Abstract
The phytopathogenic actinomycete Rhodococcus fascians drives its host to form a nutrient-rich niche by secreting a mixture of cytokinins that triggers plant cell division and shoot formation. The discrepancy between the relatively low amount of secreted cytokinins and the severe impact of R. fascians infection on plant development has puzzled researchers for a long time. Polyamine and transcript profiling of wild-type and cytokinin receptor mutant plants revealed that the bacterial cytokinins directly stimulated the biosynthesis of plant putrescine by activating arginine decarboxylase expression. Pharmacological experiments showed that the increased levels of putrescine contributed to the severity of the symptoms. Thus, putrescine functions as a secondary signal that impinges on the cytokinin-activated pathway, amplifying the hormone-induced changes that lead to the formation of a leafy gall. Exogenous putrescine and treatment with polyamine biosynthesis inhibitors combined with transcript and polyamine analyses of wild-type and mutant plants indicated that the direct target of both the bacterial cytokinins and plant putrescine was the expression of D3-type cyclins. Hence, the activated d-type cyclin/retinoblastoma/E2F transcription factor pathway integrates both external and internal hormonal signals, stimulating mitotic cell divisions and inducing pathological plant organogenesis.
Rhodococcus fascians is a biotrophic phytopathogenic actinomycete that causes the formation of multiple shoots in numerous plant hosts and poses a threat to the ornamentals industry (Putnam and Miller, 2007; Depuydt et al., 2008b). The bacteria disturb the plant’s hormone balance by producing morphogenic signals, such as auxins and cytokinins, that provoke the outgrowth of existing and the development of new shoot meristems (Eason et al., 1996; de O Manes et al., 2001; Vandeputte et al., 2005; Pertry et al., 2009), ultimately leading to the establishment of a leafy gall. In strain D188, virulence is conferred by a linear plasmid, pFiD188, that harbors the fasciation (fas) genes essential for cytokinin biosynthesis and symptom development (Crespi et al., 1992; Stange et al., 1996; Pertry et al., 2010). The bacteria secrete a mixture of synergistically acting cytokinins, some of which accumulate in infected plant tissues (Pertry et al., 2009). In Arabidopsis (Arabidopsis thaliana), a model plant adopted to study the molecular basis of this pathosystem, these cytokinins are perceived by the cytokinin receptors ARABIDOPSIS HISTIDINE KINASE3 (AHK3) and AHK4. Transduction of these signals triggers mitotic cell divisions by stimulating CYCLIN D3 (CYCD3) expression, which inhibits tissue maturation and, ultimately, establishes a nutrient-rich niche (Depuydt et al., 2008a, 2009a; Pertry et al., 2009). Although the quantity of secreted cytokinins is low, the impact of infection on plant development is severe. Therefore, secondary plant signals, such as polyamines, have been postulated to contribute to the establishment and/or the maintenance of the symptoms, a hypothesis supported by the early increase in the level of the diamine putrescine (Put) upon D188 infection of Arabidopsis (Depuydt et al., 2009b).
Diamines and polyamines are ubiquitously present in all living organisms, but in plants, they occur predominantly in young, actively growing tissues (Theiss et al., 2002). Polyamines are aliphatic polycations whose structure allows the interaction with DNA, RNA, proteins, and phospholipids. Consequently, they are important regulators of growth and development (for recent reviews, see Alcázar et al., 2010; Takahashi and Kakehi, 2010). Besides the developmental modulation of polyamine levels, their concentration also increases when plants are challenged with abiotic or biotic agents, suggesting a protective role against environmental cues (Alcázar et al., 2010; Gill and Tuteja, 2010) and pathogens (Walters, 2003).
Although their mode of action can be attributed partly to their interaction with anionic macromolecules in the cell, it has become increasingly clear that polyamines could also exert their biological activities through cross talk with almost all of the major plant hormones. Indeed, expression of polyamine biosynthesis genes and polyamine levels are altered in response to treatment with cytokinins, auxins, abscisic acid, gibberellins, and jasmonates (Altman, 1989; Biondi et al., 2001; Hanzawa et al., 2002; Imai et al., 2004a; Urano et al., 2004; Muñiz et al., 2008; Cui et al., 2010). The reverse is also true: whereas abscisic acid and cytokinin biosynthesis are induced by polyamines (Cuevas et al., 2009; Wang et al., 2009; Cui et al., 2010), ethylene and gibberellin production are down-regulated (Alcázar et al., 2005; Hu et al., 2006). Accordingly, the interaction between different hormones might be mediated in part by polyamines that would function as secondary messengers (Walters and Shuttleton, 1985; Hanzawa et al., 2002). Nevertheless, despite the wealth of circumstantial evidence and mainly correlative data, the molecular basis of polyamine action and their interplay with the other plant hormones remains unclear.
In Arabidopsis, polyamines are synthesized exclusively via the Arg pathway that starts with the decarboxylation of Arg to agmatine by arginine decarboxylases (ADC1 and ADC2). Subsequently, agmatine is converted to N-carbamoylputrescine by agmatine iminohydrolase (AIH), which is finally transformed to Put by N-carbamoylputrescine amidohydrolase (CPA). Put serves as a substrate for spermidine synthases (SPDS1 and SPDS2) to generate the polyamine spermidine (Spd), which is further modified by spermine synthase (SPMS) or by thermospermine synthase (ACL5) to yield spermine (Spm) or thermospermine (tSpm), respectively. Decarboxylated S-adenosyl-Met is the aminopropyl donor for the biosynthesis of these higher polyamines and is generated by S-adenosyl-Met decarboxylases (SAMDC1–SAMDC4) from S-adenosyl-Met (Alcázar et al., 2010; Mattoo et al., 2010). ADC and SAMDC activities are the rate-limiting steps in Put and Spd/Spm biosynthesis, respectively. Generally, the steady-state levels of Spd and Spm are more tightly regulated than those of Put through transcriptional, translational, and posttranslational control mechanisms (for recent reviews, see Handa and Mattoo, 2010; Mattoo et al., 2010). However, the intracellular pool of free polyamines depends not only on the biosynthetic rate but also on several other processes, including transport, conjugation, and degradation (Persson, 2009; Alcázar et al., 2010; Angelini et al., 2010; Takahashi et al., 2010).
Here, we evaluated whether and how polyamines play a role during the interaction of Arabidopsis with R. fascians. To get a more complete view on polyamine accumulation during symptom development, we followed the kinetics of free and conjugated forms of Put, Spd, and Spm in plants inoculated either with the virulent strain D188 or its linear plasmid-free (and hence fas−) nonpathogenic derivative D188-5. The origin of the increased polyamine levels in the symptomatic plant tissues was assessed by analyzing the amount of polyamines secreted by the bacteria and by profiling the transcription of plant genes involved in polyamine anabolism. The importance of polyamines in symptom development was investigated by monitoring the response of Arabidopsis mutants affected in polyamine biosynthesis toward R. fascians infection. In a complementary pharmacological approach, plants were treated either with polyamine biosynthesis inhibitors or with Put prior to infection. To position polyamines within the signal transduction cascade triggered by R. fascians, we identified the bacterial signal responsible for the increased polyamine production and evaluated the polyamine and molecular response of the double cytokinin receptor mutant ahk3ahk4 that is not responsive to R. fascians infection (Pertry et al., 2009) and of the triple CYCD3 mutant cycd3;1-3 that exhibits strongly reduced symptom development (Depuydt et al., 2009a). Based on our data, we propose a model on the mode of action of polyamines in regular and pathological plant development.
RESULTS
Free and Conjugated Put Accumulate during Symptom Development in R. fascians-Infected Rosettes of Arabidopsis
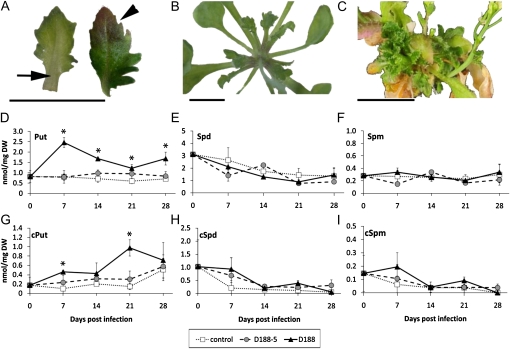
In Arabidopsis ecotype Columbia-0 (Col-0), different macroscopic stages can be discerned during symptom development upon R. fascians infection: at 7 d post infection (dpi), the onset of the disease becomes apparent by the accumulation of anthocyanins and the serrated margins and swollen vasculature of newly formed leaves (Fig. 1A); at 14 dpi, these symptoms are more pronounced, axillary meristems are activated (Fig. 1B), and new meristems are formed in the axillary regions of the plant; at 28 dpi, the pathology is fully established and plants have a bushy and stunted appearance (Fig. 1C; Depuydt et al., 2009b). To get a first indication of the possible role of polyamines in these processes, free and perchloric acid-soluble conjugated Put, Spd, and Spm titers were determined at the time points described above in rosettes of Col-0 plants infected with R. fascians strain D188. Plants infected with the nonpathogenic strain D188-5 or mock-inoculated with water were used as comparative controls. This set of inoculations was used throughout.
Figure 1.
