Abstract
Significance
The accepted effects of aging in mammalian skeletal muscle are progressive atrophy and weakening, or sarcopenia. Canonical hallmarks of aging in skeletal muscle include a reduction in muscle fiber cross-sectional area, a loss in muscle fibers through apoptosis and denervation, and infiltration of connective tissue or fibrosis. Emerging thought suggests that pro-inflammatory signaling and oxidative stress may contribute to sarcopenia.
Critical Issues
Unfortunately, most of the mammalian models used to examine and understand sarcopenia are confounded by the pervasive influence of prolonged physical inactivity. Further, the potential for underlying metabolic disorder and chronic disease (e.g., type II diabetes and cardiovascular disease) may accelerate skeletal muscle wasting. Because physical inactivity may share elevated pro-inflammatory (tumor necrosis factor-alpha and inducible nitric oxide synthase) and insufficient stress response (insulin-like growth factor-1 [IGF-1], heat-shock protein 25 [HSP25], NAD-dependent deacetylase sirtuin-3 [SIRT-3], and peroxisome proliferator-activated receptor-gamma coactivator 1[PGC-1α]) signaling with aging and chronic disease, it is critical to distinguish true aging from chronic inactivity or underlying disease. Conversely, the efficacy of exercise and caloric restrictive interventions against sarcopenia in aging populations appears highly effective when (a) conducted across the lifespan, or (b) at higher intensities when commenced in middle age or later.
Recent Advances
While the prospective mechanisms by which exercise or daily activity provide have not been elucidated, upregulation of HSPs, PGC-1α, and IGF-1 may ameliorate inflammatory signaling, apoptosis, and sarcopenia. Limited data indicate that the aging phenotype exhibited by mammals living in their natural habitat (Weddell seal and shrews) express limited apoptosis and fiber atrophy, whereas significant collagen accumulation remains. In addition, aging shrews displayed a remarkable ability to upregulate antioxidant enzymes (copper, zinc isoform of superoxide dismutase, manganese isoform of superoxide dismutase, catalase, and glutathione peroxidase).
Future Directions
It is possible that in healthy populations requiring daily activity to thrive, fibrosis and weakness, more than atrophy, may be the predominant phenotype of muscle aging until senescence. Elucidating the molecular mechanisms by which lifetime inactivity contributes to sarcopenia and chronic disease will be critical in managing the quality of life and health costs associated with our aging population. Antioxid. Redox Signal. 15, 2529–2541.
Introduction: Canonical View of Sarcopenia and Clinical Ramifications
“Sarcopenia” is derived from the Greek language and means “poverty of the flesh.” It describes skeletal muscle wasting that often accompanies the aging process. Indeed, progressive loss of muscle mass, or atrophy, is a consistent finding in humans as birthdays accumulate. Over 42 million Americans suffer from sarcopenia, including 10%–20% of the population under age 70% and 40% of those over age 80 (27, 62). Sarcopenia compromises the ability to perform the tasks of daily living necessary to maintain quality of life, health, and independence (33, 59). Sarcopenia and weakness lead to frailty, and are highly predictive of falls, physical disability, morbidity, and mortality (68, 79, 127). Loss of skeletal muscle and lean body mass have been linked with increased risk of heart disease, hypertension, and type II diabetes (11, 41, 48, 65, 66, 72, 91). Further, the presence of chronic disease exacerbates skeletal muscle dysfunction (14, 65). In 2000, the healthcare costs of sarcopenia tallied $18.5 billion and are expected to soar to $130 billion by 2030 (27).
Unfortunately, health issues related to sarcopenia will continue to mount as U.S. and global populations are growing older. By the year 2040 up to 15 million Americans will be age 85 or older (117). Indeed, the number of persons aged >65 years is expected to double from approximately 35 million in 2000 to an estimated 71 million in 2030. Correspondingly, the population aged >80 years is expected to increase from 9.3 million in 2000 to 19.5 million in 2030 (Centers for Disease Control, MMWR, 2003).
Sarcopenia is traditionally defined as a degenerative loss of muscle mass and strength. Muscle atrophy is considered a central part of an age-related process of remodeling in skeletal muscle, whereby changes in muscle architecture occur. These include (a) loss in the number of muscle fibers via apoptosis and necrosis, (b) reduction in cross-sectional area of remaining muscle fibers, and (c) accumulation of fibrotic collagen (18, 102). Sarcopenia appears to be progressive, as beyond age 50 muscle mass declines at an average rate of 1%–2% per year, a 30%–40% reduction in both skeletal muscle mass, and fiber number is typical by age 75 (27, 55, 98, 102). Further, atrophy and loss of muscle fibers preferentially affects fast-twitch or type II muscle fibers. Contractile force generation erodes with aging due to the product of fiber atrophy coupled with a diminishment of muscle quality, or active tension expressed per cross-sectional area (99). Remodeling and fibrosis are thought to play a substantial role in the impairment of muscle quality in elderly populations. When muscle atrophy is coupled with a reduction in muscle quality, joint torque production by skeletal muscles through skeletal lever systems is lessened over 30% by age 75, and in excess of 50% by age 85 (102). Muscle atrophy has been consistently documented in the gerontology literature across human and gerontological models. In humans, fiber cross-sectional area of vastus lateralis (VL), a common target of sarcopenia studies, was up to 50% lower in septuagenarians than in young adults (84, 128). A ≥30% decrease in muscle mass by age 75 has been a common occurrence in sarcopenia studies (62).
The etiology of sarcopenia in human populations is considered to be multifactorial and includes (a) endocrine alterations, (b) loss of alpha motorneurons, (c) repeated damage/repair cycling, and (d) reduction in physical activity (97). Endocrine-related changes with aging include impaired release of androgens, growth hormone, thyroxine, elevated cortisol, and increased insulin resistance (33, 59, 114). Insufficiency of androgens and growth hormone/insulin-like growth factor-1 (IGF-1) signaling exacerbate wasting and impeded repair and growth (33, 34). In addition, poor nutritional status exacerbates skeletal muscle wasting and weakness in the elderly (20).
Progressive muscle wasting and remodeling has also been demonstrated in rodent and nonhuman primates. Gastrocnemius mass decreased by 23% between 6- and 27-month-old Fischer-344 rats (105). The Fischer-344X Brown Norway (FBN) hybrid rat exhibits a large reduction in muscle mass (−45%), fiber cross-sectional area (−30%), and muscle fiber number (−27%) between 18 and 38 months (77, 105). Muscles in older FBN rats also displayed a more heterogeneous muscle morphology, characterized by fibrosis and fat infiltration (105). In C57BL/6 mice, skeletal muscle mass also decreased with aging, with percent atrophy ranging from 15% to over 30% (10). Thus, the relative progress of sarcopenia in old rodent models appears to be comparable with aging human subjects (21).
Skeletal muscles in aging populations also are susceptible to injury as a result of repetitive eccentric contractions. Remodeling is a likely explanation for age-associated weakness and enhanced risk of damage induced as muscle fiber size diminishes, and accumulation of connective tissue and fat increases. Encroaching fibrosis increases internal work of the muscle and suppresses muscle quality (98). Therefore, mechanistic approaches designed to unravel the mysteries of sarcopenia must focus on the process of skeletal muscle remodeling, in addition to atrophy. A number of molecular and cellular candidates have been linked to sarcopenia and remodeling, including IGF-1 and its splice variant mechano-growth factor (MGF) (38), disrupted mechanotransduction (94), alterations in satellite cell signaling (39, 98), mitochondrial dysfunction (53), oxidative stress (118), apoptosis (68), autophagy (126), insulin resistance (121), and dysregulation of the ubiquitin/proteosome pathway (5). Satellite cells as pluripotent nuclei may differentiate into adipocytes or fibroblasts, particularly with advancing age or disease (98). In addition, underlying mechanisms contributing to fibrosis and remodeling may include elevation of inflammatory cytokines (22, 96).
Based upon cell signaling commonalities, in this review we will address the concept that a significant portion of age-related sarcopenia may be related to chronic inactivity and underlying chronic disease.
