Abstract
Myostatin is an extracellular cytokine mostly expressed in skeletal muscles and known to play a crucial role in the negative regulation of muscle mass. Upon the binding to activin type IIB receptor, myostatin can initiate several different signalling cascades resulting in the upregulation of the atrogenes and downregulation of the important for myogenesis genes. Muscle size is regulated via a complex interplay of myostatin signalling with the insulin-like growth factor 1/phosphatidylinositol 3-kinase/Akt pathway responsible for increase in protein synthesis in muscle. Therefore, the regulation of muscle weight is a process in which myostatin plays a central role but the mechanism of its action and signalling cascades are not fully understood. Myostatin upregulation was observed in the pathogenesis of muscle wasting during cachexia associated with different diseases (i.e. cancer, heart failure, HIV). Characterisation of myostatin signalling is therefore a perspective direction in the treatment development for cachexia. The current review covers the present knowledge about myostatin signalling pathways leading to muscle wasting and the state of therapy approaches via the regulation of myostatin and/or its downstream targets in cachexia.
Keywords: Myostatin, Muscle wasting, Cachexia
Introduction
Cachexia is a syndrome occurring at terminal stages of diseases such as cancer, chronic heart failure, chronic kidney failure or AIDS [1]. This syndrome is characterized by loss of body weight as a consequence of pathological changes in different metabolic pathways. It leads to increased morbidity and mortality irrespective of the underlying disease. A major role in the development of cachexia is played by the loss of muscle mass accompanied by the loss of fat [2]. Such muscle hypotrophy is the result of multiple alterations at the molecular level, e.g. the disturbance in the balance between protein degradation and protein synthesis [1]. Proinflammatory cytokines such as interleukin-1, interleukin-6 and tumour necrosis factor-α (TNFα) were shown to play an important role in the development of muscle wasting [3]. However, there is no established treatment for cachexia based on the regulation of their signalling. Many different peptides have received therapeutic interest over the last decade, including ghrelin, leptin, melanocortins and growth hormone [4–6]. In 1997, the role of another extracellular factor in the negative regulation of muscle mass, referred to as myostatin, was discovered [7]. Myostatin upregulation was found in the pathogenesis of cancer, HIV, heart failure associated cachexia and aging [8–12]. It has become one of the main targets in the investigation of the regulation of muscle mass.
Myostatin
Myostatin, also known as growth/differentiation factor-8 (GDF-8) is a member of tumour growth factor β (TGF-β) family [7]. This protein is a homodimer with a molecular weight of 25 kDa and a disulfide bond between the monomers at the C-terminal regions [7]. Myostatin circulates in the blood in a latent form with an additional non-covalently bound propeptide at the N-terminus. Proteolytic cleavage of the propeptide by the bone morphogenetic protein (BMP)-1/tolloid family of metalloproteinases is necessary for activation of protein function [13].
The role of myostatin in skeletal muscle was discovered using the method of gene disruption in mice. Mstn null animals showed significant increase in muscle mass (up to two-fold) and decrease of fat tissue compared to the wild type [14, 15]. Similar effects were observed in the presence of natural mutations of Mstn in cattle, sheep, dogs and humans [16–19] and upon the inhibition of the protein function in adult mice [20]. At the same time, overexpression of Mstn led to the reduction of muscle mass suggesting myostatin to be a negative regulator of skeletal muscle growth. During embryogenesis, myostatin is exclusively expressed in skeletal muscle [7] to control the differentiation and proliferation of the myoblast, but in adulthood, it is not only restricted to skeletal muscle but also detected in other tissues (e.g. heart, adipose tissue, mammary gland) [7, 21–25].
The expression of myostatin in the healthy heart is low and mostly detected in Purkinje fibres but not in cardiomyocytes [22]. After myocardial infarction, increased expression of myostatin was observed in sheep, but its expression was restricted to the damaged zone [22]. Moreover, George et al. recently published new data suggesting the increase of myostatin levels in the failing heart [26]. The authors also showed an increase of its latent complex in circulation and expression of BMP-1 that could explain the development of cachexia in patients with heart failure [26]. These data are in accordance with the recent research of Heineke et al. who suggested that myostatin produced by cardiomyocytes could stimulate muscle wasting in heart failure [27].
