Abstract
Plasmid pPM114 carries the Escherichia coli 16S ribosomal RNA gene under the control of a T7 promoter. It can generate in vitro transcribed 16S rRNA that can be assembled into functional 30S ribosomal subunits. Two deletion mutants were derived from pPM114, by partial or total deletion of the conserved 900 stem/loop region of the 16S rRNA. These mutants, pMG delta 10 and pMG delta 23, respectively lack bases 895 to 904 and 889 to 911 of the 16S rRNA. The amputated 16S rRNA transcripts synthesized from these mutated plasmids were assembled into 30S subunits which were as active under the direction of an artificial or a natural messenger as subunits reconstructed with the full-length 16S rRNA transcript. They also responded as well to the stimulation of misreading by streptomycin, although the deleted region is proximal to the streptomycin binding domain. However, when we attempted to delete the 895-904 or 889-911 region from the 16S rRNA gene in plasmid pKK3535 which carries the rrnB operon, no transformants harbouring plasmids with one of these deletions could be recovered. These observations suggest that the 900 stem/loop region of the 16S rRNA is not required for the ribosomal function but is probably essential for important cell regulatory functions.
Full text
PDF




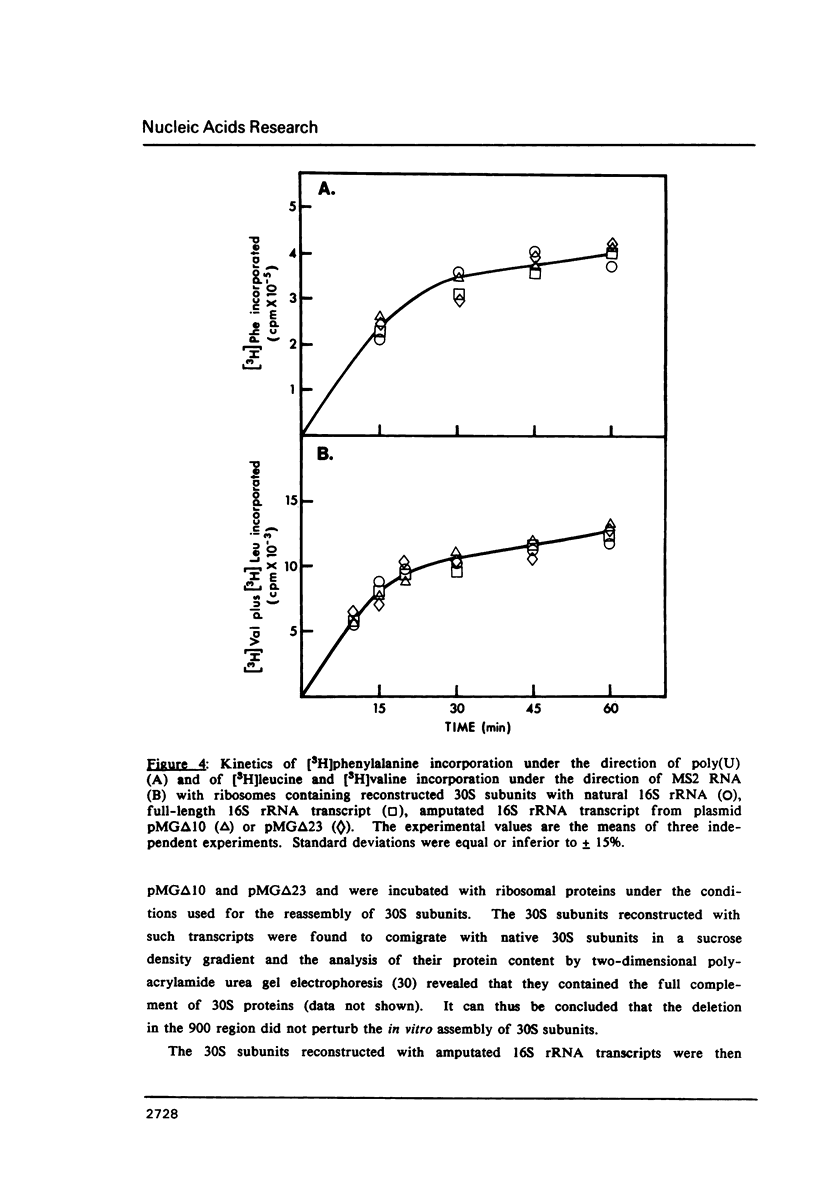



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
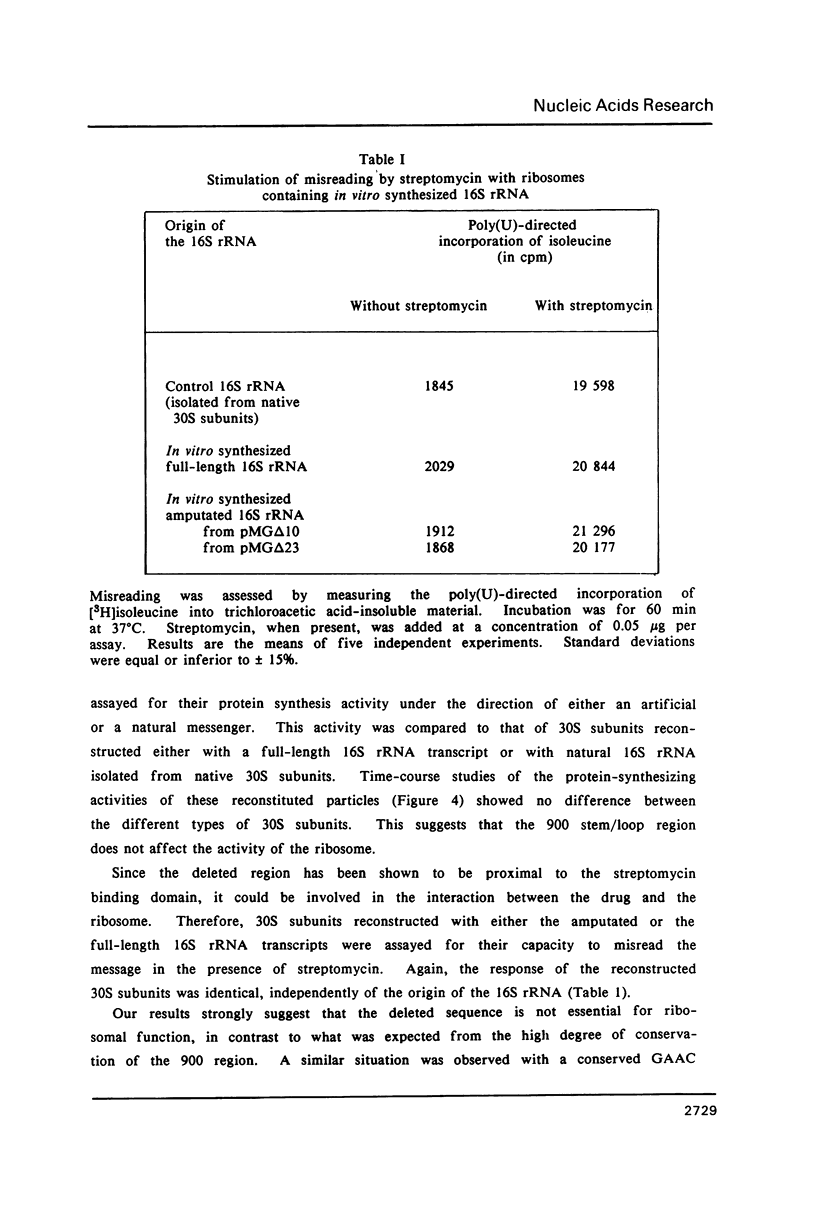
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Goldman E., Hatfield G. W. Use of purified isoacceptor tRNAs for the study of codon-anticodon recognition in vitro with sequenced natural messenger RNA. Methods Enzymol. 1979;59:292–309. doi: 10.1016/0076-6879(79)59092-2. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Stark M. J., Dahlberg A. E. Site-directed mutagenesis of ribosomal RNA. Construction and characterization of deletion mutants. J Mol Biol. 1982 Aug 15;159(3):397–416. doi: 10.1016/0022-2836(82)90291-1. [DOI] [PubMed] [Google Scholar]
