Abstract
Elevated blood pressure during hypertension has been associated with microvascular rarefaction defined by loss of microvessels. However, whether rarefaction is a result of impaired angiogenesis remains unclear. The objective of this study was to compare angiogenesis across the time course of mesenteric microvascular network remodeling in adult spontaneously hypertensive versus normotensive rats. Angiogenic responses in 15–16-week-old SHR and Wistar rats at 0, 3, 5, 10 or 25 days post 20 minute exteriorization of the mesentery were quantified. Consistent with the phenomenon of rarefaction, vascularized area in unstimulated SHR was decreased compared to Wistar. By 25 days, SHR vascular area had increased to the Wistar level and vascular length density and capillary sprouting were comparable. At 3 and 5 days, SHR and Wistar tissues displayed an increase in the capillary sprouting and vascular density relative to their unstimulated controls. At 10 days, capillary sprouting in the SHR remained elevated. The percent change in vascular density was elevated in the SHR compared to the Wistar group at 3 and 5 days and by 25 days the rate of change was more negative. Our results suggest that SHR networks undergo an increased rate of growth followed by an increased rate of pruning.
Keywords: Hypertension, Angiogenesis, Spontaneously Hypertensive Rat, Microcirculation, Mesentery
Introduction
Structural microvascular rarefaction, defined as the anatomical loss of microvessels, is a common characteristic of hypertension (1, 9, 15, 17, 27) and has been linked to increased vascular resistance (11, 13, 26). It also has been associated with altered flow distribution in microvascular networks (27), thus influencing local tissue perfusion and metabolism (18, 29, 30) and causing target organ damage (5, 14, 25). In the spontaneously hypertensive rat (SHR), vessel loss has been associated with further microvascular dysfunction including enhanced microvessel specific oxidative stress, elevated counts of activated circulating leukocytes, impairment of selection-mediated leukocyte adhesion, and extensive non-uniform endothelial cell apoptosis (11, 42, 44). Investigations aimed at elucidating the causes of rarefaction are critical for understanding the causal relationships between rarefaction and the end organ pathology associated with hypertension.
Insight can be gained into the causes of rarefaction by determining whether this phenomenon is due to impaired angiogenesis or increased vessel loss. While impaired angiogenesis, denoted as vessel growth from pre-existing vessels, has been suggested to be a contributor to rarefaction (19, 21, 26), direct evidence has relied on decreased vascular density measurements at single time point post stimulation and is not consistent across studies. In the hindlimb ischemia model, capillary density and perfusion in the ischemic limb muscles was significantly reduced at 4 weeks post-ligation in 4-week-old SHR versus normotensive control (45). At 14 days after subcutaneous sponge implantation, the level of vascular ingrowth in 6-month-old SHR was half of that in normotensive controls (48) and this impaired angiogenesis was associated with a downregulated VEGF-R2 expression. In contrast to these studies, support for increased angiogenesis during hypertension also exists. In fibrin chambers subcutaneously implanted in 4-week-old pre-hypertensive SHRs, angiogenesis and arteriogenesis were found to be increased compared to the normotensive control group (19) challenging the paradigm of impaired angiogenesis in the SHR. Insight into whether vessel growth is attenuated can be gained by further investigation of microvascular growth of hypertensive networks over a time course of remodeling.
The objective of this study was to compare angiogenesis across the time course of mesenteric microvascular network remodeling in adult spontaneously hypertensive versus normotensive rats. Using immunochemical tissue labeling method, the microvascular network growth was analyzed over the time course of the previously described mesentery exteriorization stimulation (31, 37). The model was selected because it represents a multi-factorial stimulus which cause robust network growth over a relatively short time period and thus can be used to assess the angiogenic potential of microvascular networks. Our results indicate that SHR networks undergo an increased rate of angiogenesis at early time points followed by an increased rate of vessel pruning. These dynamics resulted in comparable angiogenic metrics between the SHR and normotensive networks at the end of the observed remodeling duration. Our work suggests that differences in vessel density responses to angiogenic stimuli reported in the literature might be explained by differences in the relative time points that measurements were made and suggests that future studies investigating the mechanisms involved in rarefaction should also focus on the vessel pruning process.
Methods
Mesentery Exteriorization Model
All experiments were performed in accordance with the guidelines of Tulane University`s Institutional Animal Care and Use Committee. Age-matched (15–16 weeks) male SHR (294–313 g) and normotensive Wistar rats (407–443 g) (4 rats per group) were anesthetized with IM injection of ketamine (80 mg/kg bw, Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (8 mg/kg bw, LLOYD, Inc., Shenandoah, IA, USA). Using sterile surgery technique, 8 mesenteric tissue windows, identified as the thin translucent connective tissues attached to the ileum, were gently removed with minimal stretch from the abdominal cavity and placed on a specially designed sterile plastic stage. Specifically, the mesenteric region of interest was delicately pulled through and placed in a modified 25 mm petri dish with an elliptical hole in the center. The mesentery region was then left exposed for 20 minutes. Over the time course of the 20-minute exposure, the tissue was superfused with saline solution (sterile 0.9% sodium chloride, pH 5.6, 28 °C, Hospira, Inc., Lake Forest, IL, USA). The 2 centrally located mesenteric windows were marked with 7-0 silk sutures. At the end of the 20-minute exposure duration, the tissue was carefully returned to the abdominal cavity and the incised muscle and peritoneal layers were closed. During the process, mesentery was handled carefully with minimum stretch. After 3, 5, 10 or 25 days, rats were anesthetized again and euthanized via an intra-cardiac injection of Beuthanasia (Schering-Plough Animal Health Corp. Union, Kenilworth, NJ, USA). The previously exposed mesenteric windows were harvested and immunolabeled.
Mast Cell Degranulation Model
All experiments were performed in accordance with the guidelines of Tulane University`s Institutional Animal Care and Use Committee. Similar to a previously established protocol (10, 31, 32), single dose (0.1 mL/100 g body weight) of compound 48/80 (200, 400, 600, 800 and 1000 µg/mL in saline) (Sigma-Aldrich, St. Louis, MO, USA) was injected IP over the time course of 3 days (twice a day) in increasing concentrations into Wistar or SHR rats. At day 5 or day 13, rats were euthanized and tissues were harvested and immunolabeled.
