Abstract
We report the first demonstration of Comprehensive Two-dimensional Gas Chromatography Combustion Isotope Ratio Mass Spectrometry (GC×GCC-IRMS) for the analysis of urinary steroids to detect illicit synthetic testosterone use, of interest in sport doping. GC coupled to IRMS (GCC-IRMS) is currently used to measure the carbon isotope ratios (CIR, δ13C) of urinary steroids in anti-doping efforts; however, extensive cleanup of urine extracts is required prior to analysis to enable baseline separation of target steroids. With its greater separation capabilities, GC×GC has the potential to reduce sample preparation requirements and enable CIR analysis of minimally processed urine extracts. Challenges addressed include on-line reactors with minimized dimensions to retain narrow peaks shapes, baseline separation of peaks in some cases, and reconstruction of isotopic information from sliced steroid chromatographic peaks. Difficulties remaining include long-term robustness of on-line reactors and urine matrix effects that preclude baseline separation and isotopic analysis of low concentration and trace components. In this work, steroids were extracted, acetylated, and analyzed using a refined, home-built GC×GCC-IRMS system. 11-hydroxy-androsterone (11OHA) and 11-ketoetiocolanolone (11KE) were chosen as endogenous reference compounds (ERC) because of their satisfactory signal intensity, and their CIR was compared to target compounds (TC) androsterone (A) and etiocholanolone (E). Separately, a GC×GC-qMS system was used to measure testosterone (T)/EpiT concentration ratios. Urinary extracts of urine pooled from professional athletes, and urine from one individual that received testosterone gel (T-gel) and one individual that received testosterone injections (T-shot) were analyzed. The average precisions of δ13C and Δδ13C measurements were SD(δ13C) approximately ± 1‰ (n=11). The T-shot sample resulted in a positive for T use with a T/EpiT ratio of > 9 and CIR measurements of Δδ13C > 5‰, both fulfilling World Anti-Doping Agency criteria. These data show for the first time that synthetic steroid use is detectable by GC×GCC-IRMS without need for extensive urine cleanup.
INTRODUCTION
The detection of synthetic steroid use in sport drug testing1 has been an increasingly visible issue in competitive sports2, such as in professional sports in the U.S. and internationally in cycling events like the Tour de France3. The use of unknown exogenous steroids which mimic the anabolic effects of testosterone (T), including prescription drugs and designer steroids, can initially evade detection. However, once their existence and structures are known, straightforward analytical tests can be developed which are generally based on gas chromatography mass spectrometry (GC/MS). An example of this is the case where syringes were sent to authorities containing tetrahydrogestrinone (THG), a designer steroid known as the “clear” and synthesized by the Bay Area Lab Co-Operative (BALCO) and used by various professional athletes4. In contrast, the use of pharmaceutical preparations of endogenous steroids, which are normally in the human body, is difficult to detect based on steroid concentrations alone due to their large natural range within and between individuals. In 1983, Donike et. al.5 developed a test based on the concentration ratio of T to epitestosterone (EpiT), a non-active epimer of T, where a T/EpiT of > 4 is indicative of T use6. Unfortunately, EpiT is now often used as a masking agent along with T by athletes in order to evade this test and retain the natural T/EpiT of < 4.
The natural isotopic variability of light elements, such as C, N, O, S, and H, indicate the geographic, chemical, and biological origins of substances and is of interest in a large cross-section of scientific disciplines7, 8, such as source identification9 in ecology10, food authentication11, archaeology12, geochemistry13, environmental contaminants14, air pollution15, forensic sciences16, and as non-radioactive tracers in biomedical applications17. High precision gas isotope ratio mass spectrometry (IRMS) is used for the measurement of small differences in the stable isotopic abundances as a ratio in the sample relative to that in a traceable standard. For carbon isotope ratio (CIR) measurements, 13C/12C of sample is measured and values are reported in δ notation with respect to an international standard, expressed in units of parts per thousand (‰) according to the equation as follows
where RSPL is the CIR of the sample and RVPDB is the CIR of the international standard Vienna PeeDee Belemnite (RVPDB=0.011180)18, 19. For carbon isotopic analysis, organic molecules are combusted to CO2 gas, where the m/z 44, 45, and 46 CO2 isotopologue signals are used to calculate the 13C/12C in the parent molecule.
The total 13C/12C composition of molecules in most organisms reflects proportions of C3- and C4-plants in their diet and humans consume a varying mixture of C3- and C4-plants in most parts of the world. Due to their different photosynthetic pathways, C3-plants produce organic compounds with systematically more negative δ13C values compared to C4-plants. Since synthetic steroids are manufactured using phytosterols from only C3.plants, such as yams and soy, their use causes significant shifts in δ13C towards more negative values. Such shifts are observed for the administered steroid and steroids downstream of their metabolic pathway. In contrast, steroids upstream in the pathway of the administered substance, or steroids of other biosynthetic pathways, are not affected. These unaffected steroids can be used as endogenous reference compounds (ERC) against which the CIR of the target compound (TC) steroids can be compared20–24. The World Anti-Doping Agency (WADA) prohibits endogenous anabolic androgenic steroids (AAS) in competition25 and the criterion indicating synthetic steroid use is measured by the CIR difference between an ERC and TC steroids,
where Δδ13C > 3‰ (plus specific method error) indicates synthetic steroid use and potentially, with additional data, an adverse analytical finding26.
The integration of GC with IRMS via an online reactor interface, GC-combustion IRMS (GCC-IRMS), enabled compound-specific isotope analysis (CSIA)27. On-line systems have since been developed for 2H/1H28,29, 15N/14N30, and 18O/16O31. Position specific isotopic analysis (PSIA) of carbon isotopes has been demonstrated for the carboxyl carbon in low molecular weight organic acids by GC-pyrolysis-IRMS32, and for multiple carbon positions in individual compounds from complex mixtures by GC-py-GCC-IRMS, where separated compounds are decomposed at high temperatures by pyrolysis and the products are separated and chemically converted in a second reactor33. Recently, we demonstrated the coupling of IRMS to advanced GC separation techniques, specifically fast-GCC-IRMS34 and subsequently proof of principle for comprehensive 2D GC (GC×GCC-IRMS)35, both of which require the preservation of very narrow peak shapes where the GC and interface plumbing, especially the on-line reactor, have to be designed and implemented with minimal dead volumes and smaller i.d. tubing (i.e. 100 µm). When implementing front end online separations, IRMS requires complete separation of compounds; otherwise, measurements can result in large errors in accuracy, but not necessarily reductions in precision36.
