Abstract
It is unknown how antidepressants reverse mood-congruent memory bias, a cognitive core factor causing and maintaining depression. Using a double-blind, placebo-controlled, cross-over design, we investigated the effect of a short-term treatment (14 days) with the dual reuptake inhibitor duloxetine on neural correlates of mood-congruent and mood-incongruent memory formation and retrieval in healthy volunteers who underwent a sad mood induction procedure. Duloxetine did not affect acute mood state or memory performance, but interacted with brain processes mediating mood-congruent memory. It decreased activity related to successful memory formation for mood-congruent and -incongruent items in a set of brain regions comprising the putamen and the middle frontal gyrus, as well as the middle and the anterior cingulate cortex. Duloxetine specifically increased amygdala activity related to successful memory retrieval for mood-incongruent items. Here we show that short-term administration of duloxetine affects the neural correlates of emotional memory formation and retrieval in a set of brain regions whose processing is related to affective state and its regulation. While duloxetine suppressed the neural correlates of emotional memory formation in general, it specifically enhanced amygdala processes associated with mood-incongruent memory retrieval. This pattern of results shows how an antidepressant may reduce emotional memory formation and reverse mood-congruent processing biases at retrieval.
Keywords: depression, duloxetine, fMRI, amygdala, emotional memory, mood congruency
INTRODUCTION
The interaction between mood during learning and the emotional valence of an event leads to mood-specific memory enhancement (Leppänen, 2006). While this interaction may support adaptive behavior (McGaugh, 2004), persistent sad mood can lead to negative learning schemes (Teasdale, 1983). This so-called mood-congruent memory bias is one of the cognitive trait factors causing and maintaining depression (Hasler et al, 2004). Negative biases leading to dysfunctional attitudes have been related to chronic reductions in extracellular serotonin (Meyer et al, 2003; Bhagwagar et al, 2006), suggesting a potential reversion by antidepressants. The question that arises is how antidepressants affect these cognitive processes other than attenuating the negative affect.
Investigating an antidepressant's effect on mood-congruent memory is sparsely restricted to the behavioral effects in acutely depressed patients, where a single administration of the noradrenaline reuptake inhibitor reboxetine reversed an initial reduction of memory for positive faces (Harmer et al, 2008). In depressed patients with euthymic mood, antidepressants may even specifically affect memory formation and retrieval (Norbury et al, 2008), although this study did not dissociate neural activity during learning and retrieval as a function of (subsequent) memory success.
On a broader level, earlier positron emission tomographic studies have shown that a single administration of -fenfluramine, a serotonin agonist, induced a reduction in negative interpretation bias, which was possibly related to lower levels of extracellular serotonin as measured by the 5-HT2-binding potential in the prefrontal cortex (Meyer et al, 2003), and also in the parietal and occipital cortex (Bhagwagar et al, 2006). Functional MRI (fMRI) studies in depressed patients have shown that antidepressants acting through serotonin reuptake inhibition reverse increased neural processing of negative stimuli (Fu et al, 2004) and decreased processing of positive stimuli (Fu et al, 2007) in brain regions mediating affective regulation or higher order visual processing. While antidepressant effects in a treatment study of patients can be a mere consequence of elevated mood, fMRI studies in healthy individuals have shown that single-dose and short-term administration of an antidepressant alters affective processing and its underlying neural circuitry (Harmer et al, 2006; Murphy et al, 2009; Norbury et al, 2007). Finally, a study by Lopez-Solá et al (2010) showed that the antidepressant duloxetine altered the neural correlates of pain processing in depressed patients already after 1 week when clinical effects were still modest. Hence, whereas different types of antidepressants appear to modulate affective processing directly, the effect of the current mood state on these modulatory effects, particularly during mood-congruent memory, is unknown.
Mood-congruent memory studies in acutely depressed patients (Hamilton and Gotlib, 2008; van Wingen et al, 2010) or recovered patients under sad mood induction (Ramel et al, 2007) point to the amygdala as an important mediator. The amygdala has a central role in various aspects of affect processing and modulates the hippocampus during emotional memory (Dolcos et al, 2005). The hippocampus has an important role in the pathogenesis of depression (MacQueen and Frodl, 2011) and is a major target of antidepressant action (eg, Warner-Schmidt and Duman, 2006). Hence, amygdala and hippocampal activity should be specifically investigated when tackling the effects of antidepressants on mood-congruent memory.
Experimental sad mood induction allows assessing the neural correlates of emotional memory while aligning subjects in a reduced emotional state (Lewis et al, 2005; Fitzgerald et al, 2011). It can be combined with investigation of antidepressant effects recruiting healthy subjects in a within-subject, crossover design, which would not be feasible in a patient sample. Therefore, we combined sad mood induction with event-related fMRI to probe antidepressant effects on emotional memory processes in subjects who feel sad. Dissociating the antidepressant effects on successful and unsuccessful memory processes during encoding and retrieval further elucidates how such an antidepressant can remediate cognitive biases by affecting specific mnemonic processes. We specifically assessed the neural correlates of memory formation and retrieval of emotionally positive and negative stimuli, allowing us to dissociate the antidepressant effects on mood-congruent (ie, effects related to negative stimuli) and mood-incongruent (effects related to positive stimuli) memory.
