Abstract
Rates of obesity have been steadily increasing, along with disorders commonly associated with obesity, such as cardiovascular disease and type II diabetes. Simultaneously, average sleep times have progressively decreased. Recently, evidence from both laboratory and epidemiologic studies has suggested that insufficient sleep may stimulate overeating and thus play a role in the current epidemic of obesity and diabetes. In the human sleep laboratory it is now possible to carefully control sleep behavior and study the link between sleep duration and alterations in circulating hormones involved in feeding behavior, glucose metabolism, hunger, and appetite. This article focuses on the methodologies used in experimental protocols that have examined modifications produced by sleep restriction (or extension) compared with normal sleep. The findings provide evidence that sleep restriction does indeed impair glucose metabolism and alters the cross-talk between the periphery and the brain, favoring excessive food intake. A better understanding of the adverse effects of sleep restriction on the CNS control of hunger and appetite may have important implications for public health.
Keywords: appetite regulation, sleep manipulation, insulin sensitivity, leptin, ghrelin
The colloquia, “Medical Phenomena Susceptible to Quantitative Approaches” encompassed various areas of research that showed that it is indeed possible to rigorously manipulate human behavior and measure valuable stringent outcomes. Within the context of this volume on the “Quantification of Behavior,” the present article focuses on methods that have been used to manipulate human sleep duration and quality in the laboratory while examining the consequences on hormones, metabolism, and the control of food intake. We first briefly introduce the epidemiologic evidence in support of a link between sleep disturbances and an increased risk of obesity and diabetes. After this introduction, we provide some basic background information regarding the CNS control of eating behavior for readers of this volume who do not focus on this area of inquiry. Last, we describe the combination of quantitative methods that have been used in well-controlled laboratory studies of the impact of sleep restriction on the risks of obesity and diabetes.
Epidemiologic Evidence for a Link Between Sleep Disturbances and the Risk of Obesity and Diabetes
It is well established that the prevalence of overweight and obesity has steadily increased worldwide in recent years, with particularly alarming acceleration in the last 2 decades in the United States (1). In America, all race, sex, age, and socioeconomic groups have been affected by this epidemic. According to data collected by the Behavioral Risk Factor Surveillance System of the Centers for Disease Control and Prevention (CDC), in 1985 no participating states had obesity rates greater than 15%. By 1999, 18 states reported obesity rates of 20–24% of their population, and not a single state had obesity rates under 10%. Most concerning is that these rates have continued to increase; in 2009 only one state had obesity rates lower than 20%, whereas nine states reported a prevalence of obesity ≥30% of the state's population. Along with the rise in the prevalence of obesity, disorders commonly associated with obesity, such as cardiovascular disease and type II diabetes, have concurrently increased. Considering that obesity is associated with adverse health outcomes, elucidating potential contributing factors to the epidemic of obesity may have important implications for public health. Traditional explanations for the increase in the prevalence of overweight and obesity have focused on portion size, wide availability of inexpensive high-calorie/high-fat foods, and decreased physical activity. Experts recognize that these traditional explanations do not fully explain the epidemic. In recent years, other putative causal mechanisms have begun to emerge, including chronic partial sleep loss and poor sleep quality.
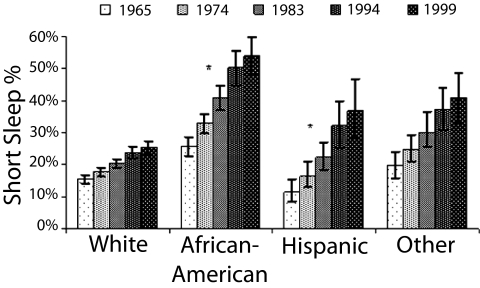
As the incidence of obesity has increased, average sleep times have indeed decreased. Polls conducted by the CDC and the National Sleep Foundation (NSF), as well as large-scale epidemiological studies, have indicated that Americans may be chronically sleep deprived because of voluntary bedtime curtailment (2–4). In 1959--1960, a study conducted by the American Cancer Society found that the majority of respondents reported sleeping 8–8.9 h per night (5). Later in the 1960s, some studies were beginning to reveal reductions in average self-reported sleep times to 7–8 h per night (6–8). Self-reported sleep times have continued to decline, as evidenced by the 2008 NSF poll in which adults reported sleeping 6 h 40 min on weeknights (4, 9). The most recent NSF poll detailed average self-reported sleep times across racial groups; all groups reported sleep times less than 7 h per weeknight, with whites reporting on average 6 h 52 min, African Americans 6 h 14 min, Asians 6 h 48 min, and Hispanics 6 h 34 min (9). Fig. 1 illustrates the intriguing finding that already in 1965 a larger proportion of short sleepers was found among African Americans, a population at increased risk for cardio-metabolic disease. Further, the proportion of short sleepers in this high-risk population and in Hispanics, an ethnic group with a higher prevalence of obesity and diabetes, has been increasing faster than in whites from 1965 to 1999 (10).
