Abstract
Because of the numerous types of neurons in the brain, and particularly the forebrain, neuron type-specific expression will benefit many potential applications of direct gene transfer. The two most promising approaches for achieving neuron type-specific expression are targeted gene transfer to a specific type of neuron and using a neuron type-specific promoter. We previously developed antibody-mediated targeted gene transfer with Herpes Simplex Virus (HSV-1) vectors by modifying glycoprotein C (gC) to replace the heparin binding domain, which mediates the initial binding of HSV-1 particles to many cell types, with the Staphylococcus A protein ZZ domain, which binds immunoglobulin (Ig) G. We showed that a chimeric gC--ZZ protein is incorporated into vector particles and binds IgG. As a proof-of-principle for antibody-mediated targeted gene transfer, we isolated complexes of these vector particles and an anti-NMDA NR1 subunit antibody, and demonstrated targeted gene transfer to neocortical cells that contain NR1 subunits. However, because most forebrain neurons contain NR1, we obtained only a modest increase in the specificity of gene transfer, and this targeting specificity is of limited utility for physiological experiments. Here, we report efficient antibody-mediated targeted gene transfer to NMDA NR2B- or NR2A-containing cells in rat postrhinal cortex, and a neuron-specific promoter further restricted recombinant expression to neurons. Of note, because NR2A-containing neurons are relatively rare, these results show that antibody-mediated targeted gene transfer with HSV-1 vectors containing neuron type-specific promoters can restrict recombinant expression to specific types of forebrain neurons of physiological significance.
Keywords: targeted gene transfer, NMDA receptor NR2B subunit, NMDA receptor NR2A subunit, herpes simplex virus vector, glycoprotein C, Staphylococcus A protein
1. Introduction
Given the complex cellular composition of the brain, and especially the forebrain, neuron type-specific recombinant gene expression is required for many potential uses of direct gene transfer into neurons. The two primary approaches for obtaining neuron type-specific expression are modifying a virus vector particle protein for targeted gene transfer to a specific type of neuron or use of a neuron type-specific promoter (Kasahara et al., 1994; Muller et al., 2003; Rasmussen et al., 2007; Song et al., 1997; Wang et al., 2005; Wickham et al., 1996a; Wickham, 2003). Importantly, targeted gene transfer supports efficient neuron type-specific expression by reducing the background of gene transfer to undesirable neuron types. Of note, these two approaches are complementary, and more restricted specificities of expression cay be obtained by using both of these approaches. Thus, targeting gene transfer to cells that contain specific NMDA receptor subunits, in combination with a neuron-specific promoter, could selectively support expression in neurons that contain specific NMDA receptor subunits. This specificity in expression would have multiple uses in neural gene transfer studies for gene therapy or basic neuroscience.
Targeted gene transfer has been developed using classical retrovirus, lentivirus, adeno-associated virus (AAV), adenovirus, and Herpes Simplex Virus (HSV-1) vectors (Buning et al., 2003; Cao et al., 2008; Cao et al., 2010; Douglas et al., 1996; Grandi et al., 2004; Kasahara et al., 1994; Laquerre et al., 1998a; Peng and Russell, 1999; Wang et al., 2005; Wickham et al., 1996a; Wickham et al., 1996b; Wickham, 2003). The most direct targeting strategy is to modify a vector particle protein to add a specific binding capability, but a limitation of this strategy is that it is specific for a particular ligand. A more general targeting strategy is to modify a vector particle to bind an antibody. This strategy theoretically supports targeting to any cell surface epitope for which an antibody exists, or can be derived. Antibody-mediated targeted gene transfer has been developed by modifying a specific vector particle protein to contain the Staphylococcus A protein ZZ domain, an immunoglobulin (Ig) G binding domain. This strategy has been used to target classical retrovirus, lentivirus, AAV, adenovirus, and sindbis virus vectors to specific peripheral cell types (Bergman et al., 2003; Morizono et al., 2001; Morizono and Chen, 2005; Morizono et al., 2005; Ohno et al., 1997; Ried et al., 2002; Tai et al., 2003; Volpers et al., 2003), and to target HSV-1 vectors to a specific cell type in the brain (Cao et al., 2010).
Helper virus-free HSV-1 plasmid (amplicon) vectors have desirable properties and can support both targeted gene transfer and use of neuron-specific promoters. These vectors have a large capacity and efficiently transduce neurons (Fraefel et al., 1996; Geller and Breakefield, 1988; Geller et al., 1991). Of note, long-term, neuron-specific expression in forebrain areas is supported by HSV-1 vectors that contain a modified neurofilament heavy gene promoter (Sun et al., 2004; Zhang et al., 2005). Importantly, targeted gene transfer is based on the entry mechanism for wt HSV-1: HSV-1 particle entry is mediated by the outermost layer of a HSV-1 particle, the envelope, a lipid bilayer containing ~10 viral-encoded glycoproteins (Roizman and Sears, 1993), and entry requires specific sequential steps (Spear and Longnecker, 2003). Initial binding to glycosaminoglycans, primarily heparin sulfate, on cell surface proteoglycans is mediated by HSV-1 glycoprotein C (gC) and gB (Laquerre et al., 1998b; Mardberg et al., 2001; Shukla and Spear, 2001; Tal-Singer et al., 1995). Next, gD binds to specific receptors, and most cells contain at least one receptor for HSV-1 (Spear and Longnecker, 2003). Entry occurs by HSV-1 envelope fusion to the cell membrane and requires gB, gD, gH, and gL. The first studies on targeted gene transfer with HSV-1 vectors modified gC to remove the heparin binding domain and add a ligand for a specific protein on the cell surface, specifically a chimeric gC--erythropoietin (Laquerre et al., 1998a) or gC--His tag (Grandi et al., 2004). We reported the first targeted gene transfer to a specific type of neuron, nigrostriatal neurons, using HSV-1 vectors that contain either gC--glial cell line-derived neurotrophic factor (GDNF) or gC--brain-derived neurotrophic factor (BDNF) protein (Cao et al., 2008; Wang et al., 2005). Next, we developed a general strategy for targeting gene transfer to many different types of neurons, based on antibody-mediated targeted gene transfer (Cao et al., 2010). We constructed a chimeric gC---Staphylococcus A ZZ domain protein (gC--ZZ), and showed this protein is incorporated into HSV-1 particles and binds IgG. Complexes of these vector particles and an anti-NMDA receptor NR1 subunit antibody supported targeted gene transfer to NR1 subunit-containing neurons in rat neocortex, with long-term expression (Cao et al., 2010). However, because a majority of neocortical neurons contain NR1 subunits, the improvement in NR-1-neuron-specific expression was a relatively modest 15 to 20 %, and this targeting specificity is of limited practical utility.