Phenotypes and polyamine kinetics during symptom development on Arabidopsis Col-0 upon R. fascians infection. A to C, Phenotypic modifications characteristic for the different stages of symptom development. A, Leaf phenotype at 7 dpi: anthocyanin accumulation (arrowhead), swollen veins (arrow), and serrated margins. B, Axillary activation at 14 dpi. C, Bushiness of a rosette at 28 dpi. Bars = 1 cm. D to F, Free levels of Put (D), Spd (E), and Spm (F). G to I, Conjugated levels of Put (G), Spd (H), and Spm (I). Statistical differences were evaluated with Student’s t tests. Error bars indicate se (n = 3). Asterisks indicate statistically significant differences between D188 and mock-infected (control) samples (P < 0.05). No statistical differences were found between D188-5 and mock-infected (control) samples. DW, Dry weight.
During development of the mock-inoculated controls, the levels of free Spd decreased with time, whereas the free Put and Spm concentrations hardly changed (Fig. 1, D–F). Similar patterns were obtained for free Spd and Spm upon interaction with both bacterial strains (Fig. 1, E and F). By contrast, during infection with strain D188, but not with strain D188-5, the free Put levels increased up to 3-fold at 7 dpi, coinciding with the onset of symptom development, and remained higher than the control throughout the interaction (Fig. 1D). Among the conjugated (c) polyamines, cSpd exhibited the strongest developmental regulation but was not affected by bacterial infection (Fig. 1H). The concentration of cSpm, on the other hand, was close to the detection limit in all samples tested (Fig. 1I). However, the amount of cPut increased up to 6-fold upon D188 infection compared with the D188-5- and mock-infected controls (Fig. 1G).
Put Accumulation Results from a Modulated Polyamine Anabolism in the Plant
Because bacteria are known to produce and secrete polyamines (Wortham et al., 2007), we determined the free polyamine profile in bacterial cell-free supernatants of R. fascians strains D188 and D188-5 grown under several culture conditions (see “Materials and Methods”). Spd and Spm levels were below the detection limit, whereas comparable, albeit very low, levels of Put (less than 10 nmol mL−1) could be measured for both strains independently of the growth condition. Therefore, it seems very unlikely that the observed increase in Put in infected plant tissues would be of bacterial origin.
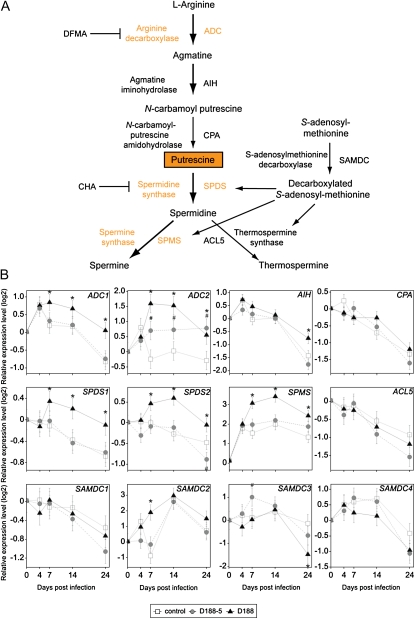
Consequently, we profiled the transcript levels of the polyamine biosynthesis genes in Arabidopsis Col-0 to test the hypothesis that R. fascians triggered Put biosynthesis in the host plant. By means of quantitative reverse transcription (qRT)-PCR, changes in gene expression were monitored in shoots sampled at 0, 4 (prior to visible symptoms), 7, 14, and 24 dpi (Fig. 2). Generally, during regular plant development, after an initial transient up-regulation, the expression of most assayed genes was down-regulated with time (Fig. 2B), which is in agreement with the observed polyamine profiles. In D188-5-infected plants, the expression patterns were comparable to those of the mock-inoculated controls, except for ADC2 (Fig. 2B). Upon D188 infection, no differential expression could be observed for AIH, CPA, ACL5, and the four SAMDC genes (Fig. 2B). By contrast, although the expression trends were comparable, from 7 dpi onward, ADC1, SPDS1, SPDS2, and SPMS expression was higher than in the control samples (Fig. 2B). Compared with the mock-infected control, the expression of ADC2 was up-regulated both after D188-5 and D188 infection, but in the D188-infected samples expression levels were higher (Fig. 2B). Importantly, when evaluating the expression patterns of these genes in Genevestigator (Hruz et al., 2008), SPMS but especially ADC2 expression appeared to be induced by a wide variety of biotic stresses, including infection with nonpathogenic bacteria. Expression of ADC1 and of both SPDS genes, on the other hand, was not up-regulated in response to biotic stress, suggesting the involvement of a more specific signal during the interaction with R. fascians.
Figure 2.
Transcript profiles of polyamine biosynthesis genes during R. fascians-induced symptom development on Arabidopsis Col-0. A, Overview of polyamine biosynthesis. Genes in orange are induced upon D188 infection. Put is boxed in orange because it accumulates during the R. fascians interaction. B, Transcript profiling. Statistical differences were evaluated with an ANOVA using the GenStat software (http://www.vsni.co.uk/software/genstat/). Error bars indicate se (n = 6). Hashes and asterisks indicate statistically significant differences (P < 0.05) between D188-5- and mock-infected (control) samples and between D188- and mock-infected (control) samples, respectively. [See online article for color version of this figure.]
Altogether, these data indicate that free Put accumulation in infected tissues results from the enhanced transcription of the rate-limiting ADC genes of the plant in response to bacterial signals that are encoded by the linear virulence plasmid pFiD188. The concomitant inactivating conjugation of Put is insufficient to undo this result.
Compromising the Functionality of ADC Reduces Symptom Development
To evaluate the significance of the increased Put concentration during R. fascians-induced symptom development, we infected the Arabidopsis polyamine biosynthesis mutants adc1-3 and adc2-3 (Cuevas et al., 2008) with strain D188 and compared the timing and level of their responsiveness with those of infected wild-type plants. Plants were considered responsive when one or more of the following reactions occurred: anthocyanin accumulation, swelling of the leaf vasculature, serration of the leaf margins, wrinkling of the lamina, activation of axillary meristems, or de novo shoot meristem formation. Symptom development on both adc mutants was only slightly delayed, and they eventually displayed symptoms that were comparable to those of wild-type plants (data not shown). Most probably, this rather modest reduction in responsiveness of the adc plants can be attributed to the functional redundancy of ADC1 and ADC2 (Urano et al., 2005) and to the ability of both mutants to still produce Put (data not shown; Cuevas et al., 2008).
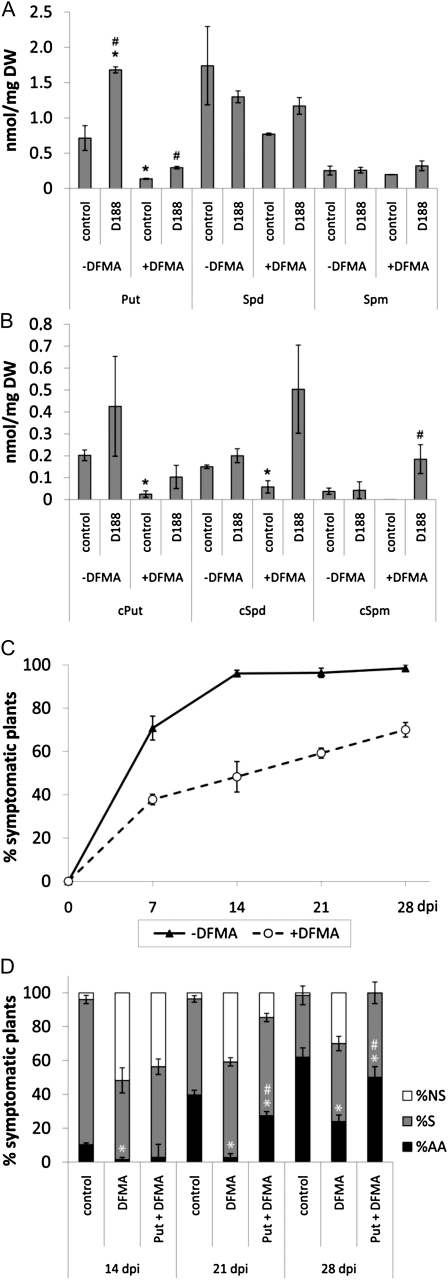
Because the data obtained with the single adc mutants were not conclusive and adc1adc2 double mutants are not viable (Urano et al., 2005), the involvement of Put in the R. fascians-plant interaction was assessed by a pharmacological approach using difluoromethyl-arginine (DFMA), which is an irreversible inhibitor of ADC (Fallon and Phillips, 1988). Arabidopsis plants were transferred to medium containing 1 mm DFMA and then immediately infected with R. fascians. Subsequently, symptom development was followed over time and compared with that of untreated plants. We first verified that the presence of DFMA in the medium did not compromise bacterial viability on the plants (36 ± 5 × 103 colony-forming units [CFU] mg−1 fresh weight [FW] in control tissue versus 34 ± 8 × 103 CFU mg−1 FW in DFMA-treated plants). Moreover, DFMA did not induce any visible alterations in the development of the D188-5- and mock-infected control plants (data not shown). Nevertheless, the activity of DFMA was apparent when we evaluated the polyamine levels in the latter. While the levels of free and conjugated Spm were not significantly modified by DFMA treatment (Fig. 3, A and B), a 7-fold decrease in free Put and cPut levels was measured at 14 d post treatment (dpt; Fig. 3, A and B). Correspondingly, this reduced amount of precursor resulted in a 2-fold decrease in free Spd and cSpd levels (Fig. 3, A and B).
