Abstract
We focused on the biological activity of the collagen extracts obtained from the giant edible jellyfish, Nemopilema nomurai. Jellyfish collagen extracts stimulates the production of immunoglobulins (Igs) and cytokines by human hybridoma cells and human peripheral blood lymphocytes. Therefore, we examined the immunoregulatory function of jellyfish collagen extracts in mice. Intake of jellyfish collagen extracts facilitated the Ig production activity of lymphocytes from spleen and Peyer’s patch. Furthermore, the levels of Igs in the serum clearly increased after the administration of jellyfish collagen extracts. Intake of bovine collagen from Achilles’ tendon also activated lymphocytes activity in mice. The activity of total and antigen-specific Ig production in splenocytes from OVA-challenged mice was also enhanced by collagen intake. However, the total and OVA-specific IgE levels in the serum were not affected by the collagen intake. These results suggested that jellyfish collagen extracts stimulates an immune response in vivo, without inducing allergic complications.
Keywords: Collagen, Immunostimulation, Immunoglobulin production, Jellyfish, Lymphocyte
Introduction
It is necessary to screen factors that enhance immunoglobulin (Ig) production by human hybridoma HB4C5 cells for increasing the production of monoclonal antibodies. Several such factors have been identified as Ig production stimulating factors (IPSFs), and some of them are found in foodstuffs. Hen egg white lysozyme (EC 3.2.1.17) was also identified as the IPSF and its mode of action was investigated (Murakami et al. 1997; Sugahara et al. 2000; Sugahara et al. 2002). We demonstrated that some basic proteins other than lysozyme stimulated Ig production by hybridomas and PBLs. Lysine-rich histones and polylysine accelerated IgM production by HB4C5 cells (Sugahara et al. 1994). Considering these results, we focused on collagen, which is a basic protein.
We chose jellyfish as the source of collagen. Jellyfish are planktonic marine invertebrates, and about 200 species of jellyfish belonging to the classes Scyphozoa (phylum Cnidaria) and Cubozoa have been described. Jellyfishes are very important foods in Asia, especially in China and Japan. Only jellyfish belonging to the order Rhizostomeae are harvested as food. Rhizostomes are favored because they are typically larger and have more rigid bodies than other scyphozoans. Edible jellyfish belong to 5–7 species, and include Lobonema smithi, Lobonemoides gracilis, Rhopilema esculentum, Rhopilema hispidum, and Stomolophus nomurai. Regardless of their size or shape, most jellyfish are very fragile. They contain more than 95% water and more than 40% of their dry weight is collagen (Miura and Kimura 1985). In recent times, explosive increases in the number of jellyfish have frequently occurred all over the world, for example in the Japan Sea, the Atlantic seaboard of the United States, and the Mediterranean littoral. In particular, swarms of giant jellyfish have caused serious fishery problems in the Japan Sea. These increases in the jellyfish population may be the result of environmental pollution in their ambient environments. Therefore, to effectively use untapped bioresourses, jellyfish were chosen as the source of collagen.
Firstly, we examined the stimulatory effect of jellyfish extracts on antibody production by HB4C5 cells. We found that jellyfish extracts enhanced IgM production by HB4C5 cells 34-fold (Sugahara et al. 2006). IgM and IgG production by hPBLs also increased 2.8- and 1.4-fold, respectively. Moreover, jellyfish extracts stimulated the production of tumor necrosis factor (TNF)-α and interferon (IFN)-γ by hPBLs. The stimulation effect of jellyfish extracts was completely lost by collagenase treatment. In addition, collagens from bovine Achilles’ tendon and rat tail showed stimulated IgM production of HB4C5 cells (Sugahara et al. 2006). These results suggest that the active substances in jellyfish extracts are not contaminants such as endotoxins, but collagen or collagen-related proteins. This also suggests that collagen facilitates the immune response, irrespectively of its source. The in vivo stimulation of the immune response by jellyfish collagen has not been reported thus far.
We proposed a putative mode of action of immunostimulation by jellyfish collagen. Jellyfish collagen stimulates the transcription of Igs and several cytokines in the immune cells, HB4C5 and hPBLs (Nishimoto et al. 2008). However, this stimulation of the transcription activity is not sufficient to explain the very high production of immune proteins by HB4C5 cells. Further investigations revealed that collagen also facilitated IgM production in HB4C5 cells in which transcription was suppressed. These findings suggest that jellyfish collagen stimulates not only transcription but also translation activity in these cells. In this study, we focused on the immunological effects of jellyfish collagen extracts in vivo.
Materials and methods
Reagents
Collagen from bovine Achilles’ tendon was purchased from Nacalai Tesque (Kyoto, Japan). Ovalbumin (OVA) was purchased from Sigma (St. Louis, MO, USA).