We now report on restricting recombinant expression to relatively rare types of neurons, of physiological significance, by using antibody-mediated targeted gene transfer in combination with a neuron-specific promoter. We developed targeted gene transfer to cells in rat postrhinal (POR) cortex that contain either NMDA NR2B or NR2A subunits. Further, the vector contained a modified neurofilament heave gene promoter that restricts expression to neurons (Zhang et al., 2000). Thus, this strategy restricts expression to neurons that contain either NMDA NR2B or NR2A subunits. Targeting increased NR2A-containing neuron-specific expression 50 %, from 25 to 75 %, demonstrating that targeting can support significant increases in specificity. The capability to selectively express genes in relatively rare types of forebrain neurons will benefit specific gene transfer studies.
2. Results
2.1. Selective expression in NMDA NR2B subunit-containing neurons in rat POR cortex is supported by antibody-mediated targeted gene transfer to NR2B-containing cells with a vector containing a neuron-specific promoter
Helper virus-free HSV-1 vector packaging was performed using a set of five cosmids that represent the HSV-1 genome, but lack the packaging site (contained in the a sequence) (Fraefel et al., 1996), and contain gC--ZZ for targeted gene transfer (Cao et al., 2010). Vector particle-antibody complexes were formed and purified using our established procedures (Cao et al., 2010). The resulting HSV-1 vector particles contain the vector, gC--ZZ+antibody for binding to specific types of neurons, and the other HSV-1 envelope glycoproteins required for subsequent cell entry (Spear and Longnecker, 2003).
The experimental design supports quantifying both targeted and untargeted gene transfer in POR cortex, in the same rats. Comparisons within the same rats are desirable because, given the heterogeneous cellular composition of neocortex, specific injections of HSV-1 vectors into neocortex likely support gene transfer to different neuron populations in different rats, complicating comparisons between rats. As HSV-1 particle entry occurs at a localized site on the cell surface, multiple HSV-1 virus particles can infect the same neuron (Javier et al., 1986; Stevens, 1975; Thompson et al., 1983), and multiple HSV-1 vector particles can infect the same neuron (Card et al., 2011; Kobiler et al., 2010; Starr et al., 1996); thus, competition between experimental, targeted vector particles and control, untargeted vector particles is unlikely to be a significant issue. Gene transfer used our established procedures for gene transfer into POR cortex (Zhang et al., 2005) and vectors containing a neuron-specific promoter, comprised of the chicken β-globin insulator (INS), an enhancer from the rat tyrosine hydroxylase (TH) promoter, and a mouse neurofilament heavy gene promoter (NFH), the INS-TH-NFH promoter (Zhang et al., 2000). Using untargeted gene transfer and a vector containing the INS-TH-NFH promoter, the vast majority of transduced cells are near the injection site in POR cortex, small numbers of transduced cells are found in specific neocortical areas that project to POR cortex (~1 % of the number in POR cortex), and no transduced cells are observed in any of the subcortical areas that were examined (Zhang et al., 2005). Further, in POR cortex, we observed >90 % neuron-specific expression, and 52 or 45 % glutamatergic- or GABAergic-specific, respectively (Zhang et al., 2005).
We targeted gene transfer to cells that contain NMDA NR2B receptors by using an anti-NMDA NR2B antibody. The targeted gene transfer vector particles were pINS-TH-NFHlac/gC--ZZ+anti-NR2B; the vector expresses β-galactosidase (β-gal) from the INS-TH-NFH promoter. The control, untargeted vector particles were pINS-TH-NFHflag-TH/ires/aadc/gC--wt; this vector uses the INS-TH-NFH promoter to express a flag-tagged TH (and aromatic amino acid decarboyxlase (AADC), not assayed here). We co-injected mixtures containing equal titers (titers in methods) of these two vector particles.