Both serotonergic and noradrenergic antidepressants have been shown to remediate cognitive biases, suggesting a final common pathway for this effect (for further discussion of this issue, see also Harmer et al, 2009). We also did not focus on specific antidepressant effects of either serotonin reuptake or noradrenaline reuptake inhibitors on mood-congruent emotional memory processing and therefore used the dual reuptake inhibitor duloxetine (Frampton and Plosker, 2007; Gupta et al, 2007). In a setting more informative for treatment (Katz et al, 2004), we applied a short-term administration of duloxetine (for 2 weeks) in a randomized, double-blind, placebo-controlled, cross-over design.
MATERIALS AND METHODS
Subjects
Eighteen healthy subjects (eight male) with a body mass index between 18.5 and 25, and between 18 and 50 years of age (mean age 26±7 years), participated in this randomized, double-blind, placebo-controlled, cross-over study approved by the local ethics committee (CMO region Arnhem-Nijmegen, The Netherlands). Participants were recruited through advertisement and screened approximately 1 week before entering the trial.
Screening
Before screening, subjects were informed about all procedures and risks, following which they signed an informed consent. The subjects underwent a general physical examination (including neurological assessment), including evaluation of medical history to exclude subjects with a neurological illness or a general medical condition, which could potentially affect the outcome of the trial. Screening for current or lifetime psychiatric illnesses was performed with the Mini International Neuropsychiatric Interview (Sheehan et al, 1998) to exclude subjects fulfilling any of the diagnoses. Medically relevant abnormalities in the ECG or laboratory parameters (general hematology and blood chemistry) taken at screening were regarded as exclusion criteria. Positive drug/alcohol and pregnancy screenings, taken at each measurement point, were additional exclusion criteria. Further exclusion criteria were a known hypersensitivity to duloxetine or contra-indications for duloxetine (hepatic impairment, severe renal impairment with a GFR <30 ml/min) as well as a history of prescribed medication within 3 months prior to the start of this trial except for oral contraceptives and paracetamol.
Drug Intervention
After screening, subjects were randomly assigned to two groups starting either with a morning dose of a capsule containing a placebo or 60 mg of duloxetine for 14 consecutive days. The treatment periods were separated by a washout period of at least 14 days (range: 14–42 days; mean±SD: 21±10 days). fMRI measurements were taken on the final day of each drug or placebo period. Serum duloxetine levels were assessed by examining a 10-ml venous blood sample collected in an EDTA anticoagulant tube at approximately 1100 hours on each scanning day. The samples were centrifuged for 10 min at 3500 r.p.m. at room temperature. The separated plasma was kept in a labeled plastic tube in a freezer at a temperature of −20 °C until the end of the study. After study de-blinding, the samples from the duloxetine session were selected for serum analysis. High-performance liquid chromatography (HPLC) was used to measure duloxetine levels (10–100 μg/l), using prothiaden (100 μg/l) as an internal standard. The HPLC system consisted of a Waters 1515 Isocratic Pump, delivering the mobile phase at a flow rate of 1.1 ml/min, and an Inertsil ODS-3 5-μm, 50 × 4.6 mm (Alltech: GL815ODS346) separation column, heated to 40 °C. The duloxetine plasma levels ranged between 7 and 247 μg/l (mean 48 μg/l).
Experimental Procedure
Assessment of emotional state
The emotional state was assessed before and after scanning, as well as before and after mood induction, as described below. Before scanning we assessed state anxiety (Dutch version of the STAI) (Kernis et al, 1997; van der Ploeg et al, 1980) and depressive symptoms (Dutch version of the BDI (Van der Does, 2002)), as well as an overall mood rating by means of the Dutch version of the shortened Profile of Moods States (POMS) (Wald and Mellenbergh, 1990), before and after scanning.
Negative mood induction procedure
Based on a previous study (Kernis et al, 1997), we induced negative mood by asking subjects to watch movie clips that were taken from the American drama film ‘Sophie's Choice' (Pakula, 1982). Prior to the first encoding session, the subjects watched an initial film segment of 12 min. The subjects were told that they would be watching a sad movie clip, and were instructed to use the situation and the emotions seen in the movie to put themselves in as strong a mood as possible. Thereafter, they underwent two study phases, each lasting 15 min. Both encoding sessions were followed by two further movie clips (lasting ∼5 min) from the same movie to boost the sad mood using the same instructions. Film fragments of equal length were interspersed between the four consecutive retrieval sessions each lasting 15 min. The subjects rated their current mood on a computer-based rating visual analog scale (ranging from −10 to 10) before and after each of the film clips.
Memory paradigm
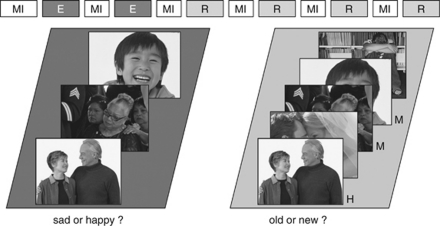
For an overview of the experimental design, see Figure 1. During scanning the participants completed a memory task, which was divided into two encoding and four test phases as mentioned before. Stimuli consisted in total of 240 emotional scenes showing one or more humans and were taken from a pool of positive and negative photographs, which had been rated during a behavioral pilot study (five-point scale ranging from ‘emotionally positive' to ‘emotionally negative' the mean valence rating of the negative photographs was ⩽2 and the mean valence rating of the positive photographs was ⩾4). Half of the photographs were chosen as study items (including 120 positive and 120 negative photographs) and the other half as lures during test. This assignment was counterbalanced across the factors test phase (ie, of which half were chosen as study items and half as lures for the test phase) and gender. The content of the positive and negative photographs (ie, individual or group, child or adult, male or female person(s)) was distributed pseudo-randomly across stimulus sets. During study and test, the photographs were presented for 0.5 s, with a jittered inter-stimulus interval of 3.7–4.7 s. At study, participants were instructed to memorize the 120 positive and 120 negative photographs, which were presented sequentially and randomly intermixed in two encoding sessions separated by mood induction as outlined above. To ensure that subjects were processing the stimuli conceptually, they had to perform an emotional valence decision task during the encoding phase.