The role of myostatin in the regulation of adipose tissue is not well understood. Myostatin and its receptor (type IIB activin receptor, ActRIIB) were shown to be expressed in adipose tissue at low levels [7, 14]. Several studies showed the negative effect of myostatin on preadipocyte differentiation and proliferation [28, 29]. Contrary, an effect of promotion of the differentiation leading to the increased adipogenesis was observed in pluripotent mesenchymal cells [30, 31]. Moreover, Mstn null mice had a decreased amount of adipose tissue [14, 32]. It is still unclear whether the effect of myostatin on adipose tissue is the direct result of regulation or it is an indirect consequence of skeletal muscle growth. Reduced adiposity was observed in different transgenic mouse models of muscle hypertrophy [33–35]. Thereby the decreased amount of adipose tissue in Mstn null mice could be an indirect drawback of significant increases in muscle mass. Such an effect can be mediated through leptin—adipose-specific hormone regulating food intake and energy homeostasis. Its level was shown to be lower in Mstn null mice [32]. Therefore, additional experiments are required to determine the role of myostatin in adipose tissue.
Binding to the cell
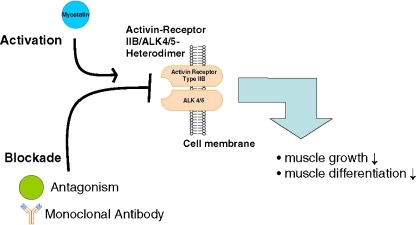
Myostatin is an extracellular cytokine, and as many other members of the TGF-β family, it mediates the signal through activin receptors [36]. Active myostatin mostly binds to the ActRIIB [36] and engages the signalling cascade leading to the inhibition of myoblast differentiation and proliferation (Fig. 1). ActRIIB can mediate other signalling pathways with diverse affinity for ligands—high for activin A and GDF11, low for BMP-2 and BMP-7 [38]. These ligands are responsible for several cell responses by activating different members of the Smad family of transcription factors.
Fig. 1.
Myostatin pathway. Myostatin is synthesized and secreted by muscle cell; it signals through the activin IIB/ALK 4/5 heterodimer to activate different pathways resulting in the decrease in muscle growth and differentiation (with permission from [37])
Activin receptors are transmembrane threonine/serine kinases divided in two types. Type I receptor (ALK 4 and ALK 5 for myostatin [28]) has the unique GS domain located closely to the intracellular space and adjacent to the kinase domain which is absent in the second type (ActRII). Binding of myostatin with ActRIIB causes its assembly with type I receptor and phosphorylation of its GS domain. Therefore, the signal of myostatin is mediated through activated complex of two receptors [39].
Myostatin signalling through ActRIIB is crucial for the regulation of muscle growth. The natural defect in ActRIIB sensitivity in humans leads to a significant increase in muscle mass [40]. The same effect was observed in the experiments that used blockade of the murine receptor [40]. In the characterization of the ActRIIB role in myostatin signalling, the soluble form of this receptor (sActRIIB) is used. sActRIIB is a fusion protein of the receptor extracellular domain with immunoglobulin Fc. It acts as a decoy receptor for myostatin. Healthy mice treated with sActRIIB showed a 60% increase in muscle mass, just 2 weeks after treatment initiation [40]. Surprisingly, in mice treated with antimyostatin antibodies (JA-16) or myostatin propeptide known to bind circulating myostatin, the hypertrophy of muscle was lower compared to sActRIIB [41, 42]. Moreover, in Mstn knockout mice treated with the sActRIIB, an increase in muscle mass by 15–25% was observed [40]. This suggests the presence of at least one more ligand that binds to the receptor thereby regulating the mass of skeletal muscle. Souza et al. proposed that such ligands could embrace BMP-11, activin A, B and AB, but this hypothesis requires more evidence [43]. However, it could explain why myostatin blockade in clinical trials had no significant effect on muscle weight [44]. The results obtained in cancer models of Lewis lung carcinoma and B16F10 melanoma are in agreement with this theory. It was shown that myostatin knockout mice had a more pronounced tendency to develop muscle wasting than wild-type animals. This finding suggests that myostatin is not the only ligand providing the signal leading to muscle wasting. On the other hand, the same study showed that myostatin could prevent muscle wasting to a certain degree [45].
Recently, Zhou et al. moved a significant step forward in development of a novel therapy [46]. The authors demonstrated that sActRIIB prevents or even reverses development of cancer cachexia. Their research showed the connection between the development of cancer cachexia and the activation of activin receptor. Blockade of ActRIIB led to the regeneration of muscle and cardiac mass [46]. These data suggest a fundamental role of ActRIIB-mediated signalling pathways in the induction of muscle wasting during cachexia and can be used in the development of treatments against cachexia.
