Abstract
During the last few decades evidence has demonstrated that adult neurogenesis is a well-preserved feature throughout the animal kingdom. In birds, ongoing neuronal addition occurs rather broadly, to a number of brain regions. This review describes adult avian neurogenesis and neuronal recruitment, discusses factors that regulate these processes, and touches upon the question of their genetic control. Several attributes make birds an extremely advantageous model to study neurogenesis. First, song learning exhibits seasonal variation that is associated with seasonal variation in neuronal turnover in some song control brain nuclei, which seems to be regulated via adult neurogenesis. Second, food-caching birds naturally use memory-dependent behavior in learning locations of thousands of food caches scattered over their home ranges. In comparison with other birds, food-caching species have relatively enlarged hippocampi with more neurons and intense neurogenesis, which appears to be related to spatial learning. Finally, migratory behavior and naturally occurring social systems in birds also provide opportunities to investigate neurogenesis. Such diversity of naturally-occurring memory-based behaviors, combined with the fact that birds can be studied both in the wild and in the laboratory, make them ideal for investigation of neural processes underlying learning. This can be done by using various approaches, from evolutionary and comparative to neuroethological and molecular. Finally, we connect the avian arena to a broader view by providing a brief comparative and evolutionary overview of adult neurogenesis and by discussing the possible functional role of the new neurons. We conclude by indicating future directions and possible medical applications.
Keywords: avian brain, neurogenesis, neuronal replacement, bird song, food hoarding
INTRODUCTION
Neurogenesis has traditionally been viewed as a strictly developmental phenomenon. The structure of the adult brain had been viewed as being stable, with each neuron morphologically fixed and irreplaceable within existing circuitry. In this sense, the adult brain was viewed as being able to process extensive information without requiring any structural changes, but liable to lose information if it is disrupted. However, during the last decades an ever-expanding body of evidence has demonstrated that new neurons are being generated in adulthood. It is now widely accepted that adult neurogenesis is a well-preserved feature throughout the animal kingdom, occurring in a variety of systematic groups, both invertebrates and vertebrates, and including humans.
Most of our knowledge on adult neurogenesis is based on studies focused on mammals and birds. In mammals, there is strong evidence that new neurons are added to the dentate gyrus (DG) of the hippocampus (HC) and the olfactory bulb (OB; for review, see Goldman 1998; Alvarez-Buylla and Garcia-Verdugo 2002; Gage 2002). In contrast, in birds, ongoing neuronal addition occurs rather broadly, to a number of regions in the neostriatum, as well as in paraolfactory and parahippocampal regions (Goldman and Nottebohm 1983; Nottebohm 1985; Alavrez-Buylla and Nottebohm 1988; Alvarez-Buylla et al. 1990; Barnea and Nottebohm 1994; Lipkind et al. 2002).
The current review tries to put together the fast accumulating knowledge concerning some aspects of adult neurogenesis in birds. It starts by presenting adult neurogenesis as a multistep process rather than a single event. This process includes cell proliferation, cell migration, and cell-fate. Adult neurogenesis differs from developmental neurogenesis in that it produces fewer neurons, it occurs in fewer brain regions, and it produces more limited selection of neuronal cell types. We then review some (although not all) of the external and internal factors that are known to regulate this dynamic process, and touch upon the yet rather poorly investigated question of its genetic control.
The fourth section of this review describes some models that are being used to study adult neurogenesis in birds. The classical and most investigated one is the song control system of songbirds that includes some regions, such as HVC, which are robustly neurogenic, exhibit well-described hormonal responsiveness with discrete anatomic concomitants, and are associated with a well-described and readily quantifiable behavioral output - song. Since song is a learned behavior in many bird species, this model enables identifying permissive conditions for migration and integration of new neurons in the adult brain and provides insights into neural mechanisms of learning. More recently, additional models such as food hoarding and social interactions have contributed to our understanding of the interplay between brain and behavior. These models are being used as a system where observations of naturally occurring behaviors delineate a series of questions of general relevance to learning in a context where it is highly tractable to elucidate neuronal mechanisms. We continue with discussing several methods that are commonly used in birds for in vivo detection of newly generated cells and for resolving stages of neuronal lineage commitment.