Preparation of jellyfish collagen extracts
The collagen from the exumbrella of edible jellyfish was denatured and solubilized by acid and heat treatment as follows: The exumbrella (5 g) from the jellyfish Nemopilema nomurai was cut into small pieces, extracted with 50 mL of dilute hydrochloric acid (pH 3.0) for 12 h, and heated at 121 °C for 20 min. Insoluble substances were removed by centrifugation at 8,000×g for 20 min, and the acid-soluble substances were collected. The collected supernatant was dialyzed against 10 mM sodium phosphate buffer (NaPB) (pH 7.4). Collagenase treatment of the extracts completely inactivated the Ig production stimulation effects on HB4C5 cells, and Achilles tendon collagen activates Ig production of the cells (Sugahara et al. 2006). These facts suggest that the active substance in jellyfish extracts is collagen, and the impurities in the extracts do not affect the Ig produced by the hybridoma cells. Cation-exchange chromatography and SDS–PAGE analysis revealed the purity of the active collagen fragments in the extracts is 88% (Nishimoto et al. 2008).
Experimental animals
Female BALB/c mice (6-week-old) were purchased from Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan). The mice were kept in specific pathogen-free facilities, and were acclimated to their housing environment for 1 week before the experiment. They were given free access to food and water, and the animal room was maintained under the following controlled conditions: temperature, 24 °C: humidity, 55%: and 12-h light/12-h dark cycle. All animal experiments were performed in accordance with protocols approved by the Ehime University Animal Care and Use Committee and the applicable guidelines and regulations.
Cells and cell culture
Mice lymphocytes from several immune tissues were used to investigate the effect of jellyfish collagen extracts on Ig production in vitro and in vivo. Mice were killed by cervical dislocation, and the spleen, Peyer’s patch (PP), and mesenteric lymph node (MLN) were excised from the cavity. The splenocytes were strained using a nylon mesh (pore size, 40 nm), and the collected cells were hemolyzed using hemolysis buffer (155 mM NH4Cl, 15 mM NaHCO3, 1 mM EDTA; pH 7.3). Splenocytes were obtained after washing twice with PBS. Lymphocytes from the PP and MLN were obtained from 5 mice and pooled. The splenocytes and PP lymphocytes were inoculated in ERDF medium (Kyokuto Pharmaceutical, Tokyo, Japan) supplemented with 5% fetal bovine serum (FBS). After cultivation, the culture supernatant was collected, and the Ig level was determined using an enzyme-linked immunosorbent assay (ELISA).
Immunostimulatory effect of jellyfish collagen on OVA-challenged mice
A specific immune reaction against ovalbumin (OVA) was induced in mice. Mice were orally given 20 μL of the jellyfish collagen extracts (10 mg·kg body weight−1·day−1) or 10 mM NaPB as vehicle control for 26 days. The mice received OVA (100 μg body−1) with an alum adjuvant (LSL Inc., Japan) by intraperitoneal (i.p.) injection on days 7 and 21 to elicit a specific IgE response (Yano et al. 2007). After 5 days of the second OVA induction (day 26), blood and splenocytes were collected. Body weight was measured on every alternate day during 26 days. The splenocytes collected were inoculated in ERDF medium supplemented with 5% FBS. After 48 h of cultivation, the culture supernatant was collected for measuring the Ig level by ELISA, as mentioned below. The total IgE and OVA-specific IgE levels in the serum were also measured using ELISA kit (DS Pharma Biochemical, Osaka Japan).
Measurement of Ig levels
To measure the Ig levels in the sera and culture media, a sandwich ELISA was performed as described previously (Sugahara et al. 2008). Briefly, 1.0 μg mL−1 of a goat anti-mouse IgA, IgG, and IgM antibodies (Invitrogen, Carlsbad, CA), and 1.0 μg mL−1 of rat anti-mouse IgE antibodies (BD Biosciences, Franklin Lakes, NJ) in 50 mM sodium carbonate-bicarbonate buffer (pH 9.6) were used to fix mouse IgA, IgG, IgM, and IgE, respectively. Each well of a 96-well ELISA plate (Nunc, Roskilde, Denmark) was treated with 100 μL of each antibody solution for 2 h at 37 °C. After washing three times with 0.05% Tween 20-PBS (T-PBS), each well was blocked with 5% skim milk in PBS for 2 h at 37 °C. After the blocking reaction, each well was washed 3 times with T-PBS, and treated with 50 μL of culture supernatant or mouse serum diluted with 5% skim milk-PBS for 1 h at 37 °C. To detect IgA and IgG, each well was treated for 1 h with 100 μL well−1 of the horseradish peroxidase (HRP)-conjugated to anti-mouse IgA and IgG antibodies (Invitrogen) diluted 1,000 times with 5% skim milk-PBS. For measuring the IgM and IgE levels, each well was treated with 100 μL of biotinylated rat anti-mouse IgE antibodies (BD Biosciences) or biotinylated goat anti-mouse IgM antibodies (Invitrogen) diluted 2,000 times with 5% skim milk-PBS, and the plate was incubated for 1 h at 37 °C. After washing 3 times with T-PBS, we added 100 μL of streptavidin-HRP conjugate (Invitrogen) diluted 4,000 times with 1% BSA-PBS to each well, and incubated the plate for 1 h. After washing 3 times with T-PBS, 100 μL of 0.6 mg mL−1 of 2,2-azino-bis (ethylbenzothazoline-6-sulfonic acid diammonium salt)(ABTS) dissolved in a 0.03% H2O2-0.05 M citrate buffer (pH 4.0) was added to each well for the enzyme reaction, and the absorbance at 415 nm was measured after adding 100 μL well−1 of 1.5% oxalic acid for the termination of the reaction. The Ig concentration was quantitatively determined using a standard curve and mouse Ig standard solution. The Ig production assay was performed three times.