Figure 1.
An overview of the experimental setup. Mood induction was interspersed throughout the entire experiment, prior to each of the encoding and recognition session. At study, subjects were required to make a valence decision. At test, participants saw the same amount of previously presented and new photographs, and were required to make an old/new judgment. For details, see section Materials and methods. E, encoding block; MI, mood induction; R, retrieval block.
At test, subjects were required to recognize the old photographs and reject the same amount of randomly intermixed new photographs (ie, 480 photographs in total). The retrieval phase was subdivided into four sessions to allow for continuous mood induction as described above. In keeping with previous studies and reducing the number of guesses during old/new recognition (Tendolkar et al, 2008; Weis et al, 2004), the participants were encouraged to make a decision between old and new, but also had the option to make an unsure decision.
Image acquisition
MRI scans were collected using a Siemens (Erlangen, Germany) Avanto 1.5-T MRI scanner equipped with a CP head coil. We obtained 326 T2*-weighted BOLD images during the task for each scan session (gradient echo EPI; TE/TR: 35/2340 ms; flip angle: 90° FOV: 212 mm; matrix size: 64 × 64; 3.5 mm slice thickness; 0.35 mm slice gap; 32 ascending slices). High-resolution, T1-weighted structural MR images were acquired for spatial normalization procedures (MP-RAGE, 176 images; TE/TR: 2.95/2250 ms; 1.0 mm slice thickness; matrix size: 256 × 256; FOV: 256 mm; flip angle: 15°).
Image analysis
We used SPM5 (Wellcome Department of Imaging Neuroscience, London, UK) for MRI data analysis. The first five EPI volumes were discarded to allow for T1 equilibration, and the remaining images were realigned to the first volume. Images were then corrected for differences in slice acquisition time, spatially normalized to the Montreal Neurological Institute (MNI) T1 template, super-sampled into 2 × 2 × 2-mm3 voxels, and spatially smoothed with a Gaussian kernel of 8 mm FWHM.
Statistical analysis of the event-related data was performed within the framework of the general linear model (Friston et al, 1995), where predictor variables were defined for each subject in the first-level analysis separately for the encoding and the retrieval session. During encoding, regressors for trials reflecting either later-remembered or later-forgotten trials were modeled, as were positive and negative stimuli. With respect to the retrieval phase, trials reflecting recognized (hits) and unrecognized stimuli (misses) were modeled, as were positive and negative stimuli. Each of the explanatory variables forming the above-mentioned factors were modeled separately.
The explanatory variables (0.5 s) were temporally convolved with the hemodynamic response function of SPM5. In addition, the realignment parameters were included to model potential movement artifacts, as was a high-pass filter (cut-off at 1/128 Hz). Data were proportionally scaled accounting for various global effects and temporal autocorrelation was modeled with an AR(1) process. Relevant parameter images contrasting each condition were entered into a random-effects, repeated-measures ANOVA with a non-sphericity correction.
In keeping with the hypotheses outlined in Introduction, exploratory analyses were performed across the entire brain using appropriate correction. Additionally, specific analyses were performed in the amygdala and hippocampus using anatomically generated masks for conducting small volume corrections. Statistical tests were family-wise error rate-corrected for multiple comparisons at the cluster level across the entire brain (p<0.05) for the exploratory analyses, or the search volumes of interest using a small volume correction (Worsley et al, 1996), both using an initial height threshold of p<0.005 uncorrected. Amygdala and hippocampus masks were constructed based on macroscopic anatomical parcellation of a canonical T1-weighted MRI scan in MNI space (Maldjian et al, 2003; Tzourio-Mazoyer et al, 2002). The peak voxels of activated clusters are reported in MNI coordinates.
RESULTS
Adverse Events
Duloxetine plasma levels ranged between 7 and 247 μg/l (mean±SD: 50±54), which confirmed compliance with drug intake. Of the 18 participants who participated in the study, five reported nausea, fatigue, and insomnia. All reported side effects were limited to the first few days of drug intake, indicating that our results do not reflect the experience of adverse effects, as the neuroimaging sessions took place only after 2 weeks of intake. Moreover, no serious adverse events were reported.
Mood and Behavior
Mood, as measured prior to scanning by the BDI and STAI state, was not affected significantly by duloxetine (maximum t=1.5, p=0.2). The pre- and post-scan measures of the POMS were compared by repeated-measures ANOVA using the factors time (before/after scanning), drug (duloxetine/placebo), and POMS subscale (five levels). There was an interaction between the factors time and subscale (F(4, 68)=33, p<0.001). Post-hoc pairwise comparisons for each subscale before and after scanning showed that depressive mood, fatigue, vigor, and tension were increased by the fMRI procedure, including the negative mood induction (minimum t=3.65, p<0.005), except for the subscale anger. Moreover, we found no interaction between the factors drug and subscale.