Figure 3.
Effect of the ADC inhibitor DFMA on polyamine levels in R. fascians-infected Arabidopsis Col-0 and on symptom development. Statistical differences were evaluated with Student’s t tests. Error bars indicate se (n = 3). A, Free polyamine levels at 14 dpi. B, Conjugated polyamine levels at 14 dpi. Asterisks and hashes indicate statistical differences (P < 0.05) between the polyamine levels of treated samples (D188 infection and/or DFMA treatment) and untreated mock-infected (control) samples and between D188- and mock-infected (control) samples either in the absence of presence of DFMA, respectively. DW, Dry weight. C and D, Kinetics of symptom development. Just before infection, plants were transferred to medium with or without 1 mm DFMA and/or 1 mm Put and scored for symptom development at different time points. C, Percentage of responsive plants. D, Quantification of different symptoms at different time points after infection. AA, Symptomatic plants exhibiting activation of the axillary meristems; NS, nonsymptomatic plants; S, symptomatic plants exhibiting anthocyanin accumulation, swollen veins, and/or serrated leaves. Statistical analysis was done on axillary activation, the most important plant response upon infection. Asterisks and hashes indicate statistical differences (P < 0.05) between DFMA-treated and untreated plants and between DFMA-treated and DFMA + Put-treated plants, respectively.
When the DFMA treatment was combined with R. fascians D188 infection, symptom development was reduced considerably: at all time points, fewer plants were symptomatic than in the untreated controls (Fig. 3C), and especially, the activation of axillary meristems was strongly impaired (Fig. 3D). In D188-infected tissues treated with DFMA, there were no significant alterations in the concentrations of free Spd and Spm (Fig. 3A), whereas unexpectedly, the levels of cSpd and cSpm increased (Fig. 3B). Although free and conjugated Put levels were 3-fold higher in D188-infected than in noninfected DFMA-treated plants (Fig. 3, A and B), it is important to point out that this enhanced level only equaled that of noninfected untreated tissues. To validate that the inability of DFMA-treated plants to fully respond to R. fascians infection was caused by their reduced Put response, DFMA was supplied in combination with 1 mm Put. From 21 dpi on, the inhibition of symptom development was alleviated, so that at 28 dpi, all plants were responsive and the repression of axillary activation was relieved (Fig. 3D). Taken together, these data support a role for Put in the establishment of the leafy gall syndrome in Arabidopsis. Additional proof stems from the observation that infection of higher polyamine biosynthesis mutants spds2 (Hewezi et al., 2010), acl5 (McElver et al., 2001), and samdc1 (Alonso et al., 2003) did not affect symptom development (data not shown).
Augmenting the Put Level in Arabidopsis Stimulates Symptom Development
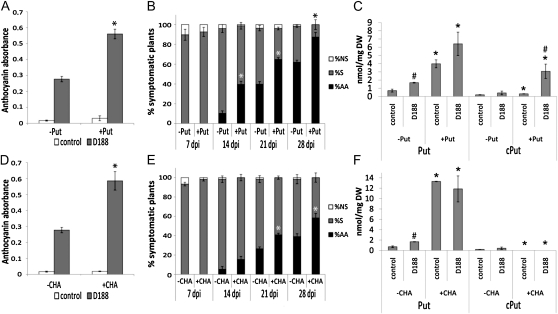
The importance of Put during the interaction with R. fascians strain D188 was further analyzed by placing Arabidopsis plants on medium supplemented with 1 mm Put 10 d prior to infection and comparing symptom formation with that of untreated plants. As anticipated, exogenous Put strengthened the response and accelerated symptom onset. Thus, in the presence of Put, anthocyanin accumulation was 2-fold higher at 28 dpi than in the absence of Put (Fig. 4A). Moreover, at 14 dpi, close to 40% of the plants grown on Put already displayed bushiness caused by the activation of axillary meristems, versus only 10% on medium without Put (Fig. 4B). The latter trend persisted at all later time points (Fig. 4B).
Figure 4.
Effects of modulating the endogenous Put pool on symptom development and on Put levels in R. fascians-infected Arabidopsis Col-0. Error bars indicate se (n = 3). Statistical differences were evaluated with Student’s t tests. Asterisks indicate statistical differences (P < 0.05) between Put-treated (A–C) or CHA-treated (D–F) and untreated plants. A to C, Put modulation accomplished by addition of 1 mm Put 10 d before infection. A, Anthocyanin accumulation at 28 dpi. B, Kinetics of symptom development. AA, Symptomatic plants exhibiting activation of the axillary meristems; NS, nonsymptomatic plants; S, symptomatic plants exhibiting anthocyanin accumulation, swollen veins, and/or serrated leaves. Statistical analysis was done on axillary activation, the most important plant response upon infection. C, Free and conjugated Put levels measured at 14 dpi. Hashes indicate statistical differences (P < 0.05) between D188- and mock-infected (control) samples either in the absence of presence of Put. DW, Dry weight. D to F, Put modulation accomplished by treatment with the SPDS inhibitor CHA (1 mm) just before infection. D, Anthocyanin accumulation at 28 dpi. E, Kinetics of symptom development. Statistical analysis was done on axillary activation. F, Free and conjugated Put levels measured at 14 dpi. Statistical analysis was done as in C.
Possibly, the stimulatory effect of exogenous Put on symptom development resulted from an increased expression of bacterial virulence. Therefore, we evaluated whether Put affected bacterial growth by following the turbidity of cultures and by counting colony-forming units present in infected Arabidopsis plants, both grown in the presence or absence of 1 mm Put. We also monitored the expression of the fasA gene involved in cytokinin production in cultures of the GUS reporter strain D188-pSPIPfasAgus (Pertry et al., 2010) incubated under optimal conditions for fas gene expression (Temmerman et al., 2000) and in bacterial cells present in plant tissues, again in the presence or absence of 1 mm Put. No significant differences were measured for either of these parameters (Supplemental Table S1), implying that the improved symptom establishment on Put is caused by an enhancement of the plant’s signal transduction process implicated in symptom induction.
The impact of exogenous Put on the endogenous polyamine profile of infected plants was also assessed at 14 dpi. Interestingly, whereas the presence of Put in the medium of uninfected plants led to a 5.5-fold increase of free Put compared with that of untreated controls, Put conjugation was hardly affected (Fig. 4C), indicating that the mere increase of endogenous Put levels does not automatically trigger conjugation. Yet, the combination of D188 infection and exogenous Put pushed the free Put to an even higher level and did activate Put conjugation (Fig. 4C), implying that bacterial signals trigger both processes. Free and conjugated Spd and Spm levels were not altered under any of these conditions (Supplemental Fig. S1A).
Finally, based on the assumption that inhibiting Spd biosynthesis would also affect Put levels (Fig. 2A), we analyzed the effect of the competitive inhibitor of SPDS activity, cyclohexylamine (CHA), on symptom development. We verified that 1 mm CHA did not compromise bacterial viability on the plants (36 ± 5 × 103 CFU mg−1 FW in control tissue versus 34 ± 8 × 103 CFU mg−1 FW in CHA-treated plants) and established that the development of CHA-treated plants was not visibly altered (data not shown). The 40-fold increase in free Put levels and the 3.5-fold decrease in free and conjugated Spd levels measured at 14 dpt in uninfected plants clearly illustrated the functionality of the inhibitor (Fig. 4F; Supplemental Fig. S1B). The concentrations of Spm and of cSpm (Supplemental Fig. S1B) were not modified by CHA. Unexpectedly, the levels of cPut dropped below the detection limit in these plants (Fig. 4F).
In agreement with the effect of exogenous Put, the increased endogenous Put levels upon CHA treatment had a stimulatory effect on symptom progression in D188-infected plants. Although the number of responsive plants was the same in the absence or presence of CHA (Fig. 4E; Supplemental Fig. S1C), the number of plants exhibiting axillary activation was consistently higher in its presence (Fig. 4E). Moreover, the combination of CHA and infection with strain D188 led to a stronger accumulation of anthocyanins at 28 dpi than bacterial infection alone (Fig. 4D). Together, these data confirm that Put, rather than Spd or Spm, has an important function in stimulating the R. fascians-triggered signal transduction cascade involved in symptom formation.
Although the very high levels of free Put resulting from CHA treatment were not further increased upon D188 infection (Fig. 4F), free and conjugated Spd and Spm did accumulate in CHA-treated D188-infected tissues (Supplemental Fig. S1B), suggesting that the homeostatic mechanism controlling the steady-state levels of these higher polyamines was overruled under these conditions. Interestingly, R. fascians infection could not restore Put conjugation in the presence of CHA (Fig. 4F), possibly indicating that CHA interferes with the activity of Put conjugation enzymes.