Living in a Box
The human genome supported a hunter-gatherer lifestyle and physiology 40,000 years ago and predominates our healthy phenotype, designed to cover large distances in acquiring and transporting food and water. Thus, it is designed to facilitate physical activity throughout the lifespan. Postmodern, Westernized societies from Japan to New York to Texas have become increasingly sedentary over the last century, “living in a box” at home, commuting, and in the office. Indeed, it has been argued that inactivity encompasses a discrete pathology that alters the expression of critical genes involved in pro-inflammatory signaling (14, 15). As a consequence, inactivity induces a phenotype that promotes the etiology and prevalence of 25 chronic diseases, including coronary artery disease, hypertension, type II diabetes, obesity, cancer, and Alzheimer's (14), all affected by pro-inflammatory signaling. Indeed, inactivity elevates risk of coronary artery disease (+45%), stroke (+60%), hypertension (+30%), type II diabetes (+50%), breast cancer (+31%), colon cancer (+41%), and osteoporosis (+59%), while contributing to 340,000 premature deaths, at a cost of $150 billion per year (15). Importantly, the chronic diseases above may also elicit muscle wasting. Indeed, emerging evidence indicates that aging, inactivity, and chronic diseases may regulate an overlapping set of genes and proteins involved in oxidative stress, inflammatory signaling, and stress protection (15, 16, 22, 95).
Although sarcopenia has been reported as a commonality among the aged since the Greek civilizations, the vast majority of inductive scientific studies involving human and mammalian subjects have been collected with sedentary, aging populations serving as the control group. Therefore, our understanding of true aging in mammalian skeletal muscle has been, unfortunately, limited by the focus on inactive human and caged animal models fed ad libitum. Logically, it could be argued that many mammalian studies involving rodents, primates, and humans have truly been evaluating the combination of aging and chronic inactivity. Further, the incidence of numerous pathologies that can elicit muscle wasting and/or weakness increases with age (37). These include insulin resistance, hypertension, coronary artery disease, peripheral vascular disease, infection, chronic obstructive pulmonary disease (COPD), and cancer that impair muscle function, many of which elicit muscle wasting, possibly distinct from the true aging process.
In addition to aging and chronic disease, disuse and inactivity in young or old animals also elicit substantial muscle atrophy (1, 56, 57). Disuse or mechanical unloading models are characterized by skeletal muscle atrophy and susceptibility to damage (57). Mounting evidence indicates that oxidative stress, NF-kappaB (NF-κB) activation, and impaired stress response are important driving mechanisms behind muscle wasting (1, 56, 57, 101). In addition, translocation of important signaling proteins such as neuronal nitric oxide synthase (nNOS), from the subsarcolemmal cytoskeleton, may elicit muscle wasting during disuse and Duchenne muscular dystrophy, possibly via activation of ubiquitin ligases (111, and [Kim, et al., unpublished observation]). In addition, there is accumulating evidence that unloading-induced myopathy is redox sensitive (1, 28, 56, 57, and [Kim, et al., unpublished observation]).
Skeletal muscles that experience mechanical unloading are also susceptible to damage upon reloading (57). Interestingly, many of the signaling pathways (insulin/IGF-1, nNOS, inflammatory cytokines, NAD(P)H oxidase, NF-κB activation, and apoptosis) contributing to muscle dysfunction during disuse and mechanical unloading are also shared with long-term physical inactivity (13), aging (94, 105, 106), and muscular dystrophies (36, 124). However, lifelong inactivity models will provide a more thorough understanding of muscle remodeling during aging than those of extreme disuse (casting, hindlimb unloading, denervation, and mechanical ventilation), as extreme disuse results in increased fast-twitch myosin isoforms and fiber-type, whereas aging is characterized by a shift from type I to type II fibers (103).
Upregulation of pro-inflammatory genes in aging skeletal muscle have been associated with sarcopenia and impaired muscle function (40). There is now substantial evidence that oxidative stress, mitochondrial function, and pro-inflammatory signaling are also central to insulin resistance (8). Mitochondria are an important source of hydroperoxides, which in turn interfere with insulin receptor substrate-1 (IRS-1) phosphorylation, and subsequent translocation of glucose transporter type 4 (GLUT4) receptors to the sarcolemma (8, 12). Disuse, sedentary lifestyle, and aging all increase the likelihood of insulin resistance, particularly as oxidative stress and pro-inflammatory signaling rise (7). Insulin resistance may exacerbate endothelial dysfunction and exert positive feedback on metabolism, thus elevating oxidative stress (116). Moreover, commonly used medications to increase insulin sensitivity such as angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, and peroxisome proliferator-activated receptor (PPAR) agonists reduce pro-oxidant genes such as Nox2 (NAD(P)H oxidase) in phagocytic and nonphagocytic cells (115). Therefore, anti-inflammatory therapy may be efficacious in attenuating sarcopenia in part by reducing insulin resistance.
Chronic Inactivity and Aging Skeletal Muscle
If many of the same genes and proteins expressed in disuse and chronic diseases (e.g., type II diabetes and cardiovascular disease) are also expressed with advancing age (13, 25), it is likely that at an interaction between aging and disuse exists. Elevated leakage of reactive oxygen species (ROS) from mitochondria, for example, is commonalities for disuse and aging (80, 103). Recent investigations that have combined aging and extreme disuse indicate that tumor protein53 (p53) and mitochondrial B-cell lymphoma 2 (Bcl-2) pathway signaling produce nonadditive effects (110). Interestingly, muscle atrophy with disuse or mechanical unloading is stimulated by increased oxidative stress and impairment of stress proteins, including heat-shock protein 70 (HSP70) and antioxidant enzymes (e.g., catalase and glutathione peroxidase) (101). Van Remmen and colleagues demonstrated that knockout of the copper, zinc isoform of superoxide dismutase (Cu,ZnSOD or SOD1) gene increased oxidative stress, while reducing muscles mass, and increasing damage in a manner mimicking accelerated sarcopenia (82). In addition, SOD1 knockouts displayed diminished contractile force, which was exacerbated with advancing aging (47, 82). It is uncertain whether short-term unloading or disuse is influence by inflammatory cytokines reported to be important in myopathy with muscular dystrophies, cachexia (COPD, cancer, chronic heart failure, and diabetes), and aging models of muscle wasting (69, 101). However, chronic inactivity may indeed elevate circulating and tissue-level inflammatory cytokines.
Underlying illness or pathology may exacerbate age-associated sarcopenia or could play a contributory role to muscle wasting associated with the aging process. The interactive nature of underlying disease and aging, particularly when coupled with chronic inactivity, is possible because of shared pro-inflammatory pathways. Indeed, Chung's Inflammatory Hypothesis of Aging, first articulated a decade ago (22), postulated that increasing loss of homeostasis at the tissue level was a function of increased pro-inflammatory signaling. Pro-inflammatory mediators proposed included inducible nitric oxide synthase (iNOS), NAD(P)H oxidase, pro-inflammatory cytokines (e.g., tumor necrosis factor-alpha [TNF-α] and interleukin-1beta), and NF-κB (22, 73, 75). In addition, caloric restriction (CR) reversed age-related increases in pro-inflammatory mediators in the kidney, heart, and brain (22). TNF-α contributes to IRS-1 phosphorylation and insulin resistance, possibly via AMP-activated protein kinase (AMPK) and p38 mitogen-activated protein kinase signaling (24, 35, 129). Chronic inflammation also increased tissue damage, and has been noted with disuse and aging (71).
Chronic elevation of pro-inflammatory as a purveyor of skeletal muscle wasting has significant global importance, as upregulation of inflammatory proteins such as TNF-α, iNOS, and NF-κB is reflective not only aging and disuse, but also disease and cachexia (2, 19, 37, 56, 57, 71 119). For example, in a series of experiments, Reid and colleagues demonstrated that elevation of TNF-α lead to mitochondrial dysfunction as well as exacerbating release of ROS (63, 64, 81). Further, TNF-α stimulated ubiquitin-driven proteolysis via Forkhead box protein O4 (FoxO4) and impaired contractile function in skeletal muscle (63, 64, 81).
While the etiology of cachexia has been categorized as metabolic and inflammatory in nature (96), increasing recognition of shared pathways between bedrest (disuse) and sarcopenia exists (34). Skeletal muscle dysfunction and weakness during chronic heart failure has also been attributed to TNF-α, NF-κB, iNOS, and oxidative stress (3, 42). In addition, recent data indicate that sepsis causes insulin resistance via iNOS-induced nitration of IRS-1 (89). Indeed, disuse, cachexia, and sarcopenia can all be argued as metabolic disorders where inflammatory signaling, mitochondrial dysfunction function, endocrine regulation, and protein turnover are shared (34), possibly distinguished by FoxO isoform activation and alterations in mechanotransduction (86).