Mood ratings before and throughout the experiments were analyzed by ANOVA using the factors drug (duloxetine/placebo) and mood rating (before/after each video). Whereas there was no significant effect of duloxetine on mood ratings, there was an expected difference between mood ratings before and after mood induction (F(11, 187)=45, p<0.001). Post-hoc pairwise comparisons showed that mood was reduced after each mood induction compared with baseline (minimum t=3.3, p<0.005). Moreover, mood at the end of the entire experiment was reduced compared with the start of the mood induction (t=6.9, p<0.001). Hence, our mood induction led, as intended, to a substantially reduced, sad mood throughout the entire fMRI experiment.
Memory performance, as measured by the difference between hit and false alarm rates for all valence and drug conditions separately, was significantly above the chance level (minimum t=6.9, p<0.001). There was no significant difference in memory performance between emotionally negative photographs and positive photographs. Duloxetine had no significant effect on memory performance. There was also no significant effect of duloxetine or valence on the reaction time data. Reaction times, however, differed as a function of memory condition (F(3, 2.7)=23.2, p<0.001), where reaction times were fastest for hits (1785 ms), followed by correct rejections (1945 ms) and almost equally slow for misses and false alarms (2118 ms and 2112 ms, respectively). A Spearman's ρ correlation was performed between the behavioral outcome measures of the memory test, but revealed no significant relationship either for the recognition scores (hits minus false alarms) or for the reaction times (minimum p⩾0.11).
Imaging Results
Encoding
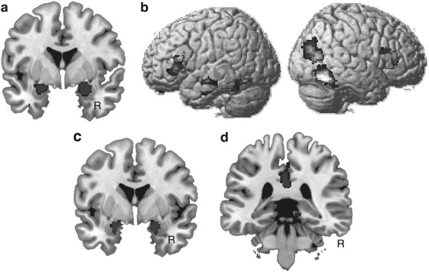
First, we conducted an exploratory whole-brain analysis looking at main effects. In line with earlier studies, a main effect of memory (ie, larger activity for subsequently remembered as compared with subsequently forgotten stimuli) gave rise to clusters in the parahippocampal gyrus extending bilaterally into the fusiform gyrus (local maximum at 46, −50, −18 and −40, −46, −18, respectively; minimum pcorr<0.001) and large clusters in the bilateral amygdala extending into the hippocampus (local maximum at 22, −4, −18 and −20, −6, −16, respectively; minimum pcorr<0.001; see Figure 2). In the right posterior cortex, an activation was found in a cluster ranging from the occipital cortex into the temporal cortex (local maximum at 48, −62, −14; pcorr<0.001; see Figure 2).
Figure 2.
Activation maps (significance threshold at p<0.001 uncorrected for displaying purposes) showing significant main effects of memory found during memory formation and memory retrieval are shown. Note that all results shown in this and the subsequent figures were family-wise error rate-corrected for multiple comparisons at the cluster level across the entire brain (p<0.05), or the search volume of interests (amygdala, hippocampus), using a small volume correction (p<0.05). (a) On the left panel, activation maps from the whole-brain analysis during encoding are superimposed on a standard T1 image provided with MRIcron, showing significantly larger activations related to successful vs unsuccessful memory formation in the bilateral amygdala. (b) Whole-brain analysis during encoding further revealed that successful memory formation was associated with significant activity in the bilateral inferior frontal regions, shown superimposed on a rendered brain provided with SPM5. (c) The results from the ROI analysis during retrieval are shown. Greater activity for recognized as compared with forgotten stimuli is shown in the bilateral amygdala/hippocampus, superimposed on a standard T1 image provided with MRIcron. (d) Significant recognition effect in the right middle cingulate cortex is shown.
Successful memory formation was associated with an activity increase in the left (local maximum at −52, 30, 0; pcorr<0.01) and the right inferior frontal gyrus (54, 32, 6; pcorr<0.01).
To probe the main effect of drug, we compared the larger activity for the duloxetine with the placebo condition. This analysis did not give rise to any significant activation, indicating that duloxetine did not generally affect the BOLD signal in this experimental setup.
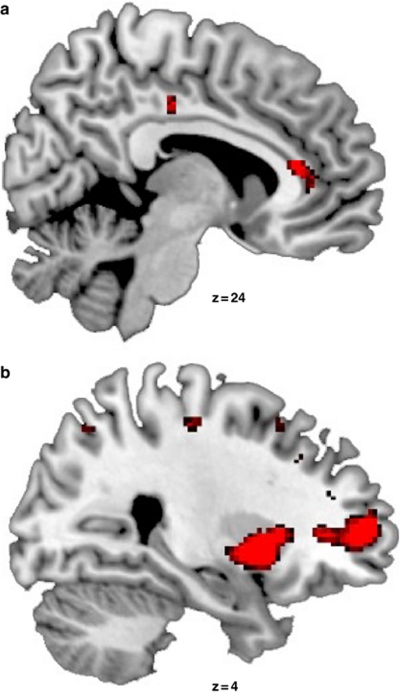
Most important with respect to our experimental question, we probed the effect of duloxetine on the neural correlates of successful memory formation by comparing the subsequent memory effect between the drug and the placebo condition. The subsequent memory effect was larger in the placebo condition than in the duloxetine condition in the right hemisphere in the putamen (local maximum at 30, −12, 0; pcorr<0.01), the anterior (local maximum at 2, 32, 12; pcorr<0.05), and the middle cingulate cortex (local maximum at 12, −24, 38; pcorr<0.05), as well as the middle frontal gyrus (local maximum at 26, 52, 6; pcorr<0.02; see Figure 3). This memory by drug interaction was not affected significantly by stimulus valence. For a summary of the significant effects see Table 1.
