Abstract
Histone H3 mRNAs were found in polyA(+) fractions of total RNA prepared from alfalfa plants, calli and somatic embryos. The sequence analysis of cDNAs revealed the presence of a polyA tail on independent alfalfa H3 mRNAs. A highly conserved sequence motif AAUGAAA identified about 20bp upstream from the 3' ends of the alfalfa H3 cDNAs was suggested to be one of the possible regulatory elements in the 3' end formation and polyadenylation. Three out of the four analysed H3 cDNAs have more than 97% homology with a genomic clone and encode the same protein. While the fourth represents a minor species with only 78.8% homology to the coding region of the genomic clone and encodes a H3 histone with four amino acid replacements. On the basis of compilation analysis we suggest a consensus sequence for plant H3 histones which differs from that of animal's by four amino acid changes.
Full text
PDF


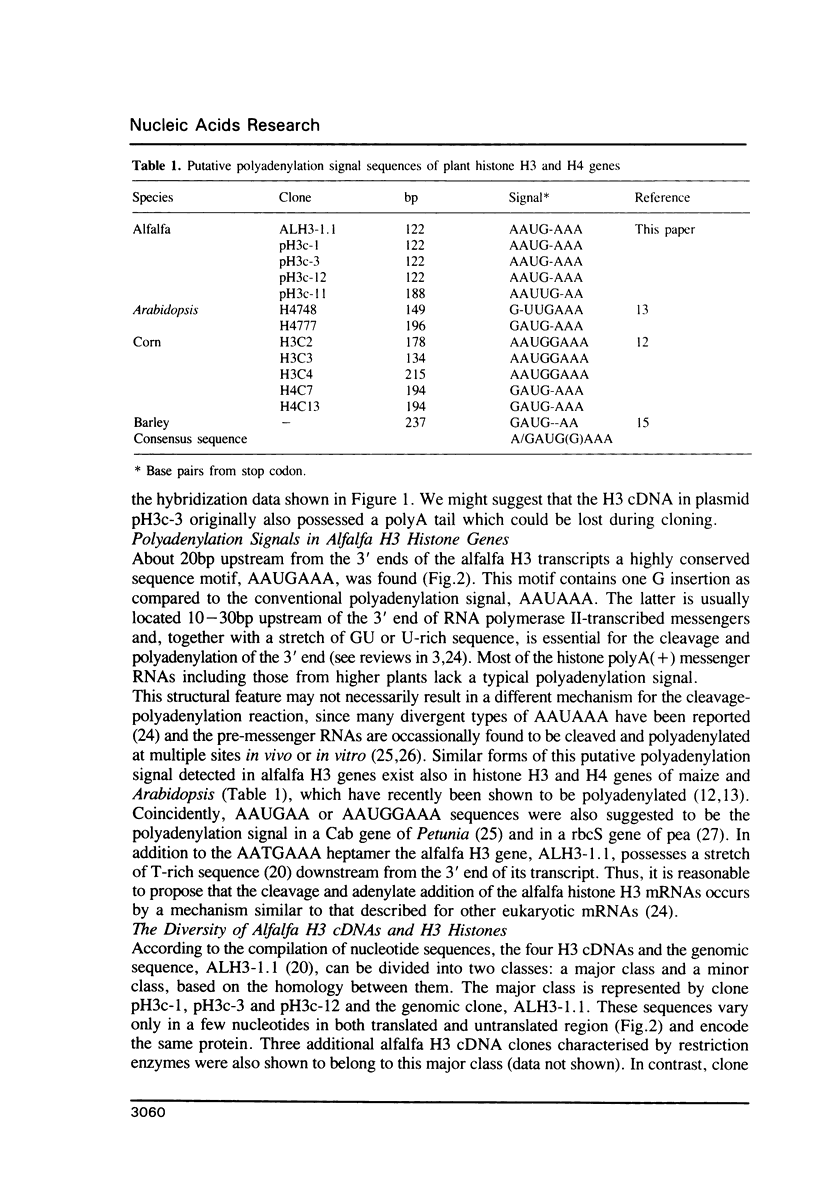


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon G. A., Calzone F. J., Bowen J. K., Allis C. D., Gorovsky M. A. Multiple, independently regulated, polyadenylated messages for histone H3 and H4 in Tetrahymena. Nucleic Acids Res. 1983 Jun 25;11(12):3903–3917. doi: 10.1093/nar/11.12.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chaboute M. E., Chaubet N., Clement B., Gigot C., Philipps G. Polyadenylation of histone H3 and H4 mRNAs in dicotyledonous plants. Gene. 1988 Nov 15;71(1):217–223. doi: 10.1016/0378-1119(88)90095-9. [DOI] [PubMed] [Google Scholar]
- Chaubet N., Chaboute M. E., Clément B., Ehling M., Philipps G., Gigot C. The histone H3 and H4 mRNAs are polyadenylated in maize. Nucleic Acids Res. 1988 Feb 25;16(4):1295–1304. doi: 10.1093/nar/16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Gick O., Vasserot A., Schaffner G., Birnstiel M. L. Specific contacts between mammalian U7 snRNA and histone precursor RNA are indispensable for the in vitro 3' RNA processing reaction. EMBO J. 1988 Mar;7(3):801–808. doi: 10.1002/j.1460-2075.1988.tb02878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner K., Yarger J., Hereford L. Yeast histone mRNA is polyadenylated. Nucleic Acids Res. 1980 Dec 11;8(23):5725–5737. doi: 10.1093/nar/8.23.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt J. S., Key J. L. Molecular cloning of a pea H1 histone cDNA. Eur J Biochem. 1987 Jul 1;166(1):119–125. doi: 10.1111/j.1432-1033.1987.tb13490.x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi H., Fusauchi Y., Iwai K. Tetrahymena histone H3. Purification and two variant sequences. J Biochem. 1984 Jun;95(6):1741–1749. doi: 10.1093/oxfordjournals.jbchem.a134788. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hunt A. G. Identification and characterization of cryptic polyadenylation sites in the 3' region of a pea ribulose-1,5-bisphosphate carboxylase small subunit gene. DNA. 1988 Jun;7(5):329–336. doi: 10.1089/dna.1.1988.7.329. [DOI] [PubMed] [Google Scholar]