Myostatin extracellular regulation
Myostatin circulates in the blood in a latent complex with non-covalently bound propeptide at the N-terminus [13, 36, 47], crucial for the correct folding of the protein [20]. The propeptide was shown to bind the active myostatin in vivo and in vitro, and its overexpression in mice results in an increase of muscle mass [36, 48].
Specific inhibitors can prevent binding of myostatin to ActRIIB in serum. One of them is follistatin, an extracellular cysteine-rich glycoprotein with a structure not similar to members of TGF-β family. Follistatin binds myostatin and inhibits its activity by preventing its binding to the receptor [11, 36]. Overexpression of follistatin in vivo leads to muscle hypertrophy similar to the one observed in Mstn null mice [36]. Mice with homozygous mutation in the Fst gene showed a decrease in muscle mass suggesting an important role of follistatin in the regulation of myogenesis [49]. Follistatin-like 3 protein has more than 30% homology with follistatin and was also shown to bind circulating myostatin [41].
Another inhibitor of myostatin is growth and differentiation factor-associated serum protein 1 (GASP-1). Unlike members of the follistatin family, GASP-1 does not have affinity to activin [42]. The structure of GASP-1 is different from previously described inhibitors: With the exception of one follistatin domain, it has additional domains typical for protease inhibitors. Interestingly, GASP-1 can also bind to the myostatin propeptide and possibly regulate the activation of myostatin through proteolytic cleavage [42]. Moreover, myostatin can be inactivated upon covalent binding to latent TGF-β binding protein 3 (LTBP3). This inactive complex of myostatin/LTBP3 is used for myostatin storage in the extracellular matrix [50]. Taken together, myostatin is regulated by at least four different inhibitors via binding of the active or latent form. The mechanisms of myostatin inhibition still remain elusive, and the crosstalk between the different intrinsic inhibitors is still unclear. The variety of inhibitors emphasizes the importance of a strict myostatin regulation in order to avoid muscle damage and wasting. The schematic mechanism of myostatin extracellular signalling is presented in Fig. 1.
Intracellular response
Skeletal muscle mass is maintained as a consequence of two main molecular mechanisms: protein synthesis and protein degradation. Despite the profound knowledge of the role of myostatin in the regulation of muscle growth, many details of the molecular mechanisms of its action are poorly understood. It has been suggested that binding of myostatin to the ActRIIB results in the phosphorylation of two serine residues of Smad2 or Smad3 at COOH domains. This leads to the assembly of Smad2/3 with Smad4 to the heterodimer that is able to translocate to the nucleus and activate transcription of target genes [51–53]. One of the known downstream targets of Smad signalling is MyoD, a transcriptional factor that is involved in skeletal muscle development and takes part in the repair of damaged skeletal muscle [54–57]. Downregulation of myoD expression was shown in vitro and in vivo during cachexia, possibly via TNFα through the induction of the NF-κB pathway. Interestingly, myostatin downregulates myoD in an NF-κB-independent way [58, 59]. Myostatin also inhibits Pax3 expression, which is possibly an upstream target of MyoD [58, 60, 61]. Moreover, Smad signalling targets other genes such as myf5 and myogenin, known to be important for myogenesis [62].
Interestingly, other Smad proteins (Smad6 and Smad7) work as agonists. They compete for the binding to the activin type I receptor and thereby inhibit signalling of TGF-β family members [63, 64]. Myostatin was also shown to activate Smad7 transcription. Smad7, in turn, is able to inhibit the association of Smad2/3 with Smad4 [65, 66]. Therefore, the Smad pathway is regulated by feedback control [67].