Assay of the OVA-specific Ig levels
The levels of the anti-OVA Igs were also measured using ELISA. Briefly, 100 μL of 200 μg mL−1 of OVA in 50 mM sodium carbonate-bicarbonate buffer (pH 9.6) was added to each well of a 96-well ELISA plate (Nunc), and the plate was incubated for 2 h at 37 °C. After washing 3 times with T-PBS, each well was blocked with 300 μL of 5% skim milk-PBS for 2 h at 37 °C. Then, 50 μL of the culture supernatant was added and the plate was incubated for 1 h at 37 °C. To detect OVA-specific IgA and IgG, each well was treated for 1 h with 100 μL well−1 of HRP-conjugated anti-mouse IgA and IgG antibodies (Invitrogen) diluted 1,000 times with 5% skim milk-PBS. For measuring the level of OVA-specific IgM, 100 μL of biotinylated goat anti-mouse IgM antibodies (Invitrogen) diluted 2,000 times with 5% skim milk-PBS was added to the wells and the plate was incubated for 1 h at 37 °C. After washing with T-PBS 3 times, 100 μL of streptavidin-HRP conjugate (Invitrogen) diluted 4,000 times with 1% BSA-PBS was added to each well, and the plate was incubated for 1 h. After washing 3 times with T-PBS, 0.1 μL of 0.6 mg mL−1 of ABTS-0.03% H2O2-0.05 M citrate buffer (pH 4.0) was added to each well, and the absorbance at 415 nm was measured after adding 100 μL well−1 of 1.5% oxalic acid. The OVA-specific IgE level was determined by using a mouse OVA-specific IgE detection kit (DS Pharma Biochemical). The detection limit of this ELISA kit was 2.7 ng mL−1.
Determination of gene expression levels
Total RNA was isolated from the PP lymphocytes obtained from mice administered jellyfish collagen extracts for 1 week by using Sepasol-RNA I Super (Nacalai Tesque, Kyoto, Japan), according to the manufacturer’s instructions. Total RNA (1 mg) was used as a template for a cDNA synthesis reaction performed using MMLV-reverse transcriptase (Promega, Madison, WI) and an oligo-(dT)20 primer (Toyobo, Osaka, Japan). Denaturation at 95 °C for 1 min was followed by primer annealing at 60 °C for 1 min, extension at 72 °C for 1 min, and a final extension phase of 7 min. The cDNA was subjected to real-time PCR with the Step One Plus System (Applied Biosystems, Foster City, CA). The amount of mRNA for IgA, IgM, TNF-α, IFN-γ, and IL-4 was normalized to that for β-actin. The specific primer sequences for each gene are as follows. Mouse β-actin: sense, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and antisense, 5′-ATGGAGCCACCGATCCACA-3′; mouse IgA: sense, 5′-TGCACAGCTTTCTTCTGCAC-3′ and antisense, 5′-TGCCAGCCTCACATGTACTC-3′; mouse IgM: sense, 5′-GGGGCACTGGTCACATACTT-3′ and antisense, 5′-CAGCTCGTGAGCAACTGAAC-3′; mouse TNF-α: sense, 5′-AAGCCTGTAGCCCACGTCGTA-3′ and antisense, 5′-GGCACCACTAGTTGGTTGTCTTTG-3′; mouse IFN-γ: sense, 5′-CGGCACAGTCATTGAAAGCCTA-3′ and antisense, 5′-GTTGCTGATGGCCTGATTGTC-3′; and mouse IL-4: sense, 5′-TCTCGAATGTACCAGGAGCCATATC-3′ and antisense, 5′-AGCACCTTGGAAGCCCTACAGA-3′.