The Infection-Induced Put Modulation Is Triggered by Cytokinins
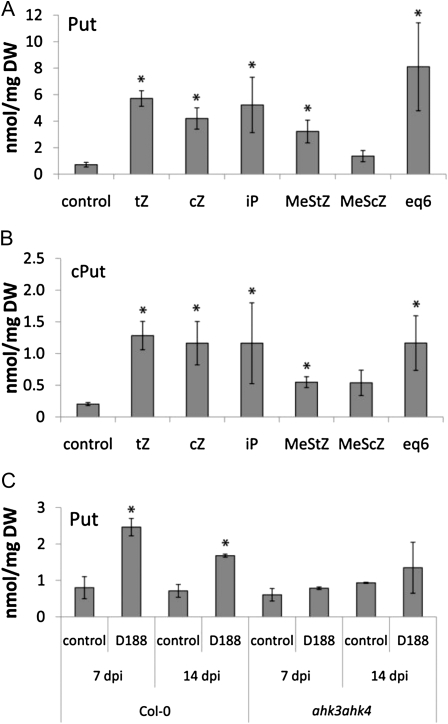
The results described above suggest that the bacterial signal responsible for the induction of Put production in Arabidopsis depends on the presence of pFiD188. Cytokinins are the main virulence factors of R. fascians, and they are encoded by the fas operon located on the linear plasmid (Pertry et al., 2010), but the bacterium also secretes indole-3-acetic acid (IAA). Although auxin production is mediated by the chromosome, the kinetics of IAA secretion are linear plasmid dependent (Vandeputte et al., 2005). Thus, to identify the bacterial signal that triggers the accumulation of Put during the interaction, Arabidopsis plants were placed on medium supplemented with 10 μm IAA, 10 μm of a single cytokinin (trans-zeatin [tZ], isopentenyladenine [iP], cis-zeatin [cZ], 2-methylthio-trans-zeatin [2MeStZ], 2-methylthio-cis-zeatin [2MeScZ]), or a mix of the six cytokinin bases produced by R. fascians (1 μm each of tZ, cZ, iP, and their 2MeS derivatives). Plants were sampled at 14 dpt for polyamine quantifications and molecular profiling.
Although it has been reported that polyamine levels are induced upon addition of IAA (Altman, 1989), under our experimental conditions, free and conjugated polyamine levels were not altered (data not shown). By contrast, all tested cytokinins generally led to free Put accumulation, albeit to different levels (Fig. 5A), whereas free Spd and Spm were hardly affected by the cytokinin treatment (Supplemental Fig. S2, A and B). The level of cSpm was close to or below the detection limit in all samples tested, and cSpd concentrations were comparable in control and cytokinin-treated plants (data not shown). However, the production of cPut was positively affected by cytokinins, with accumulation levels of up to 6-fold (Fig. 5B). Accordingly, transcript profiling of polyamine biosynthesis genes revealed an up-regulation of all tested genes upon cytokinin treatment (Supplemental Fig. S2C). These data show that the overall free and conjugated polyamine profiles and the molecular polyamine responses of cytokinin-treated plants and those of D188-infected plant tissues are very similar, implying a direct role of the bacterial cytokinins in the modification of polyamine metabolism.
Figure 5.
Importance of cytokinins on Put and cPut production. Error bars indicate se (n = 3). Statistical differences were evaluated with Student’s t tests. A and B, Free (A) and conjugated (B) Put levels in Col-0 plants treated for 14 d with 10 μm of different cytokinins or with an equimolar mix of 1 μm each of iP, tZ, cZ, and their 2MeS derivatives (eq6). Asterisks indicate statistical differences (P < 0.05) between cytokinin-treated and untreated plants. C, Free Put level in D188- and mock-infected (control) Col-0 and ahk3ahk4 plants at 7 and 14 dpi. Asterisks indicate statistical differences (P < 0.05) between D188- and mock-infected (control) plants. DW, Dry weight.
The importance of the bacterial cytokinins as the trigger for the altered Put metabolism was corroborated by evaluating the polyamine profile and the expression of polyamine biosynthesis genes in infected tissues of the ahk3ahk4 double cytokinin receptor mutant of Arabidopsis (Higuchi et al., 2004). The ahk3ahk4 mutant has been proposed to be unresponsive to R. fascians, possibly because AHK2, the only functional cytokinin receptor in this mutant, is not sufficiently sensitive to the bacterial cytokinins and/or because the downstream signaling that it controls is not involved in symptom development (Pertry et al., 2009, 2010). At 7 and 14 dpi, the levels of free Spd and Spm, which were slightly reduced upon infection with strain D188, were comparable in the receptor mutant and wild-type plants (Supplemental Fig. S2, D and E). More importantly, free Put no longer accumulated (Fig. 5C) and cPut dropped below the detection limit (data not shown) upon D188 infection of ahk3ahk4 plants. The absence of a phenotypic response of the mutant was accompanied by the absence of a molecular response, as evidenced by the loss of induction of all tested polyamine biosynthesis genes (Supplemental Fig. S2F). Overall, these results demonstrate that cytokinin perception is an essential step in the activation of Put biosynthesis during the interaction with R. fascians.
Placing Put in the Signal Transduction Cascade Triggered by R. fascians Infection
During the experiments with exogenous Put, we observed that the growth of the mock-treated and D188-5-infected plants was stimulated, confirming previous reports on polyamine-induced growth promotion (Couée et al., 2004; Farooq et al., 2007). We reasoned that the growth improvement in the presence of Put alone might reflect an activation of cell division. If so, during the R. fascians-plant interaction, the activation of the cell cycle by the bacterial cytokinins would be potentiated by the increased free Put pool in the infected plant tissues, thus leading to stronger symptom development.
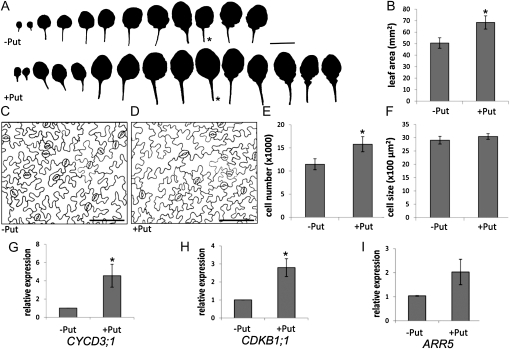
To obtain further insight into the cause of the improved growth on Put alone, we compared developmental leaf series of untreated plants and plants grown on 1 mm Put for 38 d. Plants grown in the presence of Put had two to three additional leaves (Fig. 6A). In addition, although flowering time was delayed by 1 d, the formation of extra leaves was already observed before the floral transition, suggesting that faster organ production, rather than delayed bolting, is at the basis of this Put-induced growth response. Moreover, starting from leaf 7, the leaves of plants grown on Put were larger (Fig. 6, A and B). To determine whether this enlarged leaf size resulted from an activation of cell division or from a stimulation of cell expansion, we measured the leaf area (Fig. 6B), counted the number of cells per leaf drawing (Fig. 6, C–E), and calculated the average epidermal cell area (Fig. 6F) for leaf 8. These data showed that exogenous Put did not affect cell expansion or alter the morphology of the pavement cells, indicating that leaf differentiation was not modulated. However, the number of cells was 1.5-fold higher than that of the untreated controls, implying that cell proliferation accounted exclusively for the increase in leaf size. The activation of cell division was validated by molecular profiling of Put-treated plants. Indeed, the expression of a B-type mitotic cyclin-dependent kinase, CDKB1;1, and a D-type cyclin, CYCD3;1, was significantly induced by exogenous Put (Fig. 6, G and H). Interestingly, expression of an A-type cytokinin response regulator, ARABIDOPSIS RESPONSE REGULATOR5 (ARR5), was 2-fold higher in the Put-treated plants (Fig. 6I), possibly indicating an increased cytokinin sensitivity.
Figure 6.
Phenotypic and transcriptional responses of Arabidopsis Col-0 plants treated with 1 mm Put. A, Developmental leaf series of control and treated plants. Asterisks mark leaf 8. Bar = 1 cm. B, Leaf area. C and D, Drawing-tube images of the epidermis of an untreated (C) and a Put-treated (D) plant. Bars = 0.1 mm. E, Total number of cells per leaf. F, Average epidermal cell size. Analyses were done on leaf 8 at 38 dpt. G to I, Transcript profiles of symptom-associated marker genes in untreated and Put-treated Col-0 plants at 14 dpt. Error bars indicate se (n = 3). Asterisks mark statistically significant differences upon Put treatment according to Student’s t tests (P < 0.05).
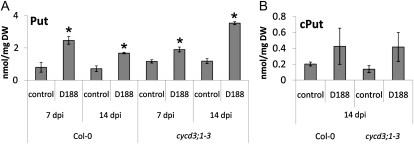
The polyamine and transcript data on the ahk3ahk4 mutant (Fig. 5; Supplemental Fig. S2) place the action of Put during the interaction with R. fascians downstream of cytokinin perception. In the current model of R. fascians-induced signal transduction, CYCD3;1 is a primary target of the bacterial cytokinins (Depuydt et al., 2009a). However, the data presented above show that CYCD3;1 expression is also induced by Put, thus positioning Put action upstream or in a parallel pathway of CYCD3;1. To confirm this finding, we analyzed the polyamine response of the cycd3;1-3 triple knockout mutant (Dewitte et al., 2007). This mutant is much less responsive to R. fascians because of the disruption of mitotic cell divisions in response to the bacterial cytokinins (Depuydt et al., 2009a). As in wild-type plants, in D188-infected cycd3;1-3 tissues, free and conjugated Put accumulated at 7 and 14 dpi (Fig. 7), whereas the free Spd and Spm levels were not affected (Supplemental Fig. S3, A and B). These data show that the CYCD3 proteins are not required for the cytokinin-induced Put accumulation.