GC×GC is a technique that employs two columns with orthogonal properties operating in tandem, where cryogenic slices collected from a first column (GC1) in few second intervals (2–8 sec), or modulation periods, are quickly released and separated rapidly within the modulation period on a second fast column (GC2)37, 38. GC×GC coupled to molecular MS systems, such as quadrupole MS and time-of-flight-MS (TOF-MS) benefit from having a third degree of separation is the unique m/z spectra of different compounds. In contrast, IRMS measures only CO2 gas resulting from the on-line combustion of the analytes, much like an FID detector, and cannot differentiate one molecule from another apart from retention times. Moreover, for GC×GC coupled to molecular MS, mass spectra of the GC2 separated peaks within a given GC1 slice are all structurally meaningful. In contrast, isotope ratios of GC2 separated peaks are not meaningful in and of themselves because of chromatographic separation of isotopologues in GC1. Summing up signal due to all isotopes from all slices is required to produce accurate isotope ratios and is feasible at high precision.35 Finally, although many studies using GC×GC show separations of 1000’s of compounds in a single chromatogram, the 2D contour plots can be represented so as to deemphasize small valleys in between peaks and thus may not reflect true baseline separation of the components in those complex mixtures. However, such baseline separation is crucial for CSIA. Consequently, stable isotope analysis with GC×GC separation of complex mixtures presents unique challenges compared to GC×GC coupled to molecular MS.
In anti-doping testing, GCC-IRMS requires extensive cleanup, involving numerous high performance liquid chromatography (HPLC) purification steps, of urine extracts prior to analysis to enable baseline separation of target steroids. GC×GC, with expanded separation capabilities, has the potential to minimize sample preparation requirements. Here we present an evaluation of a refined version of the first GC×GCC-IRMS system, a home-built system presented previously35, coupled to a refined urinary steroid acetate separation system. We demonstrate a potential approach towards CIR measurements of urinary steroids in minimally processed urine extract samples using the integrated steroid GC×GCC-IRMS system.
EXPERIMENTAL SECTION
Chemicals
N2, high purity He, high purity CO2 gas, and siphon type liquid CO2 were acquired from Airgas East (Salem, NH). 5α-androstan-3β-ol (5α-androstanol), 5β-estran-3α-ol-17-one (19-Noretiocholanolone, 19-NE), 5β-androstan-3α-ol-17-one (etiocholanolone, E), 5α-androstan-3α-ol-17-one (androsterone, A), 5-androsten-3β-ol-17-one (dehydroepiandrosterone, DHEA), 5β-androstan-3α-ol-11, 17-dione (11-ketoetiocholanolone, 11-KE), 5α-androstan-17β-ol -3-one (dihydrotestosterone, DHT), 5β-androstan-3α, 17β-diol (5βA), 5α-androstan-3α, 17β-diol (5αA), 4-androsten-17α-ol-3-one (epitestosterone, EpiT), 4-androsten-17β-ol-3-one (testosterone, T), 5α-androstan-3α, 11β-diol-17-one (11β-hydroxyandrosterone, 11OHA), 5α-androstane-3β-ol-17-one (epiandrosterone, EpiA) and 5α-cholestane (Cne) were acquired from Steraloids (Newport, RI). 5β-pregnane-3α, 20α-diol (5β-pregnanediol, 5βP) was acquired from Acros Organics (Morris Plains, NJ). HPLC grade 2-propanol and methanol were obtained from Mallinckrodt Baker (Phillipsburg, NJ), while analytical grade pyridine, acetic anhydride, tert-butylmethylether (TBME), β-glucuronidase from Escherichia coli, sodium phosphate buffer (0.2M, PH=7), and potassium carbonate buffer (K2CO3/KHCO3 1:1, w/w, 200 g/L) were obtained from Sigma-Aldrich (St. Louis, MO).
Urine Sample Collection
Urine A, representing a non-doped sample, was a pooled sample of discarded urine from an indeterminate number of male athletes. Urine B was collected from an individual male who received daily replacement therapy over 18 months as topical testosterone hydroalcoholic gel (Androgel, Abbott Laboratories) designated “T-gel”. Urine C was collected from a male who received replacement therapy over 18 months as intramuscular testosterone cypionate in oil, 200 mg every 3 weeks with the last injection 11 days prior to collection, designated “T-shot”. Urines B and C were collected as a random specimen in the morning, and both participants had serum T concentrations in the normal range for adult males at the time of urine collection. Human ethics approval was obtained from Institutional Review Boards at Cornell University and the University of Texas Southwestern Medical Center, Dallas for these samples, and participants gave written informed consent for the research study.
Urine Sample Preparation
Sample preparation procedures were adopted and slightly modified from others23. Briefly, the steroid glucuronides, in an aliquot of 20 mL of urine for each sample spiked with 100µL of 20 ng/µL EpiA Glucuronide as an internal standard, were extracted using Chromabond® C18 solid phase extraction columns (500mg, 6mL). Each sample was applied to a column, washed with 2 mL of water, eluted with 2 mL of MeOH, and evaporated to dryness under nitrogen. The dried eluate was shaken with 1 mL of sodium phosphate buffer (0.2 M, PH=7) and 5 mL of TBME, centrifuged at 1200 g for 5 min, followed by removal of the organic layer. In order to hydrolyze the steroid glucuronides, 100 µL of β-glucuronidase (50% glycerol solution, 2.3 mg protein/mL Biuret, 3.3×107 units/g protein) was added to the aqueous layer which was incubated at 50°C for 1 hour. A 500 µL aliquot of potassium carbonate buffer (K2CO3/KHCO3 1:1, w/w, 200 g/L) was added to the solution when cooled to room temperature. The solution was then extracted twice with 5 mL of TBME, where each time the mixture was shaken for 5 min and centrifuged as above, followed by collection of the organic layer. The pooled organic layers were evaporated to dryness. The residue was acetylated by adding 100 µL pyridine and 100 µL acetic anhydride and heating at 60°C for 1 hour, followed by evaporation. The derivatized residue was redissolved in 100 µL of 20 ng/µL Cne in 2-propanol. No HPLC cleanup was performed on the steroid extract samples. For GC×GCC-IRMS, 0.2 to 3 µL of the urinary extract was injected. For GC×GC-qMS, the urinary extract was diluted by a factor of 10 and 1 µL was injected.