Support for pro-apoptotic signaling as a central mediator of muscle wasting can be found in a host of pathologic conditions, including chronic heart failure, mechanical ventilation, muscle denervation, COPD, and muscular dystrophy (2, 19, 74, 100, 104, 114). Oxidative stress, disruption of Ca2+ homeostasis, and insufficient stress response can activate pro-apoptotic signaling (104, 105), a consistent outcome of aging, inactivity, muscular dystrophy, as well as wasting observed with chronic disease (104). For example, activation of apoptosis with disuse is greatest in aging skeletal muscle (104), such that a ≥25% decline in skeletal muscle mass is correlated with elevated terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling (TUNEL)–positive nuclei and DNA fragmentation.
Apoptosis is accomplished in skeletal muscle via caspase-dependent and independent signaling (6). Pro-apoptotic signaling (e.g., caspase-3 activation) may contribute to sarcopenia not only by reducing the number of myonuclei and satellite cells, but also by increasing proteolysis (6). There are 3 caspase-dependent pathways: the mitochondrial Bcl-2 pathway dependent on caspase-9 activation, the caspase-8-dependent fas ligand/cytokine pathway, and the Ca2+/endoplasmic reticulum (ER) stress pathway that activates caspase-12. Caspase-3 cleavage serves as an integrating center for the caspase-8, -9, and -12, pathways that ultimately induce caspase-3 activation clearly respond to aging (67, 68). Cleavage of caspase-3 is elevated with aging in limb muscles, associated with a 35% reduction in mean fiber cross-sectional area (105). Consistent with the “constant domain” model for myonuclei, where each myonuclei governs its own cellular volume or domain (4), apoptosis and removal of myonuclei and satellite cells in a postmitotic tissue-like skeletal muscle can result in fiber atrophy as well as fiber loss (4, 74).
Leeuwenburgh and colleagues suggested that mitochondrial damage and oxidative stress contribute to apoptosis and sarcopenia (46). Pro-apoptotic Bcl-2-associated X protein (Bax) was elevated with aging, whereas anti-apoptotic Bcl-2 protein expression declined in fast and slow-twitch skeletal muscle (105). Mitochondrial-dependent and caspase-independent signaling in skeletal muscle was activated as well. Translocation of caspase-independent endonuclease G (EndoG) and apoptosis-inducing factor (AIF) from the mitochondria to the nucleus was also present in old, but not in young animals (31, 60, and [Kim, et al., unpublished observation]). ER stress is also elevated with aging, and associated impairment of calcium homeostasis activated caspase-12 and thus caspase-3 (54, 92). ER stress may further promote cytochrome c release from the mitochondria (27). Alway and colleagues reported that aging and disuse may act in an interactive manner, with noncaspase-dependent apoptosis (AIF) amplified with aging (104).
Receptor-mediated signaling, including TNF-α, induces apoptosis by the activation of Fas-associated death domain receptors (FADD), caspase-8, and thus caspase-3 activation (90). TNF-α-driven apoptosis is believed to be critical in atrophy. Indeed, Degens and Alway proposed that low-grade inflammation is the primary contributor to skeletal muscle wasting with aging and chronic disease (25, 26). While human data remain limited, an age-related increase in apoptosis, coupled with a 60% decrease muscle fiber amounts, has been documented (109, 125). TUNEL-positive nuclei in the VL muscle stained for DNA fragmentation were 87% higher compared with muscle in young adults (125).
It is probable that some fiber loss with aging occurs in relation to α-motor neuron denervation or mitochondrial dysfunction, whereby both mechanisms may lead to elevated apoptosis and muscle dysfunction (18, 53, 104). Elevated oxidative stress results in early degeneration of skeletal muscle, producing a muscular dystrophy or amyotrophic lateral sclerosis-like phenotype (94). For example, knockout of the Cu,ZnSOD results in significant damage and atrophy of skeletal muscle reminiscent of myopathy (82). In summary, distinguishing the effects of aging on skeletal muscle function from underlying chronic inactivity and chronic disease may be challenging given their shared pro-inflammatory signaling, (114).
Exercise as a Sarcopenia Intervention in Aging Populations
Conversely, long-term exercise training appears to reduce the pro-inflammatory phenotype and reduce apoptosis (105, 106). While the mechanisms remain to be elucidated, exercise training reduces iNOS and oxidative stress, while increasing stress protective HSP70 in skeletal muscles in heart failure, coronary ischemia, and aging models (37, 56, 106). In combating sarcopenia, short- and long-term studies have largely focused on the benefits of resistive training or moderate to higher intensity level weight-bearing exercises. Indeed, the human literature suggests that resistive training is the most consistent method to combat sarcopenia in an aging population with adequate protein intake. In 10-week to 12-month intervention studies in rat and mouse models, endurance training appears to positively impact apoptosis, muscle mass, and muscle fiber cross-sectional area. Alway and colleagues have shown that treadmill exercise training elevates anti-apoptotic markers, including Bcl-2, X-chromosome-linked inhibitor of apoptosis, and apoptosis repressor with a caspase recruitment domain protein (ARC) (104). In concert, treadmill exercise reduced DNA fragmentation and caspase-3 activation (104, 105). Recent findings from Rasmussen and colleagues indicate the importance of adequate protein intake and mitigation of age-related changes in micro-RNA involved in translation repression and gene silencing (29, 30).
Sirtuin and PPAR-γ coactivator 1-alpha (PGC-1α) signaling has recently been linked to improving insulin signaling and slowing of the aging phenotype. Further, SIRT (Sirtuin) family signaling may be inducible with exercise. New data indicate that exercise training normalizes silent information regulator-6 (SIRT-6) in skeletal muscles from old rats (52). In addition, NAD-dependent deacetylase sirtuin-1 (SIRT-1) levels were upregulated in old rats by a single bout of exercise, and corresponded to an improvement in phosphorylation of IRS-1, tyrosine protein phosphatase nonreceptor type-1, and insulin signaling (87). Upregulation of SIRT family proteins may in turn elevate PGC-1α and thus may reduce oxidative stress and apoptosis (87). For example, a recent study (85) reported that knockout of NAD-dependent deacetylase sirtuin-3 (SIRT-3) prevented exercise-induced upregulation of AMPK and PGC-1α. Conversely, high fat meals reduced SIRT-3 and AMPK phosphorylation, whereas CR or fasting elevated SIRT-3 and p-AMPK. In addition, Wenz et al. (122) recently showed that overexpression of PGC-1α reduced markers of insulin resistance and sarcopenia.
When begun in middle age or later, treadmill exercise training appears to have a modest effect on reduction in muscle mass and fiber cross-sectional area (49, 105). McArdle and Jackson conducted a series of studies that demonstrated the importance of HSP70 in reducing oxidative stress and sarcopenia in aging muscles (5, 71). Starnes et al. also demonstrated that HSP70 was inducible with treadmill exercise training is retained in old age (108). While significant protection against muscle fiber atrophy has been noted in some studies, muscle mass and strength loss may persist even with maintenance of HSP70 (49).
Tarpenning et al. (112) concluded that endurance training reduced age-related decline in muscle strength and sarcopenia in human subjects. However, exercise intensity may be a critical factor when combating sarcopenia in the elderly (78). Indeed, Evans and colleagues showed that resistive training was most effective in alleviating muscle atrophy when used in aging populations, and this is still the consensus today (32, 78). Interestingly, it is thought that anti-inflammatory drugs may promote hypertrophy and strength gains in response to resistance training in older populations (40). In contrast, chronic use of anti-inflammatories blunt hypertrophy in response to resistive training in young populations (40). Additional research shows that weight training in combination with supplementation of branch-chain amino acids may be more efficacious in older populations at risk of sarcopenia than resistive training alone (99). In summary, while exercise training in an older population appears to partially reduce apoptosis and augment stress response proteins (e.g., HSP70 and SIRT-1), functional effectiveness of these interventions has been equivocal with lower-intensity exercise, unless begun at a young age.