Flow cytometric analysis of cell surface marker staining on lymphocyte
Lymphocytes were prepared from the spleen, MLN, and PP obtained from BALB/c mice after administration of jellyfish collagen for 28 days, and washed with cold PBS. The cell surface markers on the lymphocytes were stained on ice with FITC-conjugated anti-mouse B220 and PE-conjugated anti-mouse CD3 (Biolegend, San Diego, CA) antibodies for 40 min. After washing with cold PBS, the cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Statistical analysis
All results are expressed as means ± SD. Tukey’s t test was used to assess the statistical significance of the difference. p < 0.05*, p < 0.01**, and p < 0.001*** were considered to be statistically significant.
Results
Effect of jellyfish collagen extracts on Ig production by splenocytes and PP lymphocytes in vitro
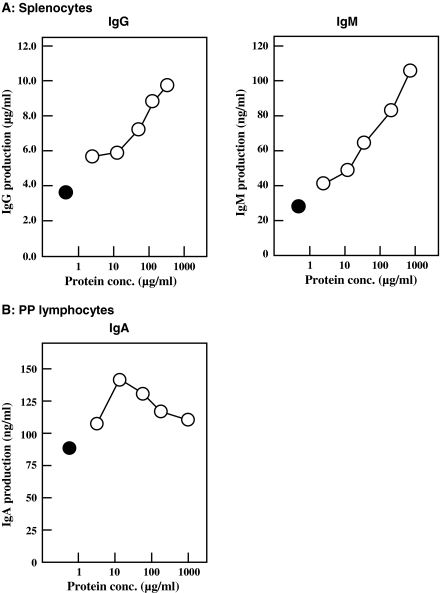
Splenocytes and PP lymphocytes (2.0 × 105 cells mL−1) from 6-week-old female BALB/c mice were inoculated into 5% FBS-ERDF medium supplemented with various concentrations of jellyfish collagen extracts. Lymphocytes were pooled from 5 mice. After cultivation for 24 h, the amount of each class of Ig in the culture media was measured using ELISA. As shown in Fig. 1a, jellyfish collagen extracts stimulated IgG and IgM production by splenocytes in a dose-dependent manner. A concentration of 750 μg mL−1 of collagen increased the production of these Igs 2.7- and 3.8-fold, respectively. IgA production of splenocytes was enhanced about 1.7-fold at a collagen concentration of 180 μg mL−1, as compared to control IgA production (data not shown). On the other hand, IgA production by PP lymphocytes was enhanced about 1.5-fold at a collagen concentration of 10 μg mL−1 (Fig. 1b). The optimum collagen concentration for stimulating IgA production was lower than that for stimulating IgG and IgM production.
Fig. 1.
Effect of jellyfish collagen extracts on Ig production by splenocytes and PP lymphocytes. Splenocytes (a) and PP lymphocytes (b) were isolated from 5 6-week-old female BALB/c mice were pooled and inoculated at 2.0 × 105 cells mL−1 in 5% FBS-ERDF medium with (open circle) and without (filled circle) jellyfish collagen extracts at varying concentrations. After 24 h of cultivation in a CO2 incubator at 37 °C under a humidified atmosphere of 5% CO2-95% air, the amount of Ig in the culture medium was measured using ELISA. The results are expressed as the means ± SD of 3 independent measurements
Effect of jellyfish collagen extracts in vivo
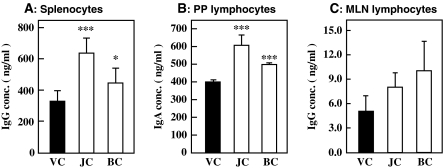
As we have reported earlier, jellyfish collagen extracts stimulates Ig production by human hybridoma cells and hPBLs (Sugahara et al. 2006). It also stimulates Ig production by mouse lymphocytes in vitro. Hence, we examined the immunostimulatory effect of jellyfish collagen extracts in vivo. An ex vivo assay to examine the activity of immune cells was performed as mentioned below. Female 6-week-old BALB/c mice (6 animals per group) were orally administered 10 mg·kg body weight−1 of jellyfish collagen extracts, 1 mg·kg body weight−1 of bovine Achilles tendon collagen or 10 mM NaPB as vehicle control for 14 days, and lymphocytes were harvested from spleen, MNL and PP. Lymphocytes from the immune tissue of each body were pooled, and inoculated at 8.0 × 105 cells mL−1 in 5% FBS-ERDF medium. After cultivation for 24 h, Ig concentration in each culture medium was measured using the ELISA. As shown in Fig. 2a, intake of jellyfish collagen extracts and bovine Achilles’ tendon collagen significantly activated IgG production by spleen lymphocytes. In addition, these collagen preparations accelerated the IgA production activity of PP lymphocytes with statistical difference against vehicle control. However, the production of IgG and IgM by PP lymphocytes was not affected by collagen intake (data not shown). IgG production activity of MLN lymphocytes was slightly activated by these collagens. These findings suggest that intake of jellyfish collagen extracts stimulates the activity of immune cells in vivo, and bovine Achilles tendon collagen also possesses the immunostimulation activity in vivo.