Immunohistochemistry
Tissues were fixed in 4% Paraformaldehyde (Fisher Scientific, Fair Lawn, NJ, USA) at 4°C for 1 hour or methanol at −20 °C for half an hour, then labeled with an antibody against PECAM (CD31). PECAM labeling protocol was as follows: 1) 1 hour incubation at room temperature with 1:200 mouse monoclonal biotinylated CD31 (BD Pharmingen, San Diego, CA, USA) antibody diluted in antibody buffer (0.1% Saponin in PBS + 2% BSA); 2) Wash with PBS with 0.1% Saponin for 30 minutes; 3) 1 hour incubation at room temperature with Streptavidin-peroxidase secondary antibody solution (VECTASTAIN Elite ABC, Vector Laboratories, Burlingame, CA, USA) for 1 hour at room temperature; and 4) 30 minutes incubation at room temperature with Vector Nova Red (Vector Laboratories) substrate.
Imaging and Quantification of Microvascular Network Structure
Images were either captured using an inverted microscope (Olympus IX70, Olympus America, Inc., Melville, NY, USA) coupled with a Pixel Fly camera (PCO, Kelheim, Germany) and a 4×/numerical aperture (NA dry) objective (Olympus, Melville, NY, USA) or a Nikon eclipse LV 100 (Nikon Instruments, Inc., Melville, NY, USA) coupled with an All Spot camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA) and a 1×/numerical aperture (NA dry) objective (Olympus, Melville, NY, USA). Four vascularized tissues were randomly selected per animal. Network montages, generated by overlaying these sequential 4× images, were used for quantification of vascular area, tissue area, and the number of capillary sprouts. Vascular area was defined as the area circumscribed by the sum of each individual network within a tissue. From two representative 4× images per tissue, capillary length, arterial and venous (A/V) length, the number of capillary segments, and the number of A/V segments were measured. All measurements were made using ImageJ software (ImageJ, U.S. National Institutes of Health, Bethesda, ML, USA). A/V length and segment density metrics included both arterioles and venules and represent indicators of remodeling of vessels larger than capillaries. The capillary sprouts were defined as blind ended vessels which were smaller than 8µm located on the periphery of the vascular networks. The percent change in vascular density metric for Figure 6 represents the percent change in vascular length density per time point relative to the mean total length density value for the previous time point.
Figure 6.
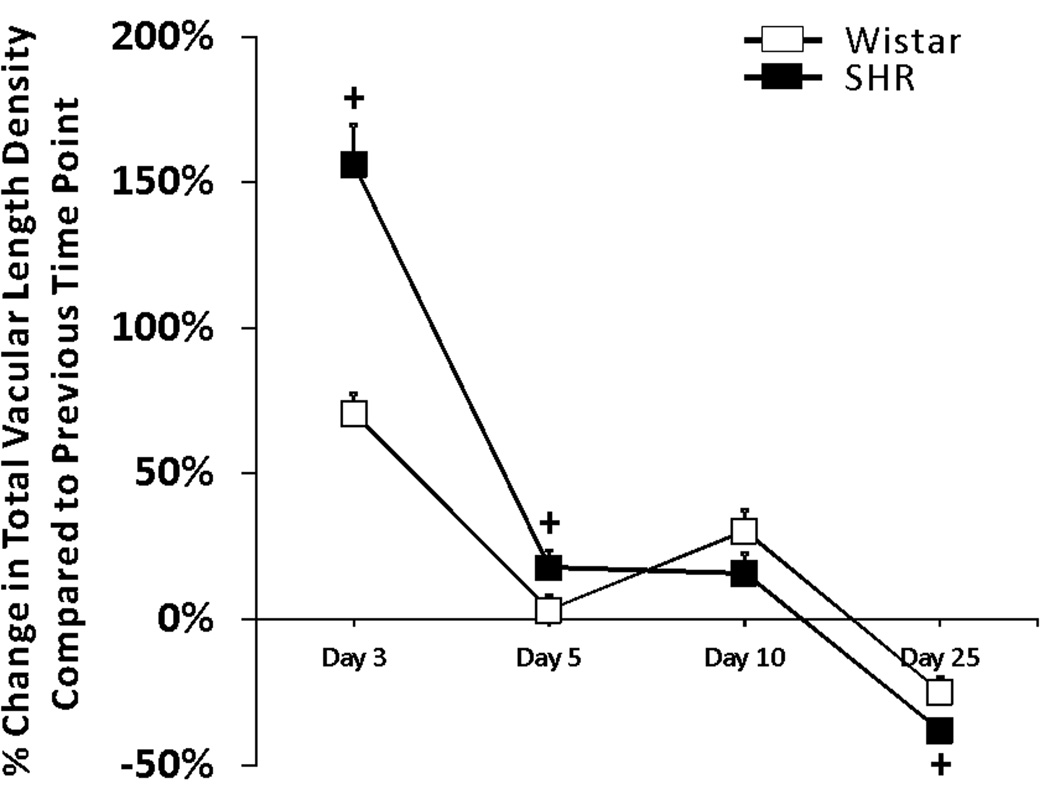
Comparison of the percent changes in total vascular length density between SHR networks (black squares) and Wistar networks (white squares) at day 3, 5, 10 and 25 post stimulation. Each square is the mean of the differences between the density of each tissue in the group and the average density in previous time point. + represents significant difference between SHR and Wistar groups (p < 0.05).
Blood Pressure Measurement
Systolic arterial pressure measurements were made, while rats were conscious, using the tail-cuff method (IITC Model 229 Blood Pressure Analyzer, IITC/Life Science Instruments, Woodland Hills, CA). To ensure reproducibility, rats were trained at the same time each day for 5 days before blood pressure values were used. The systolic arterial pressure of each animal represents the average of four repeated measurements.
