Abstract
The GH/IGF-I axis has both pre- and postpubertal metabolic effects. However, the differential effects of GH and/or IGF-I on animal physiology or the plasma proteome are still being unraveled. In this report, we analyzed several physiological effects along with the plasma proteome after treatment of mice with recombinant bovine GH or recombinant human IGF-I. GH and IGF-I showed similar effects in increasing body length, body weight, lean and fluid masses, and organ weights including muscle, kidney, and spleen. However, GH significantly increased serum total cholesterol, whereas IGF-I had no effect on it. Both acute and longer-term effects on the plasma proteome were determined. Proteins found to be significantly changed by recombinant bovine GH and/or recombinant human IGF-I injections were identified by mass spectrometry (MS) and MS/MS. The identities of these proteins were further confirmed by Western blotting analysis. Isoforms of apolipoprotein A4, apolipoprotein E, serum amyloid protein A-1, clusterin, transthyretin, and several albumin fragments were found to be differentially regulated by GH vs. IGF-I in mouse plasma. Thus, we have identified several plasma protein biomarkers that respond specifically and differentially to GH or IGF-I and may represent new physiological targets of these hormones. These findings may lead to better understanding of the independent biological effects of GH vs. IGF-I. In addition, these novel biomarkers may be useful for the development of tests to detect illicit use of GH or IGF-I.
The GH/ IGF-I axis is well known to stimulate longitudinal bone growth; however, it also is involved in several metabolic events occurring during development and beyond (1). In untreated children, GH deficiency leads to dwarfism, and GH excess can result in gigantism. For adults, GH-deficient patients exhibit weight gain, muscle weakness, lack of energy, and overall reduced quality of life (2–4). Conversely, excess GH causes acromegaly that features excess growth of the hands and feet and insulin resistance as well as cardiovascular and respiratory pathophysiologies (5, 6). Thus, normal GH/IGF-I action is essential to maintain growth and well-being. Moreover, the GH/IGF-I axis has been implicated in aging because attenuation of the axis is linked to increased lifespan in a wide range of organisms (7). Finally, in humans, disruption of GH signaling via mutations in the GH receptor (GHR) or GH-specific intracellular signaling molecules result in a state of GH resistance termed Laron syndrome, which is associated with a reduction in the incidence of cancer and diabetes (8, 9). Therefore, GH/IGF-I action has been the focus of research in many fields.
The GH/IGF-I axis is important for promoting metabolic changes in animals. However, it is difficult to differentiate between the two hormones in terms of their growth-promoting effect because they share several intracellular signaling pathways (10). To add to this complexity, GH stimulates production of IGF-I; thus, when GH levels are reduced (or elevated), IGF-I is similarly reduced (or elevated). To determine the individual contributions of GH and IGF-I to body growth, GHR and IGF-I knockout mice as well as a double-knockout mouse of both GHR and IGF-I have been compared (11). From this study, it is estimated that GH contributes 14%, IGF-I contributes 35%, and their overlapping actions contribute 34% to total growth (11). These results help to confirm the notion that GH and IGF-I have both overlapping and independent effects on growth. As a result, an intact GH/IGF-I axis is needed to achieve normal growth. For example, treatment of Laron syndrome individuals with IGF-I results in partially restored height, which does not reach the level compared with GH-deficient patients who undergo GH replacement therapy (12).
One of the most notable physiological differences induced by GH vs. IGF-I involves insulin signaling. GH is well known for its antiinsulin effect (13, 14), which can lead to insulin resistance. In contrast, IGF-I has potent insulin-like effects and directly stimulates glucose uptake. Also, GH and IGF-I are known to have differential effects on lipid metabolism. For example, GH, but not IGF-I, promotes lipolysis (15). Although several different physiological actions of GH and IGF-I have been documented, the molecular mechanisms involved in the distinct activities of these two important endocrine hormones require further study.
Another issue in the GH/IGF-I field is the misuse of recombinant human (rh) GH or more recently of IGF-I by athletes. Injected rhGH is difficult to detect directly in blood owing to its short half-life (16, 17). The current detection method for GH doping, the isoform approach, has a short window of opportunity and to date has detected only one athlete who abused rhGH (18). A second test for rhGH abuse is by detection of the plasma levels of IGF-I and procollagen type III, which are positively correlated with GH administration (19, 20). However, due to relatively large inter- and intra-individual variations in the levels of these proteins by age, gender, exercise, and diet, they have not been successfully used to detect rhGH doping. Approval of rhIGF-I for therapy of short stature due to severe primary IGF-I deficiency in the United States (21) will increase the availability of this hormone; and thus, the potential for illicit use. Therefore, biomarkers for GH and independent markers for IGF-I are needed to ensure accurate and sensitive detection of these two anabolic recombinant proteins (17).
Plasma proteins have been routinely used for the diagnosis of diseases such as cancer (22), diabetes (23), and multiple sclerosis (24). Because the plasma proteome is a composite of proteins derived from various tissues, they can reflect the systemic state of an organism, e.g. a disease state or response to a particular pharmacological treatment. To our knowledge, no study has examined the differential effects of GH and IGF-I on the mouse plasma proteome, which was one of the objectives of the current study. Recombinant bovine (rb) GH or rhIGF-I was injected into male C57BL/6J mice for 2 wk. Plasma proteins that were differentially regulated in either treatment group were identified. Additionally, two groups of mice were treated with rbGH or rhIGF-I for 2 wk followed by a 1-wk washout period to determine relatively long-lasting changes in the plasma proteome. These types of changes could be considered longer-acting biomarkers of GH/IGF-I action and may be important for future development of GH/IGF-I doping detection kits. In this report, we have identified several proteins or protein isoforms that changed acutely and others that changed over a longer time interval as a function of GH vs. IGF-I treatment.
Materials and Methods
Experimental animals and treatment protocol
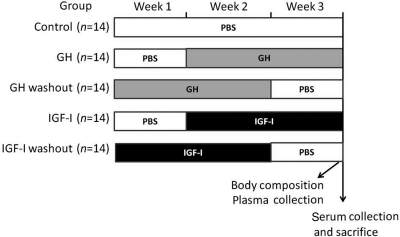
Three-month-old male C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME) were housed two to three per cage at room temperature (22 C) in a 14-h light, 10-h dark cycle with free access to food and water. rbGH and rhIGF-I were administered via sc injections. Purified rbGH was a gift from Monsanto (St. Louis, MO) and was suspended in PBS. rhIGF-I was a gift from Tercica Inc. (Brisbane, CA) and was diluted in PBS. rbGH was injected once per day at approximately 1800 h with a dose of 5 μg/g body weight as previously described (25). rhIGF-I was administered twice per day at approximately 0900 and 1800 h to prevent hypoglycemic shock induced by one large dose, with each dose at 5 μg/g body weight, resulting in a total dose of 10 μg/g body weight. The dose of rbGH was the same with a previous study by List et al. (25) and rhIGF-I was similar to a study by Tanaka et al. (26). Mice were divided randomly into five groups (n = 14, Fig. 1). The control group received PBS injection for the entire treatment period. GH and IGF-I groups received PBS injections for the first week and then rbGH or rhIGF-I for 2 wk. Two washout groups were injected with rbGH or rhIGF-I for the first 2 wk and then with PBS for 1 wk as a washout phase. Because rhIGF-I was injected twice a day, to control for stress, mice in the control and GH groups also received an additional PBS injection in the morning; therefore, each mouse was injected twice daily. During the 21 d of injections, body composition was determined on d 20 and plasma was collected on d 21. On d 22, the mice were killed, body length was measured (nose to anus), and organs were removed, weighed, and stored at −80 C for additional use. Because plasma volume was not sufficient for all assays, serum was also collected by orbital bleeding from each mouse immediately before killing on d 22.