pINS-TH-NFHlac/gC--ZZ+anti-NR2B supported targeted gene transfer to neurons that contain NMDA NR2B receptors. The rats were sacrificed at 8 days after gene transfer, and alternating sections were assayed for either β-gal-immunoreactivity (IR) and NR2B-IR or flag-IR and NR2B-IR. Photomicrographs showed that using pINS-TH-NFHlac/gC--ZZ+anti-NR2B, most of the transduced cells contained NR2B-IR (Fig. 1A–C). In contrast, using pINS-TH-NFHflag-TH/ires/aadc/gC--wt, many transduced cells contained NR2B-IR, and many of these cells lacked NR2B-IR (Fig. 1D–F). As a control for the assay, omission of the primary antibodies resulted in no IR (Fig. 1G–I). For many of the transduced cells, the β-gal-IR or flag-IR filled the cell body (see arrows in Fig. 1A and D); some cells were out of plane of focus and only a portion of the cell body contained IR. β-gal can enter processes only by passive diffusion, and most of the IR was in the cell body; we previously used an axon-targeted β-gal to fill axons (Zhang et al., 2010b). Of note, these sections were 25 μm thick, and the plane of focus for our microscope is a small fraction of 25 μm; thus, most costaining likely represented the same cell rather than two overlapping cells, especially as a neuron cell body can be 40 μm in diameter. Importantly, cell counts (Table 1) showed that pINS-TH-NFHlac/gC--ZZ+anti-NR2B supported an average of 81 % expression in NR2B-IR neurons, and pINS-TH-NFHflag-TH/ires/aadc/gC--wt supported an average of 57 % expression in NR2B-IR neurons. Of note, pINS-TH-NFHlac/gC--ZZ+anti-NR2B supported a statistically significant increase in transduction of NR2B-containing neurons compared to pINS-TH-NFHflag-TH/ires/aadc/gC--wt (p<0.001 ANOVA). Also of note, pINS-TH-NFHlac/gC--ZZ+anti-NR2B and pINS-TH-NFHflag-TH/ires/aadc/gC--wt transduced similar numbers of neurons (p>0.05), suggesting that the relatively large HSV-1 particle-antibody complexes can diffuse through the extracellular space to NR2B-containing neurons (see discussion).
Fig. 1.
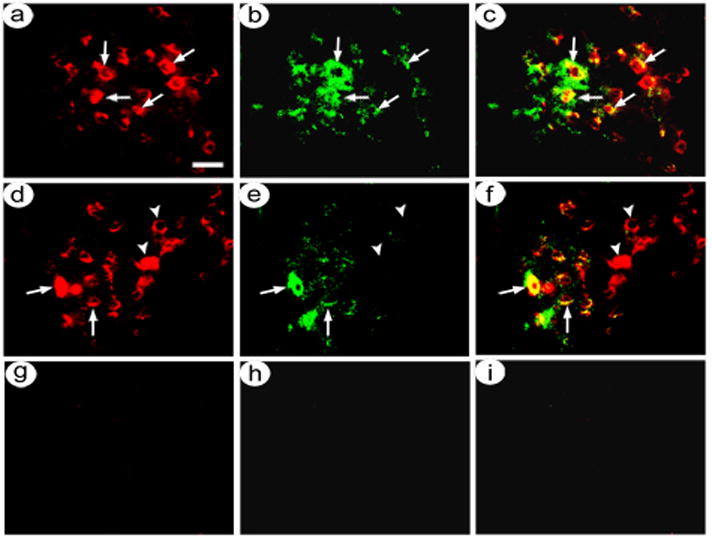
Costaining for recombinant gene expression in NMDA NR2B-containing neurons in a rat sacrificed at eight days after co-injection of pINS-TH-NFHlac/gC--ZZ+anti-NR2B and pINS-TH-NFHflag-TH/ires/aadc/gC--wt into POR cortex. Adjacent sections were costained using either mouse anti-β-gal or mouse anti-flag (detects flag-TH) and rabbit anti-NMDA NR2B, and these antibodies were visualized using rhodamine- or fluorescein-conjugated secondary antibodies, respectively. (A–C) pINS-TH-NFHlac/gC--ZZ+anti-NR2B supports β-gal expression predominantly in NR2B-containing neurons; β-gal-IR (A), NR2B-IR (B), and merge (C). Arrows, costained cells. (D–F) pINS-TH-NFHflag-TH/ires/aadc/gC--wt supports flag-TH expression in neurons that either contain or lack NR2B-IR; flag-IR (D), NR2B-IR (E), and merge (F). Arrowheads, flag-IR only. (G–I) Omitting the primary antibodies from the assay results in minimal fluorescence; rhodamine-conjugated secondary antibody-IR (G), fluorescein-conjugated secondary antibody-IR (H), and merge (I). Scale bar: 30 μm.
Table 1.
The numbers of expressing cells and the percentage of NR2B-IR or NR2A-IR costaining for rats sacrificed at 8 days after co-injection of either pINS-TH-NFHlac/gC--ZZ+anti-NR2B or +anti-NR2A and pINS-TH-NFHflag-TH/ires/aadc/gC--wt into POR cortex
| Targetinfg Antibody | pINS-TH-NFHlac/gC--ZZ+IgG | pINS-TH-NFHflag-TH/ires/aadc/gC--wt | ||
|---|---|---|---|---|
| β-gal-IR Cells | % NR2B- or NR2A-IR Costaining | Flag-IR Cells | % NR2B- or NR2A-IR Costaining | |
| anti-NR2B | 289±58 | 81±0 | 260±80 | 57±3 |
| anti-NR2A | 159±24 | 75±1 | 172±30 | 25±1 |
Three hemispheres were analyzed for each condition. Values shown are mean±s.e.m.
2.2. Antibody-mediated targeted gene transfer to a relatively rare type of neuron in rat POR cortex, NMDA NR2A subunit-containing neurons
We established targeted gene transfer to NMDA NR2A subunit-containing neurons by using an anti-NMDA NR2A subunit antibody and the experimental design described above (pINS-TH-NFHlac/gC--ZZ+anti-NR2A vector particles and control pINS-TH-NFHflag-TH/ires/aadc/gC--wt particles; 1:1 mixture of IVP). Rats were sacrificed at 8 days after gene transfer, and alternating sections were assayed for either β-gal-IR and NR2A-IR or flag-IR and NR2A-IR. Photomicrographs showed that using pINS-TH-NFHlac/gC--ZZ+anti-NR2A, the majority of the transduced neurons contained NR2A-IR (Fig. 2A–C). In contrast, using pINS-TH-NFHflag-TH/ires/aadc/gC--wt, most of the transduced neurons lacked NR2A-IR (Fig. 2D–F). Cell counts (Table 1) showed that pINS-TH-NFHlac/gC--ZZ+anti-NR2A supported an average of 75 % expression in NR2A-IR neurons, and pINS-TH-NFHflag-TH/ires/aadc/gC--wt supported an average of only 25 % expression in NR2B-IR neurons. Of note, pINS-TH-NFHlac/gC--ZZ+anti-NR2A supported a statistically significant increase in transduction of NR2A-containing neurons compared to pINS-TH-NFHflag-TH/ires/aadc/gC--wt (p<0.001). Also of note, pINS-TH-NFHlac/gC--ZZ+anti-NR2A and pINS-TH-NFHflag-TH/ires/aadc/gC--wt transduced similar numbers of neurons (p>0.05), again suggesting that the large HSV-1 particle-antibody complexes can diffuse through the extracellular space to the relatively rare NR2A-containing neurons (see discussion).