Statistical Analysis
Comparisons for angiogenic metrics of unstimulated and stimulated networks from SHR and Wistar were analyzed with a two-way ANOVA followed by a Student-Newman-Keuls multi-comparison pairwise test (SigmaStat, Systat Software, Inc., Chicago, IL, USA). Comparisons in Figure 6 of percent changes in vascular length density were made between SHR and Wistar groups per time point were analyzed with a Mann-Whitney U test. Blood pressure comparisons between Wistar and SHR were made using a Student’s t-test. Statistical significance was accepted for p < 0.05. Error bar values are mean +/− SEM.
Results
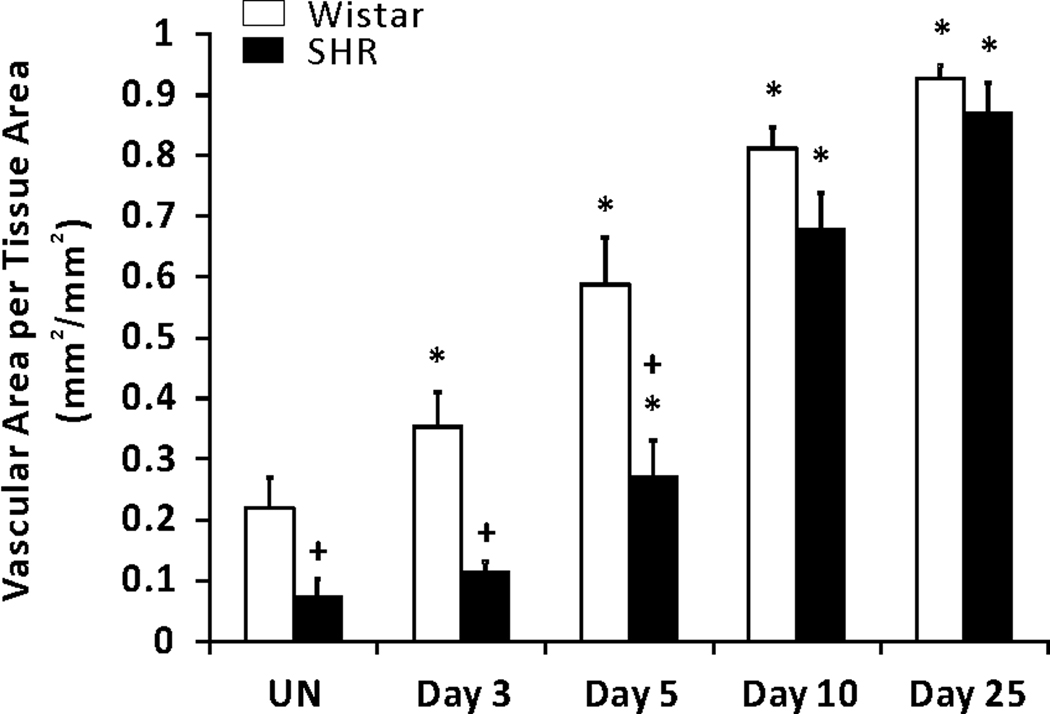
In response to the 20-minute mesentery stimulation, SHR mesenteric microvascular networks experienced dramatic angiogenesis as indicated by observation of increased capillary sprouting and vascular length density compared to unstimulated control networks (Figures 1 and 2). Consistent with previous documentation of rarefaction in this tissue (32), unstimulated SHR tissues demonstrated smaller vascular area per tissue area (7.5 % ± 1.9 %) compared with unstimulated Wistar ones (22 % ± 0.5 %) (Figures 2 and 3). The vascular area per tissue area for Wistar networks significantly increased compared to unstimulated tissues by 3 days and continued to significantly increase throughout the time course post stimulation. And at day 3, SHR microvascular networks remained comparable in area to unstimulated control. Continued subsequent significant increases in SHR network area were then observed at the 5, 10 and 25 day time points. At early time points, 3 and 5 days, SHR vascular area was remained less than the normotensive group, yet by day 10 SHR and Wistar vascular area per tissue area were comparable. The apparent lag in vascular area growth could be attributed to the initial decreased network size.
Figure 1.
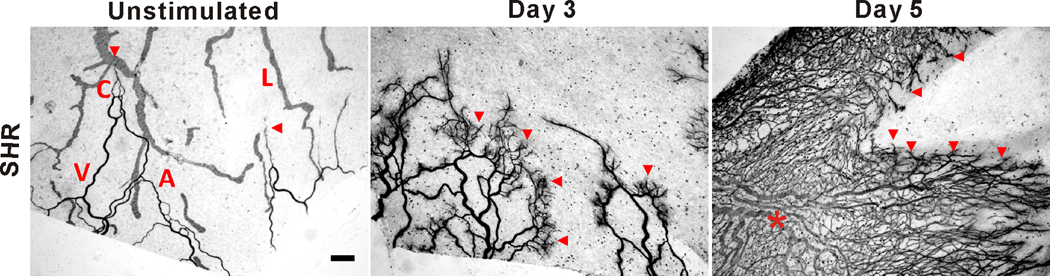
Representative images of mesenteric microvascular networks from unstimulated SHR tissues and tissues at 3 and 5 days post 20-min exteriorization of the mesentery. PECAM labeling identified the hierarchy of microvascular networks including, arterioles (A), venules (V), capillaries (C) and lymphatic (L). By 3 days and 5 days post stimulation, microvascular networks displayed an increase in capillary sprouting (arrow heads) and vascular length density (star) compared to unstimulated SHR networks. Scale bar is 200 µm.
Figure 2.