Myostatin signalling via Smad has been intensively investigated, but it is not the only feasible signalling pathway. TGF-β family members were shown to activate mitogen-activated protein kinases (MAPKs), particularly p38 and extracellular signal-regulated kinase 1/2 (ERK1/2) [68–71]. p38 MAPK is responsible for the cell response to stress factors and was shown to be activated by myostatin via TGF-β activated kinase 1 (TAK-1)/MAPK kinase (MAPKK) cascade. Its signalling results in the downregulation of myogenesis-related genes, but it does not target Smads [72]. The role of ERK1/2 in the regulation of muscle mass is controversial. On the one hand, there are studies confirming that ERK1/2 takes part in the process of satellite cell proliferation that is necessary for the maintenance of skeletal muscle weight [73] and induces protein synthesis under physiological conditions [74, 75]. On the other hand, there are experiments showing an opposite effect of ERK1/2 activation [76–79]. Thus, the data suggest that increased activity of ERK1/2 leads to the differentiation inhibition in several cell types [76, 77]. At the same time, myostatin significantly activated ERK1/2 in C2C12 cells. Similar effects were observed in mice during systematic administration of myostatin [78]. Taken together, it seems likely that myostatin mediates its signal at least partially through ERK1/2 activation. Therefore, different responses through ERK1/2 could be caused by different levels of myostatin corresponding to normal and pathological conditions. MAPK cascade normally involves the activation of Ras/Raf/MEK1. To check whether myostatin uses the same pathways to activate ERK1/2, some experiments were done. Using an inhibitor of MEK1 in C2C12 cells, Yang et al. showed that this kinase is involved in the myostatin-induced activation of ERK1/2 [78]. Moreover, such inhibition of MEK1 leads to the rescue of cell differentiation, which means that MEK-1/ERK1/2 play a role in differentiation suppression by myostatin. The presence of dominant negative form of Ras was shown to positively influence MEK1/ERK1/2 through the downstream activation of Raf. Therefore, myostatin activates ERK1/2 via Ras/Raf/MEK1 pathway [78].
Cross talk of myostatin and IGF-1
One of the main positive regulators of muscle growth is insulin-like growth factor 1 (IGF-1). Under normal conditions, IGF-1 signalling seems to be dominant and blocks the myostatin pathway [80]. However, an inhibition of IGF-1 was observed when myostatin is overexpressed [81, 82]. IGF-1 can prevent TGF-β family-mediated apoptosis [83], and it was shown that in the absence of IGF-1, the level of apoptosis in C2C12 cells treated with myostatin increased. Yang et al. speculate it may in part explain the ability of myostatin in regulating cell cycle but not apoptosis in normal conditions [84].
The mechanism by which IGF-1 regulates myostatin signalling includes the inhibition of transcription factors responsible for the induction of atrogenes via phosphorylation with phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Akt plays a significant role in different metabolic processes in the cell, particularly in the hypertrophic response to insulin and IGF-1 [85, 86]. Akt is the crossing point between IGF-1/myostatin pathways. The results of several studies suggest that the genetic loss of myostatin leads to the increase in Akt activity in skeletal muscle in vivo and in vitro [82, 87]. In contrast, a decreased level of phosphorylated Akt is associated with incubation of myotubes with myostatin [58]. It is likely that in the conditions of muscle wasting, myostatin can switch Akt/mammalian target of rapamycin (mTOR) pathway, responsible for protein synthesis, to inhibition of protein synthesis involving FoxO, GSK-3β or other unknown patterns leading to the loss of muscle mass. These pathways will be discussed below in more detail.
Akt/FoxO
The class of Forkhead box O (FoxO) transcription factors is involved in the regulation of energy metabolism. FoxOs take part in the formation of skeletal muscle and adipose tissue as major organs for energy distribution [88, 89]. During myoblast proliferation, FoxO1 and FoxO3 are localized in the cytoplasm in the latent, phosphorylated form. Upon initiation of differentiation, FoxO translocates to the nucleus where it binds to DNA and regulates transcription. Akt regulates the activity of FoxO1 and FoxO3 by phosphorylating them and thereby retaining them in the cytoplasm [58]. In myotube culture, the level of FoxO1 in the nucleus was shown to increase after treatment with myostatin [58, 90], together with FoxO3 resulting in the induction of the muscle-specific E3-ubiquitin ligases atrogin-1 (MAFbx) and MuRF-1 [91, 92]. Expression of constitutively active FoxO1 in transgenic mice led to decrease in myoblast differentiation and a reduction in muscle weight [93]. In muscle, FoxOs are known to interact with Smad3 and Smad4 inducing the protein degradation. Recently, it was shown that FoxO1 and Smad synergistically increase the expression of myostatin mRNA and its promoter activity in C2C12 myotubes but via different pathways [94]. Taken together, myostatin-mediated signalling activates FoxO, and it leads to the upregulation of proteasome ubiquitin ligases MuRF-1 and atrogin-1, which participate in protein degradation.