The last three sections try to connect the avian arena to a broader view on adult neurogenesis. We first provide a brief comparative and evolutionary overview of this phenomenon in both invertebrates and vertebrates. In both taxonomic groups adult neurogenesis occurs in brain structures that exhibit a high degree of structural plasticity and display analogies, because in both groups these structures receive numerous types of sensory information and play a central role in learning and memory processes. Despite the vast number of studies on adult neurogenesis, the functional role of newly formed neurons is still questionable, and the seventh section discusses several of the suggested hypotheses. We conclude our review by indicating future directions that might provide a better understanding of adult neurogenesis, and touch upon the issue of possible medical applications.
BASIC PROCESSES
The discovery that new neurons are born in adult brains and incorporated into functional circuits had fundamentally altered our vision of brain function. Much of what is known about the origin and migration of new neurons in the adult avian brain, from the ventricular zone (VZ; a zone lining the lateral ventricles) where they originate to the telecephalic areas where they later settle, comes from studies on the song control system of the canary (see below for details). The first study (Goldman & Nottebohm 1983) was greeted with skepticism, because it had been widely believed that in the central nervous system neurogenesis ceased soon after birth and that the same neurons, perhaps with some losses, are present throughout adult life. But over the next few years, Nottebohm and his students documented that these new cells were born in the VZ, migrated along radial glia into HVC where they differentiated into neurons which projected to RA (robust nucleus of the arcopallium) or became interneurons (Nottebohm & Alvarez-Buylla 1993).
The term “neurogenesis” is often considered as a multistep process comprised of the production of new cells from stem cell progenitors, the differentiation of these cells into neuronal phenotypes, their migration to target brain areas, and their eventual incorporation into existing neural circuits by replacing older neurons that die. Hence, neuron death and replacement are considered to be fundamental components of adult brain plasticity. Much remains unknown, however, about the mechanistic interaction between these stages. In this section we will briefly outline some of the knowledge that has been accumulated during the last few decades regarding this complex process that involves both phenotypic transformation and spatial displacement of post-mitotic cells over a time course of days to weeks. Fig. 1 provides a schematic overview for some of the information listed below. It indicates the proliferation zone, the extent of migration, and the major areas into which new neurons are finally integrated.
Figure 1.
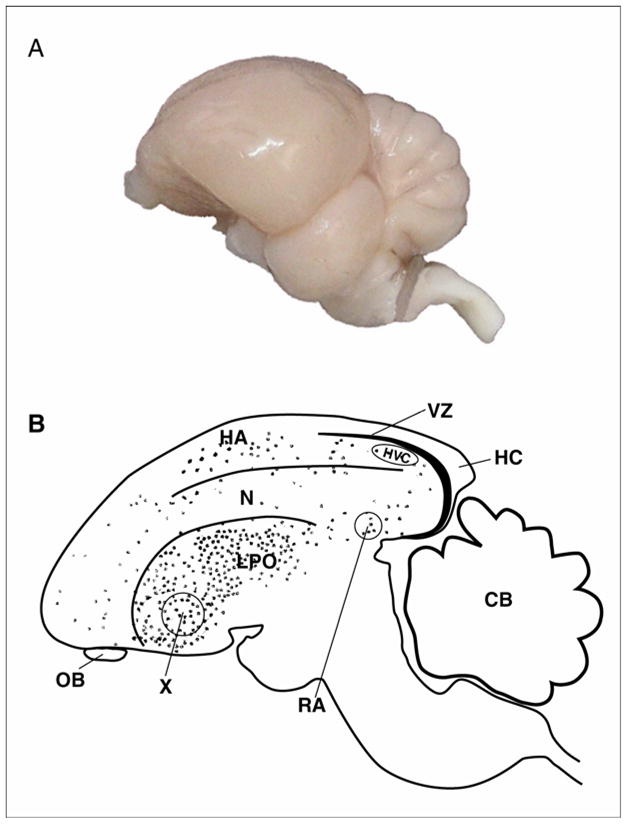
Sagittal views of the avian brain. Rostral is to the right. (A) Side view of an avian brain image (Laughing dove; Streptopelia senegalensis), Photographed by Shay Barkan. (B) Sagittal schematic overview of neurogenesis in the adult avian brain. New neurons are born in the VZ and from there they disperse widely and differentiate into neurons throughout many regions of the forebrain (dots). Regions that incorporate relatively high levels of new neurons are HVC, HC, Area X, N, HA, LPO. No new neurons are incorporated into either RA or the cerebellum (CB). HA -hyperstriatum accessorium, HC - hippocampus, LPO - lobus parolfactorius, N -nidopallium, OB - olfactory bulb, RA - robust nucleus of the arcopallium, VZ -ventricular zone, X - area X. Adapted with permission from Alvarez-Buylla et al., (1994); Doetsch & Scharff (2001).