Figure 7.
Put levels in D188- and mock-infected (control) Col-0 and cycd3;1-3 plants at 7 and 14 dpi. Free (A) and conjugated (B) Put levels are shown. Error bars indicate se (n = 3). Asterisks mark statistically significant differences between D188- and mock-infected (control) plants according to Student’s t tests (P < 0.05). DW, Dry weight.
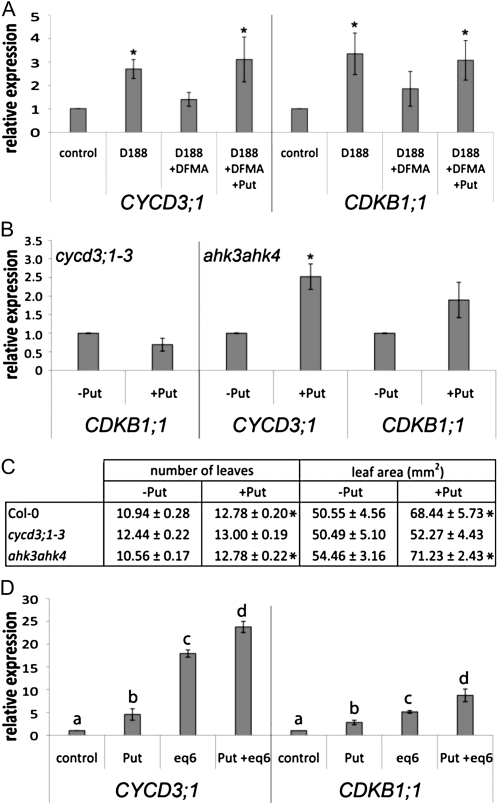
Previously, the induction of CDKB1;1 and ARR5 had been shown to be approximately 10- and 6-fold lower, respectively, upon infection of the cycd3;1-3 mutant than that of wild-type plants (Depuydt et al., 2009a). Based on these results and our findings here, we postulated a loop-shaped pathway in which the bacterial cytokinins are necessary for the activation of both CYCD3 expression and Put production. The increased Put levels would feed into the CYCD3 signal transduction pathway, further enhancing cell proliferation through the direct or indirect activation of mitotic cell cycle genes, such as CDKB1;1. This hypothesis was tested by profiling the transcript levels of CYCD3;1 and CDKB1;1 under different experimental conditions and in different mutant backgrounds. To assess the significance of the activation of the pathway via Put, we treated D188-infected plants with DFMA or with DFMA combined with Put and compared the expression profiles with those of uninfected and untreated D188-infected controls. The level of induction of both CYCD3;1 and CDKB1;1 was reduced by DFMA treatment, but this reduction was reverted in the presence of Put (Fig. 8A), implying that the fold induction obtained upon D188 infection resulted from the combined action of the bacterial cytokinins and Put. To test whether CDKB1;1 expression was activated directly by Put or indirectly through the activation of CYCD3;1, the cycd3;1-3 mutant was grown on Put and leaf number, leaf area, and CDKB1;1 expression were evaluated. CDKB1;1 expression was no longer induced by exogenous Put (Fig. 8B) and growth of the cycd3;1-3 plants was not stimulated (Fig. 8C), implying that the CYCD3 proteins may act as Put sensors. Treatment of the ahk3ahk4 mutant with exogenous Put resulted in a 2-fold induction of CYCD3;1 expression (Fig. 8B) versus 4-fold in the wild type (Fig. 6G), confirming the direct but partial activation of CYCD3;1 expression by Put. In accordance with the lower CYCD3;1 induction, expression of CDKB1;1 was less strongly activated by exogenous Put in the ahk3ahk4 background (1.9-fold; Fig. 8B) than in wild-type plants (2.8-fold; Fig. 6H). The Put-treated ahk3ahk4 plants bore on average two extra and larger leaves than the untreated plants, validating the importance of CYCD3 signaling in Put-induced growth (Fig. 8C). Finally, our model was further supported by the cumulative effect of combining the mix of bacterial cytokinins and Put on CYCD3;1 and CDKB1;1 expression in wild-type plants (Fig. 8D).
Figure 8.
Effect of modulating Put levels, R. fascians-induced signal transduction, and/or cytokinin levels on CYCD3;1 and CDKB1;1 expression. Error bars indicate se (n = 3). Asterisks mark statistically significant differences (P < 0.05) with the control samples (A) or upon Put treatment (B and C) according to Student’s t tests (A–C). A, CYCD3;1 and CDKB1;1 expression in Col-0 plants in response to D188 infection combined with DFMA and/or exogenous Put treatment. Just before infection, plants were transferred to medium with or without 1 mm DFMA and 1 mm Put. B, CDKB1;1 expression in cycd3;1-3 plants and CYCD3;1 and CDKB1;1 expression in ahk3ahk4 plants treated with exogenous Put. Ten days before infection, plants were transferred to medium with or without 1 mm Put. C, Growth effects in Col-0, cycd3;1-3, and ahk3ahk4 plants in response to Put treatment (1 mm) at 38 dpt. D, CYCD3;1 and CDKB1;1 expression in Col-0 plants in response to Put and/or eq6 (an equimolar mix of 1 μm each of iP, tZ, cZ, and their 2MeS derivatives) treatment. Plants were treated for 14 d with 1 mm Put and/or eq6. Statistical differences indicated by different letters were evaluated with the Tukey range test in conjunction with an ANOVA.
DISCUSSION
Currently, it seems impossible to assign specific roles to the different polyamines during plant development both under normal and stress conditions. In some cases, polyamines are believed to be easily biochemically interconverted; therefore, Put, Spd, and Spm would be physiologically equivalent (Applewhite et al., 2000; Mattoo et al., 2010). In other cases, Put clearly has opposite and contrasting functions when compared with Spd and Spm (Handa and Mattoo, 2010; Mattoo et al., 2010). In Arabidopsis, Spd, the most abundant polyamine, has been linked with developmental processes, Spm has no apparent function under normal growth conditions, and tSpm has been implicated in vascular development (Imai et al., 2004a, 2004b; Vera-Sirera et al., 2010). The physiological significance of Put in Arabidopsis has been associated generally with various types of abiotic stress responses (Alcázar et al., 2010; Takahashi and Kakehi, 2010).
We established that Put, rather than Spd or Spm, is an important mediator of the leafy gall syndrome induced on Arabidopsis by R. fascians. This conclusion is based on several findings: (1) Put accumulated in infected tissues as a result of the cytokinin-induced activation of the ADC genes that encode the rate-limiting step of Put biosynthesis (Urano et al., 2004); (2) interference with Put production via treatment with DFMA reduced symptom development; and (3) increasing the endogenous Put levels through exogenous addition of the diamine itself or CHA treatment enhanced symptom formation. By contrast, (1) no differential accumulation was detected for Spd and Spm in symptomatic tissues; (2) D188 infection did not induce the expression of four SAMDC genes that encode the rate-limiting step of Spd and Spm synthesis (Ge et al., 2006); and (3) infection of the higher polyamine biosynthesis mutants spds2 (Hewezi et al., 2010), acl5 (McElver et al., 2001), and samdc1 (Alonso et al., 2003) did not affect symptom development (data not shown). Moreover, also in R. fascians-infected tissues of tobacco (Nicotiana tabacum), accumulation of Put and cPut, but not of the higher polyamines, was recorded (data not shown), and treatment with difluoromethyl-ornithine, an inhibitor of Put biosynthesis active in tobacco (Burtin et al., 1991), strongly reduced symptom development (data not shown). Therefore, Put availability appears to be a rate-limiting factor for optimal cytokinin-induced symptom development during the interaction of R. fascians with different hosts.
Differential accumulation of polyamines in symptomatic versus healthy tissues has been described in several other plant-microbe interactions, especially in plant tumors or hypertrophied tissues. In crown galls induced by Agrobacterium tumefaciens on black salsify (Scorzonera hispanica; Bagni et al., 1972), cultured tobacco tissue (Srivastava, 1987), and potato (Solanum tuberosum) discs (Kulpa et al., 1985), Put and/or Spd levels are higher than in healthy tissues. Moreover, Spd is apparently essential for tumor growth on potato discs, because polyamine biosynthesis inhibitors restrain tumorigenesis (Kulpa et al., 1985). In maize (Zea mays) tumors caused by the fungus Ustilago maydis, Put levels, polyamine biosynthesis gene expression, and enzyme activity increase (Rodríguez-Kessler et al., 2008). Formation of clubroots on turnip (Brassica napus) by Plasmodiophora brassicae is associated with increased levels of Put, Spd, and Spm (Walters and Shuttleton, 1985) and on Arabidopsis with increased polyamine metabolism (Jubault et al., 2008). Moreover, high concentrations of polyamines are found in stem and root nodules of different leguminous plants. For instance, during root nodule development on Lotus japonicus, Put accumulates as a consequence of increased expression of the biosynthetic genes, suggesting the involvement of polyamines in nodule development (Efrose et al., 2008). Finally, Spd has been identified as a novel and potent molecule of parasitic success of the cyst nematode Heterodera schachtii (Hewezi et al., 2010). Altogether, these mainly correlative data support an important role of polyamines in microbially induced morphogenesis.
