: 16913–16920 [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Mossner R., Neher E., 1996. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron 16: 1209–1220 [DOI] [PubMed] [Google Scholar]
- Gracheva E. O., Burdina A. O., Touroutine D., Berthelot-Grosjean M., Parekh H., et al. , 2007. Tomosyn negatively regulates CAPS-dependent peptide release at Caenorhabditis elegans synapses. J. Neurosci. 27: 10176–10184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu-Cronin Y. M., Chen W. J., Patikoglou G., Koelle M. R., Sternberg P. W., 1999. Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G(o)alpha signaling and regulates G(q)alpha activity. Genes Dev. 13: 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Hedgecock E. M., 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: 837–847 [DOI] [PubMed] [Google Scholar]
- Hallam S. J., Jin Y., 1998. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature 395: 78–82 [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Watanabe S., Schuske K., Jorgensen E. M., 2008. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J. Cell Biol. 180: 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., Stern B. D., 1987. Genetics of cell and axon migrations in Caenorhabditis elegans. Development 100: 365–382 [DOI] [PubMed] [Google Scholar]
- Hiley E., McMullan R., Nurrish S. J., 2006. The Galpha12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 25: 5884–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg L., Kenyon C., 2000. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development 127: 4655–4668 [DOI] [PubMed] [Google Scholar]
- Husson S. J., Clynen E., Baggerman G., Janssen T., Schoofs L., 2006. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J. Neurochem. 98: 1999–2012 [DOI] [PubMed] [Google Scholar]
- Husson S. J., Mertens I., Janssen T., Lindemans M., Schoofs L., 2007. Neuropeptidergic signaling in the nematode Caenorhabditis elegans. Prog. Neurobiol. 82: 33–55 [DOI] [PubMed] [Google Scholar]
- Jacob T. C., Kaplan J. M., 2003. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23: 2122–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A., 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21: 247–269 [DOI] [PubMed] [Google Scholar]
- Jockusch W. J., Speidel D., Sigler A., Sorensen J. B., Varoqueaux F., et al. , 2007. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131: 796–808 [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237 [DOI] [PubMed] [Google Scholar]
- Kass J., Jacob T. C., Kim P., Kaplan J. M., 2001. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J. Neurosci. 21: 9265–9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P. S., Frost W. N., 1996. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci. 19: 54–61 [DOI] [PubMed] [Google Scholar]
- Keating C. D., Kriek N., Daniels M., Ashcroft N. R., Hopper N. A., et al. , 2003. Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr. Biol. 13: 1715–1720 [DOI] [PubMed] [Google Scholar]
- Kishore R. S., Sundaram M. V., 2002. ced-10 Rac and mig-2 function redundantly and act with unc-73 trio to control the orientation of vulval cell divisions and migrations in Caenorhabditis elegans. Dev. Biol. 241: 339–348 [DOI] [PubMed] [Google Scholar]
- Korswagen H. C., Park J. H., Ohshima Y., Plasterk R. H., 1997. An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev. 11: 1493–1503 [DOI] [PubMed] [Google Scholar]
- Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., et al. , 1998. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280: 2109–2111 [DOI] [PubMed] [Google Scholar]
- Kubiseski T. J., Culotti J., Pawson T., 2003. Functional analysis of the Caenorhabditis elegans UNC-73B PH domain demonstrates a role in activation of the Rac GTPase in vitro and axon guidance in vivo. Mol. Cell. Biol. 23: 6823–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner M. R., Nurrish S. J., Kaplan J. M., 1999. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346 [DOI] [PubMed] [Google Scholar]
- Lee R. Y., Sawin E. R., Chalfie M., Horvitz H. R., Avery L., 1999. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci. 19: 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V., Brigadski T., 2009. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci. Res. 65: 11–22 [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf N., Culotti J. G., 2007. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat. Neurosci. 10: 161–168 [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Wu C. H., Levine J. H., Berg H., 1980. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience 5: 967–989 [DOI] [PubMed] [Google Scholar]
- Liu T., Kim K., Li C., Barr M. M., 2007. FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J. Neurosci. 27: 7174–7182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist E. A., 2003. Rac proteins and the control of axon development. Curr. Opin. Neurobiol. 13: 384–390 [DOI] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N., 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8: 1787–1802 [DOI] [PubMed] [Google Scholar]
- Lutz S., Freichel-Blomquist A., Yang Y., Ruemenapp U., Jakobs K. H., et al. , 2005. The guanine nucleotide exchange factor p63RhoGEF—a specific link between Gq/11-coupled receptor signaling and RhoA. J. Biol. Chem. 280: 11134–11139 [DOI] [PubMed] [Google Scholar]
- Mahoney T. R., Luo S., Nonet M. L., 2006. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat. Protoc. 1: 1772–1777 [DOI] [PubMed] [Google Scholar]
- Mains R. E., Alam M. R., Johnson R. C., Darlington D. N., Back N., et al. , 1999. Kalirin, a multifunctional PAM COOH-terminal domain interactor protein, affects cytoskeletal organization and ACTH secretion from AtT-20 cells. J. Biol. Chem. 274: 2929–2937 [DOI] [PubMed] [Google Scholar]
- Marks N. J., Shaw C., Halton D. W., Thompson D. P., Geary T. G., et al. , 2001. Isolation and preliminary biological assessment of AADGAPLIRFamide and SVPGVLRFamide from Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 286: 1170–1176 [DOI] [PubMed] [Google Scholar]