Akt/GSK-3β
Cycline D1 is an important component for G1 phase of cell cycle which is also regulated via PI3K/Akt pathway. In C2C12 myoblasts treated with myostatin during the phase of active cell growth, a decrease in cycline D1 levels was shown, suggesting that myostatin targets cycline D1 for proliferation inhibition. In favour of this hypothesis, overexpression of cycline D1 was shown to rescue cell cycle. The activation of GSK-3β via dephosphorylation at Ser 9 increases proteolysis of cyclin D1 [95] in the response to myostatin-mediated dephosphorylation of Akt at Ser 473. Usage of IGF-1 or active Akt treatment blocks myostatin induced arrest of cell proliferation [84]. Thus, one more pathway activated by myostatin suggests that inhibition of Akt plays a dramatic role in the providing of myostatin signalling.
Akt/mTOR/p70s6K
This pathway is important for the differentiation of myoblasts and hypertrophy of myotubes. mTOR is a kinase downstream of Akt, which phosphorylates 4E-BP1 and p70s6k thereby inducing initiation of protein synthesis [85, 96]. In Mstn knockout mice, an increase in Akt/mTOR/p70s6K signalling was observed [87]. There are two mTOR complexes called TORC1 and TORC2, containing the proteins RAPTOR and RICTOR, respectively [97]. Activation of TORC1 leads to protein synthesis, while TORC2 plays a significant role in the feedback Akt phosphorylation leading to the blockade of FoxO signalling [98]. Inhibition of RAPTOR was shown to play an important role in myostatin signalling, whereas RICTOR is necessary for the cell differentiation itself. Inhibition of RAPTOR amplifies myostatin actions such as Smad phosphorylation [80].
Additional data confirming the connection between myostatin and Akt pathways were received in experiments with hypoxic muscles. The expression of myostatin was shown to be upregulated under these conditions [99]. At the same time, the Akt/mTOR pathway was downregulated in the rats with chronic hypoxia [100].
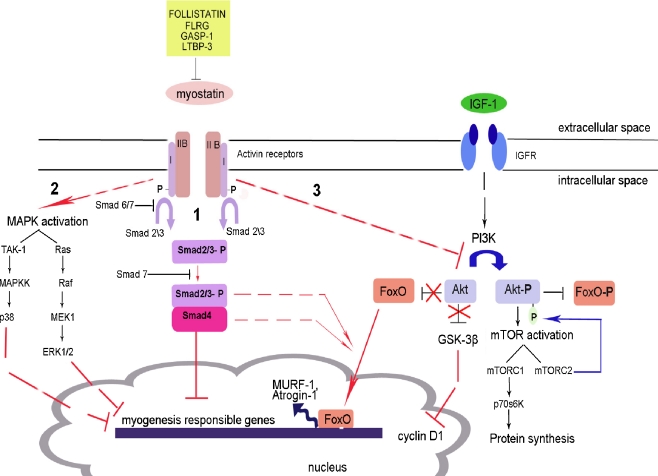
In conclusion, signal transduction of myostatin is a complex process involving activation and inhibition of several cellular signalling pathways. These pathways result in the downregulation of the expression of myogenic factors, decrease in protein synthesis and activation of proteasome–ubiquitin ligases. The schematic picture of myostatin signalling is presented in Fig. 2. There are a lot of questions still connected with the order of activation and precise importance of each pathway and its contribution in the development of muscle wasting.
Fig. 2.