In the R. fascians-Arabidopsis pathosystem, Put did not affect the level of bacterial colonization or of virulence gene expression. Therefore, we tried to unravel the molecular mechanism of Put-induced symptom enhancement by comparing polyamine profiles and marker gene expression patterns in Col-0 wild-type and ahk3ahk4 and cycd3;1-3 mutant plants in response to bacterial infection and upon treatment with exogenous Put and/or polyamine biosynthesis inhibitors. The bacterial cytokinins not only triggered the Put response, but their perception by the plant was imperative for this response, because it did not occur in infected ahk3ahk4 plants. Moreover, exogenous Put induced the expression of genes, such as CYCD3;1 and CDKB1;1, which are important markers for R. fascians-induced symptom development (Depuydt et al., 2009a), and stimulated cell division and leaf formation. Additionally, the Put-induced expression of ARR5 might reflect an increased cytokinin sensitivity (Cui et al., 2010). Interestingly, the Put-mediated growth stimulation required CYCD3 signaling but not cytokinin perception. Collectively, these data indicate that exogenous Put activates cell proliferation and organ production in a CYCD3-dependent manner and, simultaneously, might increase cytokinin sensitivity. Both these aspects would undoubtedly have a positive effect on symptom development. Finally, comparison of the expression levels of CYCD3;1 and CDKB1;1 under different experimental conditions revealed that cytokinin and Put had a cumulative effect. Based on these data, Put emerges as an amplifying signal of the cytokinin-induced stimulatory effect on the plant cell cycle provoked by R. fascians infection. Important molecular mechanisms of polyamine action in cell proliferation and cell division are their direct binding to DNA and, hence, their ability to modulate DNA conformation and DNA-protein interactions. Concordantly, when cells are stimulated to proliferate, cyclic increases of polyamine levels, mainly caused by an activation of biosynthesis, are observed at the G1-to-S and the G2-to-M transitions (Alm and Oredsson, 2009; Gemperlová et al., 2009). Interestingly, both these checkpoints are targeted by the R. fascians cytokinins through the modulation of the D-type cyclin/retinoblastoma/E2F transcription factor pathway (Depuydt et al., 2009a), and our results indicate that Put impinges on this process. The CYCD3 proteins have been identified as essential environmental sensors for growth factors, such as cytokinins and auxins (Dewitte et al., 2003, 2007), but here we show that they also respond to Put. Consequently, transcription activation of essential cell cycle genes seems to be an additional mode of action of Put to stimulate cell division.
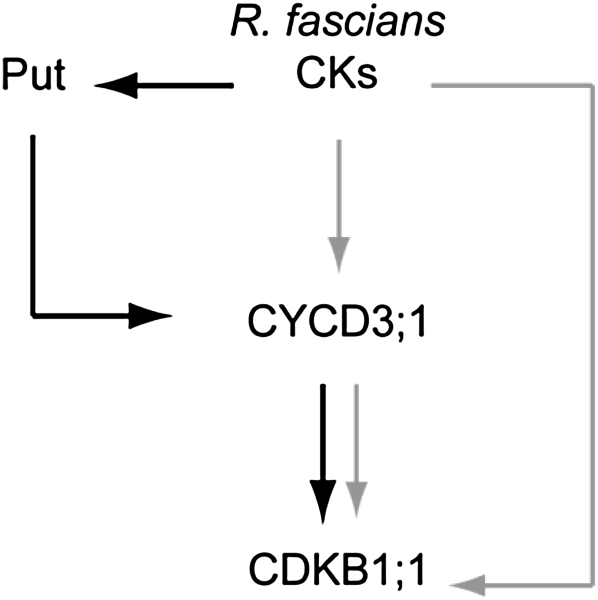
Based on our data, we propose the following model for the function of Put in R. fascians-induced symptom development in Arabidopsis (Fig. 9). Upon perception of the locally secreted bacterial cytokinins by AHK3 and AHK4, ADC expression is induced, leading to enhanced Put biosynthesis by the host. Besides the direct cytokinin-triggered CYCD3 and CDKB1;1 expression (Depuydt et al., 2009a), mitotic cell division further increases by the additional induction of CYCD3;1 by Put, which, as a secondary effect, promotes CDKB1;1 expression. Therefore, R. fascians apparently succeeds in obtaining the maximum effect with minimum effort. The bacteria secrete a relatively low amount of cytokinins, but by producing a changing mixture of cytokinins, they prevent a complete breakdown by the host (Pertry et al., 2009, 2010). Then, cytokinin-activated plant Put production acts as a secondary and amplifying messenger of the cytokinin signal. Thus, by employing plant endogenous signals, R. fascians achieves a major impact on plant development: it manipulates the host to establish a leafy gall that is a strong nutrient sink and represents a rich niche for the inhabiting bacterial population.
Figure 9.
Model of the mode of action of plant Put and bacterial cytokinins during symptom development induced by R. fascians on Arabidopsis. Gray and black arrows mark previous (Depuydt et al., 2009a) and our findings, respectively.
In conclusion, this work presents strong correlative and molecular evidence in support of a determinative role for Put in the R. fascians-Arabidopsis pathosystem. To our knowledge, we have established for the first time a direct link between Put, cell cycle gene expression, and plant development and thereby expanded the range of signals sensed by CYCD3 proteins by including polyamines. Clearly, plant-pathogen interactions leading to neoplastic hyperplasia constitute alternative, but valuable, tools to unravel the role and mode of action of hormones, polyamines, and their interactions in plant physiology and organogenesis.
MATERIALS AND METHODS
Plant Material, Sampling, and Infection Conditions
Arabidopsis (Arabidopsis thaliana) Col-0 was used throughout the experiments. Seeds of the adc1-3 (N9657), adc2-3 (N9659), spds2 (N664161), acl5 (N836360), and samdc1 (N623880) mutants were obtained from the European Arabidopsis Stock Centre. The ahk3ahk4 and cycd3;1-3 mutants were kindly provided by Tatsuo Kakimoto (Higuchi et al., 2004) and Jim Murray (Dewitte et al., 2007), respectively. The seeds were sterilized and sown on half-strength Murashige and Skoog (1/2MS) medium in a growth chamber under a 16-h/8-h light/dark photoperiod at 21°C ± 2°C. The Rhodococcus fascians strains used were the pathogenic strain D188, containing the linear virulence plasmid pFiD188, and its plasmid-free nonpathogenic derivative D188-5 (Desomer et al., 1988). These strains were grown in liquid yeast extract broth (YEB) for 2 d at 28°C under gentle agitation, then diluted 100 times in fresh medium, and allowed to grow overnight. Prior to infection, the cultures were washed and concentrated four times by resuspending the bacterial pellets in sterile distilled water. Arabidopsis plants were infected 14 d after germination by local application of a drop of bacterial culture to the shoot apical meristem. Shoot samples were collected by removing roots and flower stalks and were snap frozen in liquid nitrogen.
Chemical Treatments
The cytokinins (OlChemIm) were dissolved in dimethyl sulfoxide and applied at concentrations of 1 or 10 μm to 1/2MS medium. Plants were transferred to this medium for 14 d and sampled for further analyses. The chemical polyamine biosynthesis inhibitors DFMA (obtained from Stefania Biondi) and CHA (Sigma-Aldrich) were dissolved in sterile water and applied at concentrations of 1 mm to the 1/2MS medium. Plants, transferred to this medium just before infection, were sampled for further analyses at several time points. Put (Acros Organics) was dissolved in sterile water and added to the 1/2MS medium at a concentration of 1 mm (Kurepa et al., 1998). Four days after germination, plants were transferred to this medium, and 38 d later, the leaves were sampled for the assessment of growth parameters. To investigate whether Put had an effect on symptom development, Put-treated plants were infected with R. fascians 10 d after transfer, and aerial parts were sampled for further analyses at several time points.
Polyamine Measurements
Polyamine extractions and measurements were essentially as described by Scaramagli et al. (1999). In short, plant material (150–300 mg) was freeze dried and extracted with 10 volumes of 4% perchloric acid. The homogenate was kept on ice for 45 min and then centrifuged at 20,000g at 4°C for 30 min. The supernatant was collected and used for the determination of polyamines. Aliquots of the supernatants and standard solutions of Put, Spd, and Spm were derivatized with dansyl chloride (5 mg mL−1 acetone) at 60°C for 1 h. Dansylated derivatives were extracted with toluene, dried, and resuspended in acetonitrile. Polyamines were separated and quantified by HPLC (PU-980; Jasco) with a reverse-phase C18 column (Spherisorb ODS2, 5-μm particle diameter, 4.6 × 250 mm; Waters) and a programmed acetonitrile-water step gradient. Aliquots of the supernatants were subjected to acid hydrolysis (6 n HCl overnight at 110°C) to release polyamines from their perchloric acid-soluble conjugated forms; released polyamines were derivatized and analyzed as described above. For bacterial measurements, supernatants of strain D188 and D188-5 grown in YEB or under inducing conditions in defined medium (see below) were freeze dried, homogenized, derivatized with dansyl chloride, and analyzed for their polyamine content as described above. Three replicates were analyzed on three biological repeats.