Figure 3.
Activation maps (threshold at p<0.001 uncorrected for displaying purposes) from the whole-brain analysis, superimposed on the sagittal slices of a standard T1 image provided with MRIcron, show the significant interaction between the factors drug and memory formation. As described under Results, this interaction is based on a larger subsequent memory effect in the placebo than in the duloxetine condition in (a) the right putamen and the middle frontal gyrus, as well as in (b) the right middle and the anterior cingulate cortex. The z-coordinates shown on the figure refer to the coordinates in MNI space for the local maxima found in the analyses, where MNI space is an approximation to Talairach space.
Table 1. The Coordinates of each of the Significant Effects Found in the Statistical Analysis are shown.
| Study phase | Effect | Region | x | y | z | p-value |
|---|---|---|---|---|---|---|
| Encoding | Memory | R parahippocampal gyrus | 46 | −50 | 18 | 0.000 |
| L parahippocampal gyrus | −40 | −46 | −18 | 0.000 | ||
| R amygdala | 22 | −4 | −18 | 0.001 | ||
| L amygdala | −20 | −6 | −16 | 0.000 | ||
| L inferior frontal gyrus | −52 | 30 | 0 | 0.001 | ||
| R inferior frontal gyrus | 54 | 32 | 6 | 0.003 | ||
| R inferior temporal gyrus | 48 | −62 | −14 | 0.000 | ||
| Drug × memory | R putamen | 30 | 12 | 0 | 0.004 | |
| R anterior cingulate | 2 | 32 | 12 | 0.042 | ||
| R middle cingulate | 12 | −24 | 38 | 0.046 | ||
| R middle frontal gyrus | 26 | 52 | 6 | 0.012 | ||
| Retrieval | Memory | R middle cingulate | 4 | −36 | 36 | 0.046 |
| R amygdala (ROI) | 24 | −2 | −18 | 0.018 | ||
| L amygdala (ROI) | −22 | 2 | −18 | 0.046 | ||
| R hippocampus (ROI) | 22 | −2 | −22 | 0.042 | ||
| Drug × memory × valence | R amygdala (ROI) | 26 | 0 | −24 | 0.044 |
Abbreviations: L, left hemisphere; R, right hemisphere.
x-, y-, and z-coordinates refer to the coordinates in MNI space for the local maxima found in the analyses, where MNI space is an approximation to Talairach space. P-value refers to the significance value after correcting for multiple comparisons at the whole-brain level or within the region of interest (ROI).
Retrieval
The brain regions involved in successful recognition memory were identified by comparing the responses for hits and misses. This whole-brain analysis gave rise to a significant cluster in the right middle cingulate cortex (local maximum at 4, −36, 36; pcorr<0.05). Region of interest (ROI) analysis focusing on the amygdala and hippocampus revealed significant effects within the left (local maximum at −22, 2, −18; pcorr<0.02) and the right amygdala (local maximum at 24, −2, −18; pcorr<0.05), as well as the right hippocampus (local maximum at 22, −4, −22; pcorr<0.05). In line with the data on memory formation, there was no evidence for a significant main effect of drug in the retrieval data. Additionally, we did not find a main effect of valence.
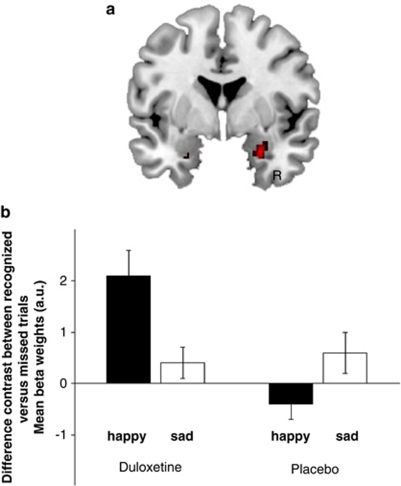
Importantly, we observed a significant three-way interaction between the factors drug, valence, and memory in the ROI analysis of the right amygdala (local maximum at 26, 0, −24; pcorr<0.05; see Figure 4). As is evident from the contrast estimates shown in Figure 4, this interaction seems to arise because of a larger recognition memory effect (ie, contrast between hits and misses) for happy stimuli only in the duloxetine as compared with the placebo condition. We therefore performed post-hoc tests within the anatomically defined ROI of the right amygdala on the difference in recognition memory effects between the drug and the placebo conditions separately for the happy and the sad pictures. Indeed, we found a significant drug-by-memory interaction only for the positive scenes (pcorr<0.001). In other words, our data show that, under sad mood induction, duloxetine specifically enhances the recognition memory effects for positive stimuli in the right amygdala. For a summary of the significant effects, see Table 1.
Figure 4.
The significant results from the three-way interaction between the factors drug, valence, and memory during retrieval within the amygdala are shown. (a) Activation maps (threshold at p<0.001 uncorrected for displaying purposes), superimposed on a coronal slice of a standard T1 image, show a significant three-way interaction in the right amygdala. (b) To visualize this interaction further, parameter estimates are shown as provided by SPM. The bar graphs show the β-estimates of the specific contrast estimates of the recognition memory effect (ie, hits minus misses ±90% confidence interval). This demonstrates that the interaction arises because of an elevated recognition effect for the happy scenes in the duloxetine condition, which was also confirmed statistically by post-hoc analyses.