Fig. 2.
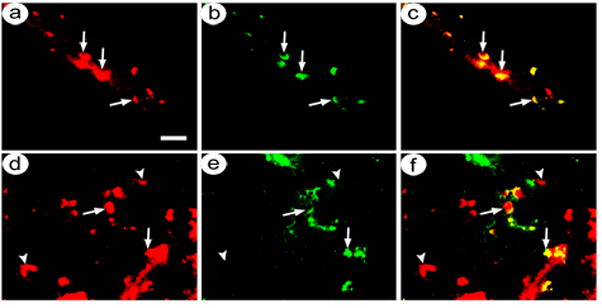
Costaining for recombinant expression in NMDA NR2A-containing neurons in a rat sacrificed at eight days after co-injection of pINS-TH-NFHlac/gC--ZZ+anti-NR2A and pINS-TH-NFHflag-TH/ires/aadc/gC--wt into POR cortex. Sections were costained using either mouse anti-β-gal or mouse anti-flag and rabbit anti-NMDA NR2A. (A–C) pINS-TH-NFHlac/gC--ZZ+anti-NR2A supports β-gal expression predominantly in NR2A-containing neurons; β-gal-IR (A), NR2A-IR (B), and merge (C). Arrows, costained cells. (D–F) pINS-TH-NFHflag-TH/ires/aadc/gC--wt supports TH expression predominantly in neurons that lack NR2A-IR; flag-IR (D), NR2A-IR (E), and merge (F). Arrowheads, flag-IR only. Scale bar: 30 μm.
3. Discussion
We have developed a general strategy for restricting recombinant gene expression to specific types of forebrain neurons. Antibody-mediated targeted gene transfer restricts expression to specific types of cells in a forebrain area; in this report, those containing either NMDA NR2B or NR2A subunits; and expression is further restricted to neurons by use of a neuron-specific promoter. Of note, the capability to obtain large increases in the specificity of gene transfer, and to target gene transfer to relatively rare types of neurons, those containing NMDA NR2A subunits, suggests that this approach will be applicable to multiple types of neurons, and shows that HSV-1 particles can diffuse significant distances through the extracellular space. The capability to restrict recombinant expression to specific types of forebrain neurons has numerous applications to both basic neuroscience and gene therapy studies.
3.1. Targeted gene transfer to NR2B- or NR2A-containing cells
We previously reported this approach to targeted gene transfer and the gC-ZZ construct; further, as a proof-of-principle, we demonstrated targeted gene transfer to cells that contain NMDA NR1 subunits (Cao et al., 2010). However, because most neocortical cells contain NR1, we obtained only a modest increase in the specificity of gene transfer (15 to 20 %), and this targeting specificity is of limited utility for physiological experiments.
Here, we report much larger increases in the specificity of gene transfer, up to 50 %, and targeted gene transfer to relatively rare types of neurons of physiological significance. Targeted gene transfer to NR2B-containing neurons supported a 24 % increase in the specificity of gene transfer, and targeted gene transfer to NR2A-containing neurons supported a 50 % increase in the specificity of gene transfer. With targeting to NR2B- or NR2A-containing neurons, 19 or 25 % of the tranduced neurons apparently lack either NR2B or NR2A, respectively, and this background of untargeted gene transfer is likely predominately due to two factors. First, although these vector particles lack the primary glycosaminoglycan binding domains on gB and gC, which represent the major glycosaminoglycan binding domains on HSV-1 particles, these modified vector particles likely retain some glycosaminoglycan binding capability. Thus, these modified vector particles likely support some nonspecific gene transfer, as reflected in their capability to inefficiently transduce fibroblast cells that lack either NR2B or NR2A, as shown in the titering assay. Second, the assays for targeted gene transfer depend on detectable levels of either NR2B or NR2A in the cell body, and some neurons likely contain a specific subunit primarily in processes, with only low levels in the cell body, and any such transduced neurons will be incorrectly scored as false negatives. Overall, competition between targeted gene transfer and the remaining background of untargeted gene transfer likely determines the efficiency of targeted gene transfer. In summary, targeting can support large increases in the specificity of gene transfer, at least 50 % increases, and such large increases in the neuron type specificity of expression will be useful in gene therapy and physiological studies.
Relatively high levels of targeted gene transfer to relatively rare neurons, specifically NR2A-containing neurons, demonstrated that HSV-1 particles can diffuse significant distances through the extracellular space, and retain biological activity. HSV-1 particles are large, ~120–280 nm diameter (Spear and Roizman, 1981), and the targeted gene transfer procedure increases the size of the vector particles by adding IgG to the surface of these particles. Thus, there was a potential issue with limited diffusion interfering with targeted gene transfer to relatively rare types of neurons. Of note, our results indicate this is not a major complication because targeting to NR2B-containing, and particularly NR2A-containing, neurons had high specificity, indicating that the vector particles could diffuse through the extracellular space to the appropriate types of neurons. Moreover, a second potential issue was reduced numbers of transduced neurons for targeted gene transfer to relatively rare types of neurons because of the limited stability of the vector particles and the time required for the vector particle-antibody complexes to diffuse to their appropriate target neurons. Also of note, our results indicate this is not a major complication because targeted gene transfer was efficient; similar numbers of neurons were transduced by targeted gene transfer to NR2B-containing, and particularly NR2A-containing, neurons as by untargeted gene transfer.