GC×GCC-IRMS system
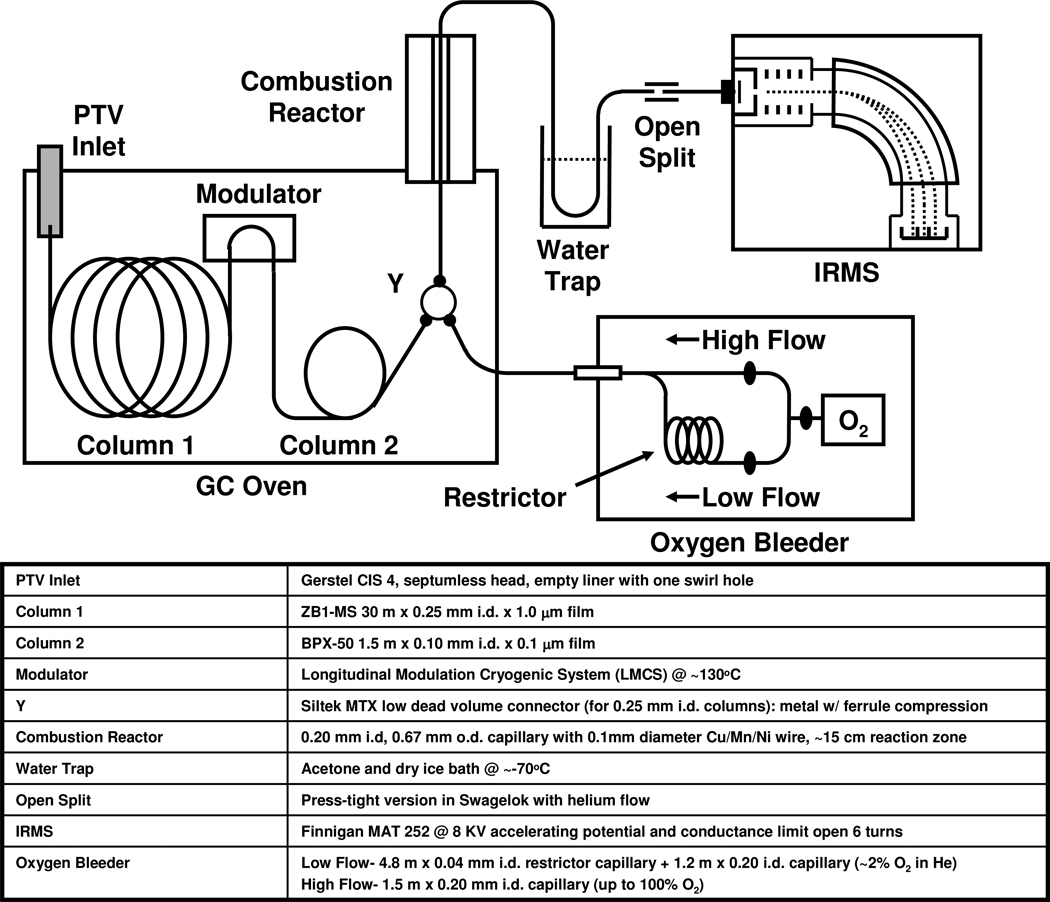
The schematic of the GC×GCC-IRMS system used in this work is depicted in Figure 1. An HP6890A GC (Agilent Technologies, Menlo Park, CA), outfitted with a longitudinally modulated cryogenic system (LMCS, Chromatography Concepts, Sandringham, Australia)39 was interfaced to a MAT 252 IRMS (Thermo Finnigan, Bremen, Germany) with a home built combustion interface. A programmable temperature vaporization (PTV) inlet (CIS 4, Gerstel US, Linthicum, MD) was used in solvent vent mode in order to divert solvent away from the GC columns and was held at 50°C for 1 min and then ramped to 300°C at 600°C/min at the beginning of the GC×GCC-IRMS runs. The PTV parameters were previously optimized for steroid acetates35, where the vent time was 0.9 min, vent pressure was 5 psi, vent flow was 100 ml/min, and purge time was 2.5 min. A 0.3 m × 0.1 mm i.d. deactivated fused silica capillary was used in the modulator to bridge GC column 1 (GC1) and GC column 2 (GC2), both held in the same GC oven. GC1 was a 30 m × 0.25 mm i.d. × 1.0 µm film ZB1-MS (100 % Dimethylpolysiloxane, Phenomenex, Torrance, California) and GC column 2 was a 1.5m × 0.1mm i.d. × 0.1µm film BPX-50 (50% Phenyl Polysilphenylene-siloxane 50% dimethyl polysiloxane, SGE, Austin, TX). The head pressure was set to 42 psi @ 70°C with a flow rate of ~1 ml/min measured at the open split and run in constant flow mode. The GC oven was initially held at 50°C for 3 min and then ramped at 40°C/min to 300°C, where it was held for 35 min. Modulation was started at 10 minutes at a temperature of 130°C and period of 6 s.
Figure 1.
Schematic and component details of the GC×GCC-IRMS system constructed and used in this work.