Fig. 1.
GH and IGF-I treatment regimes. Mice were injected at 0900 and 1800 h every day for 3 wk. The control group was injected with PBS twice a day for the entire 3 wk. The GH group was injected with PBS twice a day for the first week and then PBS at 0900 h and rbGH at 1800 h for the following 2 wk. The GHw group was injected in the same manner, but the order of PBS and GH phases was reversed. The IGF-I group received two PBS injections in the first week and then rhIGF-I injections at both 0900 and 1800 h for the following 2 wk. The IGF-I washout group received the same injections as the IGF-I group, but the order of PBS and IGF-I phases was reversed. rbGH was injected at a dose of 5 μg/g body weight. rhIGF-I was administered at a dose of 5 μg/g body weight per injection. Body composition measurement and plasma collection was performed 1 d before dissection and serum was collected on the day of the dissection.
For plasma collection, blood was drawn using heparinized capillary tubes from the tail tip as previously described (27, 28). For serum collection, blood was collected from the ocular orbit at the end of the study and allowed to clot for 30 min at room temperature. Blood was then centrifuged at 7000 × g at 4 C for 10 min, and serum was collected and stored at −80 C for use later. The plasma collected on d 21 was used for proteomic and IGF-I analyses. The serum collected on d 22 was used for cholesterol and triglyceride measurements. Animal protocols were approved by Ohio University's Institutional Animal Care and Use Committee.
Body composition measurement
Body composition was measured using the Bruker Minispec (The Woodlands, TX) as described previously (25, 28, 29). The data were recorded as grams for lean mass, fat mass, and fluid mass.
Plasma IGF-I
Mouse IGF-I (mIGF-I) levels were determined using the DSL-10-29200 mouse/rat IGF-I ELISA kit following manufacturer's instructions (Diagnostic Systems Laboratories, Inc., Webster, TX). Because rhIGF-I was used for injections into the mice, hIGF-I levels were also determined using the DSL-10-2800 IGF-I nonextraction (human) ELISA kit following manufacturer's instructions (Diagnostic Systems Laboratories). The sensitivity of the mIGF-I ELISA was 1.3 ng/ml and intraassay coefficient of variation was 4.83%. The sensitivity of the hIGF-I ELISA was 0.01 ng/ml with an intraassay coefficient of variation of 8.6%.
Serum and liver triglyceride and total cholesterol
A triglycerides-GPO (Pointe Scientific, Canton, MI) kit was used to determine glycerol concentrations. A cholesterol assay kit (Cayman Chemical Co., Ann Arbor, MI) was used to determine total cholesterol levels. Lipids were extracted from liver samples as described previously (25, 29) followed by triglyceride and cholesterol quantification.
Two-dimensional gel electrophoresis (2-DE) and quantification of individual proteins
Plasma total protein concentration was determined via the Bradford method (30) using a protein assay reagent from Bio-Rad (Hercules, CA). A total of 750 μg protein was loaded on each 2-D gel. The 2-DE procedure has been described previously (27, 28, 31–37). For quantification of plasma proteins, PDQuest software (Bio-Rad) was used. Briefly, the gels were stained using SYPRO Orange (1:5000; Molecular Probes, Eugene, OR) for 2 h. Gel images were captured using a laser-scanner Pharos FX plus (Bio-Rad) with an excitation wavelength of 488 nm and an emission wavelength of 604 nm. The intensity of each protein spot was determined according to the fluorescence signal strength and finally normalized to the quantity of total spots of each gel.
Protein identification by mass spectrometry (MS) and MS/MS
Proteins of interest were excised manually from gels and sent to Protea Biosciences, Inc. (Morgantown, WV) for MS and MS/MS analyses using matrix assisted-laser desorption ionization (MALDI)-time of flight (TOF) and MALDI-TOF-TOF as described previously (28, 34–37).
Manual confirmation of protein identification from the MS and MS/MS data
MS and MS/MS data were manually submitted to MASCOT at www.matrixscience.com as described previously (28, 34–37) for protein identification. For MS data, the searching criteria were as follows: Swiss-Prot as the database; mouse as the species; trypsin digestion; maximum one missed cleavage; fixed carbamidomethylation of cysteine; variable modifications of oxidation-M (methionine), phosphorylation of S, T, and Y; monoisotopic; and 0.5 Da of peptide mass or parent tolerance. For MS/MS ion searches, in addition to the above conditions, a peptide charge of +1 and a fragment mass tolerance of 0.5 Da were used.
Western blotting
Plasma samples were subjected to 1-D and 2-D Western blotting as described previously (28, 34). Antibodies used included goat antimouse apolipoprotein (APO) A1 (1:1000), rabbit antimouse APOA4 (1:1000), goat antimouse APOE (1:1000), rabbit antimouse transthyretin (TTR) (1:1000) and goat antimouse N- and C-terminal albumin (1:2000) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Each sample containing 50 μg proteins and 750 μg proteins was treated for 1-D and 2-D Western blotting, respectively. After blocking and treatment with the primary antibody followed by washing, the polyvinylidene difluoride membrane was incubated in horseradish peroxidase-linked donkey antigoat (Santa Cruz Biotechnology) or goat antirabbit sera (Millipore, Temecula, CA) (both 1:5000). The membrane was exposed to Pierce ECL Western blotting substrate (Thermo Scientific, Rockford, IL) for 1 min and then exposed to HyBlot CL autoradiography film (Denville Scientific Inc., Metuchen, NJ) for 1 min. SDS-PAGE gels were stained with Coomassie blue after protein transfer to the membrane to ensure equal loading of proteins.
Statistical analysis
Data were analyzed using one-way ANOVA followed by post hoc Tukey honestly significant difference test (P < 0.05 considered significant) using SPSS version 14.0 software (SPSS, Chicago, IL). All data are presented as the mean ± sem.
Results
Physiological parameters
As expected, mice injected with rbGH (GH group) had significantly elevated levels of endogenous mouse mIGF-I compared with controls (Table 1). Mice injected with rhIGF-I (IGF group) had significantly lower levels of endogenous mIGF-I. Endogenous mIGF-I levels did not differ from controls in the GH and IGF-I washout (GHw and IGFw) groups. As expected, only the group receiving rhIGF-I until the end of the study displayed detectable levels of hIGF-I (Table 1).
Table 1.