To perform a Spearman's rank correlation analysis between the neural correlates of memory and duloxetine plasma levels, β-weights per subject were extracted from the ROIs that gave rise to a significant interaction with the factor drug during encoding or during retrieval. For encoding, we correlated the drug-induced change in the subsequent memory effect (placebo condition−drug condition) with the duloxetine plasma levels in the right putamen, the right anterior and middle cingulate, and the right inferior frontal gyrus. These analyses only gave rise to a significant negative correlation between the drug-induced change in successful memory encoding in the right putamen (p=−0.007).
For retrieval, we correlated the drug-induced change in the successful memory retrieval effect (drug condition−placebo condition) in the amygdala with the duloxetine plasma levels, but did not find any significant result.
DISCUSSION
This is the first study that investigated the effect of a short-term administration of the dual serotonin and noradrenaline reuptake inhibitor duloxetine on the neural correlates of mood-congruent and mood-incongruent memory formation and retrieval in healthy volunteers. The paradigm used was designed to mimic the effect of mood on memory during depression by employing relatively long series of explicit sad mood induction using emotional movie clips during memory formation and retrieval. The experimental set-up allowed us to dissociate the effect of duloxetine on successful and unsuccessful memory formation and retrieval, and not just a global effect on memory processes.
Previous investigations of the effect of antidepressants in healthy controls often revealed changes in emotional processing in the absence of significant differences in ratings of mood and anxiety (Harmer et al, 2004, 2009). Also in the present study, duloxetine did not affect significantly subjective mood or anxiety ratings, nor our behavioral outcome measures. This limits the generalizability of our findings to the clinical state of depression at first sight. However, mood changes are not anticipated in a healthy subject population, and the number of subjects included in the present study was chosen to fulfill the power needs of a neuroimaging study, which appear more favorable than for behavioral studies. We designed the study to investigate fMRI but not behavioral differences. As such, we appreciate the fact that we did not find a behavioral difference, because that may complicate the interpretation of the imaging results as well (Price and Friston, 2002). We found a valence-unspecific effect of duloxetine on the neural correlates of emotional memory formation and a valence-specific effect on the neural correlates of emotional recognition memory. In the absence of direct effects of duloxetine on mood state, these neural effects are therefore more likely caused directly by the drug than by an indirect effect of increased mood.
Another recent fMRI study by Norbury et al (2008) also showed that a selective noradrenergic antidepressant (the noradrenaline reuptake inhibitor reboxetine) directly modulated the neural processing of emotional material in an emotional memory task in the absence of effects on mood or anxiety ratings. During a study phase consisting of a categorization task, reboxetine was associated with greater activation to positive words, relative to negative words, in the left precuneus and the right inferior frontal gyrus. However, during the test phase, recognition memory under reboxetine was associated with reduced responses to positive words in the left precuneus, the anterior cingulate, and the medial frontal gyrus. It is important to note that results from that study are not directly comparable to our results, because Norbury et al (2008) did not investigate neural activity related to memory formation by dissociating trials as to whether they were subsequently remembered or forgotten (for a review, see Fernandez and Tendolkar, 2001). Likewise, they did not investigate the neural correlates of recognition by analyzing the contrast between correctly recognized items and missed or new stimuli. Thus, Norbury et al (2008) did not directly measure the effect of antidepressants on successful memory processes, which however is crucial to understand the cognitive effects of antidepressants. Here, we provide first evidence that duloxetine affects successful memory for biological salient stimuli by acting on successful memory formation in a valence-unspecific manner and on successful memory retrieval in a valence-specific manner.
In line with previous experiments testing emotional memory, we found significant activations in the amygdala and the hippocampus during both memory formation (cf. Richardson et al, 2004) and retrieval (Dolcos et al, 2005) next to the replication of an often shown left inferior frontal activation related to successful memory formation. During successful memory formation, duloxetine decreases activation in a set of brain regions comprising the putamen, the middle frontal gyrus, as well the middle and the anterior cingulate cortex. These regions have been implicated to have an important role in a so-called anterior emotional system known to be involved in emotion regulation (Phillips et al, 2003). A recent study (Lopez-Solá et al, 2010) investigating the effect of duloxetine in acutely depressed patients also found a significant reduction of activation in the subgenual anterior cingulate, the extended medial prefrontal cortex, and the basal ganglia including the putamen. With respect to specific effects related to mood-congruent memory bias, an overactive interaction between the caudate/putamen and the hippocampus seems to account for mood-congruent memory in depression (Hamilton and Gotlib, 2008). Our data are in line with these findings and suggest that even in healthy controls the negative mood induction leads to an increased activity of this network during emotional memory formation, an effect that can be reversed by duloxetine. It has long been debated whether antidepressants can affect fundamental cognitive processes apart from their role in attenuating a pervasive negative effect (see Harmer et al, 2009). We now show that duloxetine targets the same affective neurocircuitry that is implicated in depression (Phillips et al, 2003), but without the improvement in mood that normally accompanies the drug-induced downregulation of this circuitry in depressed patients. We thus provide indirect evidence that the working mechanism of this type of antidepressants is a direct attenuation of the bottom-up processing in the (para)limbic affective neurocircuitry rather than a more indirect effect on mood.