Different pathways of myostatin signalling. → The activation of the process, -| the inhibition of the process, -- -- the presence of intermediate steps either unknown or omitted in the figure. 1 Canonical pathway of Smad activation. Myostatin binds to ActRIIB and induces its assembly with activin type I receptor. Subsequent phosphorylation of Smad2/3 leads to its binding with Smad4 and translocation of the complex to the nucleus where it blocks the transcription of genes responsible for the myogenesis. Smad6 and Smad7 compete for the binding with activin type I receptor. Smad7 can also prevent the formation of the Smad 2/3 and Smad4 complex. 2 MAPK activation. The activation of MAPKs is mediated via myostatin using different pathways: TAK-1/MAPKK for p38 MAPK or Ras/Raf/MEK1 for ERK1/2. It leads to the blockade of genes responsible for myogenesis. 3 Inhibition of Akt signalling. Akt phosphorylation occurs in the response to insulin and IGF-1. In normal case, active Akt induces mTOR signal leading to the protein synthesis; at the same time, it inhibits FoxO by phosphorylation. In the pathological conditions, dephosphorylated Akt does not inhibit FoxO. It leads to the accumulation of FoxO in the nucleus where it binds to the DNA and induces the transcription of E3 ubiquitin ligases MURF-1 and Atrogin-1. Smad3 and Smad4 possibly participate in FoxO signalling (adapted from [51, 84, 90, 101])
Treatment
Myostatin has become a main target for the development of drugs for cachexia and muscle wasting diseases. Despite the experiments using antimyostatin substances that were conducted, there are no drug discoveries against muscle wasting in cachexia so far. The information about compounds that were involved in clinical trials is scarce, but none of the known substances had a positive effect. MYO-029 is recombinant human myostatin-specific antibody that did not show a significant improvement in muscle strength and function in adult muscular dystrophies [44]. The other compound developed by Amgen, AMG 745, has an unknown composition, but its testing was stopped after phase I clinical trials [102]. The problem of using antimyostatin compounds is likely due to the ability of the ActRIIB to bind other ligands from the TGF-β family resulting in a redundancy of myostatin.
The impact of sActRIIB use in the regulation of muscle mass is intensively studied at the moment. Recently, very positive results were published by Zhou et al. [46] in C-26 tumour-bearing mice, where the use of sActRIIB resulted in the prolongation of life and reversal of muscle wasting during cachexia. In this study, a positive effect on cardiac muscle was also seen. The results of this study provide a major step forward in establishing novel cachexia therapies.
The significance of these results for the treatment of cardiac cachexia was shown by Heineke et al. [27], who observed that myostatin produced from cardiomyocytes is released into the circulation in heart failure and that myostatin subsequently activated muscle wasting. The deletion of Mstn from cardiomyocytes and inhibition of myostatin protein using antibodies in mice prevented muscle wasting in heart failure [27].
Conclusion
Myostatin is a negative regulator of myoblast proliferation and differentiation. Normally it functions to regulate hypertrophy of muscles, but a role in the induction of muscle loss was observed in muscle wasting diseases and cachexia associated with severe illnesses. The mechanism of myostatin signalling is complex and involves the activation of several downstream pathways. Additional studies are required to elucidate the cross talk between the signalling cascades and their regulation. The experiments with myostatin propeptide and antimyostatin antibodies showed a positive effect on regulation of muscle mass in different models of wasting but lacked efficacy in phase I clinical trials. The most promising target in terms of cachexia treatment seems to be the activin type II receptor. The blockade of this receptor led to a significant increase in muscle mass and even its restoration in cancer cachexia. Therefore, targeting myostatin and its receptor represent a promising direction in the development of effective treatments for cachexia and muscle wasting diseases.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [103].
Conflict of interest
The authors declare no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Moldawer LL, Copeland EM., 3rd Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer. 1997;79:1828–1839. [PubMed] [Google Scholar]
- 4.Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle. 2010;1:169–176. doi: 10.1007/s13539-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller TD, Perez-Tilve D, Tong J, Pfluger PT, Tschop MH. Ghrelin and its potential in the treatment of eating/wasting disorders and cachexia. J Cachexia Sarcopenia Muscle. 2010;1:159–167. doi: 10.1007/s13539-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 7.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 8.Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;6:343–348. [PubMed] [Google Scholar]