Although our laboratory has recently been developing micro-fabricated microreactors for increased system robustness40, currently unresolved issues with connections at input/output ports at elevated temperatures (>300°C) prompted the use of a continuous capillary combustion reactor (CCR) in this work for on-line conversion of sample effluent to CO2. Although fragile, a CCR design was successfully implemented in previous work to demonstrate the feasibility of GC×GCC-IRMS35, where a GC fused silica (FS) capillary carefully filled with a metal reactant wire, is mechanically supported in a ceramic tube with metal fittings (Valco Instruments Inc., Houston, TX) on both ends. The GC FS capillary becomes very fragile in the region where it is at 950°C, because the protective polyimide coating that gives it flexibility burns off at temperatures >400°C and is susceptible to fracturing due to vibrations, temperature fluctuations, and flexing. In a potentially more robust CCR design used in this work, we use specially obtained thicker walled and narrower i.d. GC FS capillary (0.670 mm o.d. × 0.200 mm i.d. × 45 cm, Polymicro Technologies, Phoenix AZ) than used in previous work (0.360 mm o.d. × 0.250 mm i.d. × 45 cm). In addition, the support used is a thicker ceramic tube (3.17 mm o.d. × 0.80 mm i.d. × 30.5 cm, Vesuvius, Pittsburgh, PA) than previously used (1.5 mm o.d. × 0.60 mm i.d. × 30.5 cm), making the device much more rigid. The CCR, containing one 15 cm × 0.10 mm diameter Cu/Mn/Ni (84/12/4%) wire (Alfa Aesar, Ward Hill, MA), was held at 940°C using an open tubular glass fiber furnace (Thermcraft Inc., Winston-Salem NC) containing approximately 0.15 m length of a radiatively heated region. The Cu/Mn/Ni wire was initially oxidized using pure oxygen at 600°C flowing overnight and oxidized at 940°C overnight before each use of the reactor, which was operated at 940°C during analyses. In order to prevent effluent peak tailing due to metal and metal oxides acting as significant active sites at temperatures below 850°C, the upstream end of the wire was set in the capillary so that it was well within the furnace hot zone. In addition, an oxygen bleeder was used to add ~2% O2 to He carrier before CCR to improve combustion longevity. It was made up of a low flow line (4.8 m × 0.04 mm i.d. restrictor capillary + 1.2 m × 0.20 i.d. capillary) for use during GC×GCC-IRMS runs for ~2% O2 in He and a high flow line (1.5 m × 0.20 mm i.d. capillary) for use during CCR oxidation recharging for up to 100% O2. This design seems to be less susceptible to cracking or breakage of the capillary at elevated temperatures and still provides sufficiently low dead volume connections and characteristics required for the fast GC2 peak widths and shapes resulting in ~230ms and ~350ms FWHM wide peak slices, without and with the oxygen bleeder tee in-line.
A 1.5 m × 0.10 mm i.d. deactivated fused silica capillary was used as a transfer capillary between the combustion reactor and the open split. Water vapor generated from the combustion process was trapped before the IRMS by immersing 10 cm of the transfer capillary into a dry ice and acetone bath at −78°C. Press-tight fittings (Restek, Bellafonte PA) were used for all connections, except for the oxygen bleeder Y. A fraction of the dry effluent was sampled into the IRMS by placement of the upstream end of 0.5 m × 0.190 mm × 0.075 mm i.d. IRMS sampling capillary flush with the end of the transfer capillary within a press-tight that resided in a tee fitting (Swagelok, Solon OH) swept with He gas, thereby operated as an open split. The IRMS was operated at an accelerating potential of 7.9 keV and the conductance limit was open by 6 turns in order to help with measurement linearity and minimize any peak broadening in the ion source. Absolute sensitivity of the IRMS was measured to be approximately one ion per 3000 molecules of CO2.
IRMS Data Acquisition and Analysis
A home-built LabView (National Instruments, Austin, TX) based IRMS data acquisition and home-written data reduction system (Saxicab)41, was used to monitor the m/z 44, 45, and 46 signals, for 44CO2, 45CO2, 46CO2, from the MAT 252 head amplifiers using three 24-bit National Instruments NI4351 digitizers (Austin, TX), where data was saved at an acquisition rate of 25 Hz. The standard RC time constants of the faraday cup detectors were reduced and matched by exchanging appropriate resistors (Thermo Finnigan, Bremen, Germany) and capacitors (Just Radios, Scarborough, Ontario, Canada) in order to prevent broadening of fast GC2 peaks where all three m/z arrangements were changed to R (3e8 Ω) × C (100 pF) = τ (30 ms) for m/z 44, R (3e10 Ω) × C (1 pF) = τ (30 ms) for m/z 45, and R (3e10 Ω) × C (1 pF) = τ (30 ms) for m/z 4635. The time constants are estimated and were not measured directly.
Isotope ratios were calculated in SAXICAB using the summation method with an individual background definition and a minor modification to the conventional time correction due to fast GC2 peaks, detailed previously34. The contribution of 17O to the 45CO2 signal was taken into account by the method of Santrock et al.42 The steroid isotopic standard CU/USADA 33-1, containing A–AC, 11KE–AC, and Cne with isotope ratios traceable to the international standard VPDB, was previously created in our laboratory as a steroid standard now used by many anti-doping laboratories for IRMS calibration. CU/USADA 33-1 was used here to calibrate the apparent isotope ratio of CO2 gas pulses, which in turn were used to calculate the δ13CVPDB of modulated peaks in GC×GCC-IRMS analyses, as described in detail elsewhere43. Three CO2 gas pulses were admitted from a static volume near the beginning of each run. This procedure takes into account any minor isotopic fractionation due to the difference in flow paths traversed by the CO2 calibrant gas and the steroids. Reconstruction of modulated peaks for the calculation of their δ13CVPDB was performed in Saxicab, where the peak slices for the m/z 44, 45, and 46 CO2 isotope traces were detected and integrated automatically, in a few cases manually, followed by calculation of δ13CVPDB for each individual peak slice. The peak slices belonging to a single analyte were then visually assigned (and verified by the retention times determined using a standard of the target steroids), and a weighted average of the δ13CVPDB of all the slices, based on their areas, was calculated. (The errors associated with this calculation are well within the precision of the measurement.) Visual representations of the isotopic data were constructed in GC Image software (Lincoln, NE).