- Gravel M., Melançon P., Brakier-Gingras L. Cross-linking of streptomycin to the 16S ribosomal RNA of Escherichia coli. Biochemistry. 1987 Sep 22;26(19):6227–6232. doi: 10.1021/bi00393a041. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Zimmermann R. A. Site-directed mutagenesis of the binding site for ribosomal protein S8 within 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1986 Jul 25;14(14):5761–5776. doi: 10.1093/nar/14.14.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisé-Miron L., Noreau J., Melançon P., Brakier-Gingras L. Comparison of the misreading induced by streptomycin and neomycin. Biochim Biophys Acta. 1981 Nov 27;656(1):103–110. doi: 10.1016/0005-2787(81)90032-0. [DOI] [PubMed] [Google Scholar]
- Held W. A., Mizushima S., Nomura M. Reconstitution of Escherichia coli 30 S ribosomal subunits from purified molecular components. J Biol Chem. 1973 Aug 25;248(16):5720–5730. [PubMed] [Google Scholar]
- Hershey J. W., Yanov J., Fakunding J. L. Purification of protein synthesis initiation factors IF-1, IF-2, and IF-3 from Escherichia coli. Methods Enzymol. 1979;60:3–11. doi: 10.1016/s0076-6879(79)60003-4. [DOI] [PubMed] [Google Scholar]
- Ikeda R. A., Richardson C. C. Enzymatic properties of a proteolytically nicked RNA polymerase of bacteriophage T7. J Biol Chem. 1987 Mar 15;262(8):3790–3799. [PubMed] [Google Scholar]
- Knopf U. C., Sommer A., Kenny J., Traut R. R. A new two-dimensional gel electrophoresis system for the analysis of complex protein mixtures: application to the ribosome of E. coli. Mol Biol Rep. 1975 Mar;2(1):35–40. doi: 10.1007/BF00357295. [DOI] [PubMed] [Google Scholar]
- Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C. W., Agris P. F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987 Apr 21;26(8):2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melançon P., Boileau G., Brakier-Gingras L. Cross-linking of streptomycin to the 30S subunit of Escherichia coli with phenyldiglyoxal. Biochemistry. 1984 Dec 18;23(26):6697–6703. doi: 10.1021/bi00321a064. [DOI] [PubMed] [Google Scholar]
- Melançon P., Gravel M., Boileau G., Brakier-Gingras L. Reassembly of active 30S ribosomal subunits with an unmethylated in vitro transcribed 16S rRNA. Biochem Cell Biol. 1987 Dec;65(12):1022–1030. doi: 10.1139/o87-134. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Nicolas P., Schürmann P., Stutz E. Streptomycin-resistance of Euglena gracilis chloroplasts: identification of a point mutation in the 16S rRNA gene in an invariant position. Nucleic Acids Res. 1985 Jun 25;13(12):4299–4310. doi: 10.1093/nar/13.12.