Fig. 1.
Mean prevalence of short sleep duration over race/ethnic group, adjusted for age: Alameda County Health and Ways of Living Study, 1965–1999. Sample size and response rate for each follow-up were as follows: 1974 (n = 4,864; 85%), 1983 (50% of random sample of eligible subjects, n = 1,799; 87%), 1994 (n = 2,730; 93%), and 1999 (n = 2,123; 95%). Short sleep at baseline was calculated across categories for each covariate, including frequency, prevalence, and odds ratio. A generalized estimating equation approach for statistical testing with regard to short sleep, race/ethnic group, income, and education was used owing to the repeated nature of the data (10). African Americans (P < 0.0001) and Hispanics (P < 0.001) showed significantly greater increase over time in the prevalence of short sleep duration compared with whites. (Reprinted from ref. 10, Copyright 2007, with permission from Elsevier.)
Interestingly, when measured objectively, sleep times may be lower than estimated with self-reports. A study conducted between 1990 and 1994 found that participants slept on average 6.2 h per night, as measured with wrist actigraphy (11). Recently, in the Coronary Artery Risk Development in Young Adults Sleep Study (2000–2006), sleep duration derived from several days of wrist actigraphy averaged 6.1 h per night (12), whereas self-reported sleep averaged 6.7 h per weeknight and 7.3 h per weekend night (13). Declines in sleep time can be attributed, in part, to extended work schedules including shift work, long commuting times, stressful lifestyles, modern conveniences such as 24-h television and computer/Internet use, and social and leisure activities.
There is a growing body of evidence suggesting a link between insufficient sleep duration and increased risk of obesity and type II diabetes. The epidemiological evidence for an association between short sleep and elevated body mass index (BMI) has previously been reviewed (14, 15) and established that the majority of both cross-sectional and prospective studies, whether in children or adults, had positive findings relating short sleep to increased obesity risk after controlling for multiple confounders. Importantly, the increased risk of obesity that may be attributable to insufficient sleep seems to be larger in children than in adults (15). Nonetheless, the relationship between short sleep and elevated BMI persists in both older men and women, as demonstrated in a large study that used actigraphy to assess sleep duration in more than 6,000 adults aged 67–99 y (16). There is also strong epidemiologic evidence for a link between short sleep and an increased risk of type II diabetes. Among prospective studies (which provide some indication regarding direction of causality), seven of nine studies examining the incidence of diabetes in short sleepers found a significantly elevated risk. A recent meta-analysis combining data from 90,623 individuals concluded that for short sleep duration (≤5–6 h per night), the relative risk of developing diabetes was 1.28 after controlling for multiple factors (17). Not included in this meta-analysis is the largest study to date (18), which included 174,542 adults and observed that individuals who report sleeping <5 h per night, relative to those reporting 7–8 h per night, had a 46% higher risk of developing type II diabetes, after controlling for multiple confounders, including adiposity.
Despite this increasing body of epidemiologic evidence, the mechanistic pathways linking sleep restriction with appetite dysregulation, increased food intake, and impaired glucose metabolism remain poorly understood.
Mechanistic Pathways Linking Central and Peripheral Control of Metabolism and Energy Balance
There are a plethora of mechanisms and redundancies within the CNS and periphery that work in concert to regulate feeding behavior and energy expenditure. Interestingly, despite the redundancy of these systems, alterations in a single neuroregulatory pathway can lead to modifications in food intake and energy expenditure (19). For the purpose of this article, we focus on a few circulating molecular signals that influence these outcomes.
A potential mechanism of action between chronic partial sleep loss and alterations in appetite regulation and metabolism may lie within the hypothalamus and/or within hypothalamic communication with peripheral systems. Hypothalamic nuclei have a well-established role in modulating energy homeostasis and feeding regulation; lesions of ventromedial, paraventricular, and dorsomedial nuclei or stimulation of the lateral hypothalamus (LH) produce hyperphagia, whereas lesions of the LH inhibit feeding (20). More specifically, the arcuate nucleus (Arc) lies close to the third ventricle; thus, circulating satiety and/or hunger signals, including those secreted from fat tissue, pancreas, and the gut, easily interact with this nucleus. Arc neurons produce and respond to neuropeptide Y (NPY), agouti-related protein (AgRP), proopiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript (CART) to influence feeding behavior and energy homeostasis (21, 22). In fact, discrete activation of these Arc neurons can facilitate alterations in behavior and energy expenditure; excitation of NPY/AgRP neurons increases food intake and decreases energy expenditure, and stimulation of POMC/CART receptors inhibits food intake and increases energy expenditure (20, 23). Thus, some peripherally secreted substrates modulate feeding and energy expenditure via direct action on these cells within the Arc. Moreover, cells within the Arc directly project to the LH, which in turn projects to thalamo-cortical systems, central autonomic effectors, and motor pattern generators that influence regulation of feeding (24). Thus, the Arc, LH, and the substrates that modulate these nuclei are primary candidates of study when examining the effect of sleep restriction on metabolic systems but are normally not accessible to experimental assessment in humans.