GC×GC-qMS system
GC×GC chromatography for a set of target steroids was optimized and the concentrations of steroids in the urine extracts (i.e. T/EpiT) were measured using a Shimadzu GC×GC-qMSsystem, comprised of a QP-2010 Ultra GC/MS with a Zoex ZX2 modulator system. The samples were injected splitless using an AOC-20i autoinjector into a split/splitless inlet set at 300°C. The same GC1 and GC2 column set, GC temperature program, and modulation period (6 s) was used as in the GC×GCC-IRMS system. A double focusing loop modulator, consisting of a coiled 1.5 m × 0.10 mm i.d. capillary, was operated with a continuous cold jet at a temperature of 130°C, with the chiller off, and a 325 ms pulse hot jet at a temperature of 350°C. The MS was operated using positive chemical ionization using NH3 as the reagent gas.
RESULTS AND DISCUSSION
In this work, urine samples were collected, extracted, and component steroids acetylated (AC) as described above. Using the test-bed GC×GCC-IRMS, the CIR of metabolites of testosterone, E and A, were measured relative to that of an ERC, 11OHA and 11KE, in the urinary extracts. In addition, neat standard samples of E, A, and 11OHA were analyzed native and acetylated for their CIR using the GC×GCC-IRMS without modulation, in order to perform a correction for the change in CIR of the steroids (δ13Cdcorr) in the urine extracts due to the extraneous carbon added during acetylation according to the equation:
where n=number of moles of carbon, c=compound of interest, d=derivative group, and cd=derivatized compound23, 24. The effective isotope ratio of the acetate carbon δ13Cdcorr averaged −42.73‰ in E–AC and A–AC, which was used to correct values for E, A, and 11KE that are derivatized to mono-acetates, and a δ13Cdcorr of −39.08‰ in 11OHA–AC, which was used to correct the values for 11OHA, a diol (with a known kinetic effect).
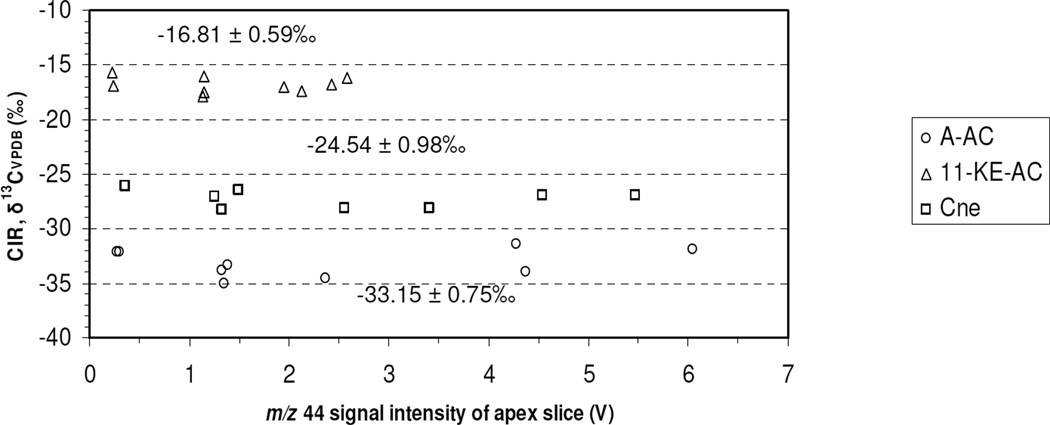
Generally, there is a large concentration difference of steroids in urine extracts, ranging from approximately 2000 ng/mL urine for E and A, to approximately 20 ng/mL urine for T and EpiT23, 44. Therefore, in order to measure a variety of target steroids in the same GC×GCC-IRMS run, the dependence of measured isotope ratio on the amount of steroid that enters the GC×GCC-IRMS system should ideally be linear, or have no dependence over the concentration range of interest. Approximately 4–80 ng each of A–AC, 11-KE–AC, and Cne in an isotopic standard, CU/USADA 33-143, was injected on column using the same analytical parameters as for the urinary steroid extract analyses by GC×GCC-IRMS with a 6s modulation period. The m/z 44 signal intensity range bracketed the signal levels measured for the steroids in the urine extract samples and approached the maximum measureable or saturation signal level by the MAT 252 IRMS, which is 10V for m/z 44, 45 and 46). The results, shown in Figure 2, reveal no obvious dependence within the reproducibility of the technique, average SD(δ13C) < ± 1‰, therefore no corrections for non-linearity based on signal intensity was performed on the data presented here. This is the first time a linearity study was performed for IRMS using GC×GCC as a front end peripheral. Generally, the m/z signal levels from the urine extract injections for E–AC and A–AC fell within the upper portion and for 11OHA–AC and 11KE–AC within the lower portion of the linearity plot.
Figure 2.
Linearity of δ13C measurements by GC×GCC-IRMS for the steroids A–AC, 11-KE–AC, and Cne contained in the isotopic standard CU/USADA 33-1 over a large mass range (4–80 ng) injected on column (n=9). The mean ± standard deviation for each steroid is also reported. (One outlier was removed).
An acetylated steroid standard mixture (SM14-AC) containing 10ng of each of the 14 steroids from those presented above, except for EpiA-AC, was analyzed and used to determine the retention times of potential target steroids during the GC×GCC-IRMS analyses. For the urine samples analyzed in this study, the best candidates for CIR measurements were found to be E–AC and A–AC as TC steroids, plus 11OHA–AC and 11KE–AC as ERC steroids, due to their signal levels falling within the linear range and favorable chromatography. While maintaining the m/z 44, 45, and 46 signal levels of E–AC and A–AC below saturation levels in the MAT 252 IRMS (<10V), signals of other steroids of anti-doping interest, such as T, 5βA, 5αA, and 5βP, were either too low in concentration or were affected by co-elutions to be useful for reliable CIR. Injecting greater amounts of extract into our GC×GCC-IRMS test-bed system to bring the analytes into useful signal ranges resulted in unfavorable chromatography due to GC×GC column set overloading effects of higher concentration components, which in turn affected lower concentration components downstream in the chromatogram, as well as unexpected urine matrix effects. Some of these effects may also be due to instrumental factors and are yet to be explored. In contrast to the routine use of selected ion monitoring to resolve overlaps when GC×GC is coupled to molecular MS, IRMS requires baseline resolution of components. This effectively limits the dynamic range of GC×GC coupled to IRMS. Isotopic analysis of trace components, such as T, in the presence of components more abundant by orders of magnitude by GC×GCC-IRMS has not been fully explored and addressed here and must be investigated in future work.