- Levine B. J., Chodchoy N., Marzluff W. F., Skoultchi A. I. Coupling of replication type histone mRNA levels to DNA synthesis requires the stem-loop sequence at the 3' end of the mRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6189–6193. doi: 10.1073/pnas.84.17.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T., Angerer L. M., Angerer R. C., Childs G. A histone H1 protein in sea urchins is encoded by a poly(A)+ mRNA. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4123–4127. doi: 10.1073/pnas.85.12.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L. Polyadenylation of mRNA precursors. Biochim Biophys Acta. 1988 May 6;950(1):1–12. doi: 10.1016/0167-4781(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Old R. W., Woodland H. R. Histone genes: not so simple after all. Cell. 1984 Oct;38(3):624–626. doi: 10.1016/0092-8674(84)90256-3. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Histone 3. V. The amino acid sequence of pea embryo histone 3. J Biol Chem. 1973 Oct 10;248(19):6834–6840. [PubMed] [Google Scholar]
- Peng Z. G., Wu R. A simple and rapid nucleotide sequencing strategy and its application in analyzing a rice histone 3 gene. Gene. 1986;45(3):247–252. doi: 10.1016/0378-1119(86)90022-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterborg J. H., Winicov I., Harrington R. E. Histone variants and acetylated species from the alfalfa plant Medicago sativa. Arch Biochem Biophys. 1987 Jul;256(1):167–178. doi: 10.1016/0003-9861(87)90435-8. [DOI] [PubMed] [Google Scholar]
- Wells D. E. Compilation analysis of histones and histone genes. Nucleic Acids Res. 1986;14 (Suppl):r119–r149. doi: 10.1093/nar/14.suppl.r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D., Bains W., Kedes L. Codon usage in histone gene families of higher eukaryotes reflects functional rather than phylogenetic relationships. J Mol Evol. 1986;23(3):224–241. doi: 10.1007/BF02115579. [DOI] [PubMed] [Google Scholar]
- Wells D., Hoffman D., Kedes L. Unusual structure, evolutionary conservation of non-coding sequences and numerous pseudogenes characterize the human H3.3 histone multigene family. Nucleic Acids Res. 1987 Apr 10;15(7):2871–2889. doi: 10.1093/nar/15.7.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]