Fig. 2.
Effect of oral administration of jellyfish collagen extracts on Ig production activity of lymphocytes. Female BALB/c mice (6 animals per group) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts (JC), 1 mg·kg body weight−1·day−1 of bovine Achilles tendon collagen (BC) or 10 mM NaPB as the vehicle control (VC) for 14 days. Lymphocytes were isolated from spleen, PP and MNL on day 14, and inoculated in 5% FBS-ERDF medium at 8.0 × 105 cells mL−1. After 24 h of cultivation, the Ig concentration in the culture medium was measured using ELISA. The results are represented as the means ± SD (n = 5). *p < 0.05 and ***p < 0.001
Effect of jellyfish collagen extracts on mRNA expression in PP lymphocytes
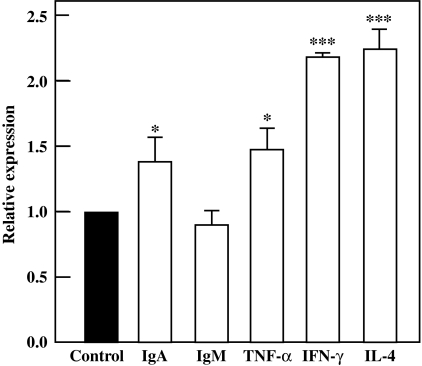
IgA production activity of PP lymphocytes was stimulated by the orally administration of jellyfish collagen extracts. Therefore, we evaluated the expression levels of mRNAs for IgA and other immune proteins in PP lymphocytes. As shown in Fig. 3, the expression level of IgA mRNA increased significantly by about 1.4-fold. This result correlated with the acceleration of IgA production in PP lymphocytes, as shown in Fig. 2. The expression levels of TNF-α, IFN-γ, and IL-4 mRNA in PP lymphocytes were also significantly enhanced by collagen administration. These facts suggest that intake of jellyfish collagen extracts activates not only IgA-producing plasma cells, but also other immune cells in PP.
Fig. 3.
Effect of jellyfish collagen extracts on mRNA expression in PP lymphocytes. Female BALB/c mice (n = 5) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts or 10 mM NaPB for 7 days. PP lymphocytes were isolated from each mouse on day 7, and total RNA was prepared. Quantitative real-time PCR assay was performed using mRNAs for IgA, IgM, TNF-α, IFN-γ, and IL-4. The results are represented as the means ± SD (n = 5). Asterisks indicate that the data are significantly different from the control data. *p < 0.05 and ***p < 0.001
Effect of jellyfish collagen extracts on Ig levels in the serum
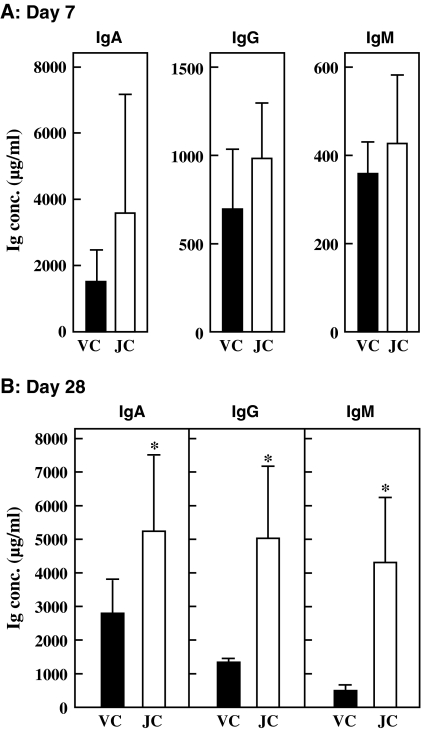
The activity of PP lymphocytes was accelerated by oral administration of jellyfish collagen extracts for 14 days. We then expanded the administration period, and evaluated the immunostimulatory effect again. Female 6-week-old BALB/c mice (6 mice per group) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts or 10 mM NaPB for 28 days. Serum was obtained from blood collected from the eye socket on days 7 and 28. The Ig level in the serum from each mouse was measured using ELISA. As indicated in Fig. 4a, on day 7, there was an increase in the levels of IgA, IgG, and IgM in the serum after the intake of jellyfish collagen extracts: this increase was not statistically significant. However, the Ig level in the serum was significantly enhanced because of collagen intake when the administration period was increased to 28 days (Fig. 4b). Thus, the immunostimulatory effect of collagen was better demonstrated after increasing the administration period.
Fig. 4.