Physiological parameters in five groups of mice
| Control | GH | GHw | IGF-I | IGF-Iw | P value (ANOVA) | |
|---|---|---|---|---|---|---|
| mIGF-I (ng/ml) | 255.2 ± 11.8a | 322.2 ± 25.7b | 224.3 ± 15.3a | 141.7 ± 7.8c | 224.0 ± 5.9a | 8.2 × 10−8 |
| hIGF-I (ng/ml) | ND | ND | ND | 34.6 ± 4.9 | ND | |
| Body length (mm) | 92.3 ± 0.6a | 94.1 ± 0.7a,b | 95.3 ± 0.6b | 96.0 ± 0.6b | 96.7 ± 0.8b | 0.001 |
| Body weight (g) | 25.74 ± 0.34a | 28.19 ± 0.28b | 26.91 ± 0.34a,b | 28.10 ± 0.57b | 27.80 ± 0.40b | 0.0001 |
| Body compositiond | ||||||
| Lean mass (g) | 20.15 ± 0.26a | 22.34 ± 0.25b | 21.16 ± 0.31a,b | 21.98 ± 0.48b | 21.90 ± 0.48b | 0.0001 |
| Fluid mass (g) | 1.10 ± 0.06a | 1.30 ± 0.05b | 1.11 ± 0.06a | 1.30 ± 0.05b | 1.21 ± 0.05a,b | 0.029 |
| Tissue weight (g) | ||||||
| Muscle (gastrocnemius) | 0.295 ± 0.004a | 0.314 ± 0.003a,b | 0.309 ± 0.002a,b | 0.324 ± 0.008b | 0.307 ± 0.006a,b | 0.006 |
| Kidney | 0.293 ± 0.005a | 0.310 ± 0.005a,b | 0.307 ± 0.004a,b | 0.323 ± 0.004b | 0.303 ± 0.005a | 0.001 |
| Spleen | 0.059 ± 0.001a | 0.066 ± 0.001b,c | 0.061 ± 0.001a,b | 0.070 ± 0.001c | 0.066 ± 0.002b,c | 0.0001 |
| Lipid measurementd | ||||||
| Serum C (mm) | 3.00 ± 0.09a | 3.67 ± 0.12b | 3.00 ± 0.13a | 3.00 ± 0.10a | 2.6 ± 0.14a | 1.1 × 10−5 |
The physiological parameters are reported as mean ± sem. C, Cholesterol; TG, triglyceride; ND, nondetectable.
Different letters denote significant differences (P < 0.05).
Fat mass, serum TG, liver TG, and liver cholesterol show nonsignificant changes among groups, and data are not shown in this table.
Body length was significantly increased in IGF-I group, whereas the GH group showed a trend (not significant) of increase for body length (Table 1). Body weight was significantly increased in the GH and IGF-I groups (Table 1). Lean mass was also increased in the GH and IGF groups. The weights of gastrocnemius muscle, kidney, and spleen were significantly larger in the IGF-I group; however, the GH group showed only significantly larger spleen weights, with gastrocnemius muscle and kidney showing a nonsignificant trend of increase. No significant changes were found in the weights of other tissues including liver, soleus muscle, heart, lungs, brain, and adipose (data not shown). Fat mass as determined by use of the nuclear magnetic resonance body composition analyzer was not significantly different among groups, although the rbGH-treated group showed a trend toward lower fat mass values (data not shown). Fluid mass was significantly increased in the GH and IGF-I groups. In the GHw group, body weight, lean and fluid masses, muscle, kidney, and spleen weights were all similar to control levels, whereas body weight, lean mass, and spleen weight remained elevated in the IGFw group.
Serum total cholesterol was significantly higher in the GH group compared with the other four groups. Liver cholesterol showed an increased trend in the GH group but did not reach significance at the P < 0.05 level (data not shown). There was no significant difference in serum and liver triglyceride levels among groups (data not shown).
Plasma proteins altered by GH and/or IGF-I
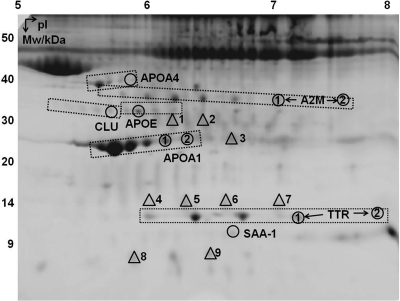
A total of 155 protein spots were reproducibly detected by 2-DE. Among them, 19 spots showed statistically significant different intensities among the five groups of mice. Figure 2 is an annotated 2-DE gel image with the selected spots of interest indicated. Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) lists the MS and MS/MS identification details of these proteins. In some cases, multiple spots were identified as the same protein, probably due to posttranslational modifications (PTM) that altered the size and/or charge of proteins. We have used the term isoforms for these different forms of the same protein. Only isoforms that are significantly altered are assigned a number and described in detail below (Fig. 2) and further documented in Table 2.
Fig. 2.
A 2-D gel image indicating proteins that are significantly altered by GH and/or IGF-I treatments. Abbreviations of the protein names are shown next to the circled spots with numbers differentiating multiple isoforms reported in this study. Triangles represent albumin, with nine different isoforms labeled 1–9. Dashed boxes encompass all isoforms identified for a particular protein; e.g. the box containing APOA1 isoforms 1 and 2 also included four other APOA1 spots. Only isoforms that were found to be significantly altered were assigned a number and described in detail in the text. Mw, Molecular weight; pI, isoelectric point.
Table 2.