There are multiple peptides and hormones that are important in the regulation of metabolism and energy balance and act on these hypothalamic nuclei, including those originating from fat tissue (such as leptin and adiponectin) and the gut (such as ghrelin, peptide YY, pancreatic polypeptide, and cholecystokinin). Although the main source of these molecules resides in the periphery, they are also present in the CNS. Studies that examined the impact of sleep disturbances on energy balance have mostly focused on leptin and ghrelin, and we will therefore limit our brief review to these two pivotal players. Leptin, discovered in 1994, is one such peptide hormone encoded by the obesity gene (ob) that is secreted by white adipose tissue into the circulatory system (25). Leptin is thought to be an index of energy sufficiency (23). It is hypothesized that a decrease in leptin levels may be a critical signal to the CNS, indicating a state of depleted energy stores in which appetite should increase and energy expenditure decrease, favoring survival. Simply put, it influences feeding behavior and energy expenditure via leptin receptors that are most abundantly found in the arcuate, ventromedial, and dorsomedial nuclei of the hypothalamus (26) as well as other extrahypothalamic CNS sites, and peripheral tissues (although less is known about the mechanism of action in these areas) (22, 26). Within the Arc, leptin directly activates, via depolarization, hypothalamic POMC/CART neurons and in contrast, inhibits (hyperpolarizes) NPY/AgRP hypothalamic neurons (21, 27). Interestingly, within the Arc, 40% of POMC neurons express the long form of the leptin receptor (28). Thus, one mechanism by which leptin mediates energy homeostasis is via its action on orexigenic NPY/AgRP and anorexigenic POMC/CART neurons within the hypothalamus. Regarding appetite regulation, individuals lacking leptin or the leptin receptor become obese (29), and leptin replacement therapy has been shown to alleviate obesity in some subjects (30). However, in obesity that is not related to a genetic defect in the leptin system, peripheral leptin levels are increased rather than decreased, indicating central resistance to leptin signaling in obesity (31). In normal-weight individuals, plasma leptin levels increase immediately after food intake and decrease in response to food deprivation (32–34). Interestingly, recent data have suggested that leptin may also play a role in modulating glucose metabolism, independent of its effects on energy balance (19). For instance, leptin administration to leptin-deficient mice and humans improves hyperinsulinemia and hyperglycemia, even during controlled food intake paradigms (35–37).
Contrasting with the role of leptin as a peripheral signal for satiety, ghrelin, a hormone produced by endocrine cells within the stomach, plays a role in stimulating appetite, as well as promoting effective storage of consumed food; ghrelin has been described as a signal of energy insufficiency (29). One mechanism by which ghrelin exerts these effects is via pathways that partly overlap leptin-sensitive pathways (23). For instance, NPY/AgRP cells within the Arc express ghrelin receptors, and ghrelin activates these neurons; ghrelin depolarizes NPY/AgRP neurons while simultaneously increasing inhibitory input to POMC/CART neurons (23, 38). With respect to appetite regulation, in humans there is a premeal rise in plasma ghrelin, suggesting a role for ghrelin in meal initiation (39). It is apparent that ghrelin is involved in mediating energy homeostasis via feeding regulation; however, the exact mechanism of ghrelin action is still unclear. The acylation of ghrelin is necessary for some, but not all, of its endocrine actions. Nonacylated ghrelin circulates in much larger amounts than acylated ghrelin and may have distinct, and perhaps antagonistic, metabolic effects.
Appetite regulation and energy homeostasis are also under the control of insulin. Insulin receptors are expressed in many areas of the body, including of course liver, muscle, and fat, but, according to most but not all studies, are also present in the CNS (40). Similar to leptin, insulin receptors are located within the Arc of the hypothalamus and may activate similar pathways (i.e., inhibit NPY/AgRP neurons) (21). Intracerebroventricular infusion of insulin decreases NPY mRNA in the Arc, and insulin-deficient rats show increased hypothalamic mRNA expression (41–43). Further, recent studies have shown that leptin and insulin both act on POMC neurons, although within distinct subpopulations within the Arc (44). Regarding food intake specifically, intraventricular infusions of insulin reduce food intake and body weight (29). As body weight increases, basal levels of insulin and insulin levels in response to a meal increase in an attempt to maintain normal glucose homeostasis.