Lifelong voluntary exercise in ameliorating sarcopenia
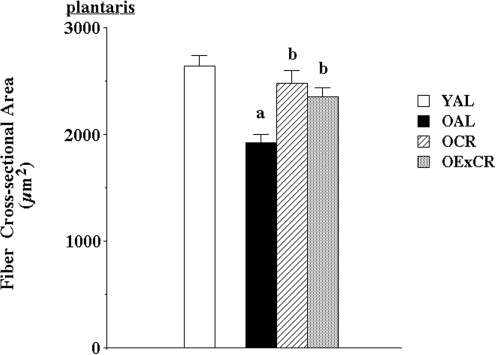
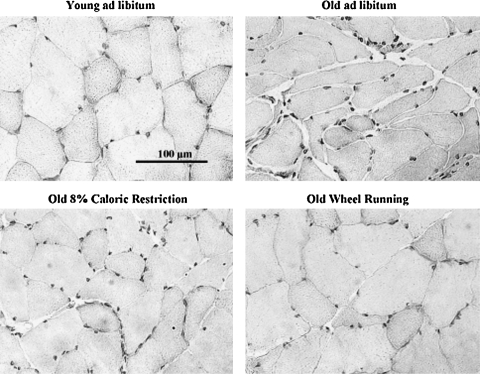
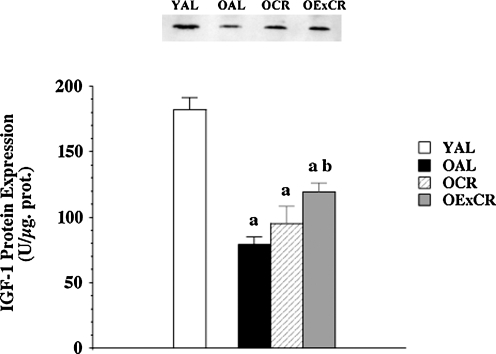
While exercise interventions in aging populations yield positive, modest results in alleviating sarcopenia, emerging data suggest that lifelong voluntary exercise provides substantially greater protection against age-associated apoptosis, muscle fiber atrophy, and remodeling (51, and [Kim, et al., unpublished observation]). Further, some of the cell signaling pathways modulated by lifelong activity and CR may be shared (55). Lifelong wheel running significantly protected against age-associated reduction in fiber cross-sectional area (51) (Figs. 1 and 2). In addition, lifelong wheel running also reduced remodeling, as visualized by attenuation of accumulation of extracellular space and collagen I (51). Wheel running also ameliorated age-associated elevation of oxidative stress. However, protection against fiber atrophy and remodeling was not related to any changes in either Cu,ZnSOD or manganese isoform of superoxide dismutase (MnSOD) protein expression. While IGF-1 levels were lowest in old, sedentary rats fed ad libitum, the most effective protection against age-related suppression of IGF-1 was provided by wheel running (Fig. 3). This is in agreement with lifelong protection of skeletal muscle mass by IGF-1 studies by Rosenthal and colleagues using mice that overexpress the IGF-1 gene (120). In addition, Goldspink presented evidence that MGF, the splice variant of IGF-1 specific for skeletal muscle, is critical in satellite cell activation and preservation of muscle mass with aging (38).
FIG. 1.
Lifelong wheel running and mild caloric restriction alleviate age-associated reduction in muscle fiber cross-sectional area (51). Plantaris muscle fiber cross-sectional area in young (6 months) Fischer-344 rats fed ad libitum (YAL), old (24 months) rats fed ad libitum (OAL), old rats that underwent lifelong 8% caloric restriction (OCR), and old rats that participated in lifelong voluntary wheel running plus 8% caloric restriction (OExCR). Values are means±SEM. (a) indicates significant difference from YAL group. (b) indicates significant difference from OAL group. SEM, standard error of the mean.
FIG. 2.
Protection of skeletal muscle morphology by lifelong mild caloric restriction and wheel running (51). Plantaris hematoxylin-stained cross sections from young (6 months) Fischer-344 rats fed ad libitum (YAL), old (24 months) rats fed ad libitum (OAL), old rats that underwent lifelong 8% caloric restriction (OCR), and old rats that participated in lifelong voluntary wheel running plus 8% caloric restriction (OExCR). Standard bar=100 μm.
FIG. 3.
Lifelong wheel running provides partial protection against age-associated downregulation of IGF-1 (51). IGF-1 protein expression in young (6 months) Fischer-344 rats fed ad libitum (YAL), old (24 months) rats fed ad libitum (OAL), old rats that underwent lifelong 8% caloric restriction (OCR), and old rats that participated in lifelong voluntary wheel running plus 8% caloric restriction (OExCR). Values are means ±SEM. (a) indicates significant difference from YAL group. (b) indicates significant difference from OAL group. IGF-1, insulin-like growth factor-1.
We recently demonstrated that lifelong wheel running ameliorates age-related elevation and translocation of pro-apoptotic EndoG to the nucleus (Kim, et al., unpublished observation). In contrast, wheel running had little effect on elevation of AIF. Further, lifelong wheel running resulted in higher levels of HSP25 and phosphorylation of HSP25 at Ser82 in the plantaris muscle of old rats that participate in lifelong, voluntary exercise. HSP25 may protect against apoptosis via death receptor pathways. However lifelong wheel running did not have a protective effect for HSP70. Lack of HSP70 response may be related to low-intensity exercise levels. Interestingly, Murlasits et al. reported that resistive training upregulated HSP25, but not HSP70 in rats (83). In contrast, Jackson and colleagues demonstrated that lifelong overexpression of HSP70 reduces muscle damage and myopathy (17, 70). Therefore, lifelong upregulation of HSPs may be a critical mechanism in reducing age-associated sarcopenia via HSP25 and HSP70.
Lifelong wheel running also reduced apoptosis and improved autophagy signaling (microtubule-associated protein 1 light chain 3, lysosomal membrane protein-1) in old Fischer-344 rats (126). In the heart long-term wheel running reduced apoptosis mediators, including cell-cycle-checkpoint kinase 2 (Chk2) and p53 via Chk2, p53, IGF-1, and endothelial nitric oxide synthase (123). Recently, Leick et al. (61) demonstrated that PGC-1α was necessary to retain exercise protection in skeletal muscle against (a) downregulation of MnSOD and citrate synthase and (b) attenuation of elevated Bcl-2 pathway signaling. Reynolds et al. (93) reported that wheel running might protect the Akt/mTOR (mammalian target of rapamycin) pathway of protein synthesis against age-related decline. In summary, lifelong exercise may limit sarcopenia and apoptosis, mediated by preservation of key stress proteins, including HSP25, IGF-1, and PGC-1α. While the efficacy of and mechanisms underlying protection by lifelong, habitual exercise are beginning to emerge, exercise may be very effective in reducing the risks of sarcopenia regardless of intensity levels when practiced over significant portions of the lifespan.
CR Delays Skeletal Muscle Dysfunction
Prolonged, moderate CR of 30%–40% has been the most consistent rodent and primate model used to increase longevity (67). Moderate (30%–40%) and mild (8%–10%) CR have also been efficacious in preserving skeletal muscle function (51, 67, and [Kim, et al., unpublished observation]). Although moderate CR may initially elicit a small reduction in muscle mass, further reduction in mass and fiber CSA with advancing age are substantially attenuated by CR (27, 76, and [Kim, et al., unpublished observation]). Further, CR may also alleviate the age-related decline in contractile function (9, 88). Interestingly, CR attenuates pro-inflammatory genes associated with oxidative stress in muscles from old and young animals (23). Kim et al. (51) found that mild CR reduced muscle fiber atrophy and partially limited reduction in IGF-1. CR was also effective in attenuating loss in muscle fiber number, oxidative stress, and mitochondria abnormalities (67). Indeed, CR significantly reduced mitochondrial proton leak and ROS generation, lipid peroxidation, and protein damage in skeletal muscle (67) and increased the expression of genes involved in ROS scavenging (107). CR also reduced the incidence of age-related abnormalities in the electron transport chain, and accumulation of mtDNA deletions in mitochondria (9, 58). Recently, CR has been found to elevate SIRT-3/PGC-1s signaling, and thus enhancing mitochondrial function and muscle mass (85).
CR may limit sarcopenia by reducing age-associated apoptosis. Activation of caspase-3 and fragmentation of DNA were significantly reduced by CR (27, 88). Specifically, CR attenuated fas/cytokine pathway signaling, reduced age-associated elevations of TNF-α, FADD, and caspase-8 cleavage (88). Further, the ER/Ca2+ stress apoptotic pathway was also downregulated by CR with a reduction in procaspase-12 and AIF (27).
Aging Mammals in Natural Habitat and Skeletal Muscle Senescence
Unlike rodent models of aging housed in laboratory animal facilities, wild mammals typically remain highly active and in mild caloric deficit over much of their lifespan. Free-living animals are susceptible to stressors such as predators, altered environmental conditions, conflict-inflicted wounds, and infectious disease. Therefore, stress protection and antioxidant strategies are paramount in skeletal muscle integrity and function. However, there is a paucity of data that characterize the existence of a senescent phenotype in skeletal muscles of aging mammals in their natural habitat.