3.2. Potential applications to basic neuroscience and gene therapy studies
The numbers of positive cells were modest, ~300 to 500 per injection site (targeted and untargeted particles combined). Of note, we have observed changes in learning after transducing similar numbers of neurons with a constitutively active protein kinase C (Zhang et al., 2005; Zhang et al., 2009; Zhang et al., 2010a). Moreover, the experimental design used here differed in two critical aspects from our study that obtained expression in ~11,400 cells at 6 months after gene transfer (Sun et al., 2004); only one injection site, rather than three injection sites, was used, and the titers (IVP/ml) were ~10 to 20-fold lower. Thus, the ~30- to 60-fold lower amount of vector injected accounts for the majority of the difference in the numbers of expressing cells between the current study and the ~11,400 positive cells observed in an earlier study (Sun et al., 2004).
We have developed a general strategy for restricting recombinant expression to specific types of neurons: Use antibody-mediated targeted gene transfer to particular types of cells in combination with vectors that contain neuron type-specific promoters. In theory, antibody-mediated targeted gene transfer can be specific for any specific cell surface protein. Although targeting to especially rare epitopes may be complicated by limitations on the diffusion of HSV-1 particles through the extracellular space and/or the stability of the vector particles, results to date suggest that the approach developed here will likely support targeted gene transfer to a wide range of specific types of cells in the forebrain.
In this study, further specificity in the type of neuron supporting recombinant expression was achieved by using a neuron-specific promoter, and additional specificity can be obtained by using a neuron type-specific promoter. HSV-1 vectors containing specific promoters restrict expression to all neurons, or catecholaminergic, enkephalinergic, GABAergic, glutamatergic, or subtypes of glutamatergic neurons (Jin et al., 1996; Kaplitt et al., 1994; Rasmussen et al., 2007; Song et al., 1997; Zhang et al., 2000; Zhang and Geller, 2010; Zhang et al., 2011). Novel specificities of expression may be obtained by performing targeted gene transfer with vectors that contain specific promoters. Restricting recombinant expression to specific types of neurons may be particularly useful in the forebrain, as it contains numerous types of neurons, and such approaches may benefit a wide range of neural gene therapy applications and basic neuroscience studies.
4. Experimental procedures
4.1. Materials
G418, lipofectamine, OPTI-MEM I, Dulbecco’s modified minimal essential medium, and fetal bovine serum were from Invitrogen. X-Gal, mouse anti-E. coli β-gal, and mouse anti-flag were purchased from Sigma. Male Long-Evans rats (initially ~6 weeks old) were from Charles River. The following antibodies were used to target gene transfer to, or detect, specific NMDA receptor subunits: Rabbit anti-NMDA NR2B (Sigma, M-265, for targeted gene transfer), rabbit anti-NMDA NR2B (Santa Cruz Biotechnology, sc-9057, for immunohistochemistry), rabbit anti-NMDA NR2A (Santa Cruz Biotechnology, sc-9056, for targeted gene transfer), and rabbit anti-NMDA NR2A (Invitrogen, A-6473, for immunohistochemistry). The secondary antibodies for immunohistochemistry were rhodamine isothiocyanate-conjugated goat anti-mouse IgG and fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma).
4.2. Cells
Maintenance of baby hamster kidney (BHK21) fibroblast cells (Sun et al., 1999) and 2-2 cells (Smith et al., 1992) have been described.
4.3. Plasmids, cosmids, and vectors
A plasmid containing the gC promoter, the prepro sequences from the human BDNF gene, the Staphylococcus A ZZ domain, gC deleted in the N-terminus (aa 153 to 511), and the gC polyadenylation site has been described (Cao et al., 2010).
Cosmid set C (cos6, cos14, cos28, cos48, cos56) represents the HSV-1 genome (Cunningham and Davison, 1993). Construction of cos56gC--ZZ has been described (Cao et al., 2010). Both cos14ΔgB and cos28ΔgB harbor deletions of the gB gene (Tang et al., 2001). The a sequence, which contains the packaging site, was deleted from cos6 and cos48, the two cosmids that contain it, to yield cos6Δa and cos48Δa (Fraefel et al., 1996). Because gB also contains glycosaminoglycan binding domains that mediate the initial non-specific binding to many cell types, we isolated (Cao et al., 2010) a plasmid that expresses a previously reported mutated gB (gBpK−) that lacks the glycosaminoglycan binding domain, but retains the essential membrane fusion capabilities of gB (Laquerre et al., 1998b).
pINS-TH-NFHlac uses the INS-TH-NFH promoter to express β-gal (Zhang et al., 2000); and pINS-TH-NFHflag-TH/ires/aadc uses the same promoter to express a flag-tagged human TH (and AADC) (Sun et al., 2003).
4.4. Vector packaging, vector particle-antibody complex formation, and titering
Helper virus-free packaging (Fraefel et al., 1996) was performed using 2-2 cells and a modified protocol for higher titers (Sun et al., 1999). For targeted gene transfer, pINS-TH-NFHlac was cotransfected with cos6Δa, cos14ΔgB, cos28ΔgB, cos48Δa, cos56gC--ZZ, and pUC18gBpK−, as previously detailed (Cao et al., 2010). Antibody binding was performed using our established procedure (Cao et al., 2010): These vector stocks were purified through the first of the two ultracentrifugations (sucrose step gradient) as for untargeted gene transfer (Lim et al., 1996), dialyzed into PBS (to remove the sucrose), incubated with a specific antibody, and purified by a second ultracentrifugation. For untargeted gene transfer, pINS-TH-NFHflag-TH/ires/aadc was cotransfected with the cosmids used for standard packaging (cos6Δa, cos14, cos28, cos48Δa, cos56gC--wt) (Fraefel et al., 1996), and these vector stocks were purified using our standard procedure (Lim et al., 1996).