Sleep deprivation affects glucose metabolism through multiple pathways. The brain uses as much as 50% of total glucose utilization in the fasting state (20% in the fed state), which is 10 times more than predicted on a mass basis. Imaging studies have shown that the sleep-deprived brain uses 7–8% less glucose than the well-rested brain. A limitation of these imaging studies is that they have been conducted under conditions of total sleep deprivation. Thus, the impact of recurrent partial sleep restriction, a much more common condition, on brain glucose metabolism remains to be elucidated. Another major pathway linking sleep loss and peripheral function is the autonomous nervous system. Alterations of cardiac sympatho-vagal balance, with higher sympathetic nervous activity and lower vagal tone, have been documented in a number of studies of human sleep loss, including studies of partial sleep restriction (45). Whether similar alterations of autonomic nervous system control in conditions of sleep loss occur at multiple organ levels is not known. A few studies have shown that norepinephrine levels are elevated during sleep deprivation (46) and in individuals suffering from obstructive sleep apnea (47). Peripheral organs that play a major role in glucose regulation and are strongly affected by sympathetic activity include the liver, pancreas, adipose tissue, and the gastrointestinal tract. Sleep loss also dysregulates counter-regulatory hormones in a manner consistent with the promotion of hyperglycemia and/or insulin resistance. Indeed, elevations of evening cortisol levels (48–51), increased release of growth hormone and ghrelin during daytime hours (52–54), and elevated daytime levels of epinephrine (55) have all been observed under sleep debt conditions.
Orexins A and B, two peptides that are synthesized by neurons concentrated in the LH, constitute a molecular link between sleep–wake regulation and the neuroendocrine control of appetite. Orexin-containing neurons play a central role in the maintenance of arousal. There is evidence that orexins may stimulate food intake, particularly the early part of the usual sleep period, which is when voluntary sleep deprivation most often occurs in humans (56, 57). Feeding requires the maintenance of wakefulness, and the orexin system seems to play a central role in this interaction between feeding and arousal (58). Orexin-containing neurons are active during waking and quiescent during sleep. Deficiencies in the orexin system are associated with sleep disorders that involve chronic excessive daytime sleepiness, including narcolepsy and obstructive sleep apnea. In contrast, when sleep deprivation is enforced behaviorally, the orexin system is overactive, most likely to maintain wakefulness against the increased sleep pressure (59–61). The orexin system activates the appetite-promoting NPY neurons in the Arc (62). Orexinergic activity is in turn influenced by both central and peripheral signals, with glucose and leptin exerting inhibitory effects, whereas ghrelin promotes further activation. Furthermore, orexin neurons have dense projections to the dopaminergic ventrotegmental area and nucleus accumbens, which are involved in the hedonic control of food intake, and thus orexin may serve as an interface between metabolic and motivational systems with motor outputs to modulate food intake (58). A profound impact of sleep loss on appetite regulation should therefore be expected when considering the associations of these systems. Although peripheral levels of circulating orexin may not be representative of central orexinergic activity, they would nonetheless be an interesting exploratory outcome measure in studies involving manipulations of sleep behavior. Of note, in a 2005 study from Japan, plasma orexin levels were found to decrease with the severity of obstructive sleep apnea and to increase after treatment with continuous positive airway pressure (63), suggesting that peripheral orexin levels may track daytime alertness.
Some of the alterations that have been observed in conditions of sleep loss, such as elevated sympatho-adrenal activity should be associated with increased, rather than decreased, energy expenditure. Although extreme sleep deprivation in rodent models results in weight loss despite increased caloric intake, this does not seem to be the case in humans, in whom the stimulation of food intake seems to be excessive in relation to the energy demands of extended wakefulness under comfortable sedentary conditions. A recent study quantified the caloric requirement of staying awake vs. sleeping in healthy young adults and found that the energy cost of a night of total sleep deprivation averaged a modest 134 calories (64). The large body of epidemiologic evidence linking short sleep duration and obesity suggests that in humans, the increase in energy intake resulting from sleep restriction exceeds the modest increase in energy needed to sustain extended wakefulness.