Comparison of the effects of GH or IGF-I
| Immediate effect of rbGH | Immediate effect of rhIGF-I | Delayed or sustained effect of rbGH | Delayed or sustained effect of rhIGF-I | |
|---|---|---|---|---|
| Physiological parameters | ||||
| Endogenous IGF-I | ↑ | ↓ | ⇆ | ⇆ |
| Body weight | ↑ | ↑ | ⇆ | ↑ |
| Lean mass | ↑ | ↑ | ⇆ | ↑ |
| Fluid mass | ↑ | ↑ | ⇆ | ⇆ |
| Gastrocnemius muscle mass | ⇆a | ↑a | ⇆ | ⇆ |
| Kidney mass | ⇆a | ↑a | ⇆ | ⇆ |
| Spleen mass | ↑ | ↑ | ⇆ | ↑ |
| Serum total cholesterol | ↑a | ⇆a | ⇆ | ⇆ |
| Plasma proteins | ||||
| APOE | ↑a | ⇆a | ⇆ | ⇆ |
| SAA-1 | ↓a | ⇆a | ⇆ | ⇆ |
| CLU | ↓a | ⇆a | ⇆ | ⇆ |
| APOA4 | ⇆a | ↓a | ↓ | ↓ |
| TTR (1) | ↓a | ⇆a | ↓ | ↓ |
| TTR (2) | ⇆ | ⇆ | ↓ | ↓ |
| APOA1 (1 and 2) | ⇆ | ⇆ | ↓ | ↓ |
| A2m (1 and 2) | ↓ | ↓ | ↓ | ↓ |
| Albumin (1) | ⇆ | ⇆ | ↑ | ⇆ |
| Albumin (2) | ⇆a | ↑a | ⇆ | ⇆ |
| Albumin (3) | ↓a | ⇆a | ⇆ | ⇆ |
| Albumin (4) | ⇆a | ↑a | ↑ | ↑ |
| Albumin (5) | ⇆ | ⇆ | ↑ | ↑ |
| Albumin (6) | ↓a | ⇆a | ↑ | ↑ |
| Albumin (7) | ⇆ | ⇆ | ↑ | ↑ |
| Albumin (8) | ↓a | ↑a | ↑ | ↑ |
| Albumin (9) | ↓a | ↑a | ⇆ | ⇆ |
Proteins that were differentially altered by GH and IGF-I
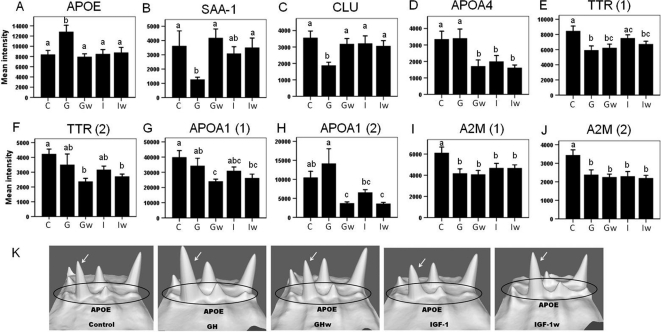
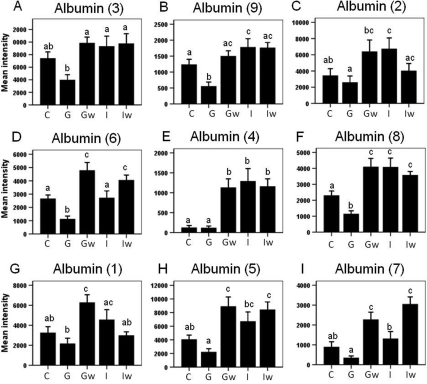
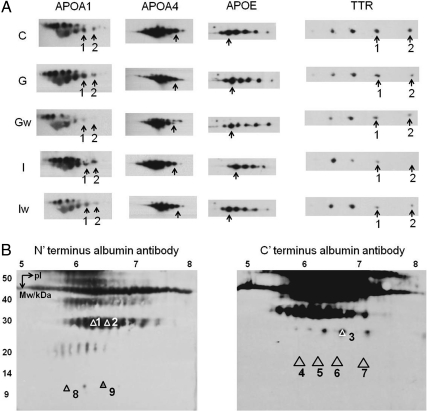
Among the 19 spots of interest, 11 were differentially regulated by GH and IGF-I (Fig. 3 A–E, and Fig. 4 A–F). Among these, one isoform of APOE was up-regulated by GH but not IGF-I (Fig. 3A). Serum amyloid protein A-1 (SAA-1) (Fig. 3B) and one isoform of clusterin (CLU) (Fig. 3C) were down-regulated by GH but not IGF-I; one isoform of APOA4 was down-regulated in the IGF-I group but not the GH group (Fig. 3D), and TTR isoform 1 was significantly down-regulated in the GH but showed only a nonsignificant trend of down-regulation in the IGF-I group (Fig. 3E). Six albumin isoforms were also differentially regulated. Albumin isoforms 3 (Fig. 4A) and 6 (Fig. 4D) were down-regulated by GH but were unchanged in the IGF-I group; isoforms 9 (Fig. 4B) and 8 (Fig. 4F) were down-regulated in the GH group but up-regulated by IGF-I; isoforms 2 (Fig. 4C) and 4 (Fig. 4E) were unchanged in the GH group but up-regulated in the IGF-I group. To better visualize the protein level change, 3-D views of protein spot intensities were generated using the PDQuest software. An illustrative example is shown in Fig. 3K where the APOE peak is more pronounced in the GH group than the other groups.
Fig. 3.
2-DE quantification of proteins that changed significantly due to GH or IGF-I treatment. Panels A–E, Proteins that show differential effects by GH vs. IGF-I; panels D–J, proteins that show significantly delayed or sustained changes by GH. Proteins isoforms correspond to those labeled in Fig. 2. Different letters denote significant differences (P < 0.05). Error bars represent sem from n = 14 mice per group. The x-axis represents five groups: C, control; G, GH; Gw, GHw; I, IGF-I; Iw, IGF-Iw. Panel K, 3-D view of spot intensities for APOE isoforms. Four peaks representing four APOE isoforms are circled, and the isoform corresponding to Figs. 2 and 3A is indicated by an arrow. The 3-D view is generated by PDQuest software (Bio-Rad).
Fig. 4.
2-DE quantification of albumin isoforms that changed significantly with GH or IGF-I treatment. Panels A–F, Albumin isoforms that show differential effects of GH vs. IGF-I treatment; panels D–I, albumin isoforms that show significant delayed effects of GH treatment. Albumin isoforms correspond to those labeled in Fig. 2. Different letters denote significant differences (P < 0.05). Error bars represent sem from n = 14 mice per group. The x-axis represents five groups: C, control; G, GH; Gw, GHw; I, IGF-I; Iw, IGF-Iw.
Proteins that showed delayed or sustained changes by GH
Of the 19 spots of interest, 13 showed significant changes induced by GH after a 7-d washout period (Fig. 3, D–J, and Fig. 4, D–I). Of these, the APOA4 isoform (Fig. 3D), TTR isoform 2 (Fig. 3F), and APOA1 isoforms 1 and 2 (Fig. 3, G and H) showed delayed down-regulation in the GHw group, whereas TTR isoform 1 (Fig. 3E) and α2-macroglobulin (A2M) isoforms 1 and 2 (Fig. 3, I and J) showed a sustained decrease in both GH and GHw groups. Finally, seven albumin isoforms showed delayed up-regulation by GH in the GHw group (Fig. 4, D–I).
Western blotting confirmation
The 1-D Western blotting was performed on APOA1, APOA4, APOE, and TTR, and no difference in protein band intensity was found among the five groups of mice (data not shown). The 2-D Western blotting on these proteins showed that the intensities of the specific isoforms had the same trend of change as that found by 2-DE quantification. For example, APOA1 isoforms 1 and 2 showed reduced intensity in the GHw group compared with control and GH groups (Fig. 5A, first column), confirming a delayed reduction of these two isoforms. The APOA4 isoform showed decreased intensity in GHw, IGF, and IGFw groups (Fig. 5A, second column). The APOE isoform was increased in the GH group (Fig. 5A, third column). TTR isoforms 1 and 2 were both decreased in the GHw group (Fig. 5A, last column). Additionally, the identities of the nine albumin isoforms were confirmed by 2-D Western blotting using either N- or C-terminal-specific albumin antibodies (Fig. 5B for 2-D Western blotting compared with Fig. 2 for isoform distribution on a 2-D gel). In Supplemental Table 1, we show the amino acid sequences of the isoforms that matched either the N or C terminus of albumin.
Fig. 5.
2-D Western blotting. Panel A, 2-D Western blotting of APOA1, APOA4, APOE, and TTR. C, Control; G, GH; Gw, GHw; I, IGF-I; Iw, IGF-Iw. Arrows indicate specific isoforms found to be significantly altered in this study. Panel B, 2-D Western blotting confirmation of albumin isoforms using N- and C-terminal-specific albumin antibodies.