We initially chose mammalian species of disparate size and longevity to examine naturally occurring senescent phenotoypes. Two were diving mammals, and thus experience repeated hypoxia-reoxygenation during breath-hold over the course of their lifespan. One such species was the Weddell seal (Leptonychotes weddelli). In the Ross Sea region of Antarctica, a local population of seals has been under scientific investigation since the 1980s, resulting in a high percentage of known-aged, old (20+ years) individuals (113). We thought that these initial experiments might shed light on the influence of chronic inactivity, and ad libitum feeding on skeletal muscle senescence, including sarcopenia. Seals were captured, sedated, and biopsies taken (43). A second breath-holding mammal, the diminutive water shrew (Sorex palustris), was obtained in woodlands of Manitoba, Canada. The third species, the short-tailed terrestrial shrew (Blarina brevicauda), is also a carnivore subjected largely to cold stress and altered ambient gas partial pressures associated with underground burrows, rather than breath-holding during hunting.
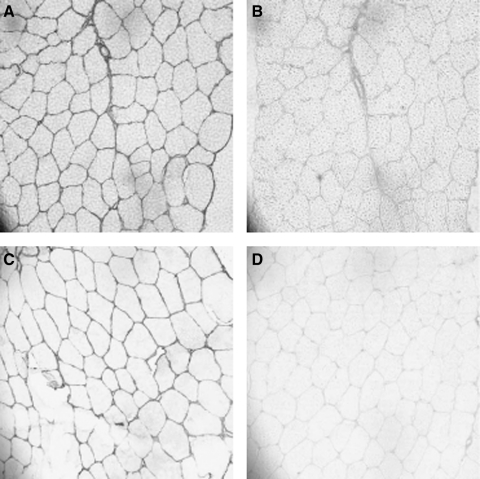
In the long-lived Weddell seal, muscle fiber cross-sectional areas of longissimus dorsi and pectoralis major showed either an increase or no change compared with muscle samples from young adults (43) (Table 1). Collagen content increased by 115% in longissimus dorsi and 65% in pectoralis. In addition, a shift of the collagen isoform profile from type III to the stiffer type I also occurred with age, with an 79% increase of the collagen type I/III ratio in pectoralis and 49% in longissimus dorsi (Fig. 4). In older adult water and terrestrial shrews, muscle fiber cross-sectional areas increased, rather than losing size, whereas increases in extracellular space and collagen I staining were observed (44). The ratio of type I/type III collagen also was elevated with age in both species of shrews. However, the ratio of muscle fiber to collagen levels was lower in the terrestrial shrew compared with its aquatic sympatric species. Thus, aging mammals that are active in their natural habitat may exhibit fibrosis as a primary characteristic of the aging phenotype in skeletal muscle.
Table 1.
Morphological Characteristics of Gracilis Muscle Sampled from Two Species of Wild-Caught Shrew
| |
Water shrew (Sorex palustris) |
Short-tailed shrew (Blarina brevicauda) |
||
|---|---|---|---|---|
| Old (n=9) | Young (n=10) | Old (n=8) | Young (n=7) | |
| Cross-sectional area (μm2) | 1036±139* | 829±62* | 632±52 | 669±125 |
| Extracellular space (μm2) | 60236±5012 | 54467±3523 | 69225±4163 | 40398±9126** |
Muscle was transversely sectioned at 7–9 μm and stained with hematoxylin before analyses. A single asterisk denotes significant differences between species (α=0.05); double asterisks indicate significant differences between age classes. Values are means±standard error.
FIG. 4.
Aging elevates collagen I staining (A, C) without an increase in collagen III isoform (B, D). Immunohistochemical staining for collagen subtypes in pectoralis muscle biopsies of female Weddell seals, Leptonychotes weddelli (7–9 μm transverse sections; 200× magnification). Samples are presented for young (collagen type I and III isoforms shown in A and B) and old (collagen types I and III shown in C and D) individuals (43).
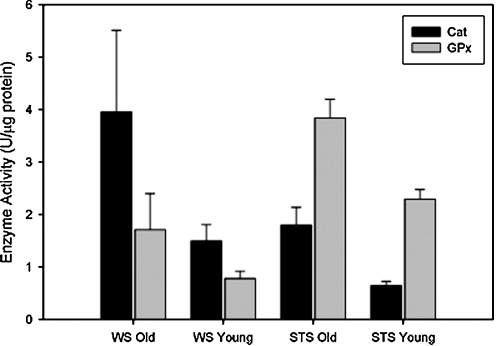
Although hydroperoxide levels increased, markers of oxidative damage (4-hydroynonenal) showed no increase with aging in any shrew species (44). There was also no increase in DNA fragmentation or TUNEL+ nuclei in old shrews (44). Interestingly, Cu,ZnSOD was dramatically upregulated with age, with particularly high levels discovered in the water shrew (60%) compared with terrestrial shrew (25%) (45). Protection against hydroperoxides was thus augmented with age. Catalase levels doubled in the old water shrews, whereas glutathione peroxidase was elevated by 120% in its terrestrial counterpart with age (Fig. 5). It is possible that the remarkable upregulation of stress protection and antioxidant enzymes in the shrews with aging may limit oxidative damage and apoptosis, reflective of lifelong activity and/or adaptation to their unique environments. In short, these data suggest that senescence in muscles of old mammals living in their natural habitats may not be characterized by atrophy and apoptosis as much as by fibrosis. Fibrosis elevates internal work of aging muscles, reducing force production and increasing susceptibility to damage. Because of the early stage of these studies and potential influence of natural selection and removal of weaker senescent animals by predators or disease, extrapolation to senior human patients is cautioned at this time.
FIG. 5.
Upregulation of catalase and glutathione peroxidase in terrestrial and water shrews. Protein content of Mn-SOD (black bars) and CuZn-SOD (gray bars) isoforms, expressed in arbitrary units, in skeletal muscle hindlimb homogenate from two species of shrew (45). Mn-SOD, manganese isoform of superoxide dismutase; CuZn-SOD, copper, zinc isoform of superoxide dismutase.
Conclusion
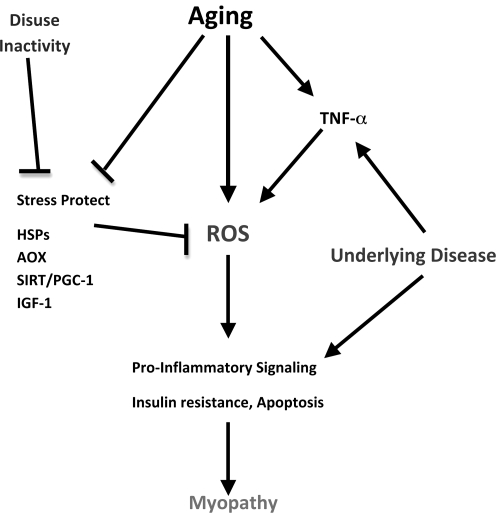
We have taken an integrative approach to re-examining the notion of understanding age-related sarcopenia in mammals by using sedentary, caged rodents. CR, short-term and lifelong exercise, human patients, and aging mammals in the natural habitat were used as models to provide a cell signaling perspective on the interactive nature of aging and chronic inactivity. Given that much of the cage-restricted rodent and human aging literature includes inactivity as part of the aging approach, the use of such subjects as control groups is cautioned. In addition to inactivity, underlying disease (e.g., cardiovascular disease and insulin resistance) may also impact the expression of the aging phenotype observed in skeletal muscle. Indeed, models that involved maintenance of lifelong activity expressed less muscle atrophy and apoptosis into old age, consistent with an ability to maintain or increase key stress-protective proteins (e.g., HSPs, IGF-1, SIRT-3, PGC-1α, Cu,ZnSOD, and catalase). The literature also supports the notion that lifelong activity is far more effective in mitigating the effects of sarcopenia than treatments commencing in middle age or beyond. A prospective integrative model is found in Figure 6.
FIG. 6.
Interactive signaling pathways for chronic inactivity with aging and underlying disease. Stress protective pathways, including HSPs, IGF-1, and PGC-1, may be targets of both aging and inactivity exacerbating sarcopenia and myopathy. HSPs, heat-shock proteins; PGC-1, PPAR-gamma coactivator 1; SIRT, sirtuin; ROS, reactive oxygen species; AOX, antioxidant enzymes.