Quantitative Methods to Assess the Impact of Bedtime Restriction on the Cross-Talk Between Brain and Peripheral Metabolism
Laboratory studies in human volunteers are generally limited by a relatively small sample size (10–20 subjects) but have the advantage of rigorously imposing different sleep conditions (normal bedtimes, restricted bedtimes, and extended bedtimes) and allowing for a multi-level assessment of components of energy balance and glucose metabolism. A few studies involving experimental manipulation of sleep quality without change in sleep duration have also been conducted (65, 66). Typically, the participants are young healthy lean volunteers, caloric intake is rigorously controlled, and sedentary conditions are enforced to control for activity levels. Each sleep condition involves 2–15 d, with stringently controlled bedtimes and light–dark cycles. In our laboratory the center of the bedtime period is kept constant, and light exposure is reduced in the evening and morning to avoid a shift of circadian phase. Within-subject comparisons can be made between conditions; therefore, any alterations observed are most likely due to the experimental intervention instead of other confounding factor(s). Moreover, the amount of sleep (hours per night and length of restriction) can be carefully managed in the laboratory setting; sleep is recorded nightly by polysomnography. During each sleep condition, participants complete questionnaires about mood, subjective sleepiness, alertness, hunger, and appetite multiple times per day. Each sleep condition involves at least one 24-h period when blood is frequently sampled, which allows for profiles of circulating peptides including leptin, ghrelin, glucose, insulin, and C-peptide (a marker of insulin secretion), cortisol, and growth hormone. Intravenous glucose tolerance tests (ivGTT) are conducted to examine glucose tolerance (kg, rate of decrease of glucose levels after glucose injection), glucose effectiveness (Sg, a marker of non–insulin-dependent glucose utilization and therefore an indirect measure of brain glucose metabolism), insulin sensitivity (SI, which quantifies the amount of insulin needed to metabolize a given amount of glucose), acute insulin response to glucose (AIRg, the acute insulin response to glucose in the first 20 min after glucose injection), and the disposition index (a validated marker of diabetes risk; DI = SI × AIRg). The response to oral glucose, which involves the gastrointestinal tract and the so-called incretin effect, may be assessed on a separate day.
Although these studies allow for thorough evaluation of alterations produced by sleep restriction, translating these results to real-life conditions is not straightforward. Indeed, laboratory studies of sleep restriction are generally limited in duration and thus may not fully mimic changes in appetite and metabolism produced by more chronic partial sleep loss. The stress of sleep loss under the demands of normal life conditions, including professional and social demands, is also not replicated by laboratory conditions. Furthermore, although cross-sectional and prospective studies have reported associations between shortened sleep duration and increased weight irrespective of sex differences, the vast majority of laboratory studies in both humans and animals have only been conducted on males. Thus, further examination of potential sex differences with regard to this relationship is necessary.
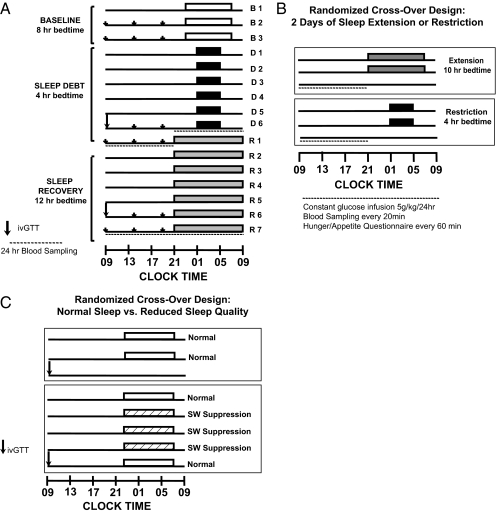
In the remainder of this article, we summarize the methodology and findings of three laboratory studies examining the metabolic impact of reduced sleep duration or quality. The protocol design of each study is schematically illustrated in Fig. 2 A–C.
Fig. 2.
(A) Experimental protocol included three baseline nights of 8 h in bed (white bars), followed by six night of sleep restriction (4 h in bed; black bars), ending with a seven night period of sleep extension (12 h in bed; light gray bars). The last 2 d of the sleep-debt and sleep-extension periods included identical meals for breakfast, lunch, and dinner (small arrows), an ivGTT to assess glucose metabolism (large arrow), and 24-h blood sampling to examine circulating hormonal profiles (dashed lines). (B) In a randomized cross-over design, two nights of either bedtime extension (10 h in bed; dark gray bars) or sleep restriction (4 h in bed; black bars) were followed by 12 h of blood sampling, coupled with a constant glucose infusion and hourly questionnaires regarding hunger/appetite, mood, and sleepiness. (C) In a randomized cross-over design, two nights of normal sleep (8 h in bed; white bars) or three nights of slow-wave sleep suppression (hatched bars) were followed by an ivGTT (arrow) to assess glucose metabolism.