- 9.Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, et al. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11:342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, et al. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA. 1998;95:14938–14943. doi: 10.1073/pnas.95.25.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 12.Costelli P, Muscaritoli M, Bonetto A, Penna F, Reffo P, Bossola M, et al. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest. 2008;38:531–538. doi: 10.1111/j.1365-2362.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 13.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, et al. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamrick MW, Pennington C, Webb CN, Isales CM. Resistance to body fat gain in ‘double-muscled’ mice fed a high-fat diet. Int J Obes (Lond) 2006;30:868–870. doi: 10.1038/sj.ijo.0803200. [DOI] [PubMed] [Google Scholar]
- 16.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 18.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38:813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 21.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, et al. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res. 2006;99:15–24. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006;36:713–719. doi: 10.1111/j.1365-2362.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 24.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, et al. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294:E918–E927. doi: 10.1152/ajpendo.00798.2007. [DOI] [PubMed] [Google Scholar]
- 25.Ji S, Losinski RL, Cornelius SG, Frank GR, Willis GM, Gerrard DE, et al. Myostatin expression in porcine tissues: tissue specificity and developmental and postnatal regulation. Am J Physiol. 1998;275:R1265–R1273. doi: 10.1152/ajpregu.1998.275.4.R1265. [DOI] [PubMed] [Google Scholar]
- 26.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, et al. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail. 2010;12:444–453. doi: 10.1093/eurjhf/hfq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Liang L, Dean RG, Hausman DB, Hartzell DL, Baile CA. Inhibition of preadipocyte differentiation by myostatin treatment in 3T3-L1 cultures. Biochem Biophys Res Commun. 2001;281:902–906. doi: 10.1006/bbrc.2001.4435. [DOI] [PubMed] [Google Scholar]
- 30.Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146:3547–3557. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 31.Feldman BJ, Streeper RS, Farese RV, Jr, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA. 2006;103:15675–15680. doi: 10.1073/pnas.0607501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002;291:701–706. doi: 10.1006/bbrc.2002.6500. [DOI] [PubMed] [Google Scholar]
- 33.Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, et al. Conditional activation of Akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized IGF-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kung T, Szabo T, Springer J, Doehner W, Anker SD, von Haehling S. Cachexia in heart disease: highlights from the ESC 2010. J Cachexia Sarcopenia Muscle. 2011;2:63–69. doi: 10.1007/s13539-011-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sako D, Grinberg AV, Liu J, Davies MV, Castonguay R, Maniatis S, et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J Biol Chem. 2010;285:21037–21048. doi: 10.1074/jbc.M110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- 42.Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 43.Souza TA, Chen X, Guo Y, Sava P, Zhang J, Hill JJ, et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22:2689–2702. doi: 10.1210/me.2008-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, et al. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 45.Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391:1548–1554. doi: 10.1016/j.bbrc.2009.12.123. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Thies RS, Chen T, Davies MV, Tomkinson KN, Pearson AA, Shakey QA, et al. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors. 2001;18:251–259. doi: 10.3109/08977190109029114. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Ratovitski T, Brady JP, Solomon MB, Wells KD, Wall RJ. Expression of myostatin pro domain results in muscular transgenic mice. Mol Reprod Dev. 2001;60:351–361. doi: 10.1002/mrd.1097. [DOI] [PubMed] [Google Scholar]
- 49.Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- 50.Anderson SB, Goldberg AL, Whitman M. Identification of a novel pool of extracellular pro-myostatin in skeletal muscle. J Biol Chem. 2008;283:7027–7035. doi: 10.1074/jbc.M706678200. [DOI] [PubMed] [Google Scholar]
- 51.Joulia-Ekaza D, Cabello G. The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol. 2007;7:310–315. doi: 10.1016/j.coph.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol. 2008;104:579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 53.Patel K, Amthor H. The function of myostatin and strategies of myostatin blockade-new hope for therapies aimed at promoting growth of skeletal muscle. Neuromuscul Disord. 2005;15:117–126. doi: 10.1016/j.nmd.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- 55.Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol. 2000;224:122–137. doi: 10.1006/dbio.2000.9682. [DOI] [PubMed] [Google Scholar]
- 56.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 57.Montarras D, Lindon C, Pinset C, Domeyne P. Cultured myf5 null and myoD null muscle precursor cells display distinct growth defects. Biol Cell. 2000;92:565–572. doi: 10.1016/s0248-4900(00)01110-2. [DOI] [PubMed] [Google Scholar]
- 58.McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, et al. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209:501–514. doi: 10.1002/jcp.20757. [DOI] [PubMed] [Google Scholar]
- 59.Bakkar N, Wackerhage H, Guttridge D. Myostatin and NF-κB regulate skeletal myogenesis through distinct signaling pathways. Signal Transduction. 2005;5:202–210. [Google Scholar]