Effect of oral administration of jellyfish collagen extracts on Ig level in serum. Female 6-week-old BALB/c mice (6 animals per group) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts or 10 mM NaPB as vehicle control for 28 days. The serum was obtained by drawing blood from the eye socket on days 7 (a) and day 28 (b). IgA, IgG, and IgM concentrations in the serum were measured using ELISA. Solid bars and open bars show the Ig levels in the serum of mice administered the vehicle control (VC) and jellyfish collagen extracts (JC), respectively. The results are presented as mean ± SD (n = 6). Asterisks indicate that the data are significantly different as compared to control data. *p < 0.05
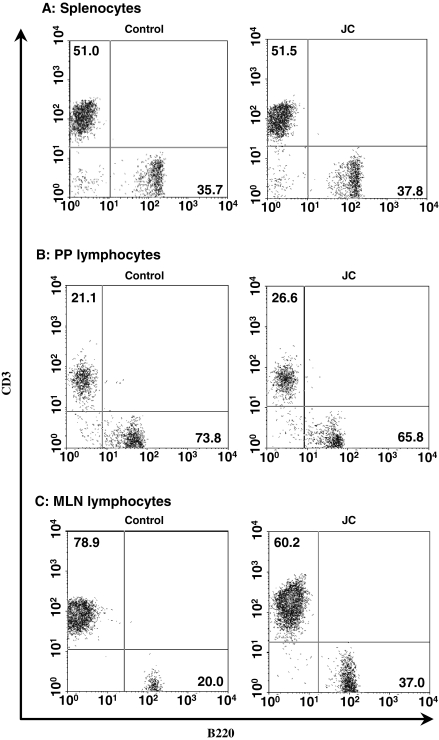
Effect of jellyfish collagen extracts on the population of lymphocytes in immune tissues
We next examined the effect of collagen extracts on the B/T cell proportion in immune tissues. Lymphocytes were prepared from the spleen, MLN, and PP of 5 BALB/c mice after the administration of jellyfish collagen extracts for 28 days. The ratio of B220+ cells (B cells) and CD3+ cells (T cells) of the pooled lymphocytes from each tissue were determined. As shown in Fig. 5, jellyfish collagen extracts did not affect the balance of B and T cells in the spleen. T cells in PP were slightly activated by the collagen uptake. This fact was consistent with activation of cytokine gene expression levels in PP lymphocytes as shown in Fig. 3. On the other hand, ratio of B cells in MLN was increased by collagen intake.
Fig. 5.
Effect of jellyfish collagen on the cell population of lymphocytes in immune tissues. Cell population analysis of lymphocytes in spleen, PP, and MLN from 5 collagen-administered mice was performed. Splenocytes and lymphocytes from 5 mice were pooled, and the cell surface markers on the lymphocytes were stained on ice with FITC-conjugated anti-mouse B220 and PE-conjugated anti-mouse CD3 antibodies for 30 min. After washing with cold PBS, the cells were analyzed by using a FACSCalibur flow cytometer
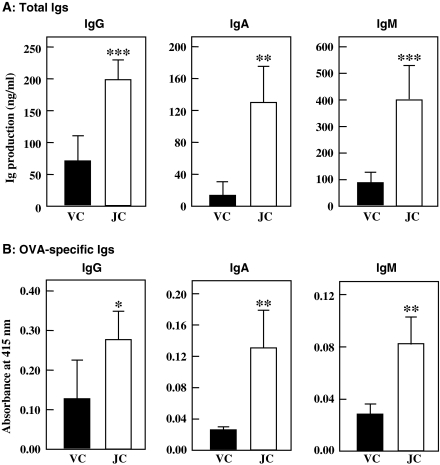
Effect of jellyfish collagen extracts on Ig production by splenocytes from OVA-challenged mice
To examine the in vivo effect of jellyfish collagen extracts on the specific immune response, the mice were immunized with OVA-alum. OVA-alum administration induces a specific IgE response in mice (Sugahara et al. 2008). Female BALB/c mice (6 mice per group) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts or vehicle (10 mM NaPB) for 26 days, and immunized 2 times with OVA-alum (on days 7 and 21). The splenocytes were harvested on day 26 and cultured in 5% FBS-ERDF medium at 1.0 × 106 cells mL−1 for 48 h. As indicated in Fig. 6, total Ig production activity of each Ig class was significantly stimulated by collagen intake. This fact correlated with the increase in serum Ig level shown in Fig. 4a. Thus, the increase in the serum Ig level was caused by the activation of Ig production activity of the splenocytes. In addition, the administration of jellyfish collagen extracts enhanced the production of OVA-specific Igs (Fig. 6b). This means that collagen accelerates antigen-specific immune reactions.
Fig. 6.