Methodology and Findings of Previous Laboratory Studies
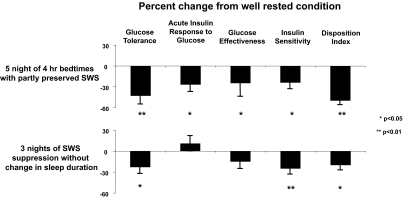
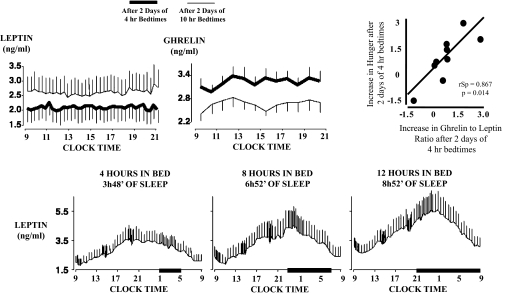
The first controlled study to examine the effect of sleep restriction on metabolic and endocrine function involved 11 healthy young men who were allowed 8 h in bed for three nights (baseline), restricted to 4 h in bed for six nights (sleep debt), followed by 12 h in bed for seven nights (sleep recovery) (49). The protocol is shown in Fig. 2A. During the last 2 d of each experimental condition, the participants were given identical carbohydrate-rich meals and were subjected to bed rest while an ivGTT was conducted, followed by a 24-h period of frequent blood sampling. Sleep restriction was successful in that the mean total sleep time during the period of sleep debt was 3 h and 49 min in comparison with the 9 h and 3 min attained during the period of sleep recovery. The proportion of slow-wave sleep [stages III and IV of non-REM (rapid eye movement) sleep] was higher during the sleep-debt conditions in comparison with baseline and sleep recovery, revealing an increased pressure for slow-wave sleep. However, the state of sleep debt was primarily characterized by a loss of REM sleep, which was reduced from ≈90 min at baseline to 40 min during the last two short nights. After the period of sleep restriction, glucose and insulin responses to the ivGTT and breakfast were consistent with an impairment of carbohydrate tolerance consistent with a prediabetic state. As illustrated in Fig. 3 (Upper), the ivGTT revealed 40% lower glucose clearance, a 25–30% reduction in glucose effectiveness and in insulin sensitivity, and a disposition index 40% lower in the sleep-restricted than in the sleep-extension condition (49, 67). Findings of decreased insulin sensitivity without adequate compensation by increased insulin release have been replicated in other studies of sleep restriction (55, 68). In the initial “sleep debt study,” postbreakfast homeostatic model assessment values (a marker of insulin resistance) increased 56% in the sleep-debt condition compared with sleep extension, owing to elevated glucose levels in response to breakfast despite the fact that insulin levels also tended to be higher. Evening cortisol and sympathetic nervous system activation were increased; both are risk factors for the development of insulin resistance and obesity. The 24-h profile of blood constituents revealed that leptin levels are particularly sensitive to sleep duration (45), confirming an early report (69). Mean leptin levels were 19% lower in the sleep-debt vs. sleep-extension condition and intermediate with 8-h bedtimes (Fig. 4, Lower). Further, both the nocturnal acrophase and amplitude of the diurnal variation of leptin were decreased (26% and 20%, respectively). Interestingly, these changes occurred despite similar caloric intake, activity, and without changes in BMI (45). These observed leptin concentrations are somewhat larger than those observed in young adults after 3 d of dietary restriction by ≈900 kcal/d (70). Thus, not only does sleep debt alter metabolic systems promoting diabetes risk, but leptin seems to be signaling a state of caloric deficit during the period of sleep debt, potentially leading to hyperphagia.
Fig. 3.
Adapted from refs. 49, 65, 67, and 74. Percentage change relative to the well-rested condition for measures of glucose metabolism in young healthy lean adults subjected to either sleep restriction (Upper; five nights of 4-h bedtimes, n = 11) or slow-wave sleep (SWS) suppression (Lower; three nights, n = 9). In each study, comparisons between well-rested condition and sleep restriction (Upper) or SWS suppression (Lower) were made by paired t test.
Fig. 4.
Adapted from refs. 54 and 74. Upper: Mean (±SEM) daytime profiles of plasma leptin and ghrelin observed in 12 healthy lean subjects after 2 d of 4-h bedtimes or 2 d with 10-h bedtimes. Caloric intake was exclusively administered via a constant glucose infusion. Comparisons between the two sleep conditions were performed using the Wilcoxon test for matched pairs; leptin decrease 18% (P = 0.04) and ghrelin increase 28% (P < 0.04). Correlation of the change in hunger ratings and the ghrelin/leptin ratio were calculated using the Spearman rank test. Values were calculated using data from 4 h in bed minus the value obtained after 10 h in bed. The calculated value was negative when the variable measured after 10 h in bed was higher than that measured after 4 h in bed. Lower: Mean (±SEM) 24-h plasma leptin profiles obtained from nine healthy lean men studied at bed rest who ate three identical carbohydrate-rich meals after 6 d of 4 h, 8 h, and 12 h in bed. Comparisons of variables obtained during 8-h, 4-h, and 12-h bedtime conditions were performed using ANOVA for repeated measures. Time spent asleep in each condition was as follows; 3 h 48 min in 4-h bedtime condition, 6 h 52 min in 8-h bed condition, and 8 h 52 min in the 12-h condition. Note that the characteristics of the 24-h leptin profile (amplitude, nocturnal maximum, and overall mean) increased from the 4-h to the 12-h bedtime condition. Dark bars denote sleep periods.