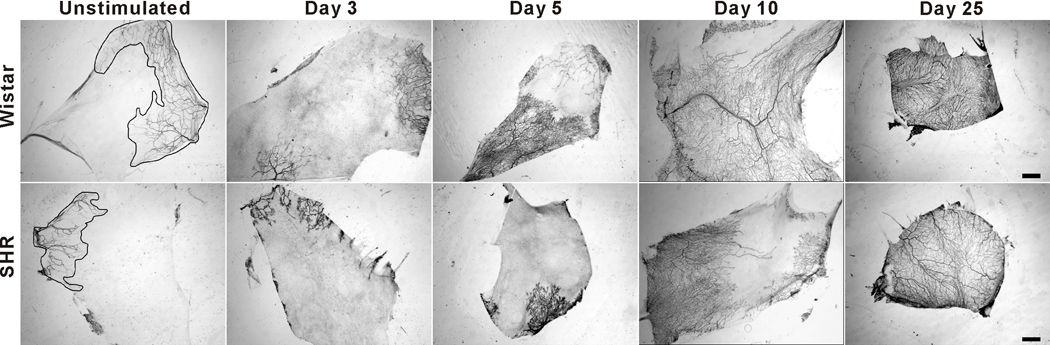
Representative mesenteric tissues immunolabeled for PECAM expression harvested from adult normotensive Wistar rats (top row) and SHRs (bottom row) over a 25-day-time course of microvascular growth post 20-min exteriorization of the mesentery. In the unstimulated groups, the phenomenon of rarefaction is supported by the presence of smaller vascularized regions (circumscribed areas) in the SHR relative to Wistar network tissues. Over the experimental time course, both SHR and Wistar tissues displayed microvascular network growth resulting in comparable vascular area density, capillary sprouts and vascular length density at 25 days post stimulation. Scale bar is 2 mm.
Figure 3.
Comparison of total vascular area per tissue area in adult SHR and normotensive Wistar rats in response to a 20-min exteriorization of the mesentery. * represents significant difference between SHR or Wistar groups and the unstimulated control group the same rat strain. + represents significant difference between SHR and Wistar groups at the same day post stimulation (p<0.05). UN, unstimulated.
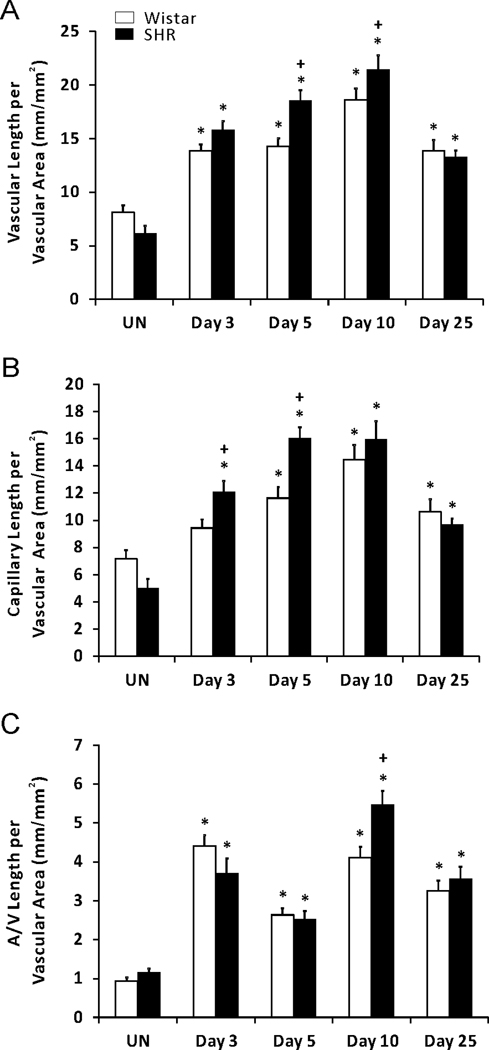
During the time course of remodeling, network growth in both hypertensive and normotensive networks was further indicated by increases in vascular length density, capillary sprouting, and vessel segments compared to unstimulated networks (Figures 4 and 5). The SHR networks displayed higher total vascular length density at day 5 and 10 post stimulation compared to Wistar networks (Figure 4A). At day 5, the increase in SHR total vascular density versus the Wistar group was attributed to increased capillary length per vascular area, where as at day 10 the increased density was attributed to increased length of arterioles and venules (Figure 4). By the end of the remodeling, both the capillary and arterial and venous (A/V) length density were comparable between SHR and Wistar networks.
Figure 4.
Comparison of microvascular length density in adult SHR and normotensive Wistar rats in response to a 20-min exteriorization of the mesentery. (A) Total vascular length per vascular area. (B) Capillary length per vascular area. (C) Arterial and venous (A/V) length per vascular area. * represents significant difference between SHR or Wistar groups and the unstimulated control group the same rat strain. + represents significant difference between SHR and Wistar groups at the same day post stimulation (p < 0.05). UN, unstimulated.
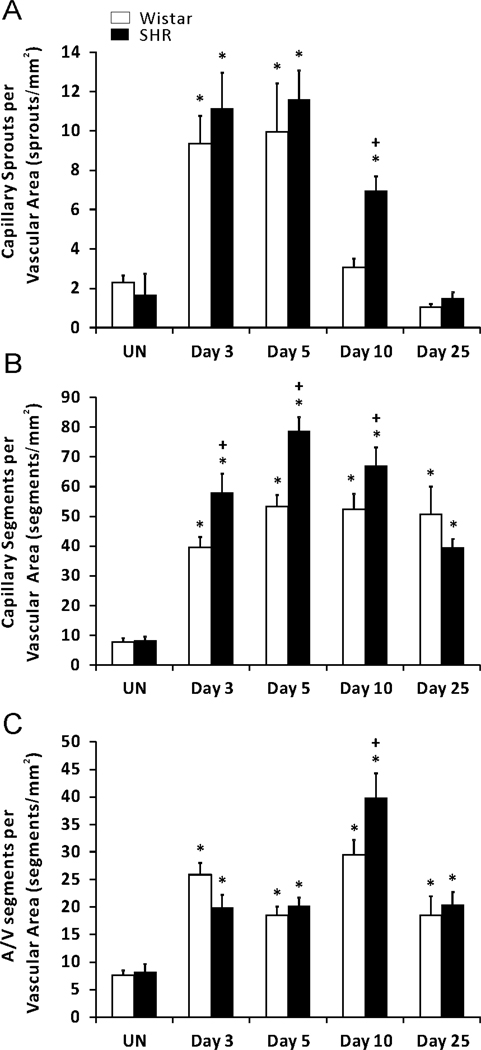
Figure 5.