Discussion
The GH/IGF-I axis is an important endocrine regulator of growth and metabolism. To date, there are limited data that differentiate the actions of GH and IGF-I. Also, to the best of our knowledge, this is the first study to analyze the effects of GH vs. IGF-I on the plasma proteome. Here we report that specific isoforms of APOE, CLU, SAA-1, APOA4, TTR, and albumin are differentially regulated by GH and IGF-I in mice.
Both GH and IGF-I treatments resulted in increased body weight. Further analysis of body composition showed that this increase was due to increases in lean body mass and fluid mass. These results are expected because both GH and IGF-I have known anabolic effects on lean body mass (5, 38), and both are known to increase fluid retention (39, 40). GH possesses lipolytic activities and reduces fat mass (41). We have previously shown that obese mice fed on high-fat diet have reduced fat mass when treated with GH (25). In the current study, we found a trend but nonsignificant decrease of fat mass of normal mice. This is likely due to the fact that the mice used in the current study were relatively lean (normal) at the start of the experiment.
rbGH treatment significantly increased endogenous IGF-I levels as expected. The decreased endogenous mIGF-I in the rhIGF-I-treated group was expected because an increase in total IGF-I will result in a decrease of endogenous GH and ultimately mIGF-I via the long-loop IGF-I feedback system. Also, the mIGF-I ELISA used to measure endogenous IGF-I does not cross-react with human IGF-I. Further analysis using a human-specific IGF-I ELISA verified the presence of the rhIGF-I in the IGF-I group. As expected, hIGF-I was not detectable after the 7-d washout phase following rhIGF-I administration because the half-life of free IGF-I is about 12 min and that of IGFBP-bound IGF-I is about 15 h (42).
The present study showed a differential effect of GH and IGF-I on lipid parameters. Serum total cholesterol was higher in the GH group but not the IGF-I group. It has been shown that chronic GH excess results in elevated serum triglyceride and total cholesterol levels in acromegalic individuals (43–45), although this has been controversial because other studies have shown decreased total cholesterol in acromegaly (46). Also, GH transgenic mice have higher total serum cholesterol levels but, interestingly, lower serum and liver triglyceride levels than control mice (29, 47, 48). The mechanism of the latter discrepancy is unknown, although it is perhaps partly due to the fact that GH transgenic mice have elevated GH levels for their entire lifespan, whereas acromegalic patients do not. We have also found an increased trend of serum triglyceride in the GH group, consistent with the elevated triglyceride levels in human acromegalic individuals (43–45). This increased trend in circulating triglyceride levels is also seen in human subjects treated with rhGH (49). Our results also indicated a lack of an IGF-I effect on lipid levels, suggesting that the elevated cholesterol levels seen in acromegaly and GH transgenic mice are likely due to a direct action of GH rather than mediated through IGF-I. Similar to mIGF-I levels and body composition, the changes of total serum cholesterol normalized 1 wk after discontinuation of GH treatment, indicating that these physiological effects of GH/IGF-I were reversible.
We used 2-DE to uncover plasma proteins responsive to GH and IGF-I. One challenge is the dynamic range of detection of the plasma protein levels. Albumin and a few other abundant proteins make up the majority of the plasma protein mass (50). To prevent background smearing of albumin at the 60- to 80-kDa region, we employed a high concentration (15%) of polyacrylamide. Using this method, we did not deplete albumin and were still able to resolve smaller albumin fragments (<40 kDa), which have been previously shown to be regulated by aging (28) and therefore may be affected by GH. A limitation of this approach is that proteins larger than 50 kDa are not resolved. However, this method is quick and reproducible for the purpose of screening relatively low molecular weight plasma biomarkers.
We have found isoforms of several proteins to be differentially regulated by GH and IGF-I. These isoforms usually have the same molecular weight but different isoelectric points, which suggests PTM. The chemical nature of these PTM is not known, and algorithms to identify these changes are still being developed. We have tried two different algorithms developed by MASCOT (freely available at www.matrixscience.com) and PEAKS (commercial software by Bioinformatics Solutions, Inc.) for PTM identification and were not able to identify them. One possibility is that peptides with the actual PTM may not be selected for MS/MS because only the most prominent precursor peptides are used in the MS/MS analysis. Future studies will be directed toward the identification of the PTM as newer and better algorithms are developed.
The present study showed a differential effect of GH and IGF-I on several isoforms of APOA4, CLU, APOE, and SAA-1. These proteins are all synthesized by the liver, which is a primary target of GH but not IGF-I because GH receptors are expressed in the liver where IGF-I receptors are not (51). These proteins are all associated with high-density lipoprotein (HDL) cholesterol, which is thought to have an antiatherogenic role (52). Down-regulation of CLU and SAA-1 and up-regulation of APOE resulted from a direct effect of GH because IGF-I did not alter the levels of these proteins, whereas the APOA4 isoform was reduced by IGF-I and not GH. However, APOA4 was also down-regulated in the GHw group, and this delayed effect may be an indirect action of IGF-I. On the other hand, isoforms of APOA1, TTR, and A2M were all down-regulated by both GH and IGF-I, indicating that GH acts on these proteins via IGF-I. The same changes in the levels of CLU, APOE, TTR, and A2M isoforms are also found in bGH transgenic mice of similar ages (4 months) (34) further confirming the regulation of these proteins by GH. In bGH mice, the total levels of CLU, APOE, and TTR show the same changes as individual isoforms (34). However, in the present study, 1-D Western blotting showed no difference in total levels (data not shown), likely due to short-term vs. chronic GH exposure.
GH, but not IGF-I, results in a decrease in CLU, which has been implicated in many disorders, including heart disease, diabetes, kidney disease, cancer, and Alzheimer's disease. As an HDL-associated protein, CLU is reduced in humans with coronary heart disease but increased in type 2 diabetic patients (53). CLU has been shown to be up-regulated in glomerulopathy (54, 55). Also, CLU acts as a tumor suppressor by inhibiting nuclear factor-κB activity at the early stage of tumorigenesis, but at later stages it may increase tumor survival by increasing tumor resistance to cancer drugs (56). Finally, as a chaperone protein, CLU is increased in Alzheimer's disease and facilitates the clearance of amyloid-β peptides (57), and sc injection of recombinant human CLU in mice has shown beneficial effects on peripheral neuropathies (58).
GH, but not IGF-I, decreases SAA-1. SAA-1 is an acute-phase protein that is highly induced in the liver under acute inflammation, infection, or injury. Unlike the other HDL-associated proteins, it has a proatherogenic effect. SAA-1 is present in HDL usually under acute inflammation conditions (59, 60). It is thought that displacement of APOA1 in HDL by SAA attenuates the antiatherogenic activity of HDL (52). Interestingly, SAA-1 is positively correlated with body mass index in humans (61) and with atherosclerotic lesion size in rabbits fed cholesterol (62). Recently, it has been suggested as a possible mediator of insulin resistance (63). In addition to HDL transportation, SAA-1 is also involved in amyloid formation (64). Interestingly, SAA-1 is decreased during normal mouse aging (28). GHR knockout (GHR−/−) mice (65) that lack GH signaling show a 3.6-fold increase in Saa2 RNA expression in the liver (66). SAA-2 protein is highly homologous (93% identical) to SAA-1. These data together with ours suggest that GH inhibits the expression of SAA. However, it is interesting that GHR null mice, which are known to be insulin sensitive, should have increased Saa2 gene expression, which is a possible mediator of insulin resistance in mice. More studies are needed to determine the role of SAA in insulin resistance and its interaction with GH.