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Wagner R., Stutz E. E. coli ribosomes with a C912 to U base change in the 16S rRNA are streptomycin resistant. EMBO J. 1986 Dec 20;5(13):3705–3708. doi: 10.1002/j.1460-2075.1986.tb04703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Stern S., Moazed D., Powers T., Svensson P., Changchien L. M. Studies on the architecture and function of 16S rRNA. Cold Spring Harb Symp Quant Biol. 1987;52:695–708. doi: 10.1101/sqb.1987.052.01.079. [DOI] [PubMed] [Google Scholar]
- Pace B., Matthews E. A., Johnson K. D., Cantor C. R., Pace N. R. Conserved 5S rRNA complement to tRNA is not required for protein synthesis. Proc Natl Acad Sci U S A. 1982 Jan;79(1):36–40. doi: 10.1073/pnas.79.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs P. A., Thompson J., Cundliffe E. Methylation of 16S ribosomal RNA and resistance to aminoglycoside antibiotics in clones of Streptomyces lividans carrying DNA from Streptomyces tenjimariensis. Mol Gen Genet. 1985;200(3):415–421. doi: 10.1007/BF00425725. [DOI] [PubMed] [Google Scholar]
- Steen R., Jemiolo D. K., Skinner R. H., Dunn J. J., Dahlberg A. E. Expression of plasmid-coded mutant ribosomal RNA in E. coli: choice of plasmid vectors and gene expression systems. Prog Nucleic Acid Res Mol Biol. 1986;33:1–18. doi: 10.1016/s0079-6603(08)60018-5. [DOI] [PubMed] [Google Scholar]
- Stern S., Changchien L. M., Craven G. R., Noller H. F. Interaction of proteins S16, S17 and S20 with 16 S ribosomal RNA. J Mol Biol. 1988 Mar 20;200(2):291–299. doi: 10.1016/0022-2836(88)90241-0. [DOI] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. Interaction of ribosomal proteins S5, S6, S11, S12, S18 and S21 with 16 S rRNA. J Mol Biol. 1988 Jun 20;201(4):683–695. doi: 10.1016/0022-2836(88)90467-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. L., Gregory R. J., Winslow G., Muto A., Zimmermann R. A. Mutations within the decoding site of Escherichia coli 16S rRNA: growth rate impairment, lethality and intragenic suppression. Nucleic Acids Res. 1988 Aug 25;16(16):8129–8146. doi: 10.1093/nar/16.16.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska L., Van Duin J., Noller H. F., Pace B., Johnson K. D., Pace N. R. The conserved 5 S rRNA complement to tRNA is not required for translation of natural mRNA. J Biol Chem. 1984 Mar 10;259(5):2798–2802. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]