Comparison of microvascular segments in adult SHR and normotensive Wistar rats in response to a 20-min exteriorization of the mesentery. (A) Number of capillary sprouts per vascular area. (B) Total capillary segments per vascular area. (C) Arterial and venous (A/V) segments per vascular area. * represents significant difference between SHR or Wistar groups and the unstimulated control group the same rat strain. + represents significant difference between SHR and Wistar groups at the same day post stimulation (p < 0.05). UN, unstimulated.
By day 25, capillary sprouting had returned to unstimulated levels in both SHR and Wistar microvascular networks, indicating that these tissues were no longer undergoing angiogenesis (Figure 5A). However, the duration of angiogenesis indicated by capillary sprouting was shorter in the Wistar networks compared to SHR networks. At day 10, capillary sprouting remained elevated in SHR, while in Wistar networks sprouting had already returned to unstimulated level. Additional support for altered network growth dynamics is provided by the increased capillary and A/V segments at day 10 in SHR versus Wistar networks (Figure 5B, 5C). Interestingly, by 25 days, capillary and arteriole/venous segments were comparable across the rat strains. The differences in angiogenic metrics per specific time point prior to 25 days can be explained by the apparent differences in the rate of growth. Over the time course of network remodeling, the percent change in vascular density was elevated in the SHR compared to the Wistar group at 3 and 5 days and by 25 days the rate of change was more negative (Figure 6).
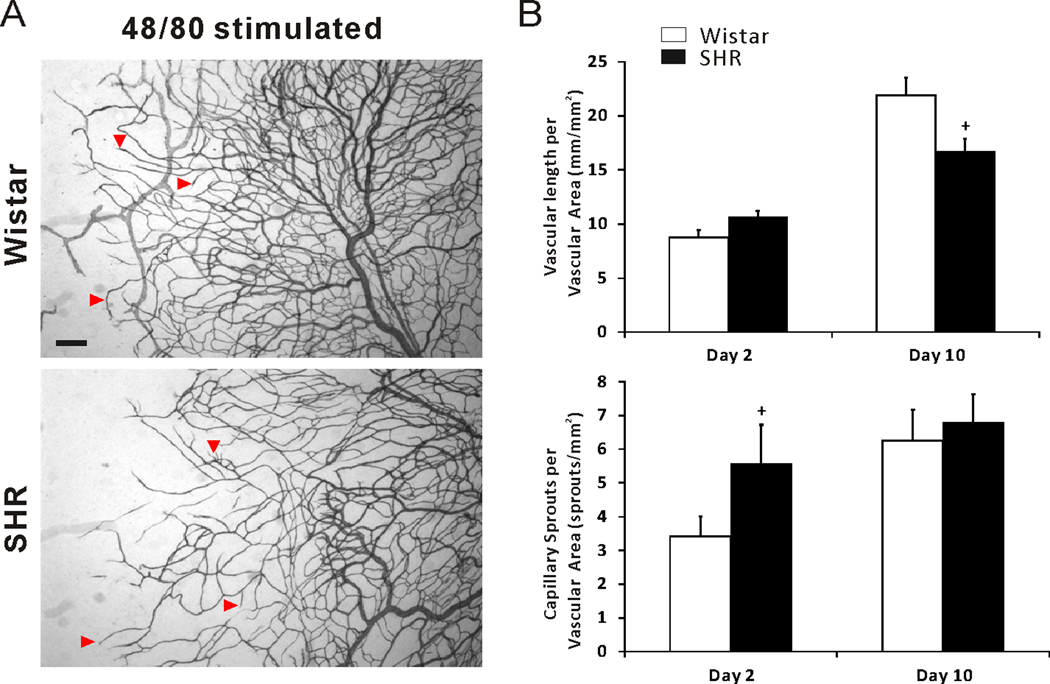
To compare angiogenic responses between hypertensive and normotensive microvascular networks in the rat mesentery using an alternative stimuli, we also examined mesenteric tissues from animals injected with 48/80, a mast cell degranulator. In SHR tissues, 48/80 induced microvascular growth was confirmed by observation of increased capillary sprouting and vessel length density at day 2 and day 10 post stimulation compared to unstimulated networks (Figure 7). At the day 2 post 48/80 injections, capillary sprouts per vascular area of SHR was increased compared to Wistar (Figure 7B). These results support that at different time points post stimulation, the conclusions based on comparisons of specific angiogenic metrics between hypertensive and normotensive groups can be different.
Figure 7.
(A) Representative mesenteric adult SHR and Wistar microvascular networks during 48/80 mast cell degranulation induced microvascular growth at day 2 and 10 post stimulation. (B) Comparison of vascular length per vascular area and capillary sprouts per vascular area in Wistar versus SHR mesenteric tissues. Arrowheads indicate examples of capillary sprouting. + represents significant difference between SHR and Wistar groups (p < 0.05).
Systolic blood pressure in unstimulated Wistar rats (n = 6) and SHRs (n = 6) was 124.2 ± 4.2 mmHg and 189.5 ± 9.5 mmHg (p<0.05), respectively.
Discussion
The primary finding of this study is that angiogenesis in adult SHR mesenteric microvasculature is not impaired during the network remodeling post stimulation. Microvascular growth induced by exteriorization of the mesentery was characterized by growth in vascular area and increased capillary sprouts and vascular length density. As an indicator of angiogenesis accompanied by arteriogenesis, we observed increases in total arterial and venous (A/V) length and segments per vascular area. By 25 days post stimulation, vascularized area, vascular density, vessel segments and capillary sprouting in SHR networks were comparable to Wistar networks. Since the initial network size of SHR networks was smaller than the size of Wistar networks, the hypertensive networks experienced an increased growth rate during the early stage of the remodeling process. Evidence of an increased angiogenesis was also supported by increased vascular density in the SHR compared to Wistar tissues at 5 and 10 days and increased capillary sprouting at 10 days.
Comparison of the percent change in vascular density between time points also indicated altered rates of remodeling in the SHR. At 3 and 5 days post our tissue exteriorization stimulation, the percent change in vascular length density was increased in the SHR relative to Wistar networks supporting an increased initial rate of angiogenesis. By 25 days, SHR networks experienced an increased rate of vessel pruning or dropout. Our characterization of network growth over a time course of remodeling indicates that mechanisms associated with vessel loss contribute to rarefaction in hypertension.