GH, but not IGF-I, increases APOE. APOE is a major protein associated with very-low-density lipoprotein and low-density lipoprotein. It is also known to be involved in Alzheimer's disease (67) as well as modulating immune response and inflammation (68). APOE levels are increased in patients with acromegaly (69). It is also increased in rats treated with GH. This effect is not mediated by IGF-I because IGF-I administration to hypophysectomized female rats did not change APOE total levels (70). These results are consistent with ours. Finally, GH has been shown to increase APOE by increasing mRNA translation and the secretion rate of APOE from hepatocytes (71).
Interestingly, the levels of six albumin isoforms were altered by GH and IGF-I in different manners. These albumin isoforms varying in molecular mass from 8–30 kDa are likely the proteolytic products of intact serum albumin, which is 66 kDa. In plasma proteomic analysis using 2-DE, albumin is usually removed because this highly abundant protein masks the presence of other proteins. However, we have discovered multiple albumin isoforms to be significantly altered during aging in mice (28). In the present study, albumin was not depleted, which allowed for the identification of nine albumin isoforms that were significantly regulated by GH and IGF-I. Overall, our study showed that GH either had no effect or suppressed the levels of plasma albumin isoforms, whereas IGF-I tended to increase them. GH also showed delayed up-regulation of six albumin isoforms in the washout group. This may indicate that the delayed effect of GH was mediated by IGF-I. Although mIGF-I levels normalized in both GHw and IGFw groups and hIGF-I was nondetectable in the IGFw group, the up-regulation of albumin isoforms persisted in both washout groups, perhaps due to differences in half-lives of IGF-I vs. these albumin isoforms. As mentioned earlier, the isoforms observed in this study are likely the proteolytic products of intact albumin. If this is due to one or more proteases that cleave albumin, then GH and IGF-I may affect the albumin isoforms by regulating the activity of the proteases. In fact, GH and IGF-I both down-regulated two isoforms of A2M, which is a nonspecific protease inhibitor. In this sense, one possible cause of increased albumin fragments could be decreased A2M in GHw, IGF, and IGFw groups. The decreased albumin fragments in the GH group may be due to other unknown targets specific to GH. Currently, it is not known how cleavage of albumin is regulated or the functions of the albumin fragments. Interestingly, other isoforms of albumin fragments not described in this study decrease as a function of age in mice (28).
In summary, the present study examined the differential effects of GH vs. IGF-I on the mouse plasma proteome (Table 2). We have discovered protein/isoforms that are differentially regulated by GH and IGF-I, including one isoform of APOE that was specifically up-regulated by GH and one isoform of CLU, SAA-1, and TTR that was specifically down-regulated by GH. These proteins are regulated by GH but not IGF-I. In contrast, one isoform of APOA4 was down-regulated by IGF-I but not GH. In addition, a differential effect of GH and IGF-I was found on six albumin isoforms. On the other hand, two isoforms of APOA1, one isoform of TTR, and two isoforms of A2M were down-regulated both by GH and IGF-I, suggesting the effect of GH on these proteins was mediated by IGF-I. Together, the various changes in the levels of these plasma proteins may indicate differential physiological actions of GH and IGF-I on lipoprotein metabolism, inflammation, insulin resistance, diabetes, cancer, Alzheimer's disease, cardiovascular diseases, amyloid formation, and kidney functions. Discovery of these plasma proteins provides a basis for future studies on differential actions of GH and IGF-I.
Because plasma proteins are routinely used for disease diagnosis, our results may have an impact on diagnosis of GH- and IGF-I-related diseases such as acromegaly or GH or IGF-I deficiency. For example, this may be important in the subset of acromegalic patients whose levels of GH and IGF-1 do not concur (72, 73). The proteins described in this report are not used for GH/IGF-I-related diseases. However, in the future, these novel biomarkers may be useful in situations where GH and/or IGF-I levels are either elevated or suppressed. Additionally, some of the proteins found in this study are already implicated in disease diagnosis. For example, APOE allele ε4 is associated with increased risks for Alzheimer's disease, and APOE genotyping, although not used alone, can be used to facilitate the diagnosis of Alzheimer's disease (74). Another example is that a low level of serum albumin is one diagnostic measurement of liver cirrhosis (75). Importantly, the current methods detect total level of these proteins and not isoforms.
As an added benefit, our experimental design also allows the identification of biomarkers of GH or IGF-I in a doping scenario. Our data show that two isoforms of APOA1, one isoform of APOA4, two isoforms of TTR, two isoforms of A2M, and six isoforms of albumin fragments were significantly altered by GH after a 1-wk washout period. All of these proteins show the same changes by IGF-I treatment following the washout phase except for albumin isoform 1. In contrast, mIGF-I levels were the same in the GHw group and control group, suggesting that these novel biomarkers of GH have longer-lasting changes than IGF-I. The window of opportunity by using these new biomarkers certainly surpasses the current standard, which detects GH isoforms within a 36-h time window. If similar results are found in human studies, new detection assays for GH or IGF-I may be developed.
Acknowledgments
We posthumously thank the late Dr. Ross Clark for generously donating the rhIGF-I used in this study.
This work was supported by the World Anti-Doping Agency and by the State of Ohio's Eminent Scholars Program that includes a gift by Milton and Lawrence Goll. J.J.K. and E.O.L. are also supported by grants from the National Institutes of Health (DK083729, AG019899, and AG031736).
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- A2M
- α2-Macroglobulin
- APO
- apolipoprotein
- CLU
- clusterin
- 2-DE
- two-dimensional gel electrophoresis
- GHR
- GH receptor
- GHw and IGF-Iw
- GH and IGF-I washout
- HDL
- high-density lipoprotein
- mIGF-I
- mouse IGF-I
- MS
- mass spectrometry
- PTM
- posttranslational modifications
- rb
- recombinant bovine
- rh
- recombinant human
- SAA-1
- serum amyloid protein A-1
- TTR
- transthyretin.