Proliferation
When Goldman & Nottebohm (1983) injected adult canaries with the birth-date marker [3H]thymidine, they found many HVC labeled neuron-like cells when the birds were killed 30 days later, but not when the birds were killed one or two days after the injections. However, in those short time intervals there were many labeled cells on the wall of the VZ overlying HVC. Goldman & Nottebohm inferred that, as during the embryonic stages, new neurons are born in the VZ and migrate into the telencephalon, where they settle and differentiate. Indeed, to date we know that neurons formed post hatching in songbirds arise exclusively from the cells in the VZ (Alvarez-Buylla & Nottebohm 1988). In their work, Alvarez-Buylla et al. (1998) described the architecture of the adult avian VZ and identified three main cell types: primary precursors (Type B cells), which maintain an end foot on the ventricular surface and move towards the ventricle to undergo mitosis. Type B cells give rise to Type A cells, which seem to correspond to young migrating neurons, move away from the ventricle and become oriented parallel to this wall. Ependymal cells (Type E cells) shared the ventricular surface with Type B cells but did not divide.
The birth of new cells that eventually mature into neurons occurs throughout the avian VZ, but Alvarez-Buylla et al. (1990) noticed that the labeled VZ cells were particularly abundant in “hot spots” in the dorsal and ventral reaches of the lateral ventricle wall, and showed that these cells were radial glia. They suggested that these cells are neuronal stem cells and that new neurons are formed when radial glia divide and one of the daughter cells assumes the identity of a young migrating neuron. This view was later confirmed by ultrastructural work (Alvarez-Buylla et al. 1998). We do not yet know how often a given stem cell can divide and give rise to new neurons. This information is important when tracking a cohort of cells labeled with a birth-date marker.
Migration
Most neurons are born quite far from where they will ultimately reside and therefore need to be able to travel to their final destination. Alvarez-Bullya et al. (1987) revealed that many radial glia cells with small cell bodies, which are lodged in the wall of the VZ, have long, unbranching processes that penetrate the adjacent parenchyma. They found that young neurons, which are small and elongated at this stage, migrate along these radial fibers for considerable distances through mature brain tissue and reach various regions in the telencephalon (Alvarez-Bullya et al. 1988). Neuronal migration also follows a path perpendicular to that associated with radial glia, known as tangential migration. In this mode, cells migrate near or within the walls of the lateral ventricles for varying distances before dispersing along radial glial fibers (Doetsch & Scharff 2001).
Alvarez-Buylla & Nottebohm (1988) described the migration process and the eventual settling and differentiation of a wave of young neurons that are born in the adult canary brain on a particular day. They showed that three days after birth the young elongated cells start their journey and that at day 20 some of them reach the farthest corners of the telencephalon (up to a distance of 5mm from the VZ) and differentiate into neurons. Migration rates are highest (28μm/h−1) when young neurons migrate through regions which are rich in radial glia. Additionally, as during embryogeny, the majority of the migrating neurons were culled, so that by day 40 only one third of the initial cohort survived. Those neurons that survived and reached their final destination form elaborate dendritic and axonal arbors and establish connections. Depending on specific location within the brain, the process from birth of a young neuron to its post-migratory differentiation may take from seven days in the HC (Hoshooley et al. 2007), or eight days in HVC (Kirn et al. 1999), to 20–40 days elsewhere in the telencephalon (Alvarez-Buylla & Nottebohm 1988).
Brain regions that recruit new neurons
The most detailed information about adult neuron addition comes from studies of zebra finches and canaries. The first target region for the new neurons that has been studied in detail was the HVC, which is involved in the control of vocal behavior. Although Burd & Nottebohn (1985) confirmed the neuronal identity of the cells that migrated from the VZ into the HVC, questions remained as to what was a reliable proof of neuronal identity and adult birth, and whether these cells were indeed functional neurons. Paton & Nottebohm (1984) provided the first evidence by showing that the [3H]thymidine cells in HVC had neurophysiological profiles and clear neuronal anatomy, and that they were incorporated into functional neural circuits. HVC has two types of projection neurons. The first type is HVC neurons which project to Area X; these are produced before hatching and are not replaced in adulthood (Alvarez-Buylla et al. 1988; Gahr 1990; Kirn et al. 1999; Scharff et al. 2000). The second type of HVC projecting neurons is adult-formed ones, which send long axonal projections to RA (a song-control nucleus which projects to the motor neurons that innervate the vocal organ). The rest of the HVC neurons are interneurons (see also review by Nottebohm 2008).