Since microvascular rarefaction during hypertension has been attributed to impaired angiogenesis (8, 21), our observations over a time course of remodeling offer an alternative perspective and an explanation for inconsistent results in the literature. In 4-week-old SHRs, angiogenesis has been documented to be impaired in response to hind limb ischemia (45) and increased in a subcutaneous fibrin gel chamber implantation model (19). Kiefer et al. examined the angiogenic capacity of serum from SHR and Wistar-Kyoto (WKY) rats at 6 weeks and 12 weeks old (21). The results showed that at the age of 5 months, the angiogenic potential of serum of SHR is decreased but not in 6-week adult stage. They proposed that in SHR, there is transient period in which the vascular growth stimulating capacity is smaller than control rats. While the inconsistent results could be attributed to different models, tissues, ages, our results suggest that differences in angiogenic metrics also depend on the end time point in the study. For example, in our study comparisons of vascular areas at 3, 5, and 10 days would suggest that angiogenesis was impaired in the SHR. However, this result is not consistent when comparing vascular area at 25 days. In contrast, comparing microvascular length density or the number of vessel segments at early time points might suggest that angiogenesis is increased in the SHR; yet again at 25 days this result would not be supported. Based on our evidence of increased pruning of vessels in later stage of network remodeling, decreased vascular density measurement comparisons in other hypertension angiogenic studies might be explained by measurements made at time points after any peak in angiogenesis.
The importance of examining angiogenesis in hypertension versus normotensive networks over the time course of growth is emphasized by our characterization of angiogenesis in response to another inflammatory stimulus via 48/80 induced mast cell degranulation. At the day 2 time point for 48/80 induced angiogenesis in this study, capillary sprouts per vascular area of SHR was increased compared to normotensive control and comparable at 10 days. In contrast, the vascular density between groups was comparable at day 2 and decreased in the SHR at day 10. The data from our 48/80 studies further support the notion that comparisons from the literature depend on the angiogenic metric and the specific time point. Additionally, the 48/80 stimulation results at day 2 and day 10 are inconsistent with the comparisons made at similar time points in our exteriorization model. While further investigations would be required to fully characterize the difference of angiogenic responses in the two models, these inconsistencies suggest that angiogenic response comparisons between hypertensive and normotensive groups might also be model specific.
In the SHR, systolic blood pressure begins to rise at 5–6 weeks of age and may reach 180 mmHg by 15 weeks old, while the pressure of a normotensive rat is around 130mmHg (23, 35). By this age, microvascular rarefaction has been documented in numerous tissues including skeletal muscle, cardiac muscle, kidney and the brain of SHR (22, 28, 32, 36, 38, 39). Microvascular rarefaction has also been documented in the SHR mesentery (4, 16, 32). While mesenteric blood flow rates average about 10% of the cardiac output or 40ml/min/100 g in the human body (12, 24), a limitation associated with mesenteric tissue is its unknown function and contribution to overall resistance (9). In 15–16 weeks old SHRs, microvascular rarefaction has been correlated with microvascular dysfunction in the mesentery including increased wall-to-lumen ratio, enhanced microvessel specific oxidative stress, elevated MMP levels, elevated microvascular tone, deficient leukocyte-endothelial interaction and extensive non-uniform endothelial cell apoptosis (20, 22, 33, 38, 40, 42). Because these characteristics and vessel growth mechanisms in mesentery are similar to those in other tissues, the rat mesentery represents a model system for examining the microvascular network growth during hypertension. Future studies will be required to determine if the angiogenic response is different in older or younger hypertensive animals.
The SHR represents a genetic model of essential hypertension and the different angiogenic responses in this study could be explained by related strain differences between SHR and Wistar animals. However, the importance of the local environment on vessel growth is supported by the ability of microvascular networks in the SHR to reacquire normal vessel densities compared to normotensive strains. Rarefaction has been reversed with antioxidant treatment (22), MMP inhibition (48), VEGF gene transfer (8) or chronic hypoxia stimulation (47). In addition, treatment with angiotensin converting enzyme (ACE) inhibitor and angiotensin 2 type 1 receptor antagonist can reverse functional capillary rarefaction in muscle and skin of SHR (45, 46). Collectively, these studies along with our results support that microvascular networks in the SHR are able to undergo microvascular remodeling in response to local changes in the environment.
The importance of the rarefaction phenomenon in humans with hypertension is supported by the documentation of a reduced number of vessels in various from hypertensive patients including skin (2), conjunctival circulation (43), intestine (41) and skeletal muscles (17). The ability of the microcirculation in hypertensive patients to undergo normal remodeling is supported by the reversal of rarefaction with antihypertensive therapies (3, 6, 7, 39). Still, the cause-effect relationship between rarefaction and elevated blood pressure remains debated and the recent characterization of rarefaction in the skin of borderline essential patients and normotensive offspring of individuals with essential hypertension suggest that microvascular rarefaction could be a primary indicator and contributor to elevated blood pressure (1, 2, 34). These results suggest that pre-hypertensive therapies targeted at reversing rarefaction might represent alternative treatment strategies and emphasize the importance of investigations aimed at understanding the mechanisms of rarefaction. Our results suggest that an emphasis on the pruning process is necessary for the design of long lasting rarefaction reversing treatments. For adults with developed hypertension, the development of multi-combination based therapies targeted at blood pressure regulation and preventing local end-organ damage require understanding how microvascular rarefaction is linked to microvascular dysfunction. While this is not the objective of our study, this issue does motivate the need for more fully understanding rarefaction dynamics and the ability of hypertensive networks to respond to tissue remodeling stimulus.