Effect of oral administration of jellyfish collagen extracts on Ig production by splenocytes from OVA-challenged mice. Female 6-week-old BALB/c mice (6 animals per group) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts or 10 mM NaPB for 26 days, and immunized with OVA-alum on days 7 and 21. Splenocytes were harvested on day 26, inoculated in 5% FBS-ERDF medium at 1.0 × 106 cells mL−1 and cultured for 48 h. The amounts of total Igs (a) and OVA-specific Igs (b) in the culture media were measured using ELISA. Solid bars and open bars show the Ig production activity of splenocytes obtained from each mouse administered the vehicle control (VC) and jellyfish collagen extracts (JC), respectively. The results are presented as mean ± SD (n = 6). Asterisks indicate that the data are significantly different from the control data. *p < 0.05, **p < 0.01, and ***p < 0.001
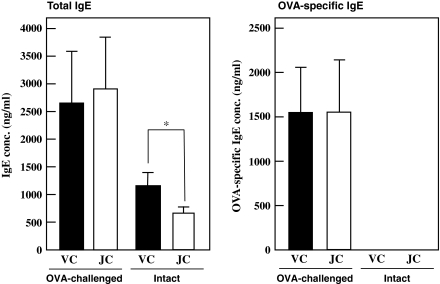
To determine whether jellyfish collagen extracts enhances allergic responses, we determined the IgE level in the serum from OVA-challenged mice. As shown in Fig. 7, both total IgE and OVA-specific IgE levels in serum from OVA-challenged mice were not affected by collagen intake. On the centrally, jellyfish collagen decreased serum IgE level in intact mice.
Fig. 7.
Effect of oral administration of jellyfish collagen extracts on IgE level in serum. Female 6-week-old BALB/c mice (6 animals per group) were orally administered 10 mg·kg body weight−1·day−1 of jellyfish collagen extracts or 10 mM NaPB for 26 days, and were immunized twice with OVA-alum (on days 7 and 21). The serum was obtained by drawing blood from the eye socket of OVA-challenged mice on day 26. The amounts of total IgE and OVA-specific IgE in the serum of each mouse were measured using ELISA. Intact mice were not injected OVA and orally administered 10 mg·kg body weight−1·day−1 of jellyfish extracts or 10 mM NaPB for 26 days. Solid and open bars show the IgE levels in serum obtained from each mouse administered the vehicle control (VC) and jellyfish collagen extracts (JC), respectively. The results are presented as mean ± SD (n = 6)
Discussion
Collagen is a well-characterized protein. It belongs to a group of fibrous proteins with very high tensile strength that form the main component of connective tissues in animals. Type 2 collagen is widely used to induce arthritis in mouse models of rheumatoid arthritis (Courtenay et al. 1980). In these models, collagen may induce immunological effects, leading to autoimmune and inflammatory responses (Firestein and Corr 2005). Moreover, collagen is an important platelet agonist thought to be involved in the early stages of platelet activation during both hemostasis and thrombosis (Sugiyama et al. 1987; Pignatelli et al. 1998). However, collagen-induced stimulation of the immune response has not been reported thus far. We have previously reported the immunostimulatory effect of jellyfish collagen extracts. Jellyfish collagen extracts enhanced the production of IgM by human hybridoma HB4C5 cells, and that of IgM, IgG, IFN-γ, and TNF-α by hPBLs (Sugahara et al. 2006). In this study, we revealed that jellyfish collagen extracts also stimulates the in vitro production of Ig by mouse lymphocytes from the spleen and PP. We investigated the mode of action of jellyfish collagen extracts in vitro. Collagen from jellyfish stimulates the transcription of IgM mRNA in HB4C5 cells and that of the mRNAs of several cytokines in hPBLs. However, the level of stimulation of transcription activity was insufficient to explain the high amount of proteins produced by these cells. Indeed, collagen also facilitated IgM production by HB4C5 cells in which transcription was suppressed. Thus, our findings suggest that jellyfish collagen extracts stimulates not only transcription but also translation activity (Nishimoto et al. 2008).
The immunostimulatory effect of jellyfish collagen extracts is advantageous for its use as functional food, and therefore, we investigated this effect of jellyfish collagen extracts in vivo. The intake of jellyfish collagen extracts for 14 days significantly stimulated IgG production activity in splenocytes and IgA production activity in PP lymphocytes. IgA is a major class of immunoglobulins mediating the intestinal immune response. Along with IgA mRNA, the mRNA expression levels of some cytokines also increased in the PP lymphocytes. These facts correlate with our previous findings that jellyfish collagen accelerates transcription activity in HB4C5 cells (Nishimoto et al. 2008). Oral administration of collagen from bovine Achilles’ tendon also activated Ig production of lymphocytes in spleen and PP. This fact suggests the active substance in jellyfish extracts is collagen, and immunostimulation effect of collagen in vitro and in vivo is not exclusive activity of jellyfish collagen.