In a subsequent randomized cross-over study (Fig. 2B), developed in part to control for putative order effects in the “sleep debt” study, metabolic parameters were examined after two nights of either 4 or 10 h in bed (54). Hormonal findings pertinent to appetite regulation are illustrated in Fig. 4 (Upper). Consistent with the seminal study, sleep restriction was associated with decreased leptin concentration, even though meals were replaced by constant glucose infusion. Furthermore, this study revealed a concomitant increase in the orexigenic factor ghrelin (24%), along with increased hunger and appetite ratings (24% and 23%, respectively) in the short-sleep condition compared with the well-rested condition (54). Interestingly, appetite ratings tended to be greatest for carbohydrate-rich, calorie-dense foods. The change in the ghrelin/leptin ratio between the two experimental conditions was significantly and positively correlated with changes in hunger ratings, suggesting that sleep restriction-induced alterations in these hormones may, in part, be driving observed increases in hunger ratings. These results are consistent with findings from two population-based studies that have also reported associations between short sleep duration, leptin, and ghrelin levels consistent with an up-regulation of appetite (71, 72). Moreover, these associations were independent of BMI and other possible confounding factors. Thus, sleep restriction or partial chronic sleep loss produces modifications in the profile of appetite-regulating hormones, favoring hyperphagia.
In a more recent study of the metabolic impact of experimental reductions of sleep quality (65), suppression of slow-wave activity (non-REM stages III and IV) was accomplished without altering total sleep time for three consecutive nights (Fig. 2C). A control condition of normal undisturbed sleep with 8.5 h in bed was presented in a randomized cross-over design. As summarized in Fig. 3 (Lower), metabolic alterations qualitatively similar to those observed after reductions in sleep duration emerged (65). In this study, slow-wave suppression was achieved with acoustic stimulation; as soon as slow-waves were identified on the EEG recording, a sound was sent to amplifiers on both sides of the subject's bed to elicit a microarousal and subsequent suppression of slow-wave activity. This resulted in an increase in the lighter sleep stages, stages I and II, without changing total sleep time or the amount of REM sleep. After three nights of slow-wave sleep suppression, insulin sensitivity, glucose tolerance, and the disposition index decreased compared with baseline (by 25%, 23%, and 20%, respectively). These values are typical of individuals who have impaired glucose tolerance and are at risk for developing frank diabetes. Notably, the magnitude of the changes for both insulin sensitivity and acute insulin response to glucose were correlated with the decreases in slow-wave sleep (65). Similar to the previous studies, elevated cardiac sympathetic nervous activity was apparent after 3 d of slow-wave suppression. Selective suppression of slow-wave activity thus produces clear reductions in glucose tolerance and increases in markers of diabetes risk. Therefore, it seems that not only the duration of sleep but also the quality of sleep is important for metabolic function. Consistent with this notion, an independent study of sleep fragmentation across all stages (which, however, led to a greater decrease of slow-wave sleep than of other sleep stages) obtained similar findings as in the study of selective sleep suppression (66). Considering that there are marked age-related changes in sleep quality, particularly reductions in slow-wave activity, it is possible that the sleep disturbances of normal aging may also play a role in the increased diabetes risk in older populations. In contrast, a current ongoing clinical trial may offer interesting insight into the association between sleep duration and BMI. The authors are examining whether extending sleep duration and/or quality may positively affect body weight (i.e., decrease in overweight individuals), although findings have yet to be reported (73).
Conclusions
As rates of overweight and obesity continue to increase, chronic partial sleep loss and poor sleep quality have also become endemic conditions. A growing body of epidemiologic and laboratory evidence suggests that the behavior of bedtime curtailment may be contributing to the epidemics of obesity and type II diabetes. Whereas epidemiologic studies have revealed an association between short sleep times and increased BMI and increased diabetes risk, controlled laboratory studies are able to demonstrate a causal relationship between bedtime restriction and metabolic alterations. Sleep restriction leads to increases in hunger and appetite ratings, along with modifications in hormonal profiles—lower leptin and higher ghrelin levels—that favor hyperphagia. Moreover, a state of sleep debt causes disturbances in glucose regulation, potentially leading to increased risk for developing type II diabetes. Similarly, experimental reduction of sleep quality without change of sleep duration has also been shown to be associated with impairment of glucose tolerance. These findings have important implications for public health and well being. Increased awareness of the deleterious effects of chronic partial sleep loss and poor sleep quality may be important in regulating the prevalence of overweight, obesity, and associated adverse health outcomes.
Acknowledgments
The work reviewed in this article was partly supported by National Institutes of Health Grants PO1 AG-11412, RR-024999, RO1-HL-075079, P50-HD-057796, and P60-DK-20595 and by Department of Defense Grant PR064727.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Quantification of Behavior” held June 11–13, 2010, at the AAAS Building in Washington, DC. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/quantification.
This article is a PNAS Direct Submission.