References
- 1. Frisch H. 1999. Growth hormone and body composition in athletes. J Endocrinol Invest 22:106–109 [PubMed] [Google Scholar]
- 2. Laursen T, Jørgensen JO, Christiansen JS. 2008. The management of adult growth hormone deficiency syndrome. Expert Opin Pharmacother 9:2435–2450 [DOI] [PubMed] [Google Scholar]
- 3. Biller BM. 2007. Concepts in the diagnosis of adult growth hormone deficiency. Horm Res 68(Suppl 5):59–65 [DOI] [PubMed] [Google Scholar]
- 4. Svensson J, Johannson G. 2003. Long-term efficacy and safety of somatropin for adult growth hormone deficiency. Treat Endocrinol 2:109–120 [DOI] [PubMed] [Google Scholar]
- 5. Møller N, Jørgensen JO. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- 6. Chanson P, Salenave S. 2008. Acromegaly. Orphanet J Rare Dis 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fontana L, Partridge L, Longo VD. 2010. Extending healthy life span–from yeast to humans. Science 328:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. 2011. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3:70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steuerman R, Shevah O, Laron Z. 2011. Congenital IGF-I deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol 164:485–489 [DOI] [PubMed] [Google Scholar]
- 10. Dominici FP, Argentino DP, Muñoz MC, Miquet JG, Sotelo AI, Turyn D. 2005. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res 15:324–336 [DOI] [PubMed] [Google Scholar]
- 11. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. 2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- 12. Rosenbloom AL. 2008. Insulin-like growth factor-I (rhIGF-I) therapy of short stature. J Pediatr Endocrinol Metab 21:301–315 [DOI] [PubMed] [Google Scholar]
- 13. De Bodo RC, Kurtz M, Ancowitz A, Kiang SP. 1950. Loss of insulin hypersensitivity and development of diabetes in hypophysectomized dogs produced by purified growth hormone. Proc Soc Exp Biol Med 74:524–526 [DOI] [PubMed] [Google Scholar]
- 14. Rabinowitz D, Zierler KL. 1963. A metabolic regulating device based on the actions of human growth hormone and of insulin, singly and together, on the human forearm. Nature 199:913–915 [DOI] [PubMed] [Google Scholar]
- 15. Yuen KC, Dunger DB. 2007. Therapeutic aspects of growth hormone and insulin-like growth factor-I treatment on visceral fat and insulin sensitivity in adults. Diabetes Obes Metab 9:11–22 [DOI] [PubMed] [Google Scholar]
- 16. Holl RW, Schwarz U, Schauwecker P, Benz R, Veldhuis JD, Heinze E. 1993. Diurnal variation in the elimination rate of human growth hormone (GH): the half-life of serum GH is prolonged in the evening, and affected by the source of the hormone, as well as by body size and serum estradiol. J Clin Endocrinol Metab 77:216–220 [DOI] [PubMed] [Google Scholar]
- 17. Ding J, List EO, Okada S, Kopchick JJ. 2009. Perspective: Proteomic approach to detect biomarkers of human growth hormone. Growth Horm IGF Res 19:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Travis J. 2010. Growth hormone test finally nabs first doper. Science 327:1185. [DOI] [PubMed] [Google Scholar]
- 19. Dall R, Longobardi S, Ehrnborg C, Keay N, Rosén T, Jørgensen JO, Cuneo RC, Boroujerdi MA, Cittadini A, Napoli R, Christiansen JS, Bengtsson BA, Sacca L, Baxter RC, Basset EE, Sönksen PH. 2000. The effect of four weeks of supraphysiological growth hormone administration on the insulin-like growth factor axis in women and men. GH-2000 Study Group. J Clin Endocrinol Metab 85:4193–4200 [DOI] [PubMed] [Google Scholar]
- 20. Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosén T, Dall R, Boroujerdi MA, Bassett EE, Healy ML, Pentecost C, Wallace JD, Powrie J, Jørgensen JO, Saccà L. 2000. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. J Clin Endocrinol Metab 85:1505–1512 [DOI] [PubMed] [Google Scholar]
- 21. Fintini D, Brufani C, Cappa M. 2009. Profile of mecasermin for the long-term treatment of growth failure in children and adolescents with severe primary IGF-1 deficiency. Ther Clin Risk Manag 5:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bates SE. 1991. Clinical applications of serum tumor markers. Ann Intern Med 115:623–638 [DOI] [PubMed] [Google Scholar]
- 23. Dworacka M, Winiarska H. 2005. The application of plasma 1,5-anhydro-d-glucitol for monitoring type 2 diabetic patients. Dis Markers 21:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Angelucci F, Mirabella M, Frisullo G, Caggiula M, Tonali PA, Batocchi AP. 2005. Serum levels of anti-myelin antibodies in relapsing-remitting multiple sclerosis patients during different phases of disease activity and immunomodulatory therapy. Dis Markers 21:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. 2009. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia 52:1647–1655 [DOI] [PubMed] [Google Scholar]
- 26. Tanaka N, Ryoke T, Hongo M, Mao L, Rockman HA, Clark RG, Ross J., Jr 1998. Effects of growth hormone and IGF-I on cardiac hypertrophy and gene expression in mice. Am J Physiol 275:H393–H399 [DOI] [PubMed] [Google Scholar]
- 27. Okada S, List EO, Sankaran S, Kopchick JJ. 2010. Plasma protein biomarkers correlated with the development of diet-induced type 2 diabetes in mice. Clin Proteomics 6:6–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding J, Kopchick JJ. 15 September 2010. Plasma biomarkers of mouse aging. Age (Dordr) 10.1007/s11357-010-9179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE. 2009. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology 150:1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- 31. List EO, Berryman DE, Palmer AJ, Qiu L, Sankaran S, Kohn DT, Kelder B, Okada S, Kopchick JJ. 2007. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics 7:1140–1149 [DOI] [PubMed] [Google Scholar]
- 32. Qiu L, List EO, Kopchick JJ. 2005. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics 4:1311–1318 [DOI] [PubMed] [Google Scholar]
- 33. Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. 2009. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res 19:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding J, Berryman DE, Kopchick JJ. 2 March 2011. Plasma proteomic profiles of bovine growth hormone transgenic mice as they age. Transgenic Res 10.1007/s11248-011-9499-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen B, Sackmann-Sala L, Cruz-Topete D, Jørgensen JO, Jessen N, Lundby C, Kopchick JJ. 2011. Novel serum biomarkers for erythropoietin use in humans: a proteomic approach. J Appl Physiol 110:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cruz-Topete D, List EO, Okada S, Kelder B, Kopchick JJ. 2011. Proteomic changes in the heart of diet-induced pre-diabetic mice. J Proteomics 74:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, Jorgensen JO, Kopchick JJ. 2011. Serum proteome changes in acromegalic patients following transsphenoidal surgery: novel biomarkers of disease activity. Eur J Endocrinol 164:157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Velloso CP. 2008. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moller J, Frandsen E, Fisker S, Jorgensen JO, Christiansen JS. 1996. Decreased plasma and extracellular volume in growth hormone deficient adults and the acute and prolonged effects of GH administration: a controlled experimental study. Clin Endocrinol (Oxf) 44:533–539 [DOI] [PubMed] [Google Scholar]