Vector stocks were titered on BHK cells; at 1 day after transduction, positive cells were visualized using X-gal. Titering using BHK cells is the best available assay, as these fibroblast cells form a monolayer; in contrast, most neuronal cell lines do not form a monolayer, and the titers obtained using BHK cells are higher than those observed using PC12 cells (Yang et al., 2001; Zhang et al., 2000). The activity of the INS-TH-NFH promoter in fibroblast cells represents ectopic activity that declines at longer times after gene transfer (not shown). Further, for vector particle-antibody complexes, the gene transfer is due to a remaining low level of untargeted gene transfer, as BHK cells lack significant levels of NMDA receptors. For both of these reasons, the observed titers likely underestimate the titers. The titers of the vector stocks were pINS-TH-NFHlac/gC--ZZ+anti-NR2B, 1.0 × 106 infectious vector particles (IVP)/ml; pINS-TH-NFHlac/gC--ZZ+anti-NR2A, 2.4 × 106 IVP/ml; and pINS-TH-NFHflag-TH/ires/aadc/gC--wt, 6.0 × 107 IVP/ml. Further, we determined the titers of vector genomes using a previously established PCR assay (Yang et al., 2001). The titers of the vector stocks were pINS-TH-NFHlac/gC--ZZ+anti-NR2B, 2.4 × 107 vector genomes (VG)/ml; pINS-TH-NFHlac/gC--ZZ+anti-NR2A, 7.0 × 107 VG/ml. Thus, the ratio of VG/IVP for these vector stocks were pINS-TH-NFHlac/gC--ZZ+anti-NR2B, 24 VG/ml/IVP/ml, and pINS-TH-NFHlac/gC--ZZ+anti-NR2A 29 VG/ml/IVP/ml. These ratios of VG/ml/IVP/ml are similar to those previously observed in vector stocks for untargeted gene transfer for a number of vectors, in multiple studies (Gao et al., 2007; Liu et al., 2005; Liu et al., 2009; Yang et al., 2001). No wt HSV-1 was detected in these vector stocks (<10 plaque forming units (pfu)/ml).
4.5. Gene transfer and immunofluorescent costaining
These studies were approved by the West Roxbury VA Hospital IACUC; Male Long-Evans rats (initially ~6 weeks old) were used. Appropriate volumes of either pINS-TH-NFHlac/gC--ZZ+anti-NR2B or pINS-TH-NFHlac/gC--ZZ+anti-NR2A and pINS-TH-NFHflag-TH/ires/aadc/gC--wt were mixed together to yield the same IVP/ml for each set of two vectors. Specific mixtures of two vector stocks were delivered into POR cortex by stereotactic injections (2 sites/rat, 1/hemisphere, 3 μl/site; anterior-posterior (AP) −8.0, medial-lateral (ML) ±6.0, dorsal-ventral (DV) −5.2) (Zhang et al., 2005); AP is relative to bregma, ML is relative to the sagittal suture, and DV is relative to the bregma-lambda plane (Paxinos and Watson, 1986)). All injections were performed using a micropump (Model 100, KD Scientific); vector stocks were injected over five minutes, and after five more minutes, the 33 gauge needle was slowly retracted.
Rat perfusions, brain sectioning, and immunofluorescent costaining were performed as previously described (Zhang et al., 2000). Alternating sections were costained using either mouse anti-β-gal (1:200 dilution) or mouse monoclonal anti-flag (1:400 dilution) and either rabbit anti-NMDA NR2B (1:50 dilution) or rabbit anti-NMDA NR2A (1:1,000 dilution). The primary antibodies were visualized using both rhodamine isothiocyanate-conjugated goat anti-mouse IgG and fluorescein isothiocyanate-conjugated goat anti-rabbit IgG.
4.6. Cell counts and statistical analyses
Twenty-five μm coronal sections from POR cortex were prepared; recombinant proteins were found in ~20 sections per hemisphere. Alternating sections were analyzed by costaining for either β-gal-IR or flag-IR (TH-flag) and either NR2B-IR or NR2A-IR. Photomicrographs were captured using a video camera, under 60x magnification. The positive cells (β-gal-IR or flag-IR) in each section were scored for costaining with either NR2B-IR or NR2A-IR. All the cells containing recombinant proteins in a section were scored; each section was counted at least twice, on different days, and the values differed by <10 % for each section.
Statistical comparisons were performed using ANOVAs (Excel).
Highlights.