References
- 1.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. QuickStats: Percentage of adults who reported an average of < 6 hours of sleep per 24-hour period, by sex and age group—United States, 1985 and 2004. MMWR. 2005;54:933. [Google Scholar]
- 4.National Sleep Foundation. Sleep in America Poll. Washington, DC: National Sleep Foundation; 2008. [Google Scholar]
- 5.Kripke D, Simons R, Garfunkel L, Hammon E. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 6.Tune GS. Sleep and wakefulness in normal human adults. BMJ. 1968;2:269–271. doi: 10.1136/bmj.2.5600.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tune GS. Sleep and wakefulness in a group of shift workers. Br J Ind Med. 1969;26:54–58. doi: 10.1136/oem.26.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health Nations Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Sleep Foundation. Sleep in America Poll. Washington, DC: National Sleep Foundation; 2010. [Google Scholar]
- 10.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: Population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17:948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: Effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 12.Lauderdale DS, et al. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: The CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauderdale DS, et al. Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 14.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159(Suppl 1):S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SR, et al. Osteoporotic Fractures in Men Research Group; Study of Osteoporotic Fractures Research Group. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Q, et al. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept. 2008;149:3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 22.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 23.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity—the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 24.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 26.Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 27.Elias CF, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 29.Badman MK, Flier JS. The gut and energy balance: Visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 30.O'Rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: Human obesity-lessons from monogenic disorders. Endocrinology. 2003;144:3757–3764. doi: 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MW. Brain pathways controlling food intake and body weight. Exp Biol Med (Maywood) 2001;226:978–981. doi: 10.1177/153537020122601103. [DOI] [PubMed] [Google Scholar]
- 32.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 33.Frederich RC, et al. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 34.Maffei M, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 35.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 36.Hedbacker K, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MW, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 38.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 39.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 40.Brüning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MW, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Leibowitz KL. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 1997;777:231–236. doi: 10.1016/s0006-8993(97)00963-3. [DOI] [PubMed] [Google Scholar]
- 43.Abe M, Saito M, Ikeda H, Shimazu T. Increased neuropeptide Y content in the arcuato-paraventricular hypothalamic neuronal system in both insulin-dependent and non-insulin-dependent diabetic rats. Brain Res. 1991;539:223–227. doi: 10.1016/0006-8993(91)91624-a. [DOI] [PubMed] [Google Scholar]
- 44.Williams KW, Elmquist JK. Lighting up the hypothalamus: Coordinated control of feeding behavior. Nat Neurosci. 2011;14:277–278. doi: 10.1038/nn0311-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegel K, et al. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 46.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: Clinical implications. J Clin Endocrinol Metab. 1999;84:1979–1985. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 47.Tasali E, Chapotot F, Leproult R, Whitmore H, Ehrmann DA. Treatment of obstructive sleep apnea improves cardiometabolic function in young obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:365–374. doi: 10.1210/jc.2010-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 49.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 50.Chapotot F, Buguet A, Gronfier C, Brandenberger G. Hypothalamo-pituitary-adrenal axis activity is related to the level of central arousal: Effect of sleep deprivation on the association of high-frequency waking electroencephalogram with cortisol release. Neuroendocrinology. 2001;73:312–321. doi: 10.1159/000054648. [DOI] [PubMed] [Google Scholar]
- 51.Kumari M, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandenberger G, Gronfier C, Chapotot F, Simon C, Piquard F. Effect of sleep deprivation on overall 24 h growth-hormone secretion. Lancet. 2000;356:1408. doi: 10.1016/S0140-6736(00)02847-6. [DOI] [PubMed] [Google Scholar]
- 53.Spiegel K, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol. 2000;279:R874–R883. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- 54.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 55.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–3250. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 57.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonnavion P, de Lecea L. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 2010;10:174–179. doi: 10.1007/s11910-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 59.Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1079–R1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Estabrooke IV, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Increasing length of wakefulness and modulation of hypocretin-1 in the wake-consolidated squirrel monkey. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1736–R1742. doi: 10.1152/ajpregu.00460.2007. [DOI] [PubMed] [Google Scholar]
- 62.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 63.Sakurai S, et al. Low plasma orexin-A levels were improved by continuous positive airway pressure treatment in patients with severe obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:731–737. doi: 10.1378/chest.127.3.731. [DOI] [PubMed] [Google Scholar]
- 64.Jung CM, et al. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 69.Guilleminault C, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4:177–184. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 70.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85:2685–2691. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 71.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 73.Cizza G, et al. Treatment of obesity with extension of sleep duration: A randomized, prospective, controlled trial. Clin Trials. 2010;7:274–285. doi: 10.1177/1740774510368298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Cauter E, Tasali E. Endocrine physiology in relation to sleep and sleep disturbances. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. , 5th Ed. Philadelphia: Elsevier-Saunders; 2011. [Google Scholar]