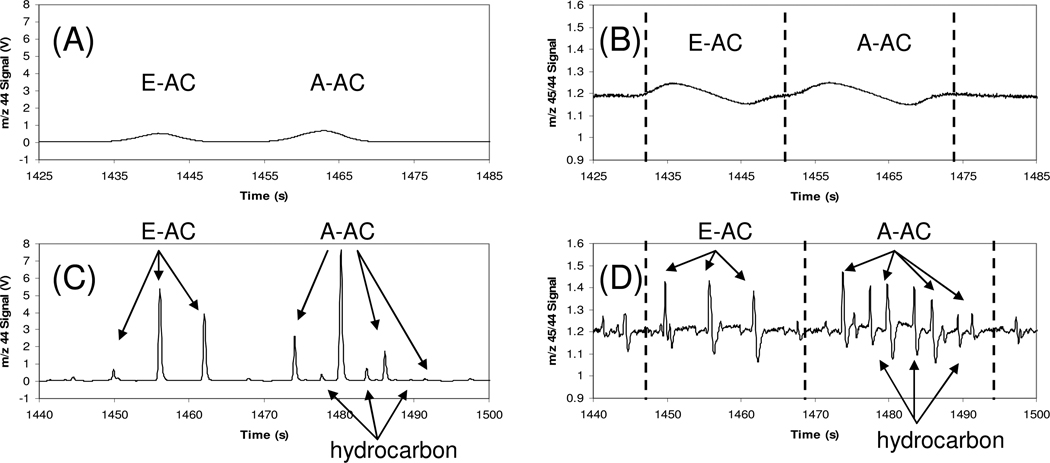
Due to the differences in urine specific gravities, various volumes (0.4 to 1.6 µL) of the urine extracts (20 mL urine in 100µL extract) were injected for GC×GCC-IRMS analyses, where the mass injected on column ranged approximately from <10 ng to 100 ng, depending on analyte, as roughly estimated based on the IRMS m/z 44 signals. The m/z 44 traces of the E–AC and A–AC region of the non-modulated and 6 sec modulated chromatograms of the professional athletes’ pooled Urine A sample are presented in Figure 3A and 3C, respectively. A benefit of using GC×GC as a front end is illustrated by the resolution of a contaminating hydrocarbon (identified by GC×GC-qMS), which co-elutes with A–AC in the first dimension (GC1), but is separated in the second dimension (GC2). The CIR of this co-eluter in GC1 was measured to be δ13C = −35.44 ± 0.68‰ (standard deviation where n=4), which would alter the CIR of A by −1.32‰, to δ13C = −21.25 ± 0.42‰ (n=4), if measured along with the co-eluting peak. For the specific purpose of anti-doping analysis this result would not change the ultimate test result, the co-elution nevertheless is detrimental to the measured CIR accuracy.
Figure 3.
The m/z 44 trace GC×GCC-IRMS chromatogram in the E–AC and A–AC region of the Urine A extract (from athletes’ pooled urine) with a (A) no modulation and (C) 6s modulation. The m/z 45/44 ratio trace of the same chromatogram with (B) no modulation and (D) 6s modulation.
The m/z 45/44 isotope ratio traces of the same region of the chromatograms are presented in Figure 3B and 3D. The rise and fall of the isotope ratio over the length of the GC1 peak (Figure 3B) and each peak slice (Figure 3D) due to modulation shows the known isotope fractionation where each peak and peak slice is enriched in 13C at the front and depleted at the tail. Moreover, this trend is seen among the peak slices associated with a single component, where the amplitude of the swing is greater in the positive direction in the first slice and greater in the negative direction in the last slice. Figure 4 depicts the reconstructed m/z 44 and m/z 45/44 isotope ratio 2D contour and 3D plots. The above side plane and under side plane of the isotope ratio 3D plot (Figure 4D and 4E) are shown to aid in visualization of enrichment and depletion peaks. The large dynamic range of components seen in the 3D plot of absolute m/z 44 signal is visually flattened in the plot of the m/z 45/44 ratio trace, as also observed in Figure 3D, and highlights potential interferences for isotope ratio measurement by low level components not readily observed by the m/z 44 signal intensity alone.
Figure 4.
The (A) m/z 44 and (B) m/z 45/44 ratio trace 2D contour chromatograms of the GC×GCC-IRMS of Urine A (from athletes’ pooled urine) with a 6s modulation rate. The (C) m/z 44 and (D, E) m/z 45/44 ratio trace 3D chromatograms of the GC×GCC-IRMS of Urine A (from athletes’ pooled urine) with a 6s modulation rate.