RNA Isolation, cDNA Synthesis, and Gene Expression Analysis
For each sample, 100 mg of shoot tissue was collected and ground in liquid nitrogen. Total RNA was extracted with the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer's protocol. Genomic DNA was removed by DNase treatment, and the RNA samples were purified through NH4Ac (5 m) precipitation. Samples were quality controlled and quantified with a NanoDrop Spectrophotometer (Isogen). Two micrograms of RNA was reverse transcribed into cDNA with the SuperScript Reverse Transcriptase Kit (Invitrogen), subsequently diluted 50 times, and stored at −20°C until further use. Real-time qRT-PCR was done on a LightCycler 480 (Roche Diagnostics) with SYBR Green for detection in a 5-μL volume (2.5 μL of Master Mix, 0.25 μL of 2.5 μm of each forward and reverse primer, and 2 μL of cDNA) in triplicate on a 384-multiwell plate to determine mean and sd. Cycle threshold (Ct) values were obtained with the accompanying software and analyzed with the 2−ΔΔCT method (Livak and Schmittgen, 2001). The obtained values were normalized against those of ACTIN2 (ACT2), which was used as an internal standard. Depending on the experiment, six (Fig. 2) or three biological repeats (Figs. 6 and 9; Supplemental Fig. S2) were sampled. Primer sequences are given in Supplemental Table S2.
Anthocyanin Quantification
Anthocyanin was quantified as described (Feinbaum and Ausubel, 1988) with slight modifications. Aerial plant parts were collected at 28 dpi and ground in liquid nitrogen. From 100 mg of material, total pigments were extracted for 10 min in 0.75 mL of 1% HCl/methanol supplemented with 0.5 mL of distilled water. Chlorophyll was removed by chloroform extraction, and the anthocyanin pigments in the aqueous/methanol phase were quantified by measuring the A530 minus A657. The mean was calculated from the values of three independent biological repeats.
Analysis of Leaf Growth Parameters
The leaf area was determined by photographing the leaf and analyzing the image with the image-analysis program ImageJ (version 1.41; http://rsbweb.nih.gov/ij/). For cell density and cell area, the leaves were cleared overnight in ethanol (70%, v/v) and stored in lactic acid prior to analysis on a microscope stage fitted with differential interference contrast optics (Leica). Images of at least 30 cells of the abaxial epidermis located at 25% and 75% from the leaf base, halfway between the midrib and the leaf margin, were drawn with a drawing tube mounted on the microscope. These images were scanned and processed with ImageJ. The total number of cells per leaf was derived by extrapolating the number of cells in the drawing tube images to the full leaf surface area. The average cell area was estimated by dividing the leaf area by the number of cells. At least three independent biological repeats were analyzed.
In Vitro Determination of Bacterial Growth and Virulence Gene Expression
R. fascians strain D188-pSPIPfasAgus carrying a fasA-uidA fusion (Pertry et al., 2010) was grown in 5 mL of YEB medium for 2 d at 28°C, diluted in 100 mL of fresh medium, and allowed to grow overnight. Bacteria were collected by centrifugation, washed, and diluted to an optical density at 600 nm (OD600) of 2.0 in B5 medium (Gamborg’s B5 salts, 0.5 g L−1 MES, 0.25 g L−1 NH4NO3, and 0.001% thiamine, pH 5.5). Upon addition of pyruvate (10 mm) as a carbon source and/or His (5 mm) to induce virulence gene expression, bacteria were incubated overnight at 28°C in a rotary shaker. To test the effect of Put on the bacteria, the cultures were either supplemented with 1 mm Put or not. GUS activity was measured with the substrate 4-methylumbelliferyl-β-d-glucuronide (MUG). Cells were collected by centrifugation after overnight induction and resuspended in 1 mL of MUG buffer (50 mm sodium phosphate buffer, pH 7.0, 0.1% SDS, 10 mm EDTA, 10 mm β-mercaptoethanol, and 0.1% Triton X-100). A sample of 50 μL was taken to measure the cell density (OD600). The MUG substrate was added to a final concentration of 0.1 mm, and the sample was incubated at 37°C. At 15, 30, 60, and 120 min, samples of 50 μL were taken and added to 200 μL of 200 mm Na2CO3 on a black microtiter plate (F16 Black Polysorb Fluoronunc plates; Nunc) to stop the reaction. The fluorescence was measured by excitation at 365 nm and emission at 460 nm with the Fluostar optima reader. GUS activity was calculated as the measured fluorescence × 1,000/OD600 × time (min).
In Planta Determination of Bacterial Growth and Virulence Gene Expression
Arabidopsis plants were coinfected with the R. fascians strains D188 and D188-pSPIPfasAgus as described (Pertry et al., 2010), and aerial plant tissue was collected at 7 dpi. Extracts were prepared by crushing the plants in an Eppendorf tube with a pestle, after which 500 μL of sterile water was added immediately. Ten microliters of each sample was used for a set of 10-fold dilutions to determine the colony-forming units. The samples were diluted to 1 mL with 2-fold-concentrated MUG buffer. The MUG substrate was added to a final concentration of 0.1 mm, and the GUS activity was analyzed as described above.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of modulating the Put content on polyamine levels and symptom development in R. fascians-infected Arabidopsis Col-0.
Supplemental Figure S2. Importance of cytokinins on Spd and Spm production.
Supplemental Figure S3. Free Spd (A) and Spm (B) levels in D188- and mock-infected (control) Col-0 and cycd3;1-3 plants at 14 dpi.
Supplemental Table S1. In vitro and in planta bacterial growth and fasA gene expression in the presence and absence of Put.
Supplemental Table S2. Primers used for qRT-PCR amplifications.
Supplementary Material
Acknowledgments
We thank Marnik Vuylsteke for help with the statistical analyses of the results, Karel Spruyt for photography, Mondher Jaziri for fruitful discussions, and Martine De Cock for help in preparing the manuscript.
References
- Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF. (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231: 1237–1249 [DOI] [PubMed] [Google Scholar]
- Alcázar R, García-Martínez JL, Cuevas JC, Tiburcio AF, Altabella T. (2005) Overexpression of ADC2 in Arabidopsis induces dwarfism and late-flowering through GA deficiency. Plant J 43: 425–436 [DOI] [PubMed] [Google Scholar]
- Alm K, Oredsson S. (2009) Cells and polyamines do it cyclically. Essays Biochem 46: 63–76 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657; erratum Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Science 301: 1849 [DOI] [PubMed] [Google Scholar]
- Altman A. (1989) Polyamines and plant hormones. Bachrach U, Heimer YM, eds, The Physiology of Polyamines. CRC Press, Boca Raton, FL, pp 121–145 [Google Scholar]
- Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A. (2010) Plant amine oxidases “on the move”: an update. Plant Physiol Biochem 48: 560–564 [DOI] [PubMed] [Google Scholar]
- Applewhite PB, Kaur-Sawhney R, Galston AW. (2000) A role for spermidine in the bolting and flowering of Arabidopsis. Physiol Plant 108: 314–320 [Google Scholar]
- Bagni N, Serafini Fracassini D, Corsini E. (1972) Tumours of Scorzonera hispanica: their content in polyamines. Z Pflanzenphysiol 67: 19–23 [Google Scholar]
- Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P. (2001) Methyl jasmonate upregulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot 52: 231–242 [PubMed] [Google Scholar]
- Burtin D, Martin-Tanguy J, Tepfer D. (1991) α-dl-Difluoromethylornithine, a specific, irreversible inhibitor of putrescine biosynthesis, induces a phenotype in tobacco similar to that ascribed to the root-inducing, left-hand transferred DNA of Agrobacterium rhizogenes. Plant Physiol 95: 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couée I, Hummel I, Sulmon C, Gouesbet G, El Amrani A. (2004) Involvement of polyamines in root development. Plant Cell Tissue Organ Cult 76: 1–10 [Google Scholar]
- Crespi M, Messens E, Caplan AB, van Montagu M, Desomer J. (1992) Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J 11: 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas JC, López-Cobollo R, Alcázar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A. (2008) Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol 148: 1094–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas JC, López-Cobollo R, Alcázar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A. (2009) Putrescine as a signal to modulate the indispensable ABA increase under cold stress. Plant Signal Behav 4: 219–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Ge C, Wang R, Wang H, Chen W, Fu Z, Jiang X, Li J, Wang Y. (2010) The BUD2 mutation affects plant architecture through altering cytokinin and auxin responses in Arabidopsis. Cell Res 20: 576–586 [DOI] [PubMed] [Google Scholar]
- de O Manes CL, Van Montagu M, Prinsen E, Goethals K, Holsters M. (2001) De novo cortical cell division triggered by the phytopathogen Rhodococcus fascians in tobacco. Mol Plant Microbe Interact 14: 189–195 [DOI] [PubMed] [Google Scholar]