Moreover, some of the above-mentioned brain regions also fulfill other functions when the brain is at rest. The anterior cingulate cortex, the ventromedial prefrontal cortex, and the dorsal medial prefrontal cortex belong to the so-called default mode network, a set of brain regions, which may fulfill self-referential tasks involved in the evaluation of potentially survival-salient information from the body and the world (Buckner et al, 2008; Raichle et al, 2001). Evidence is accumulating that there seems to be a dysregulation of the default mode network in depression (Greicius et al, 2007; Sheline et al, 2009). Whereas activity within this network is decreased in healthy controls in order to allow a shift to a goal-oriented behavior, activity of the default mode network is not decreased equally in depressed patients during cognitive tasks. Sheline et al (2009) suggested that these abnormalities might contribute to deficits in the ‘automatic' and controlled processing of affective stimuli. Our data suggest that sad mood induction leads to increased activity of the default mode network in the placebo condition comparable to that found in depression, and that this effect is reversed by duloxetine.
During retrieval, duloxetine specifically enhanced mood-incongruent neural activity in the amygdala related to the successful recognition of positive scenes. The amygdala has a central role in various aspects of affect processing and mood regulation, and has been shown to have a modulatory effect on the hippocampus during emotional memory (Dolcos et al, 2005). Harmer et al (2009) proposed that antidepressants reverse affective biases in depression and anxiety. Our data add to this hypothesis by suggesting that at least during memory retrieval a mood-congruent memory bias reversal can be related to a processing enhancement for positive stimuli rather than an attenuation of processing for negative stimuli. Although the consequence of such effects on behavior needs to be tested in larger samples, an enhancement of positive memories seems more favorable than a mere decrease of negative memories. By these means, antidepressants do not only serve to decrease negative memories, but improve a positive memory bias, which is usually found in healthy subjects. In terms of clinical impact, this could mean that depressed patients under antidepressant treatment are allowed to gain again from the positive aspects of their environment.
Antidepressants acting through serotonergic reuptake inhibition appear to have a more general blunting effect, where emotional reactivity to both negative and positive experiences can be reduced (Opbroek et al, 2002; Price et al, 2009). The more general effect of duloxetine decreasing neural activity in brain regions known to be involved in emotion regulation could therefore rely on a more ‘inhibitory' serotonergic effect during emotional memory formation, where decreased effects of mood are likely to normalize biased processing. However, previous studies have also shown that SSRIs can increase the neural processing related to happy faces in depressed patients in the course of 8 weeks of treatment (Fu et al, 2007), which, potentially, could explain the effect we found during retrieval. It has been postulated that the amygdala contributes to emotional memory through noradrenergic activation (Cahill et al, 1994; McGaugh, 2004; Strange and Dolan, 2004). While this at first sight could account for the significantly larger recognition effect under duloxetine during retrieval, it has also been suggested that the effect of noradrenergic activation is more likely to affect memory formation than retrieval. A recent study by McCabe et al (2010) directly compared the effect of an SSRI and an NRI on the neural correlates of reward processing and found that the SSRI had reduced activation in the ventral striatum and the ventral medial/orbitofrontal cortex, whereas the NRI increased neural responses within the medial orbitofrontal cortex to reward. Finally, on the one hand, Harmer et al (2009) in a recent review suggested that at least in healthy volunteers a common overlapping mechanism may account for the effects of conventional antidepressant drugs with different neurochemical actions on emotional processing. In light of these aforementioned findings, it is certainly relevant to disentangle the specific effects of noradrenergic and serotonergic treatment on remediating mood-congruent memory bias. On the other hand, knowledge of common antidepressant effects on cognitive biases is important when attuning the combination of antidepressant and cognitive behavioral therapy.
A couple of limitations have to be taken into account. First, we chose to investigate the effect of duloxetine on mood-congruent and mood-incongruent memory processes with the largest contrast possible (negatively vs positively valenced stimuli). Thus, we did not include neutral stimuli to keep an efficient design. However, that decision precludes the conclusion that duloxetine specifically affects emotional memory. Although the sample size appears quite optimal for a within-subject design and in balance with the sample sizes of other fMRI studies with similar questions (Norbury et al, 2007; McCabe et al, 2010; Murphy et al, 2009), we cannot rule out the fact that the number of subjects included in the present study prohibited us from finding higher-order interactions such as a triple interaction with the factors drug, memory, and valence not only during retrieval but also during encoding. Given that no previous fMRI study has combined pharmacological challenge with such an extensive emotional memory setup, further studies certainly are needed to support our conclusion also with respect to negative findings namely, that there is a more general effect of duloxetine during encoding but not retrieval. For now we can only compare our results with a previous study of our own group (Urner et al, 2011) that used the same experimental setup except for the fact that that study investigated the effect of a genetic variation instead of a drug on the neural correlates of emotional memory. In that study also, no interaction with both memory and valence was found during encoding, but during retrieval. Regardless, the present results might not generalize to a euthymic or positive mood, but the experimental procedure was set up as mimicking acutely reduced mood as it may occur during depression. Finally, although each subject showed an increased duloxetine level, we observed a relative large variation in the plasma duloxetine levels. Higher plasma levels could be caused by late drug intake so that the intake–test delay was shortened. Also, some of the subjects might have been poor metabolizers (Cyp1A2 en Cyp2D6). In turn, lower than expected plasma levels were observed rarely in the present sample. They might have been the consequence of a longer intake–test delay or rapid metabolization.