Targeted gene transfer to NMDA receptor NR2B-containing neurons
Targeted gene transfer to NMDA receptor NR2A-containing neurons
Transduction of specific neocortical neuron types by targeting and specific promoters
Acknowledgments
We gratefully thank Dr. A. Davison for HSV-1 cosmid set C, Dr. K. O’Malley for the TH promoter, Dr. W. Schlaepfer for the NFH promoter, and Dr. G. Felsenfeld for β-globin insulator. This work was supported by AG025894 (G.Z.) and AG021193, NS043107, NS045855, and NS057558 (A.I.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergman I, Whitaker-Dowling P, Gao Y, Griffin JA, Watkins SC. Vesicular stomatitis virus expressing a chimeric Sindbis glycoprotein containing an Fc antibody binding domain targets to Her2/neu overexpressing breast cancer cells. Virology. 2003;316:337–47. doi: 10.1016/j.virol.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Buning H, Ried MU, Perabo L, Gerner FM, Huttner NA, Enssle J, Hallek M. Receptor targeting of adeno-associated virus vectors. Gene Ther. 2003;10:1142–51. doi: 10.1038/sj.gt.3301976. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang GR, Wang X, Kong L, Geller AI. Enhanced nigrostriatal neuron-specific, long-term expression by using neural-specific promoters in combination with targeted gene transfer by modified helper virus-free HSV-1 vector particles. BMC Neurosci. 2008;9:37. doi: 10.1186/1471-2202-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang GR, Geller AI. Antibody-mediated targeted gene transfer to NMDA NR1-containing neurons in rat neocortex by helper virus-free HSV-1 vector particles containing a chimeric HSV-1 glycoprotein C--Staphylococcus A protein. Brain Res. 2010;1351:1–12. doi: 10.1016/j.brainres.2010.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Kobiler O, McCambridge J, Ebdlahad S, Shan Z, Raizada MK, Sved AF, Enquist LW. Microdissection of neural networks by conditional reporter expression from a Brainbow herpesvirus. Proc Natl Acad Sci U S A. 2011;108:3377–82. doi: 10.1073/pnas.1015033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Davison AJ. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–24. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–8. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Sun M, Wang X, Geller AI. Isolation of an enhancer from the rat tyrosine hydroxylase promoter that supports long-term, neuronal-specific expression from a neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 2007;1130:1–16. doi: 10.1016/j.brainres.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–9. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AI, During MJ, Neve RL. Molecular analysis of neuronal physiology by gene transfer into neurons with herpes simplex virus vectors. Trends Neurosci. 1991;14:428–32. doi: 10.1016/0166-2236(91)90040-2. [DOI] [PubMed] [Google Scholar]
- Grandi P, Wang S, Schuback D, Krasnykh V, Spear M, Curiel DT, Manservigi R, Breakefield XO. HSV-1 virions engineered for specific binding to cell surface receptors. Mol Ther. 2004;9:419–27. doi: 10.1016/j.ymthe.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Javier RT, Sedarati F, Stevens JG. Two avirulent herpes simplex viruses generate lethal recombinants in vivo. Science. 1986;234:746–8. doi: 10.1126/science.3022376. [DOI] [PubMed] [Google Scholar]
- Jin BK, Belloni M, Conti B, Federoff HJ, Starr R, Son JH, Baker H, Joh TH. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum Gene Ther. 1996;7:2015–24. doi: 10.1089/hum.1996.7.16-2015. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Kwong AD, Kleopoulos SP, Mobbs CV, Rabkin SD, Pfaff DW. Preproenkephalin promoter yields region-specific and long-term expression in adult brain after direct in vivo gene transfer via a defective herpes simplex viral vector. Proc Natl Acad Sci USA. 1994;91:8979–83. doi: 10.1073/pnas.91.19.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara N, Dozy AM, Kan YW. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266:1373–6. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- Kobiler O, Lipman Y, Therkelsen K, Daubechies I, Enquist LW. Herpesviruses carrying a Brainbow cassette reveal replication and expression of limited numbers of incoming genomes. Nat Commun. 2010;1:146. doi: 10.1038/ncomms1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laquerre S, Anderson DB, Stolz DB, Glorioso JC. Recombinant herpes simplex virus type 1 engineered for targeted binding to erythropoietin receptor-bearing cells. J Virol. 1998a;72:9683–97. doi: 10.1128/jvi.72.12.9683-9697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998b;72:6119–30. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, Hartley D, Starr P, Lang P, Song S, Yu L, Wang Y, Geller AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. Biotechniques. 1996;20:460–9. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- Liu M, Tang J, Wang X, Yang T, Geller AI. Enhanced long-term expression from helper virus-free HSV-1 vectors packaged in the presence of deletions in genes that modulate the function of VP16, UL46 and UL47. J Neurosci Methods. 2005;145:1–9. doi: 10.1016/j.jneumeth.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Liu M, Wang X, Geller AI. Helper virus-free HSV-1 vectors packaged in the presence of combinations of mutations in the protein kinase UL13 and the VP16 transcriptional complex enhance long-term expression. BMC Molecular Biology. 2009;10:58. doi: 10.1186/1471-2199-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardberg K, Trybala E, Glorioso JC, Bergstrom T. Mutational analysis of the major heparan sulfate-binding domain of herpes simplex virus type 1 glycoprotein C. J Gen Virol. 2001;82:1941–50. doi: 10.1099/0022-1317-82-8-1941. [DOI] [PubMed] [Google Scholar]
- Morizono K, Bristol G, Xie YM, Kung SK, Chen IS. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–20. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Chen IS. Targeted gene delivery by intravenous injection of retroviral vectors. Cell Cycle. 2005;4:854–6. doi: 10.4161/cc.4.7.1789. [DOI] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–52. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, Trepel M. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat Biotechnol. 2003;21:1040–6. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- Ohno K, Sawai K, Iijima Y, Levin B, Meruelo D. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat Biotechnol. 1997;15:763–7. doi: 10.1038/nbt0897-763. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sidney: 1986. [DOI] [PubMed] [Google Scholar]