The B cell population in the MLN was increased after collagen intake. Jellyfish collagen also stimulated not only IgA production, but also helper T cells in PP. It is supposed from these results that intake of jellyfish collagen activates helper T cells and induces cytokine production in PP. This induces B cells to produce Igs. The mode of action of the stimulatory effect of jellyfish collagen on intestinal immune response has not been revealed yet. Since collagen administration clearly stimulated the immune response, we increased the administration period to 4 weeks. This caused an increase in the Ig level in the serum along with the activation of Ig production in the splenocytes.
The OVA-specific Ig production by splenocytes was activated by the intake of jellyfish collagen extracts. However, the total and OVA-specific IgE levels in the serum were not affected by the intake of jellyfish collagen extracts. It is supposed from these facts that collagen does not aggravate the IgE-mediated allergic response in vivo. These findings indicate that jellyfish collagen extracts are useful for activating the immune response in vivo, and it is valuable as a functional food. The exact mechanism underlying the immunostimulatory effect of jellyfish collagen extracts in vivo is not completely clear, and the detailed effects of collagen are being investigated.
Our previous data show that purified collagen from bovine Achilles’ tendon stimulated IgM production by human hybridoma HB4C5 cells (Sugahara et al. 2006). In addition, oral administration of bovine Achilles’ tendon collagen also stimulated Ig production by oral administration. These facts suggest that the immunostimulatory effect of collagen is not specific for that from jellyfish, and that the effect is irrespective to its origin. Hence, it is possible that the immunostimulation effect is a common feature of collagen molecule, especially Type-I collagen. This means that the immunological functions of collagen should be considered when collagen is used for studies of the autoimmune and inflammatory responses.
Abbreviations
- BSA
Bovine serum albumin
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- IFN-γ
Interferon-γ
- Ig
Immunoglobulin
- IL-4
Interleukin 4
- IPSF
Immunoglobulin production stimulating factor
- MLN
Mesenteric lymph node
- NaPB
Sodium phosphate buffer
- OVA
Ovalbumin
- PBL
Peripheral blood lymphocyte
- PBS
Phosphate buffered saline
- PP
Peyer’s patch
- TNF-α
Tumor necrosis factor-α
References
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Corr M. Common mechanisms in immune-mediated inflammatory disease. J Rheumatol Suppl. 2005;73:8–13. [PubMed] [Google Scholar]
- Miura S, Kimura S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. J Biol Chem. 1985;260:15352–15356. [PubMed] [Google Scholar]
- Murakami F, Sasaki T, Sugahara T. Lysozyme stimulates immunoglobulin production by human–human hybridoma and human peripheral blood lymphocytes. Cytotechnology. 1997;24:177–182. doi: 10.1023/A:1007936629501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Goto Y, Morishige H, Shiraishi R, Doi M, Akiyama K, Yamauchi S, Sugahara T. The mode of action of the immunostimulatory effect of collagen from jellyfish. Biosci Biotechnol Biochem. 2008;72:2806–2814. doi: 10.1271/bbb.80154. [DOI] [PubMed] [Google Scholar]
- Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood. 1998;91:484–490. [PubMed] [Google Scholar]
- Sugahara T, Sasaki T, Murakami H. Enhancement of immunoglobulin productivity of human–human hybridoma HB4C5 cells by basic proteins and poly-basic amino acids. Biosci Biotechnol Biochem. 1994;58:2212–2214. doi: 10.1271/bbb.58.2212. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Murakami F, Yamada Y, Sasaki T. The mode of actions of lysozyme as an immunoglobulin production stimulating factor. Biochim Biophys Acta. 2000;1475:27–34. doi: 10.1016/s0304-4165(00)00041-6. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Yamada Y, Yano S, Sasaki T. Heat denaturation enhanced immunoglobulin production stimulating activity of lysozyme from hen egg white. Biochim Biophys Acta. 2002;1572:19–24. doi: 10.1016/s0304-4165(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Ueno M, Goto Y, Shiraishi R, Doi M, Akiyama K, Yamauchi S. Immunostimulation effect of the jellyfish collagen. Biosci Biotechnol Biochem. 2006;70:2131–2137. doi: 10.1271/bbb.60076. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Nishimoto S, Miyazaki Y. Effects of polyamines on proliferation and IgM productivity of human–human hybridoma, HB4C5 cells. Cytotechnology. 2008;57:115–122. doi: 10.1007/s10616-007-9115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Okuma M, Ushikubi F, Sensaki S, Kanaji K, Uchino H. A novel platelet aggregating factor found in a patient with defective collagen-induced platelet aggregation and autoimmune thrombocytopenia. Blood. 1987;69:1712–1720. [PubMed] [Google Scholar]
- Yano S, Umeda D, Yamashita T, Yamada K, Tachibana H. Dietary flavones suppresses IgE and Th2 cytokines in OVA-immunized BALB/c mice. Eur J Nutr. 2007;46:257–263. doi: 10.1007/s00394-007-0658-7. [DOI] [PubMed] [Google Scholar]