Abbreviations Used
- AIF
apoptosis-inducing factor
- AMPK
AMP-activated protein kinase
- AOX
antioxidant enzymes
- ARC
apoptosis repressor with a caspase recruitment domain protein
- Bax
Bcl-2–associated X protein
- Bcl-2
B-cell lymphoma 2
- Chk2
cell-cycle-checkpoint kinase 2
- COPD
chronic obstructive pulmonary disease
- CR
caloric restriction
- Cu,Zn-SOD
copper, zinc isoform of superoxide dismutase
- EndoG
endonuclease G
- ER
endoplasmic reticulum
- FADD
Fas-associated death domain receptor
- FBN
Fischer-344X Brown Norway cross rats
- FoxO4
Forkhead box protein O4
- GLUT4
glucose transporter type 4
- HSP25
heat shock protein 25
- HSP70
heat-shock protein 70
- IGF-1
insulin-like growth factor-1
- iNOS
inducible nitric oxide synthase
- IRS-1
insulin-substrate receptor-1
- MGF
mechano-growth factor
- MnSOD
manganese isoform of superoxide dismutase
- NF-κB
nuclear factor-kappaB, a transcription factor
- nNOS
neuronal nitric oxide synthase
- Nox2
NAD(P)H oxidase isoform containing gp91phox subunit
- p53
tumor protein53
- PGC-1α
PPAR-gamma coactivator 1
- PPAR-γ
peroxisome proliferator-activated receptor-gamma
- ROS
reactive oxygen species
- SEM
standard error of the mean
- SOD1
copper, zinc isoform of superoxide dismutase
- SIRT
sirtuin
- SIRT-1
NAD-dependent deacetylase sirtuin-1
- SIRT-3
NAD-dependent deacetylase sirtuin-3, mitochondrial
- TNF-α
tumor necrosis factor-alpha
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling
- VL
vastus lateralis
Acknowledgments
This work has been supported by grants from NIH (AR054084), NSF (055185F), AHA (0555064Y, 0855158F), and The Sydney and J.L. Huffines Institute at Texas A&M University.
Author Disclosure Statement
There are no existing financial conflicts.
References
- 1.Abrogast S. Smith J. Matuszczak JY. Hardin BJ. Moylan JS. Smith JD. Ware J. Kennedy AR. Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102:956–964. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- 2.Adams V. Jiang H. Yu J. Möbius-Winkler S. Fiehn E. Linke A. Weigli C. Schuler G. Hambrecht R. Apoptosis in skeletal muscle myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33:959–965. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 3.Adams V. Späte U. Kränkel N. Schulze PC. Linke A. Schuler G. Hambrecht R. Nuclear factor-kappa B activation in skeletal muscle of patients with chronic heart failure: correlation with the expression of inducible nitric oxide synthase. Eur J Cardiovasc Prev Rehabil. 2003;10:273–277. doi: 10.1097/00149831-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Allen DL. Linderman JK. Roy RR. Bigbee AJ. Grindeland RE. Makku Y. Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 5.Altun M. Besche HC. Overkleeft HS. Piccirillo R. Edelmann MJ. Kessler BM. Goldberg AL. Ulfhake B. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem. 2010;285:39597–39608. doi: 10.1074/jbc.M110.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alway SE. Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev. 2008;36:51–57. doi: 10.1097/JES.0b013e318168e9dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amati F. Dubé JJ. Coen PM. Stefanovic-Racic M. Toledo FG. Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32:1547–1549. doi: 10.2337/dc09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EJ. Lustig ME. Boyle KE. Woodlief TL. Kane DA. Lin CT. Price JW., 3rd Kang L. Rabinovitch PS. Szeto HH. Houmard JA. Cortright RN. Wasserman DH. Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspnes L. Lee C. Weindruch R. Chung S. Roecker E. Aiken J. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1999;11:573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- 10.Barton-Davis ER. Shoturma DI. Musaro A. Rosenthal N. Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bijnen FC. Caspersen CJ. Mosterd WL. Physical inactivity as a risk factor for coronary artery disease: WHO and International Society and Federation of Cardiology position statement. Bull World Health Org. 1994;72:1–4. [PMC free article] [PubMed] [Google Scholar]
- 12.Bikman BT. Zheng D. Kane DA. Anderson EJ. Woodlief TL. Price JW. Dohm GL. Neufer PD. Cortright RN. Metformin improves insulin signaling in obese rats via reduced IKKbeta action in a fiber-type specific manner. J Obes. 2010;pii:970865. doi: 10.1155/2010/970865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth FW. Criswell DS. Molecular events underlying skeletal muscle atrophy and the development of effective countermeasures. Int J Sports Med. 1997;18:S265–S269. doi: 10.1055/s-2007-972723. [DOI] [PubMed] [Google Scholar]
- 14.Booth FW. Gordon SE. Carlson CJ. Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 15.Booth FW. Laye MJ. Lees SJ. Rector RS. Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol. 2008;102:381–390. doi: 10.1007/s00421-007-0606-5. [DOI] [PubMed] [Google Scholar]
- 16.Bronikowski AM. Morgan TJ. Garland T., Jr. Carter PA. The evolution of aging and age-related physical decline in mice selectively bred for high voluntary exercise. Evol Int J Org Evol. 2006;60:1494–1508. [PubMed] [Google Scholar]
- 17.Broome CS. Kayani AC. Palomero J. Dillmann WH. Mestril R. Jackson MJ. McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- 18.Bua EA. McKiernan SH. Wanagat J. McKenzie D. Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol. 2007;92:2617–2624. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- 19.Buck M. Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1998;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 20.Burton LA. Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217–228. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cartee GD. What insights into age-related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci. 1995;50:137–141. doi: 10.1093/gerona/50a.special_issue.137. [DOI] [PubMed] [Google Scholar]
- 22.Chung HY. Kim HJ. Kim KW. Yi BP. Molecular inflammation hypothesis of aging based on the antiaging mechanism of caloric restriction. Microsc Res Tech. 2002;59:264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- 23.Chung L. Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762:103–109. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.de Alvaro C. Teruel T. Hernandez R. Lorenzo M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem. 2004;279:17070–17078. doi: 10.1074/jbc.M312021200. [DOI] [PubMed] [Google Scholar]
- 25.Degens H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports. 2010;20:28–38. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 26.Degens H. Alway SE. Control of muscle size during disuse, disease, and aging. Int J Sports Med. 2006;27:94–99. doi: 10.1055/s-2005-837571. [DOI] [PubMed] [Google Scholar]
- 27.Dirks AJ. Leeuwenburgh C. Tumor necrosis factor alpha signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem. 2006;17:501–508. doi: 10.1016/j.jnutbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Dodd SL. Gagnon BJ. Senf SM. Hain BA. Judge AR. ROS-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 2010;41:110–113. doi: 10.1002/mus.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond MJ. McCarthy JJ. Fry CS. Esser KA. Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol. 2008;295:E1333–E1340. doi: 10.1152/ajpendo.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond MJ. Miyazaki M. Dreyer HC. Pennings B. Dhanani S. Volpi E. Esser KA. Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2008;106:1403–1411. doi: 10.1152/japplphysiol.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont-Versteegden EE. Strotman BA. Gurley CM. Gaddy D. Knox M. Fluckey JD. Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol. 2006;291:R1730–R1740. doi: 10.1152/ajpregu.00176.2006. [DOI] [PubMed] [Google Scholar]
- 32.Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci. 1995;50:147–150. doi: 10.1093/gerona/50a.special_issue.147. [DOI] [PubMed] [Google Scholar]
- 33.Evans WJ. Functional and metabolic consequences of sarponenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 34.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 35.Friedman JE. Kirwan JP. Jing M. Presley L. Catalano PM. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008;57:606–613. doi: 10.2337/db07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gervasio OL. Whitehead N. Yeung EW. Philips WD. Allen DG. TRPC1 binds to caveolin-3 and its regulated by Src kinase: role in Duchenne muscular dystrophy. J Cell Sci. 2008;121:2246–2255. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- 37.Gielen S. Adams V. Linke A. Erbs S. Möbius-Winkler S. Schubert A. Schuler G. Hambrecht R. Exercise training in chronic heart failure: correlation between reduced local inflammation and improved oxidative capacity in the skeletal muscle. Eur J Cardiovasc Prev Rehabil. 2005;12:393–400. doi: 10.1097/01.hjr.0000174824.94892.43. [DOI] [PubMed] [Google Scholar]
- 38.Goldspink G. Impairment of IGF-I gene splicing and MGF expression associated with muscle wasting. Int J Biochem Cell Biol. 2006;38:481–489. doi: 10.1016/j.biocel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Gopinath SD. Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 40.Greig CA. Atherton PJ. Rennie MJ. Can an NSAID a day keep muscle wasting way? J Physiol. 2009;587:5799–5800. doi: 10.1113/jphysiol.2009.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groop LC. Insulin resistance: the fundamental trigger of type-2 diabetes. Diabet Obes Metab. 1999;1:S1–S7. doi: 10.1046/j.1463-1326.1999.0010s1001.x. [DOI] [PubMed] [Google Scholar]
- 42.Hambrecht R. Schulze PC. Gielen S. Linke A. Mobius-Winkler S. Wu J. Kratsch JJ. Baldauf G. Busse MW. Schubert A. Adams V. Schuler G. Reduction of insulin-like growth factor-1 expression in the skeletal muscle of non-cachectic patients with chronic heart failure. J Am Coll Cardiol. 2002;39:1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 43.Hindle AG. Horning M. Melish JE. Lawler JM. Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii) J Exp Biol. 2009;212:790–796. doi: 10.1242/jeb.025387. [DOI] [PubMed] [Google Scholar]
- 44.Hindle AG. Lawler JM. Campbell KL. Horning M. Muscle senescence in short-lived wild mammals, the soricine shrews Blarina brevicauda and Sorex palustris. J Exp Zool. 2009;311A:358–367. doi: 10.1002/jez.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hindle AG. Lawler JM. Campbell KL. Horning M. Muscle aging and oxidative stress in wild-caught shrews. Comp Physiol Biochem Part B. 2010;155:427–434. doi: 10.1016/j.cbpb.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiona A. Sanz A. Kujoth GC. Pamplona R. Seo AY. Hofer T. Someya S. Miyakawa T. Nakayama C. Samhan-Arias AK. Servais S. Barger JL. Portero-Otín M. Tanokura M. Prolla TA. Leeuwenburgh C. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang YC. Lustgarten MS. Liu Y. Muller FL. Bhattacharya A. Liang H. Salmon AB. Brooks SV. Larkin L. Hayworth CR. Richardson A. Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61:157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- 49.Kayani AC. Close GL. Jackson MJ. McArdle A. Prolonged treadmill training increases HSP70 in skeletal muscle but does not affect age-related functional deficits. Am J Physiol. 2008;294:R568–R576. doi: 10.1152/ajpregu.00575.2007. [DOI] [PubMed] [Google Scholar]
- 50. This reference has been deleted.