Table 1 lists the results of the analysis of the urine extract samples. The T/EpiT concentration ratio for each sample was measured using GC×GC-qMS and is generally indicative of the amount of synthetic testosterone used by and remaining in the two individuals dosed with the T-gel and T-shot treatments. The T-shot, Urine C, had the largest T/EpiT (> 9) and the athletes’ pooled urine, Urine A, had the lowest T/EpiT (< 2). According to WADA guidelines26, the A/E concentration ratio can also be indicative of T use, where a ratio > 4 is suspicious; here it was > 4 for the T-shot sample (Urine C). The δ13C values for target steroids E and A, and ERC’s 11OHA and 11KE were measured using GC×GC-C-IRMS and corrected for the contributions due to acetylation. The average precision for SD(δ13C) was ± 0.97‰ and SD(Δδ13C) was ± 1.07‰. According to the guidelines set by WADA26, a Δδ13C > 3‰ (plus the error associated with the measurement) is indicative of synthetic steroid use. Taking into account the largest 95% confidence interval for the measured Δδ13C in this work, 1.56‰, we used a cutoff of Δδ13C > 4.6‰ to indicate synthetic T use. The Urine A, the athletes’ pooled urine, sample was analyzed as a control and its results are indicative of a normal, non-doped individual. The Urine B, T-gel, sample resulted in a suspicious T/EpiT concentration ratio (> 4); however, the Δδ13C values did not indicate an adverse analytical finding. On the other hand, the Urine C, T-shot, sample resulted in elevated T/EpiT (> 9) and A/E (> 4) concentration ratios and Δδ13C of 11OHA–E > 7‰, 11OHA–A > 5‰, and 11KE–E > 5‰, all indicating an adverse analytical finding for T use based on WADA criteria26. For these urine samples, the Δδ13C using 11OHA as the ERC was more sensitive to synthetic T use than the Δδ13C using 11KE when E and A were the TC steroids.
Table 1.
Results of urinary steroid extract analysis by GC×GC-qMS for measurement of T/EpiT and A/E concentration ratios and by GC×GCC-IRMS for measurement of δ13C and Δδ13C and the corresponding standard deviations. The same sample preparation procedure was used for both techniques.
| GC×GC-qMS (CI NH3) |
GC×GCC-IRMS (CIR, δ13CVPDB)a | GC×GCC-IRMS (Δδ13CVPDB, ERC-TC)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine Extract |
Treatment | T/EpiT Conc. Ratio |
A/E Conc. Ratio |
E | A | 11OHA | 11KE | 11OHA–E | 11OHA–A | 11KE–E | 11KE–A |
| Urine A | None | 1.9 ± 0.3% (n=2) | 1.8 ± 4.8% (n=2) | −21.26 ± 0.57‰ (n=4) | −19.93 ± 0.28‰ (n=4) | −21.35 ± 0.53‰ (n=4) | −21.86 ± 1.29‰ (n=4) | −0.10 ± 0.92‰ (n=4) | −1.42 ± 0.44‰ (n=4) | −0.37 ± 1.58‰ (n=4) | −1.69 ± 1.28‰ (n=4) |
| Urine B | T Gel | 4.6 ± 3.1%c (n=2) | 2.0 ± 3.6% (n=2) | −26.17 ± 0.57‰ (n=4) | −25.58 ± 1.78‰ (n=4) | −22.41 ± 0.81‰ (n=4) | −24.68 ± 1.37‰ (n=4) | +3.76 ± 0.45‰ (n=4) | +3.17 ± 1.39‰ (n=4) | +1.25 ± 0.98‰ (n=4) | +1.10 ± 1.06‰ (n=4) |
| Urine C | T Shot | 9.2 ± 6.4%c (n=2) | 4.1 ± 6.1%c (n=2) | −34.27 ± 0.58‰ (n=3) | −32.22 ± 0.52‰ (n=3) | −26.32 ± 1.50‰ (n=3) | −29.36 ± 1.83‰ (n=3) | +7.96 ± 1.03‰d (n=3) | +5.90 ± 1.04‰d (n=3) | +5.06 ± 1.28‰d (n=3) | +3.00 ± 1.38‰ (n=3) |
corrected for AC groups
mean of the differences
these values (T/EpiT > 4 and A/E > 4) trigger the requirement for an IRMS measurement to confirm synthetic steroid (i.e. T) use
along with the T/EpiT and A/E concentration ratios, these values indicate an adverse analytical finding for synthetic steroid (i.e. T) use using World Anti-Doping Agency (WADA) criteria (Δδ13C>3‰ plus error, in this case using a cutoff of Δδ13C>4.6‰)
CONCLUSIONS
Using a refined, home-built GC×GCC-IRMS instrument with home-written software, we demonstrate for the first time the measurement of the CIR of urinary steroids in minimally processed extracts. A T/EpiT concentration ratio of >9 and Δδ13C values ≥5‰ were measured in the urine of an individual that was given a T-shot, both fulfilling World Anti-Doping Agency (WADA) criterion confirming an adverse analytical finding for T use. These data show for the first time that exogenous steroid use is detectable by GC×GCC-IRMS without the need for extensive sample cleanup. Further refinements to achieve analysis of trace target steroids are under investigation to determine the usefulness of GC×GCC-IRMS toward more complete steroid analysis.
ACKNOWLEDGMENTS
This work was supported by the United States Anti-Doping Agency (USADA), the Partnership for Clean Competition (PCC, <http://www.cleancompetition.org/>), and the National Institutes of Health (NIH) grant RR031264. We thank Xavier de la Torre, Gavin Sacks and Larry Bowers for helpful discussions.