- 60.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 61.Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- 62.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Lebrun JJ, Vale W. Regulation of transforming growth factor beta- and activin-induced transcription by mammalian Mad proteins. Proc Natl Acad Sci USA. 1996;93:12992–12997. doi: 10.1073/pnas.93.23.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao J, Shi W, Chen H, Warburton D. Smad7 and Smad6 differentially modulate transforming growth factor beta-induced inhibition of embryonic lung morphogenesis. J Biol Chem. 2000;275:23992–23997. doi: 10.1074/jbc.M002433200. [DOI] [PubMed] [Google Scholar]
- 65.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 67.Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol. 2006;206:264–272. doi: 10.1002/jcp.20477. [DOI] [PubMed] [Google Scholar]
- 68.Hartsough MT, Mulder KM. Transforming growth factor beta activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- 69.Mulder KM. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 70.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 71.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Philip B, Lu Z, Gao Y. Regulation of GDF-8 signaling by the p38 MAPK. Cell Signal. 2005;17:365–375. doi: 10.1016/j.cellsig.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Nicolas N, Marazzi G, Kelley K, Sassoon D. Embryonic deregulation of muscle stress signaling pathways leads to altered postnatal stem cell behavior and a failure in postnatal muscle growth. Dev Biol. 2005;281:171–183. doi: 10.1016/j.ydbio.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 74.Sale EM, Atkinson PP, Arnott CH, Chad JE, Sale GJ. Role of ERK1/ERK2 and p70S6K pathway in insulin signalling of protein synthesis. FEBS Lett. 1999;446:122–126. doi: 10.1016/s0014-5793(99)00193-3. [DOI] [PubMed] [Google Scholar]
- 75.Felton-Edkins ZA, Fairley JA, Graham EL, Johnston IM, White RJ, Scott PH. The mitogen-activated protein (MAP) kinase ERK induces tRNA synthesis by phosphorylating TFIIIB. EMBO J. 2003;22:2422–2432. doi: 10.1093/emboj/cdg240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Penn BH, Berkes CA, Bergstrom DA, Tapscott SJ. How to MEK muscle. Mol Cell. 2001;8:245–246. doi: 10.1016/s1097-2765(01)00331-8. [DOI] [PubMed] [Google Scholar]
- 77.Kang CD, Do IR, Kim KW, Ahn BK, Kim SH, Chung BS, et al. Role of Ras/ERK-dependent pathway in the erythroid differentiation of K562 cells. Exp Mol Med. 1999;31:76–82. doi: 10.1038/emm.1999.13. [DOI] [PubMed] [Google Scholar]
- 78.Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D. Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res. 2006;66:1320–1326. doi: 10.1158/0008-5472.CAN-05-3060. [DOI] [PubMed] [Google Scholar]
- 79.Penna F, Costamagna D, Bonetto A, Minero VG, Baccino FM, Costelli P. Extracellular signal-regulated kinase (ERK) inhibition prevents muscle atrophy in experimental cancer cachexia. J Cachexia Sarcopenia Muscle. 2010;1:67. [Google Scholar]
- 80.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 81.Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, et al. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009;150:286–294. doi: 10.1210/en.2008-0959. [DOI] [PubMed] [Google Scholar]
- 82.Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol. 2009;297:C1124–C1132. doi: 10.1152/ajpcell.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen RH, Su YH, Chuang RL, Chang TY. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene. 1998;17:1959–1968. doi: 10.1038/sj.onc.1202111. [DOI] [PubMed] [Google Scholar]
- 84.Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem. 2007;282:3799–3808. doi: 10.1074/jbc.M610185200. [DOI] [PubMed] [Google Scholar]
- 85.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 86.Zdychova J, Komers R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]
- 87.Lipina C, Kendall H, McPherron AC, Taylor PM, Hundal HS. Mechanisms involved in the enhancement of mammalian target of rapamycin signalling and hypertrophy in skeletal muscle of myostatin-deficient mice. FEBS Lett. 2010;584:2403–2408. doi: 10.1016/j.febslet.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hribal ML, Nakae J, Kitamura T, Shutter JR, Accili D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol. 2003;162:535–541. doi: 10.1083/jcb.200212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 90.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 94.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol. 2007;292:C188–C199. doi: 10.1152/ajpcell.00542.2005. [DOI] [PubMed] [Google Scholar]
- 95.Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- 96.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 97.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 98.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Hayot M, Rodriguez J, Vernus B, Carnac G, Jean E, Allen D, et al. Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol Cell Endocrinol. 2011;332:38–47. doi: 10.1016/j.mce.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 100.Favier FB, Costes F, Defour A, Bonnefoy R, Lefai E, Bauge S, et al. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1659–R1666. doi: 10.1152/ajpregu.00550.2009. [DOI] [PubMed] [Google Scholar]
- 101.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Springer J, Adams V, Anker SD. Myostatin: regulator of muscle wasting in heart failure and treatment target for cardiac cachexia. Circulation. 2010;121:354–356. doi: 10.1161/CIR.0b013e3181d0ba8b. [DOI] [PubMed] [Google Scholar]
- 103.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]