- 51.Kim JH. Kwak HB. Leeuwenburgh C. Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress, and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koltai E. Szabo Z. Atalay M. Boldogh I. Naito H. Goto S. Nyakas C. Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kujoth GC. Hiona A. Pugh TD. Someya S. Panzer K. Wohlgemuth SE. Hofer T. Seo AY. Sullivan R. Jobling WA. Morrow JD. Van RH. Sedivy JM. Yamasoba T. Tanokura M. Weindruch R. Leeuwenburgh C. Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 54.Larner SF. Hayes RL. McKinsey DM. Pike BR. Wang KK. Increased expression and processing of caspase-12 after traumatic brain injury in rats. J Neurochem. 2004;88:78–90. doi: 10.1046/j.1471-4159.2003.02141.x. [DOI] [PubMed] [Google Scholar]
- 55.Lawler JM. Exercise protection against oxidative stress, apoptosis, and remodeling in aging skeletal muscle. In: Gutiérrez-Merino C, editor. Free Radicals in Biology and Medicine. Boca Raton, FL: CRC Press; 2008. pp. 1–19. [Google Scholar]
- 56.Lawler JM. Song W. Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Rad Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 57.Lawler JM. Song W. Kwak HB. Differential regulation of heat shock proteins by hindlimb unloading and reloading in the rat soleus. Muscle Nerve. 2006;33:200–207. doi: 10.1002/mus.20454. [DOI] [PubMed] [Google Scholar]
- 58.Lee CM. Aspnes LE. Chung SS. Weindruch R. Aiken JM. Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann N Y Acad Sci. 1998;854:182–191. doi: 10.1111/j.1749-6632.1998.tb09901.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee CE. McArdle A. Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Leeuwenburgh C. Gurley CM. Strotman BA. Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- 61.Leick L. Lyngby SS. Wojtasewski JF. Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 63.Li YP. Atkins CM. Sweatt JD. Reid MB. Mitochondria mediate tumor necrosis factor-alpha/NF-kappaB signaling in skeletal muscle myotubes. Antioxid Redox Signal. 1999;1:97–104. doi: 10.1089/ars.1999.1.1-97. [DOI] [PubMed] [Google Scholar]
- 64.Li YP. Chen Y. John J. Moylan J. Jin B. Mann DL. Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lunde PK. Sjaastad J. Schiotz Thorod HM. Sejersted OM. Skeletal muscle disorders in heart failure. Acta Physiol Scand. 2001;171:277–294. doi: 10.1046/j.1365-201x.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 66.Macnair AL. Is inactivity the origin of essential hypertension: should we all be runners? Nephrol Dial Transplant. 2000;15:1751–1754. doi: 10.1093/ndt/15.11.1751. [DOI] [PubMed] [Google Scholar]
- 67.Marzetti E. Lawler JM. Hiona A. Manini T. Seo AY. Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and caloric restriction in skeletal muscle. Free Radic Biol Med. 2007;15:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 68.Marzetti E. Privitera G. Simili V. Wohlgemuth SE. Aulisa L. Pahor M. Leeuwenburgh C. Multiple pathways to the same end: mechanisms of myonuclear apoptosis in sarcopenia of aging. Sci World J. 2010;10:340–349. doi: 10.1100/tsw.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayer B. Oberbauer R. Mitochondrial regulation of apoptosis. News Physiol Sci. 2003;18:89–94. doi: 10.1152/nips.01433.2002. [DOI] [PubMed] [Google Scholar]
- 70.McArdle A. Dillmann WH. Mestril R. Faulkner JA. Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 71.McArdle A. Jackson MJ. Exercise, oxidative stress and ageing. J Anat. 2000;197:539–542. doi: 10.1046/j.1469-7580.2000.19740539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McArdle A. Vasilaki A. Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1:79–93. doi: 10.1016/s0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- 73.McCann SM. Licino J. Wong ML. Yu WH. Karanth S. Rettorri V. The nitric oxide hypothesis of aging. Exp Gerontol. 1998;33:813–826. doi: 10.1016/s0531-5565(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 74.McClung JM. Kavazis AN. DeRuisseau KC. Falk DJ. Deering MA. Lee Y. Sugiura T. Powers SK. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med. 2007;175:150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGeer EG. McGeer PL. Brain inflammation in Alzheimer disease and the therapeutic implications. Curr Pharm Des. 1999;5:821–836. [PubMed] [Google Scholar]
- 76.McKiernan S. Bua E. McGorray J. Aiken J. Early-onset caloric restriction conserves fiber number in aging rat skeletal muscle. FASEB J. 2004;18:580–581. doi: 10.1096/fj.03-0667fje. [DOI] [PubMed] [Google Scholar]
- 77.McKiernan SH. Colman RJ. Lopez M. Beasley TM. Aiken JM. Anderson RM. Weindruch R. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2010;46:23–29. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melov S. Tarnopolsky MA. Beckman K. Felkey K. Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One. 2007;2:e465. doi: 10.1371/journal.pone.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metter EJ. Talbot LA. Schrager M. Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 80.Milne KJ. Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002;93:561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- 81.Moylan JS. Smith JD. Chambers MA. McLoughlin TJ. Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol. 2008;295:C986–C993. doi: 10.1152/ajpcell.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller FL. Song W. Liu Y. Chaudhuri A. Pieke-Dahl S. Strong R. Huang TT. Epstein CJ. Roberts LJ., 2nd Csete M. Faulkner JA. Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 83.Murlasits Z. Cutlip RG. Geronilla KB. Rao KM. Wonderlin WF. Alway SE. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp Gerontol. 2006;41:398–406. doi: 10.1016/j.exger.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 84.Overend TJ. Cunningham DA. Paterson DH. Lefcoe MS. Thigh composition in young and elderly men determined by computed tomography. Clin Physiol. 1992;12:629–640. doi: 10.1111/j.1475-097x.1992.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 85.Palacios OM. Carmona JJ. Michan S. Chen KY. Manabe Y. Ward JL., 3rd Goodyear LJ. Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pardo PS. Lopez MA. Boriek AM. FOXO transcription factors are mechanosensitive and their regulation is altered with aging in the respiratory pump. Am J Physiol. 2008;294:C1056–C1066. doi: 10.1152/ajpcell.00270.2007. [DOI] [PubMed] [Google Scholar]
- 87.Pauli JR. Ropelle ER. Cintra DE. De Souza CT. da Silva AS. Moraes JC. Prada PO. de Almeida Leme JA. Luciano E. Velloso LA. Carvalheira JB. Saad MJ. Acute exercise reverses aged-induced impairments in insulin signaling in rodent skeletal muscle. Mech Age Dev. 2010;131:323–329. doi: 10.1016/j.mad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Phillips T. Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 89.Pilon G. Charbonneau A. White PJ. Dallaire P. Perreault M. Kapur S. Marette A. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO− induced tyrosine nitration of IRS-1 in skeletal muscle. PLoS One. 2010;5:e15912. doi: 10.1371/journal.pone.0015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pistilli EE. Jackson JR. Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis. 2006;11:2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polidori MC. Mecocci P. Cherubini A. Senin L. Physical inactivity and oxidative stress during aging. Int J Sports Med. 2000;21:154–157. doi: 10.1055/s-2000-8881. [DOI] [PubMed] [Google Scholar]
- 92.Rao RV. Poksay KS. Castro-Obregon S. Schilling B. Row RH. del Rio G. Gibson BW. Ellerby HM. Bredesen DE. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem. 2004;279:177–187. doi: 10.1074/jbc.M304490200. [DOI] [PubMed] [Google Scholar]