- Depuydt S, De Veylder L, Holsters M, Vereecke D. (2009a) Eternal youth, the fate of developing Arabidopsis leaves upon Rhodococcus fascians infection. Plant Physiol 149: 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Doležal K, Van Lijsebettens M, Moritz T, Holsters M, Vereecke D. (2008a) Modulation of the hormone setting by Rhodococcus fascians results in ectopic KNOX activation in Arabidopsis. Plant Physiol 146: 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Putnam M, Holsters M, Vereecke D. (2008b) Rhodococcus fascians, an emerging threat for ornamental crops. Teixeira JA, da Silva, ed, Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, Ed 1, Vol V. Global Science Books, London, pp 480–489 [Google Scholar]
- Depuydt S, Trenkamp S, Fernie AR, Elftieh S, Renou J-P, Vuylsteke M, Holsters M, Vereecke D. (2009b) An integrated genomics approach to define niche establishment by Rhodococcus fascians. Plant Physiol 149: 1366–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desomer J, Dhaese P, Van Montagu M. (1988) Conjugative transfer of cadmium resistance plasmids in Rhodococcus fascians strains. J Bacteriol 170: 2401–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, Riou-Khamlichi C, Scofield S, Healy JMS, Jacqmard A, Kilby NJ, Murray JAH. (2003) Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3. Plant Cell 15: 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V.et al (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA 104: 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason JR, Morris RO, Jameson PE. (1996) The relationship between virulence and cytokinin production by Rhodococcus fascians (Tilford 1936) Goodfellow 1984. Plant Pathol 45: 323–331 [Google Scholar]
- Efrose RC, Flemetakis E, Sfichi L, Stedel C, Kouri ED, Udvardi MK, Kotzabasis K, Katinakis P. (2008) Characterization of spermidine and spermine synthases in Lotus japonicus: induction and spatial organization of polyamine biosynthesis in nitrogen fixing nodules. Planta 228: 37–49 [DOI] [PubMed] [Google Scholar]
- Fallon KM, Phillips R. (1988) Polyamines in relation to growth in carrot cell cultures. Plant Physiol 88: 224–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Basra SMA, Hussain M, Rehman H, Saleem BA. (2007) Incorporation of polyamines in the priming media enhances the germination and early seedling growth in hybrid sunflower (Helianthus annuus L.). Int J Agric Biol 9: 868–872 [Google Scholar]
- Feinbaum RL, Ausubel FM. (1988) Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol 8: 1985–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Cui X, Wang Y, Hu Y, Fu Z, Zhang D, Cheng Z, Li J. (2006) BUD2, encoding an S-adenosylmethionine decarboxylase, is required for Arabidopsis growth and development. Cell Res 16: 446–456 [DOI] [PubMed] [Google Scholar]
- Gemperlová L, Cvikrová M, Fischerová L, Binarová P, Fischer L, Eder J. (2009) Polyamine metabolism during the cell cycle of synchronized tobacco BY-2 cell line. Plant Physiol Biochem 47: 584–591 [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5: 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa AK, Mattoo AK. (2010) Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem 48: 540–546 [DOI] [PubMed] [Google Scholar]
- Hanzawa Y, Imai A, Michael AJ, Komeda Y, Takahashi T. (2002) Characterization of the spermidine synthase-related gene family in Arabidopsis thaliana. FEBS Lett 527: 176–180 [DOI] [PubMed] [Google Scholar]
- Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ. (2010) Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiol 152: 968–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, et al. (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA 101: 8821–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W-W, Gong H, Pua E-C. (2006) Modulation of SAMDC expression in Arabidopsis thaliana alters in vitro shoot organogenesis. Physiol Plant 128: 740–750 [Google Scholar]
- Imai A, Akiyama T, Kato T, Sato S, Tabata S, Yamamoto KT, Takahashi T. (2004a) Spermine is not essential for survival of Arabidopsis. FEBS Lett 556: 148–152 [DOI] [PubMed] [Google Scholar]
- Imai A, Matsuyama T, Hanzawa Y, Akiyama T, Tamaoki M, Saji H, Shirano Y, Kato T, Hayashi H, Shibata D, et al. (2004b) Spermidine synthase genes are essential for survival of Arabidopsis. Plant Physiol 135: 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubault M, Hamon C, Gravot A, Lariagon C, Delourme R, Bouchereau A, Manzanares-Dauleux MJ. (2008) Differential regulation of root arginine catabolism and polyamine metabolism in clubroot-susceptible and partially resistant Arabidopsis genotypes. Plant Physiol 146: 2008–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa JM, Galsky AG, Lipetz P, Stephens R. (1985) Polyamines and crown gall tumor growth. Plant Cell Rep 4: 81–83 [DOI] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Van Montagu M, Inzé D. (1998) Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiol 39: 987–992 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Minocha SC, Minocha R, Handa AK. (2010) Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 38: 405–413 [DOI] [PubMed] [Google Scholar]
- McElver J, Tzafrir I, Aux G, Rogers R, Ashby C, Smith K, Thomas C, Schetter A, Zhou Q, Cushman MA, et al. (2001) Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 159: 1751–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñiz L, Minguet EG, Singh SK, Pesquet E, Vera-Sirera F, Moreau-Courtois CL, Carbonell J, Blázquez MA, Tuominen H. (2008) ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development 135: 2573–2582 [DOI] [PubMed] [Google Scholar]
- Persson L. (2009) Polyamine homoeostasis. Essays Biochem 46: 11–24 [DOI] [PubMed] [Google Scholar]
- Pertry I, Václavíková K, Depuydt S, Galuszka P, Spíchal L, Temmerman W, Stes E, Schmülling T, Kakimoto T, Van Montagu MC, et al. (2009) Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proc Natl Acad Sci USA 106: 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertry I, Václavíková K, Gemrotová M, Spíchal L, Galuszka P, Depuydt S, Temmerman W, Stes E, De Keyser A, Riefler M, et al. (2010) Rhodococcus fascians impacts plant development through the dynamic fas-mediated production of a cytokinin mix. Mol Plant Microbe Interact 23: 1164–1174 [DOI] [PubMed] [Google Scholar]
- Putnam ML, Miller ML. (2007) Rhodococcus fascians in herbaceous perennials. Plant Dis 91: 1064–1076 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Kessler M, Ruiz OA, Maiale S, Ruiz-Herrera J, Jiménez-Bremont JF. (2008) Polyamine metabolism in maize tumors induced by Ustilago maydis. Plant Physiol Biochem 46: 805–814 [DOI] [PubMed] [Google Scholar]
- Scaramagli S, Franceschetti M, Michael AJ, Torrigiani P, Bagni N. (1999) Polyamines and flowering: spermidine biosynthesis in the different whorls of developing flowers of Nicotiana tabacum L. Plant Biosyst 133: 229–237 [Google Scholar]
- Srivastava BIS. (1987) Polyamine changes during senescence and tumorogenesis in plants. Mech Ageing Dev 40: 17–30 [DOI] [PubMed] [Google Scholar]
- Stange RR, Jeffares D, Young C, Scott DB, Eason JR, Jameson PE. (1996) PCR amplification of the fas-1 gene for detection of virulent strains of Rhodococcus fascians. Plant Pathol 45: 407–417 [Google Scholar]
- Takahashi T, Kakehi J-i. (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot (Lond) 105: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Cong R, Sagor GHM, Niitsu M, Berberich T, Kusano T. (2010) Characterization of five polyamine oxidase isoforms in Arabidopsis thaliana. Plant Cell Rep 29: 955–965 [DOI] [PubMed] [Google Scholar]
- Temmerman W, Vereecke D, Dreesen R, Van Montagu M, Holsters M, Goethals K. (2000) Leafy gall formation is controlled by fasR, an AraC-type regulatory gene in Rhodococcus fascians. J Bacteriol 182: 5832–5840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss C, Bohley P, Voigt J. (2002) Regulation by polyamines of ornithine decarboxylase activity and cell division in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol 128: 1470–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano K, Hobo T, Shinozaki K. (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579: 1557–1564 [DOI] [PubMed] [Google Scholar]
- Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K. (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313: 369–375 [DOI] [PubMed] [Google Scholar]
- Vandeputte O, Oden S, Mol A, Vereecke D, Goethals K, El Jaziri M, Prinsen E. (2005) Biosynthesis of auxin by the gram-positive phytopathogen Rhodococcus fascians is controlled by compounds specific to infected plant tissues. Appl Environ Microbiol 71: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Sirera F, Minguet EG, Singh SK, Ljung K, Tuominen H, Blázquez MA, Carbonell J. (2010) Role of polyamines in plant vascular development. Plant Physiol Biochem 48: 534–539 [DOI] [PubMed] [Google Scholar]
- Walters DR. (2003) Polyamines and plant disease. Phytochemistry 64: 97–107 [DOI] [PubMed] [Google Scholar]
- Walters DR, Shuttleton MA. (1985) Polyamines in the roots of turnip infected with Plasmodiophora brassicae Wor. New Phytol 100: 209–214 [Google Scholar]
- Wang Y, Luo J-P, Wu H-Q, Jin H. (2009) Conversion of protocorm-like bodies of Dendrobium huoshanense to shoots: the role of polyamines in relation to the ratio of total cytokinins and indole-3-acetic acid. J Plant Physiol 166: 2013–2022 [DOI] [PubMed] [Google Scholar]
- Wortham BW, Patel CN, Oliveira MA. (2007) Polyamines in bacteria: pleiotropic effects yet specific mechanisms. Adv Exp Med Biol 603: 106–115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.