Taken together, our data show that the antidepressant duloxetine has specific effects on memory formation and retrieval when subjects are sad. Whereas during emotional memory formation it seems to downregulate more globally a network involved in affect regulation, during retrieval it specifically acts upon a brain region known to modulate emotional memory in a valence-specific manner. Although, of course, this finding has to be replicated with other antidepressants, we suggest that these effects can be seen as final common pathways of antidepressants reversing mood-congruent processing biases.
Acknowledgments
This work was supported in part by a grant from Eli Lilly Nederland BV (who also provided the study medication) to Dr Tendolkar, Professor Fernández, and Dr Verkes. We thank Sabine Kooijman for assistance in recruiting and monitoring the participants.
The authors declare that over the past three years RJV has served on the advisory boards of Eli Lilly, Servier, and Organon, and has received grants from Astra Zeneca, Eli Lilly, Pfizer, Wyeth, and Organon, and honoraria and/or speaker's fees from Astra Zeneca, Eli Lilly, Lundbeck, Wyeth, and Organon. All other authors report no biomedical financial interests or potential conflict of interest.
References
- Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: human declarative memory formation at the system level. Brain Res Bull. 2001;55:1–9. doi: 10.1016/s0361-9230(01)00494-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Arnold JF, Becker ES, Speckens AE, Rinck M, Rijpkema M, et al. How mood challenges emotional memory formation: an fMRI investigation. Neuroimage. 2011;56:1783–1790. doi: 10.1016/j.neuroimage.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Frampton JE, Plosker GL. Duloxetine: a review of its use in the treatment of major depressive disorder. CNS Drugs. 2007;21:581–609. doi: 10.2165/00023210-200721070-00004. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Nihalani N, Masand P. Duloxetine: review of its pharmacology, and therapeutic use in depression and other psychiatric disorders. Ann Clin Psychiatry. 2007;19:125–132. doi: 10.1080/10401230701333319. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195:102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Heinzen J, O'Sullivan U, Ayres RA, Cowen PJ. Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology (Berl) 2008;199:495–502. doi: 10.1007/s00213-007-1058-7. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive vs negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, et al. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology. 2004;29:566–579. doi: 10.1038/sj.npp.1300341. [DOI] [PubMed] [Google Scholar]
- Kernis M, Greenier K, Herlocker C, Whisenhunt C, Abend T. Self-perceptions of reactions to doing well or poorly: the roles of stability and level of self-esteem. Person Individ Diff. 1997;22:845–854. [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 2005;25:1214–1223. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Lopez-Solá M, Pujol J, Hernandez-Ribas R, Harrison BJ, Contreras-Rodriguez O, Soriano-Mas C, et al. Effects of duloxetine treatment on brain response to painful stimulation in major depressive disorder. Neuropsychopharmacology. 2010;35:2305–2317. doi: 10.1038/npp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research. Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010;67:439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol Psychiatry. 2007;13:1011–1020. doi: 10.1038/sj.mp.4002091. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol Psychiatry. 2008;13:1011–1020. doi: 10.1038/sj.mp.4002091. [DOI] [PubMed] [Google Scholar]
- Opbroek A, Delgado PL, Laukes C, McGahuey C, Katsanis J, Moreno FA, et al. Emotional blunting associated with SSRI-induced sexual dysfunction. Do SSRIs inhibit emotional responses. Int J Neuropsychopharmacol. 2002;5:147–151. doi: 10.1017/S1461145702002870. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase. 2002;8:345–354. doi: 10.1076/neur.8.4.345.16186. [DOI] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195:211–217. doi: 10.1192/bjp.bp.108.051110. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. 1998The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 J Clin Psychiatry 59(Suppl 2022–33.quiz 34–57. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD. Negative thinking in depression: cause, effect, or reciprocal relationship. Advances Beh Res Ther. 1983;5:3–25. [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Brockhaus-Dumke A, van Eijndhoven P, et al. Contributions of the medial temporal lobe to declarative memory retrieval: manipulating the amount of contextual retrieval. Learn Mem. 2008;15:611–617. doi: 10.1101/lm.916708. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urner M, van Wingen G, Franke B, Rijpkema M, Fernández G, Tendolkar I.2011Genetic variation of the α2b-adrenoceptor affects neural correlates of successful emotional memory formation Hum Brain Mappe-pub ahead of print 21 January 2011. [DOI] [PMC free article] [PubMed]
- Van der Does AJ.2002BDI-II-NL. De Nederlandse versie van de Beck Depression Inventory2nd edn.Zwets en Zeitlinger: Lisse [Google Scholar]
- van der Ploeg HM, Defares PB, Spielberger CD. Handleiding bij de Zelf-Beoordelings Vragenlijst, ZBV: Een Nederlandse vertaling van de Spielberger State-Trait Anxiety Inventory. Swets & Zeitlinger: Lisse; 1980. [Google Scholar]
- van Wingen GA, van Eijndhoven P, Cremers HR, Tendolkar I, Verkes RJ, Buitelaar JK, et al. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. J Psychiatr Res. 2010;44:527–534. doi: 10.1016/j.jpsychires.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Wald FDM, Mellenbergh GJ. De verkorte versie van de Nederlandse vertaling van de Profile of Moods State (POMS). [The short Dutch version of the Profile of Moods State (POMS) questionnaire] Nederlands Tijdschrift voor de Psychologie. 1990;45:86–90. [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, Ruhlmann J, et al. Process dissociation between contextual retrieval and item recognition. Neuroreport. 2004;15:2729–2733. [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]