- 40. Møller J. 2003. Effects of growth hormone on fluid homeostasis. Clinical and experimental aspects. Growth Horm IGF Res 13:55–74 [DOI] [PubMed] [Google Scholar]
- 41. Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. 2006. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology 147:2801–2808 [DOI] [PubMed] [Google Scholar]
- 42. Guler HP ZJ, Schmid C, Froesch ER. 1989. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 121:753–758 [DOI] [PubMed] [Google Scholar]
- 43. Colao A, Spinelli L, Cuocolo A, Spiezia S, Pivonello R, di Somma C, Bonaduce D, Salvatore M, Lombardi G. 2002. Cardiovascular consequences of early-onset growth hormone excess. J Clin Endocrinol Metab 87:3097–3104 [DOI] [PubMed] [Google Scholar]
- 44. Boero L, Manavela M, Gomez Rosso L, Insua C, Berardi V, Fornari MC, Brites F. 2009. Alterations in biomarkers of cardiovascular disease (CVD) in active acromegaly. Clin Endocrinol (Oxf) 70:88–95 [DOI] [PubMed] [Google Scholar]
- 45. Vilar L, Naves LA, Costa SS, Abdalla LF, Coelho CE, Casulari LA. 2007. Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 13:363–372 [DOI] [PubMed] [Google Scholar]
- 46. Parkinson C, Drake WM, Wieringa G, Yates AP, Besser GM, Trainer PJ. 2002. Serum lipoprotein changes following IGF-I normalization using a growth hormone receptor antagonist in acromegaly. Clin Endocrinol (Oxf) 56:303–311 [DOI] [PubMed] [Google Scholar]
- 47. Frick F, Bohlooly-Y M, Lindén D, Olsson B, Törnell J, Edén S, Oscarsson J. 2001. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. Am J Physiol Endocrinol Metab 281:E1230–E1239 [DOI] [PubMed] [Google Scholar]
- 48. Olsson B, Bohlooly-Y M, Fitzgerald SM, Frick F, Ljungberg A, Ahrén B, Törnell J, Bergström G, Oscarsson J. 2005. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology 146:920–930 [DOI] [PubMed] [Google Scholar]
- 49. Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, Jørgensen JO. 2007. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab 292:E920–E927 [DOI] [PubMed] [Google Scholar]
- 50. Anderson NL, Anderson NG. 2002. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol Cell Proteomics 1:845–867 [DOI] [PubMed] [Google Scholar]
- 51. LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr 1995. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 16:143–163 [DOI] [PubMed] [Google Scholar]
- 52. Kontush A, Chapman MJ. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58:342–374 [DOI] [PubMed] [Google Scholar]
- 53. Kujiraoka T, Hattori H, Miwa Y, Ishihara M, Ueno T, Ishii J, Tsuji M, Iwasaki T, Sasaguri Y, Fujioka T, Saito S, Tsushima M, Maruyama T, Miller IP, Miller NE, Egashira T. 2006. Serum apolipoprotein J in health, coronary heart disease and type 2 diabetes mellitus. J Atheroscler Thromb 13:314–322 [DOI] [PubMed] [Google Scholar]
- 54. Ghiggeri GM, Bruschi M, Candiano G, Rastaldi MP, Scolari F, Passerini P, Musante L, Pertica N, Caridi G, Ferrario F, Perfumo F, Ponticelli C. 2002. Depletion of clusterin in renal diseases causing nephrotic syndrome. Kidney Int 62:2184–2194 [DOI] [PubMed] [Google Scholar]
- 55. Rosenberg ME, Girton R, Finkel D, Chmielewski D, Barrie A, 3rd, Witte DP, Zhu G, Bissler JJ, Harmony JA, Aronow BJ. 2002. Apolipoprotein J/Clusterin Prevents a Progressive Glomerulopathy of Aging. Mol Cell Biol 22:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rizzi F, Bettuzzi S. 2010. The clusterin paradigm in prostate and breast carcinogenesis. Endocr Relat Cancer 17:R1–R17 [DOI] [PubMed] [Google Scholar]
- 57. Nuutinen T, Suuronen T, Kauppinen A, Salminen A. 2009. Clusterin: a forgotten player in Alzheimer's disease. Brain Res Rev 61:89–104 [DOI] [PubMed] [Google Scholar]
- 58. Dati G, Quattrini A, Bernasconi L, Malaguti MC, Antonsson B, Nicoletti F, Alliod C, Di Marco R, Sagot Y, Vitte PA, Hiver A, Greco B, Roach A, Zaratin PF. 2007. Beneficial effects of r-h-CLU on disease severity in different animal models of peripheral neuropathies. J Neuroimmunol 190:8–17 [DOI] [PubMed] [Google Scholar]
- 59. Artl A, Marsche G, Lestavel S, Sattler W, Malle E. 2000. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol 20:763–772 [DOI] [PubMed] [Google Scholar]
- 60. Kisilevsky R, Subrahmanyan L. 1992. Serum amyloid A changes high density lipoprotein's cellular affinity. A clue to serum amyloid A's principal function. Lab Invest 66:778–785 [PubMed] [Google Scholar]
- 61. Zhao Y, He X, Shi X, Huang C, Liu J, Zhou S, Heng CK. 2010. Association between serum amyloid A and obesity: a meta-analysis and systematic review. Inflamm Res 59:323–334 [DOI] [PubMed] [Google Scholar]
- 62. Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM. 2007. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res 48:2344–2353 [DOI] [PubMed] [Google Scholar]
- 63. Scheja L, Heese B, Zitzer H, Michael MD, Siesky AM, Pospisil H, Beisiegel U, Seedorf K. 2008. Acute-phase serum amyloid A as a marker of insulin resistance in mice. Exp Diabetes Res 2008:230837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Merlini G, Bellotti V. 2003. Molecular mechanisms of amyloidosis. N Engl J Med 349:583–596 [DOI] [PubMed] [Google Scholar]
- 65. Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. 1997. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG, Waters MJ. 2005. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 25:66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim J, Basak JM, Holtzman DM. 2009. The role of apolipoprotein E in Alzheimer's disease. Neuron 63:287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Finch CE. 2010. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA 107(Suppl 1):1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wildbrett J, Hanefeld M, Fücker K, Pinzer T, Bergmann S, Siegert G, Breidert M. 1997. Anomalies of lipoprotein pattern and fibrinolysis in acromegalic patients: relation to growth hormone levels and insulin-like growth factor I. Exp Clin Endocrinol Diabetes 105:331–335 [DOI] [PubMed] [Google Scholar]
- 70. Sjöberg A, Oscarsson J, Olofsson SO, Edén S. 1994. Insulin-like growth factor-I and growth hormone have different effects on serum lipoproteins and secretion of lipoproteins from cultured rat hepatocytes. Endocrinology 135:1415–1421 [DOI] [PubMed] [Google Scholar]
- 71. Sjöberg A, Oscarsson J, Edén S, Olofsson SO. 1994. Continuous but not intermittent administration of growth hormone to hypophysectomized rats increases apolipoprotein-E secretion from cultured hepatocytes. Endocrinology 134:790–798 [DOI] [PubMed] [Google Scholar]
- 72. Popovic V. 2005. Are there alternative tests for diagnosis of acromegaly? J Endocrinol Invest 28:73–74 [PubMed] [Google Scholar]
- 73. Clemmons DR. 2006. Clinical utility of measurements of insulin-like growth factor 1. Nat Clin Pract Endocrinol Metabol 2:436–446 [DOI] [PubMed] [Google Scholar]
- 74. Tsuang D, Larson EB, Bowen J, McCormick W, Teri L, Nochlin D, Leverenz JB, Peskind ER, Lim A, Raskind MA, Thompson ML, Mirra SS, Gearing M, Schellenberg GD, Kukull W. 1999. The utility of apolipoprotein E genotyping in the diagnosis of Alzheimer disease in a community-based case series. Arch Neurol 56:1489–1495 [DOI] [PubMed] [Google Scholar]
- 75. Rothschild MA, Oratz M, Zimmon D, Schreiber SS, Weiner I, Van Caneghem A. 1969. Albumin synthesis in cirrhotic subjects with ascites studied with carbonate-14C. J Clin Invest 48:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]