- 93.Reynolds TH., 4th Reid P. Larkin LM. Dengel DR. Effects of aerobic exercise training on the protein kinase B (PKB)/mammalian target of rapamycin (mTOR) signaling pathway in aged skeletal muscle. Exp Gerontol. 2004;39:379–385. doi: 10.1016/j.exger.2003.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rice KM. Preston DL. Neff D. Norton M. Blough ER. Age-related dystrophin-glycoprotein complex structure and function in the rat extensor digitorum longus and soleus muscle. J Gerontol A Biol Sci Med Sci. 2006;61:1119–1129. doi: 10.1093/gerona/61.11.1119. [DOI] [PubMed] [Google Scholar]
- 95.Ridker PM. Rifai N. Rose L. Buring JE. Cook NR. Comparison of c-reactive protein and low density cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 96.Rolland Y. Van Kan GA. Gillette-Guyonnet S. Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14:15–21. doi: 10.1097/MCO.0b013e328340c2c2. [DOI] [PubMed] [Google Scholar]
- 97.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–S47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 98.Ryall JG. Schertzer JD. Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 99.Sakuma K. Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 2010;3:90–101. doi: 10.2174/1874609811003020090. [DOI] [PubMed] [Google Scholar]
- 100.Sandri M. El Meslemani AH. Sandri C. Schjerling P. Vissing K. Andersen JL. Rossini K. Carraro U. Angelini C. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J Neuropathol Exp Neurol. 2001;60:302–312. doi: 10.1093/jnen/60.3.302. [DOI] [PubMed] [Google Scholar]
- 101.Senf SM. Dodd SL. McClung JM. Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22:3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Short KR. Nair KS. Muscle protein metabolism and the sarcopenia of aging. Int J Sports Nutr Metab. 2001;11:S119–S127. doi: 10.1123/ijsnem.11.s1.s119. [DOI] [PubMed] [Google Scholar]
- 103.Siu PM. Bryner RW. Martyn JK. Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004;18:1150–1152. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- 104.Siu PM. Pistilli EE. Butler DC. Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol. 2005;288:C338–C449. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- 105.Song W. Kwak HB. Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antiox Redox Signal. 2006;8:517–528. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- 106.Song W. Kwak HB. Lawler JM. Exercise training modulates the NOS profile in skeletal muscle from old rats. J Gerontol Ser A Biol Sci Med Sci. 2009;64:540–549. doi: 10.1093/gerona/glp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sreekumar R. Unnikrishnan J. Fu A. Nygren J. Short KR. Schimke J. Barazzoni R. Nair KS. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol. 2002;283:E38–E43. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- 108.Starnes JW. Choilawala AM. Taylor RP. Nelson MJ. Delp MD. Myocardial heat shock protein 70 expression in young and old rats after identical exercise programs. J Gerontol A Biol Sci Med Sci. 2005;60:963–969. doi: 10.1093/gerona/60.8.963. [DOI] [PubMed] [Google Scholar]
- 109.Strasser H. Tiefenthaler M. Steinlechner M. Bartsch G. Konwalinka G. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet. 1999;354:918–919. doi: 10.1016/S0140-6736(99)02588-X. [DOI] [PubMed] [Google Scholar]
- 110.Susin SA. Lorenzo HK. Zamzami N. Marzo I. Snow BE. Brothers GM. Mangion J. Jacotot E. Costantini P. Loeffler M. Larochette N. Goodlett DR. Aebersold R. Siderovski DP. Penninger JM. Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki N. Motohashi N. Uezumi A. Fukada S. Yoshimura T. Itoyama Y. Aoki M. Miyagoe-Suzuki Y. Takeda S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest. 2007;117:2468–2476. doi: 10.1172/JCI30654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tarpenning KM. Hamilton-Wessler M. Wiswell RA. Hawkins SA. Endurance training delays age of decline in leg strength and muscle morphology. Med Sci Sports Exerc. 2004;36:74–78. doi: 10.1249/01.MSS.0000106179.73735.A6. [DOI] [PubMed] [Google Scholar]
- 113.Testa JW. Siniff DB. Population dynamics of Weddell seals (Leptnoychotes weddelli) in McMurdo Sound, Antarctica. Ecol Monog. 1987;57:149–165. [Google Scholar]
- 114.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 115.Toba H. Miki S. Shimizu T. Yoshimura A. Inoue R. Sawai N. Tsukamoto R. Murakami M. Morita Y. Nakayama Y. Kobara M. Nakata T. The direct antioxidative and anti-inflammatory effects of peroxisome proliferator-activated receptors ligands are associated with the inhibition of angiotensin converting enzyme expression in streptozotocin-induced diabetic rat aorta. Eur J Pharmacol. 2006;549:124–132. doi: 10.1016/j.ejphar.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 116.Tomiyama H. Motobe K. Zaydun G. Koji Y. Yambe M. Arai T. Kushiro T. Yamashina A. Insulin sensitivity and endothelial function in hypertension: a comparison of temocapril and candesartan. Am J Hypertens. 2005;18:178–182. doi: 10.1016/j.amjhyper.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 117.US Census Bureau Decennial Census, Population Estimates and Projections. 2006.
- 118.Vasilaki A. Van Der Meulen JH. Larkin L. Harrison DC. Pearson T. Van Remmen H. Richardson A. Brooks SV. Jackson MJ. McArdle A. Vasilaki A, Van Der Meulen JH, Larkin L, Harrison DC, Pearson T, Van Remmen H, Richardson A, Brooks SV, Jackson MJ, and McArdle A. The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu, Zn superoxide dismutase. Aging Cell. 2010;9:979–990. doi: 10.1111/j.1474-9726.2010.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaziri ND. Ding IY. Sangha DS. Purdy RE. Upregulation of NOS by simulated microgravity, potential cause of orthostatic intolerance. J Appl Physiol. 2000;89:338–344. doi: 10.1152/jappl.2000.89.1.338. [DOI] [PubMed] [Google Scholar]
- 120.Vinciguerra M. Musaro A. Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol. 2010;694:211–233. doi: 10.1007/978-1-4419-7002-2_15. [DOI] [PubMed] [Google Scholar]
- 121.Wenz T. Diaz F. Hernandez D. Moraes CT. Endurance exercise is protective for mice with mitochondrial myopathy. J Appl Physiol. 2009;106:1712–1719. doi: 10.1152/japplphysiol.91571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Wenz T. Rossi SG. Rotundo RL. Spiegelman BM. Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Werner C. Hanhoun M. Widmann T. Kazakov A. Semenov A. Pöss J. Bauersachs J. Thum T. Pfreundschuh M. Müller P. Haendeler J. Böhm M. Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 124.Whitehead NP. Pham C. Gervasio OL. Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586:2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Whitman SA. Wacker MJ. Richmond SR. Godard MP. Contributions of the ubiquitin-proteasome pathway, apoptosis to human skeletal muscle wasting with age. Pflugers Arch. 2005;450:437–446. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- 126.Wohlgemuth SE. Seo AY. Marzetti E. Lees HA. Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xue QL. Beamer BA. Chaves PH. Guralnik JM. Fried LP. Heterogeneity in Rate of Decline in Grip, Hip, and Knee Strength and the Risk of All-Cause Mortality: The Women's Health and Aging Study II. J Am Geriatr Soc. 2010;58:2076–2084. doi: 10.1111/j.1532-5415.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Young A. Stokes M. Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5:145–154. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Z. Zhao M. Li Q. Zhao H. Wang J. Li Y. Acetyl-l-carnitine inhibits TNF-alpha-induced insulin resistance via AMPK pathway in rat skeletal muscle cells. FEBS Lett. 2009;583:470–474. doi: 10.1016/j.febslet.2008.12.053. [DOI] [PubMed] [Google Scholar]