- Peng KW, Russell SJ. Viral vector targeting. Curr Opin Biotechnol. 1999;10:454–7. doi: 10.1016/s0958-1669(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Kong L, Zhang G, Liu M, Wang X, Szabo G, Curthoys NP, Geller AI. Glutamatergic or GABAergic neuron-specific, long-term expression in neocortical neurons from helper virus-free HSV-1 vectors containing the phosphate-activated glutaminase, vesicular glutamate transporter-1, or glutamic acid decarboxylase promoter. Brain Res. 2007;1144:19–32. doi: 10.1016/j.brainres.2007.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried MU, Girod A, Leike K, Buning H, Hallek M. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J Virol. 2002;76:4559–66. doi: 10.1128/JVI.76.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Sears AE. Herpes simplex viruses and their replication. In: Roizman B, Whitley RJ, Lopez C, editors. The human herpesviruses. Raven Press; New York: 1993. pp. 11–68. [Google Scholar]
- Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–10. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak SY, Lang P, Ullrey D, Neve RL, O’Malley KL, Geller AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J Neurochem. 1997;68:1792–803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- Spear P, Roizman B. DNA Tumor Viruses. In: Tooze J, editor. DNA Tumor Viruses. Cold Spring Harbor Laboratory; Cold Spring Harbor NY: 1981. pp. 615–746. [Google Scholar]
- Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77:10179–85. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Lim F, Grant FD, Trask L, Lang P, Yu L, Geller AI. Long-term persistence of defective HSV-1 vectors in the rat brain is demonstrated by reactivation of vector gene expression. Gene Ther. 1996;3:615–23. [PubMed] [Google Scholar]
- Stevens JG. Latent herpes simplex virus and the nervous system. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhang GR, Yang T, Yu L, Geller AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (previral DNA replication enveloped particles) to the packaging procedure. Hum Gene Ther. 1999;10:2005–11. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhang G, Kong L, Holmes C, Wang X, Zhang W, Goldstein DS, Geller AI. Correction of a rat model of Parkinson’s disease by coexpression of tyrosine hydroxylase and aromatic amino acid decarboxylase from a helper virus-free herpes simplex virus type 1 vector. Hum Gene Ther. 2003;14:415–24. doi: 10.1089/104303403321467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Holmes C, Gao Q, Zhang W, Pfeilschifter J, Goldstein DS, Geller AI. Coexpression of Tyrosine Hydroxylase, GTP Cyclohydrolase I, Aromatic Amino Acid Decarboxylase, and Vesicular Monoamine Transporter-2 from a Helper Virus-Free HSV-1 Vector Supports High-Level, Long-Term Biochemical and Behavioral Correction of a Rat Model of Parkinson’s Disease. Hum Gene Ther. 2004;15:1177–1196. doi: 10.1089/hum.2004.15.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CK, Logg CR, Park JM, Anderson WF, Press MF, Kasahara N. Antibody-mediated targeting of replication-competent retroviral vectors. Hum Gene Ther. 2003;14:789–802. doi: 10.1089/104303403765255174. [DOI] [PubMed] [Google Scholar]
- Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–83. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Yang T, Ghosh HP, Geller AI. Helper Virus-Free HSV-1 Vectors Packaged Both in the Presence of VSV G Protein and in the Absence of HSV-1 Glycoprotein B Support Gene Transfer into Neurons in the Rat Striatum. J NeuroVirol. 2001;7:548–55. doi: 10.1080/135502801753248132. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Wagner EK, Stevens JG. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983;131:180–92. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Volpers C, Thirion C, Biermann V, Hussmann S, Kewes H, Dunant P, von der Mark H, Herrmann A, Kochanek S, Lochmuller H. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin g-binding domain in the capsid. J Virol. 2003;77:2093–104. doi: 10.1128/JVI.77.3.2093-2104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kong L, Zhang G, Sun M, Geller AI. Targeted gene transfer to nigrostriatal neurons in the rat brain by helper virus-free HSV-1 vector particles that contain either a chimeric HSV-1 glycoprotein C--GDNF or a gC--BDNF protein. Molec Brain Res. 2005;139:88–102. doi: 10.1016/j.molbrainres.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996a;14:1570–3. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Segal DM, Roelvink PW, Carrion ME, Lizonova A, Lee GM, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996b;70:6831–8. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ. Ligand-directed targeting of genes to the site of disease. Nat Med. 2003;9:135–9. doi: 10.1038/nm0103-135. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang G, Zhang W, Sun M, Wang X, Geller AI. Enhanced reporter gene expression in the rat brain from helper virus-free HSV-1 vectors packaged in the presence of specific mutated HSV-1 proteins that affect the virion. Molec Brain Res. 2001;90:1–16. doi: 10.1016/s0169-328x(01)00059-6. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Yang T, Sun M, Zhang W, Wang Y, Geller AI. A tyrosine hydroxylase--neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Molec Brain Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Kong L, Lu X, Lee B, Liu M, Sun M, Franklin C, Cook RG, Geller AI. Genetic enhancement of visual learning by activation of protein kinase C pathways in small groups of rat cortical neurons. J Neurosci. 2005;25:8468–81. doi: 10.1523/JNEUROSCI.2271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Liu M, Cao H, Kong L, Wang X, Cook RG, Geller AI. Improved spatial learning in aged rats by genetic activation of protein kinase C in small groups of rat hippocampal neurons. Hippocampus. 2009;19:413–23. doi: 10.1002/hipo.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cao H, Kong L, O’Brien J, Baughns A, Jan M, Zhao H, Wang X, Lu X, Cook RG, Geller AI. Identified circuit in rat postrhinal cortex encodes essential information for performing specific visual shape discriminations. Proc Natl Acad Sci USA. 2010a;107:14478–14483. doi: 10.1073/pnas.0912950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cao H, Li X, Zhao H, Geller AI. Genetic labeling of both the axons of transduced, glutamatergic neurons in rat postrhinal cortex and their postsynaptic neurons in other neocortical areas by Herpes Simplex Virus vectors that coexpress an axon-targeted β-galactosidase and wheat germ agglutinin from a vesicular glutamate transporter-1 promoter. Brain Res. 2010b;1361:1–11. doi: 10.1016/j.brainres.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Geller AI. An HSV-1 vector containing the VGLUT1 promoter is expressed only in VGLUT1-, and not VGLUT2-, containing glutamatergic neurons. Brain Res. 2010;1331:12–19. doi: 10.1016/j.brainres.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li X, Cao H, Zhao H, Geller AI. The vesicular glutamate transporter-1 upstream promoter and first intron each support glutamatergic-specific expression in rat postrhinal cortex. Brain Research. 2011;1377:1–12. doi: 10.1016/j.brainres.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