This particular model of angiogenesis was selected because it produced a robust angiogenic response over a relatively short time course via inherent mechanisms in a tissue and without the invasion of a foreign material. Another advantage of this mesentery exteriorization model is that it reflects physiological tissue response inside the body (10). While the exteriorization of the mesentery has been linked to mast cell activation and an increase of local histamine level (10), the exact mechanisms involved in this model have not yet been identified. Still, we selected this model because it represented a multi-factorial stimulus that could be used to assess the angiogenic potential of microvascular networks. Our exteriorization model also allows us to examine a local angiogenic response. Because tissue windows outside the exteriorized mesentery region do not exhibit angiogenic responses (data not shown), we speculate that systemic blood pressure is not influenced. Future studies would be required to assess the impact of angiogenesis on local blood pressure per network.
In summary, our work suggests that impaired angiogenesis may not be a contributor to rarefaction during hypertension. Over the time course of microvascular network remodeling, we observed both apparent increases in initial growth and subsequent increases in vessel loss. These altered rates suggest that inconsistent results in the literature regarding whether angiogenesis is impaired during hypertension might be explained by comparisons between hypertensive and normotensive groups at different relative time points during the angiogenic response.
Acknowledgements
This work was supported by the Tulane Hypertension and Renal Center of Excellence funded by NIH grant P20RR017659-08 (PI: L. Gabriel Navar).
Abbreviations
- SHR
Spontaneously Hypertensive Rat
- (WKY) Rat
Wistar-Kyoto
- IM
Intramuscular
- IP
Intraperitoneal
- bw
Body Weight
- PECAM
Platelet Endothelial Cell Adhesion Molecule-1
- VEGF
Vascular Endothelial Growth Factor
- PBS
Phosphate Buffered Saline
- BSA
Bovine Serum Albumin
- NA
Numerical Aperture
- SEM
Standard Error of the Mean
- A/V
Arterial/venous
- UN
Unstimulated
References
- 1.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003;89:175–178. doi: 10.1136/heart.89.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–658. doi: 10.1161/01.hyp.34.4.655. [DOI] [PubMed] [Google Scholar]
- 3.Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr.Opin.Pharmacol. 2007;7:151–157. doi: 10.1016/j.coph.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Bohlen HG. Intestinal microvascular adaptation during maturation of spontaneously hypertensive rats. Hypertension. 1983;5:739–745. doi: 10.1161/01.hyp.5.5.739. [DOI] [PubMed] [Google Scholar]
- 5.Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol.Ther. 2006;111:81–98. doi: 10.1016/j.pharmthera.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Debbabi H, Bonnin P, Levy BI. Effects of blood pressure control with perindopril/indapamide on the microcirculation in hypertensive patients. Am.J.Hypertens. 2010;23:1136–1143. doi: 10.1038/ajh.2010.115. [DOI] [PubMed] [Google Scholar]
- 7.Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibirica E. Increased skin capillary density in treated essential hypertensive patients. Am.J.Hypertens. 2006;19:477–483. doi: 10.1016/j.amjhyper.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Emanueli C, Salis MB, Stacca T, Gaspa L, Chao J, Chao L, Piana A, Madeddu P. Rescue of impaired angiogenesis in spontaneously hypertensive rats by intramuscular human tissue kallikrein gene transfer. Hypertension. 2001;38:136–141. doi: 10.1161/01.hyp.38.1.136. [DOI] [PubMed] [Google Scholar]
- 9.Folkow B. Physiological aspects of primary hypertension. Physiol.Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 10.Franzen L, Ghassemifar R, Malcherek P. Experimental mast cell activation improves connective tissue repair in the perforated rat mesentery. Agents Actions. 1991;33:371–377. doi: 10.1007/BF01986588. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Yasu T, Kobayashi N, Ikeda N, Schmid-Schonbein GW. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ.Res. 2004;95:100–108. doi: 10.1161/01.RES.0000133677.77465.38. [DOI] [PubMed] [Google Scholar]
- 12.Grayson J, Parratt JR. Observations on blood flow in the myocardium. Bibl.Anat. 1965;7:416–422. [PubMed] [Google Scholar]
- 13.Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW., Jr Microvascular rarefaction and tissue vascular resistance in hypertension. Am.J.Physiol. 1989;256:H126–H131. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- 14.Greene AS, Tonellato PJ, Zhang Z, Lombard JH, Cowley AW., Jr Effect of microvascular rarefaction on tissue oxygen delivery in hypertension. Am.J.Physiol. 1992;262:H1486–H1493. doi: 10.1152/ajpheart.1992.262.5.H1486. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto H, Prewitt RL, Efaw CW. Alterations in the microvasculature of one-kidney, one-clip hypertensive rats. Am.J.Physiol. 1987;253:H933–H940. doi: 10.1152/ajpheart.1987.253.4.H933. [DOI] [PubMed] [Google Scholar]
- 16.Henrich H, Hertel R, Assmann R. Structural differences in the mesentery microcirculation between normotensive and spontaneously hypertensive rats. Pflugers Arch. 1978;375:153–159. doi: 10.1007/BF00584238. [DOI] [PubMed] [Google Scholar]
- 17.Henrich HA, Romen W, Heimgartner W, Hartung E, Baumer F. Capillary rarefaction characteristic of the skeletal muscle of hypertensive patients. Klin.Wochenschr. 1988;66:54–60. doi: 10.1007/BF01713011. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez N, Torres SH, Vera O, De Sanctis JB, Flores E. Muscle fiber composition and capillarization in relation to metabolic alterations in hypertensive men. J.Med. 2001;32:67–82. [PubMed] [Google Scholar]