- Maruyama I. N., Brenner S., 1991. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 88: 5729–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan R., Hiley E., Morrison P., Nurrish S. J., 2006. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 20: 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Alfonso A., Nguyen M., Crowell J. A., Johnson C. D., et al. , 1996. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA 93: 12593–12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Emerson M. D., Rand J. B., 1999. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 24: 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Jacques K. M., Stauffer S., Kubosaki A., Zhu K., et al. , 2002. ARAP1: a point of convergence for Arf and Rho signaling. Mol. Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Morita K., Hirono K., Han M., 2005. The Caenorhabditis elegans ect-2 RhoGEF gene regulates cytokinesis and migration of epidermal P cells. EMBO Rep. 6: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R., Hall D. H., Miller D. M., 3rd, Blakely R. D., 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99: 3264–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. S., Rosoff M. L., Li C., 1998. Disruption of a neuropeptide gene, flp-1, causes multiple behavioral defects in Caenorhabditis elegans. Science 281: 1686–1690 [DOI] [PubMed] [Google Scholar]
- Nguyen M., Alfonso A., Johnson C. D., Rand J. B., 1995. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., 1999. Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J. Neurosci. Methods 89: 33–40 [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Saifee O., Zhao H., Rand J. B., Wei L., 1998. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J. Neurosci. 18: 70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurrish S., Segalat L., Kaplan J. M., 1999. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242 [DOI] [PubMed] [Google Scholar]
- Ory S., Gasman S., 2011. Rho GTPases and exocytosis: What are the molecular links? Semin. Cell Dev. Biol. 22: 27–32 [DOI] [PubMed] [Google Scholar]
- Park E. C., Horvitz H. R., 1986. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics 113: 821–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mansilla B., Nurrish S., 2009. A network of G-protein signaling pathways control neuronal activity in C. elegans. Adv. Genet. 65: 145–192 [DOI] [PubMed] [Google Scholar]
- Price L. S., Norman J. C., Ridley A. J., Koffer A., 1995. The small GTPases Rac and Rho as regulators of secretion in mast cells. Curr. Biol. 5: 68–73 [DOI] [PubMed] [Google Scholar]
- Renden R., Berwin B., Davis W., Ann K., Chin C. T., et al. , 2001. Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron 31: 421–437 [DOI] [PubMed] [Google Scholar]
- Reynolds N. K., Schade M. A., Miller K. G., 2005. Convergent, RIC-8-dependent Galpha signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics 169: 651–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J. S., Betz A., Pyott S., Reim K., Varoqueaux F., et al. , 2002. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 108: 121–133 [DOI] [PubMed] [Google Scholar]
- Rogers C. M., Franks C. J., Walker R. J., Burke J. F., Holden-Dye L., 2001. Regulation of the pharynx of Caenorhabditis elegans by 5-HT, octopamine, and FMRFamide-like neuropeptides. J. Neurobiol. 49: 235–244 [DOI] [PubMed] [Google Scholar]
- Rojas R. J., Yohe M. E., Gershburg S., Kawano T., Kozasa T., et al. , 2007. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J. Biol. Chem. 282: 29201–29210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M., Kreft M., Sikdar S. K., Grilc S., Romih R., et al. , 2000. Rapid regulated dense-core vesicle exocytosis requires the CAPS protein. Proc. Natl. Acad. Sci. USA 97: 5627–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade M. A., Reynolds N. K., Dollins C. M., Miller K. G., 2005. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics 169: 631–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S., Shibasaki T., 2005. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85: 1303–1342 [DOI] [PubMed] [Google Scholar]
- Sieburth D., Madison J. M., Kaplan J. M., 2007. PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 10: 49–57 [DOI] [PubMed] [Google Scholar]
- Speese S., Petrie M., Schuske K., Ailion M., Ann K., et al. , 2007. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 27: 6150–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel D., Bruederle C. E., Enk C., Voets T., Varoqueaux F., et al. , 2005. CAPS1 regulates catecholamine loading of large dense-core vesicles. Neuron 46: 75–88 [DOI] [PubMed] [Google Scholar]
- Spencer A. G., Orita S., Malone C. J., Han M., 2001. A RHO GTPase-mediated pathway is required during P cell migration in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 98: 13132–13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven R., Kubiseski T. J., Zheng H., Kulkarni S., Mancillas J., et al. , 1998. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 92: 785–795 [DOI] [PubMed] [Google Scholar]
- Steven R., Zhang L., Culotti J., Pawson T., 2005. The UNC-73/Trio RhoGEF-2 domain is required in separate isoforms for the regulation of pharynx pumping and normal neurotransmission in C. elegans. Genes Dev. 19: 2016–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F., Sullivan J. M., 1998. Regulation of the readily releasable vesicle pool by protein kinase C. Neuron 21: 885–893 [DOI] [PubMed] [Google Scholar]
- Sudhof T. C., 2008. Neurotransmitter release. Handb. Exp. Pharmacol. 184: 1–21 [DOI] [PubMed] [Google Scholar]
- Sumakovic M., Hegermann J., Luo L., Husson S. J., Schwarze K., et al. , 2009. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J. Cell Biol. 186: 897–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walent J. H., Porter B. W., Martin T. F., 1992. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell 70: 765–775 [DOI] [PubMed] [Google Scholar]
- Watari-Goshima N., Ogura K., Wolf F. W., Goshima Y., Garriga G., 2007. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat. Neurosci. 10: 169–176 [DOI] [PubMed] [Google Scholar]
- Williams S. L., Lutz S., Charlie N. K., Vettel C., Ailion M., et al. , 2007. Trio’s Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev. 21: 2731–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. C., Cheng T. W., Lee M. C., Weng N. Y., 2002. Distinct rac activation pathways control Caenorhabditis elegans cell migration and axon outgrowth. Dev. Biol. 250: 145–155 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Grant B., Hirsh D., 2001. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol. Biol. Cell 12: 2011–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. M., Dong Y. M., Ge Q., Zhu D., Zhou W., et al. , 2007. PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56: 657–669 [DOI] [PubMed] [Google Scholar]