REFERENCES
- 1.Thevis M, Kuuranne T, Geyer H, Schanzer W. Drug Test. Anal. 2010;3:1–14. doi: 10.1002/dta.245. [DOI] [PubMed] [Google Scholar]
- 2.Walker J, Adams B. Int. J. Derm. 2009;48:1044–1048. doi: 10.1111/j.1365-4632.2009.04139.x. [DOI] [PubMed] [Google Scholar]
- 3.WADA. Coordinating Investigations and Sharing Anti-Doping Information and Evidence. 2011. [Google Scholar]
- 4.Catlin DH, Sekera MH, Ahrens BD, Starcevic B, Chang YC, Hatton CK. Rapid Commun. Mass Spectrom. 2004;18:1245–1249. doi: 10.1002/rcm.1495. [DOI] [PubMed] [Google Scholar]
- 5.Donike M, Barwald KR, Klostermann K, Schanzer W, Zimmermann J. Int. J. Sports Med. 1983;4:68–68. [Google Scholar]
- 6.WADA. Guideline Reporting and Management of Elevated T/E Ratios. 2006. [Google Scholar]
- 7.Meier-Augenstein W. J. Chromatogr. A. 1999;842:351–371. doi: 10.1016/s0021-9673(98)01057-7. [DOI] [PubMed] [Google Scholar]
- 8.Lichtfouse E. Rapid Commun. Mass Spectrom. 2000;14:1337–1344. doi: 10.1002/1097-0231(20000815)14:15<1337::AID-RCM9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Oulhote Y, Le Bot B, Deguen S, Glorennec P. Trends Anal. Chem. 2011;30:302–312. [Google Scholar]
- 10.Gessler A. Progress in Botany 72. Berlin: Springer-Verlag; 2011. pp. 227–248. [Google Scholar]
- 11.Primrose S, Woolfe M, Rollinson S. Trends Food Sci. Technol. 2011;21:582–590. [Google Scholar]
- 12.Regert M. Mass Spectrom. Rev. 2011;30:177–220. doi: 10.1002/mas.20271. [DOI] [PubMed] [Google Scholar]
- 13.Hoefs J. Eur. J. Mineral. 2010;22:3–15. [Google Scholar]
- 14.Hofstetter TB, Berg M. Trends Anal. Chem. 2011;30:618–627. [Google Scholar]
- 15.Mladenov N, Alados-Arboledas L, Olmo FJ, Lyamani H, Delgado A, Molina A, Reche I. Atmos. Environ. 2011;45:1960–1969. [Google Scholar]
- 16.Daeid NN, Buchanan HAS, Savage KA, Fraser JG, Cresswell SL. Aust. J. Chem. 2011;63:3–7. [Google Scholar]
- 17.Stellaard F, Elzinga H. Isot. Environ. Health Stud. 2005;41:345–361. doi: 10.1080/10256010500384333. [DOI] [PubMed] [Google Scholar]
- 18.Chang T-L, Li W-J. Chin. Sci. Bull. 1990;35:290–296. [Google Scholar]
- 19.Wieser ME, Coplen TB. Pure Appl. Chem. 83:359–396. [Google Scholar]
- 20.Aguilera R, Catlin DH, Becchi M, Phillips A, Wang C, Swerdloff RS, Pope HG, Hatton CK. J. Chromatogr. B. Biomed. Sci. Appl. 1999;727:95–105. doi: 10.1016/s0378-4347(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 21.Aguilera R, Chapman TE, Starcevic B, Hatton CK, Catlin DH. Clin. Chem. 2001;47:292–300. [PubMed] [Google Scholar]
- 22.Aguilera R, Becchi M, Casabianca H, Hatton CK, Catlin DH, Starcevic B, Pope HG., Jr J. Mass Spectrom. 1996;31:169–176. doi: 10.1002/(SICI)1096-9888(199602)31:2<169::AID-JMS276>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Piper T, Mareck U, Geyer H, Flenker U, Thevis M, Platen P, Schanzer W. Rapid Commun. Mass Spectrom. 2008;22:2161–2175. doi: 10.1002/rcm.3601. [DOI] [PubMed] [Google Scholar]
- 24.Saudan C, Emery C, Marclay F, Strahm E, Mangin P, Saugy M. J. Chromatogr. B. 2009;877:2321–2329. doi: 10.1016/j.jchromb.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 25.WADA. The 2011 Prohibited List International Standard. 2011. [Google Scholar]
- 26.WADA. Endogenous Anabolic Androgenic Steroids Testing, Reporting and Interpretive Guidance. 2009. [Google Scholar]
- 27.Matthews DE, Hayes JM. Anal. Chem. 1978;50:1465–1473. [Google Scholar]
- 28.Tobias HJ, Brenna JT. Anal. Chem. 1997;69:3148–3152. doi: 10.1021/ac970332v. [DOI] [PubMed] [Google Scholar]
- 29.Burgoyne TW, Hayes JM. Anal. Chem. 1998;70:5136–5141. [Google Scholar]
- 30.Merritt DA, Hayes JM. J. Am. Soc. Mass Spectrom. 1994;5:387–397. [PubMed] [Google Scholar]
- 31.Werner RA, Kornexl BE, Rossmann A, Schmidt HL. Anal. Chim. Acta. 1996;319:159–164. [Google Scholar]
- 32.Dias RF, Freeman KH, Franks SG. Org. Geochem. 2002;33:161–168. [Google Scholar]
- 33.Corso TN, Brenna JT. Proc. Nat. Acad. Sci. USA. 1997;94:1049–1053. doi: 10.1073/pnas.94.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks GL, Zhang Y, Brenna JT. Anal. Chem. 2007;79:6348–6358. doi: 10.1021/ac0706325. [DOI] [PubMed] [Google Scholar]
- 35.Tobias HJ, Sacks GL, Zhang Y, Brenna JT. Anal. Chem. 2008;80:8613–8621. doi: 10.1021/ac801511d. [DOI] [PubMed] [Google Scholar]
- 36.Goodman KJ, Brenna JT. Anal. Chem. 1994;66:1294–1301. doi: 10.1021/ac00080a015. [DOI] [PubMed] [Google Scholar]
- 37.Adahchour M, Beens J, Vreuls RJJ, Brinkman UAT. Trends Anal. Chem. 2006;25:540–553. [Google Scholar]
- 38.Adahchour M, Beens J, Vreuls RJJ, Brinkman UAT. Trends Anal. Chem. 2006;25:438–454. [Google Scholar]
- 39.Marriott PJ, Kinghorn RM. Anal. Chem. 1997;69:2582–2588. doi: 10.1021/ac961310w. [DOI] [PubMed] [Google Scholar]
- 40.Tobias HJ, Brenna JT. Microfluid. Nanofluid. 2010;9:461–470. [Google Scholar]
- 41.Zhang Y, Sacks GS, Tobias HJ, Brenna JT. 2011 submitted for publication. [Google Scholar]
- 42.Santrock J, Studley SA, Hayes JM. Anal. Chem. 1985;57:1444–1448. doi: 10.1021/ac00284a060. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Tobias HJ, Brenna JT. Steroids. 2009;74:369–378. doi: 10.1016/j.steroids.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Van Renterghem P, Van Eenoo P, Geyer H, Schänzer W, Delbeke FT. Steroids. 2010;75:154–163. doi: 10.1016/j.steroids.2009.11.008. [DOI] [PubMed] [Google Scholar]