- 19.Hudlett P, Neuville A, Miternique A, Griffon C, Weltin D, Stephan D. Angiogenesis and arteriogenesis are increased in fibrin gel chambers implanted in prehypertensive spontaneously hypertensive rats. J.Hypertens. 2005;23:1559–1564. doi: 10.1097/01.hjh.0000174607.18780.62. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M. Role of oxidative stress in health and disease. Rinsho Byori. 1996;44:911–914. [PubMed] [Google Scholar]
- 21.Kiefer FN, Neysari S, Humar R, Li W, Munk VC, Battegay EJ. Hypertension and angiogenesis. Curr.Pharm.Des. 2003;9:1733–1744. doi: 10.2174/1381612033454540. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N, DeLano FA, Schmid-Schonbein GW. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler.Thromb.Vasc.Biol. 2005;25:2114–2121. doi: 10.1161/01.ATV.0000178993.13222.f2. [DOI] [PubMed] [Google Scholar]
- 23.Kundu S, Rao P. The story of spontaneously hypertensive rat (SHR): A Review. Al Ameen J Med. 2008;1:65–66. [Google Scholar]
- 24.Lanciault G, Jacobson ED. The gastrointestinal circulation. Gastroenterology. 1976;71:851–873. [PubMed] [Google Scholar]
- 25.Larouche I, Schiffrin EL. Cardiac microvasculature in DOCA-salt hypertensive rats : effect of endothelin ET(A) receptor antagonism. Hypertension. 1999;34:795–801. doi: 10.1161/01.hyp.34.4.795. [DOI] [PubMed] [Google Scholar]
- 26.le Noble FA, Stassen FR, Hacking WJ, Struijker Boudier HA. Angiogenesis and hypertension. J.Hypertens. 1998;16:1563–1572. doi: 10.1097/00004872-199816110-00001. [DOI] [PubMed] [Google Scholar]
- 27.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 28.Levy BI, Duriez M, Samuel JL. Coronary microvasculature alteration in hypertensive rats. Effect of treatment with a diuretic and an ACE inhibitor. Am.J.Hypertens. 2001;14:7–13. doi: 10.1016/s0895-7061(00)01212-7. [DOI] [PubMed] [Google Scholar]
- 29.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 30.McGuire BJ, Secomb TW. A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J.Appl.Physiol. 2001;91:2255–2265. doi: 10.1152/jappl.2001.91.5.2255. [DOI] [PubMed] [Google Scholar]
- 31.Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation. 2006;13:261–273. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- 32.Murfee WL, Schmid-Schonbein GW. Chapter 12. Structure of microvascular networks in genetic hypertension. Methods Enzymol. 2008;444:271–284. doi: 10.1016/S0076-6879(08)02812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson H, Folkow B. Vasoconstrictor nerve influence on isolated mesenteric resistance vessels from normotensive and spontaneously hypertensive rats. Acta Physiol.Scand. 1982;116:205–208. doi: 10.1111/j.1748-1716.1982.tb07131.x. [DOI] [PubMed] [Google Scholar]
- 34.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J.Clin.Invest. 1997;99:1873–1879. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto KAK. Development of a strain of spontaneously hypertensive rats. Jpn Cir J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 36.Paiardi S, Rodella LF, De Ciuceis C, Porteri E, Boari GE, Rezzani R, Rizzardi N, Platto C, Tiberio GA, Giulini SM, Rizzoni D, Agabiti-Rosei E. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin.Hemorheol.Microcirc. 2009;42:259–268. doi: 10.3233/CH-2009-1195. [DOI] [PubMed] [Google Scholar]
- 37.Ponce AM, Price RJ. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc.Res. 2003;65:45–48. doi: 10.1016/s0026286202000146. [DOI] [PubMed] [Google Scholar]
- 38.Prewitt RL, Chen II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am.J.Physiol. 1982;243:H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- 39.Sabino B, Lessa MA, Nascimento AR, Rodrigues CA, Henriques MG, Garzoni LR, Levy BI, Tibirica E. Effects of antihypertensive drugs on capillary rarefaction in spontaneously hypertensive rats: intravital microscopy and histologic analysis. J.Cardiovasc.Pharmacol. 2008;51:402–409. doi: 10.1097/FJC.0b013e3181673bc5. [DOI] [PubMed] [Google Scholar]
- 40.Schmid-Schonbein GW, Zweifach BW, DeLano FA, Chen PC. Microvascular tone in a skeletal muscle of spontaneously hypertensive rats. Hypertension. 1987;9:164–171. doi: 10.1161/01.hyp.9.2.164. [DOI] [PubMed] [Google Scholar]
- 41.Short DS, Thomson AD. The arteries of the small intestine in systemic hypertension. J.Pathol.Bacteriol. 1959;78:321–334. doi: 10.1002/path.1700780202. [DOI] [PubMed] [Google Scholar]
- 42.Suematsu M, Suzuki H, Delano FA, Schmid-Schonbein GW. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation. 2002;9:259–276. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan JM, Prewitt RL, Josephs JA. Attenuation of the microcirculation in young patients with high-output borderline hypertension. Hypertension. 1983;5:844–851. doi: 10.1161/01.hyp.5.6.844. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Schmid-Schonbein GW, Suematsu M, DeLano FA, Forrest MJ, Miyasaka M, Zweifach BW. Impaired leukocyte-endothelial cell interaction in spontaneously hypertensive rats. Hypertension. 1994;24:719–727. doi: 10.1161/01.hyp.24.6.719. [DOI] [PubMed] [Google Scholar]
- 45.Takeshita S, Tomiyama H, Yokoyama N, Kawamura Y, Furukawa T, Ishigai Y, Shibano T, Isshiki T, Sato T. Angiotensin-converting enzyme inhibition improves defective angiogenesis in the ischemic limb of spontaneously hypertensive rats. Cardiovasc.Res. 2001;52:314–320. doi: 10.1016/s0008-6363(01)00372-8. [DOI] [PubMed] [Google Scholar]
- 46.Tran HA, Schwartzbard A, Weintraub HS. Role of RAAS Inhibition in the Prevention of Cardiovascular Disease. Curr.Treat.Options Cardiovasc.Med. 2011 doi: 10.1007/s11936-011-0126-9. [DOI] [PubMed] [Google Scholar]
- 47.Vilar J, Waeckel L, Bonnin P, Cochain C, Loinard C, Duriez M, Silvestre JS, Levy BI. Chronic hypoxia-induced angiogenesis normalizes blood pressure in spontaneously hypertensive rats. Circ.Res. 2008;103:761–769. doi: 10.1161/CIRCRESAHA.108.182758. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Olszewski B, Rosebury W, Wang D, Robertson A, Keiser JA. Impaired angiogenesis in SHR is associated with decreased KDR and MT1-MMP expression. Biochem.Biophys.Res.Commun. 2004;315:363–368. doi: 10.1016/j.bbrc.2004.01.059. [DOI] [PubMed] [Google Scholar]