Soon after neuronal recruitment was discovered in the adult HVC (Goldman & Nottebohm 1983), it became clear that it also occurs throughout most of the songbird telencephalon (Nottebohm 1985; reviewed by Gahr et al. 2002). Alvarez-Buylla et al. (1994) carried out a comprehensive study that determined the contribution of neurons born in adulthood (as well as during development) to different regions throughout the canary brain. Concerning adult-born neurons, they recorded similar patterns to those observed at the later stages of juvenile development. Adult neurogenesis was restricted to the telencephalon. From a functional point of view, the avian areas that protracted neurogenesis include the vocal control system and the HC, areas involved in the control of learned behaviors (vocal learning and signing and spatial memory, respectively; for review: Gahr et al. 2002). As to lobus parolfactorius (LPO), similar to juvenile development, it continued to receive a large number of neurons in adulthood. However, unlike juvenile neurogenesis, the density of new neurons in LPO in adults was only slightly higher compared with other regions of the telencephalon, indicating that the decrease in neurogenesis with age is more pronounced in LPO. Below, we focus in more details only on some of the brain regions in which new neuronal recruitment has been previously recorded.
Another vocal control nucleus in the adult avian brain that recruits new neurons is Area X, which is known to exhibit large-scale neuron addition both after hatching and throughout adulthood. This region is part of the anterior forebrain pathway and it is critical for the acquisition of song in juveniles (Nottebohm et al. 1976; Bottjer et al. 1984; Sohrabji et al. 1990; Scharff & Nottebohm 1991) and may also play a role in song maintenance in adults (Reviewed in Brainard 2008). Area X neurons, which are produced after hatching, are exclusively interneurons (Sohrabji et al. 1990).
New neuronal recruitment during adulthood is not restricted only to song control nuclei. For example, the NC, an auditory region that has indirect projections to the vocal control system (Mello & Jarvis 2008), also receives new neurons in adulthood (Alvarez-Borda 2002; Lipkind et al. 2002). NC appears to store song-specific auditory information of potential use by juveniles during song acquisition and, perhaps, song maintenance by adults (Bolhuis & Gahr 2006; Phan et al. 2006). For more details about NC neuronal recruitment and conditions that influence it see below, in ‘Models to study neurogenesis – Sociality’.
The HC, an important forebrain region for spatial learning (Krebs et al. 1989; Sherry & Vaccarino 1989), also exhibits adult neuronal recruitment. So far, neuronal plasticity in this region in birds has been investigated mostly in relation to seasonal food-hoarding behavior, and for details see below, in ‘Models to study neurogenesis–Food hoarding”. Additionally, since HC is thought to play an important role in the processing of spatial information, it has been suggested that migratory behavior might also be associated with increased hippocampal neurogenesis and that migratory birds might provide another model to study the interactions between behavior and neuronal plasticity. For more on this subject see below, in ‘Models to study neurogenesis–Migration’.
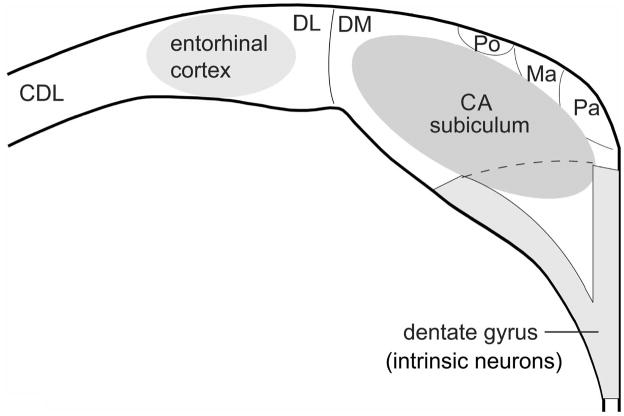
Avian and mammalian hippocampus are generally considered homologous based on developmental gene expression studies, which have shown that the same embryonic neural tissue gives rise to both avian and mammalian hippocampus (see references in Rattenborg et al. 2011). Despite being homologous, avian and mammalian hippocampi look distinctly different (Fig. 2) and it is currently unclear which regions of the avian hippocampus correspond to the much better described regions of the mammalian hippocampus (Rattenborg et al. 2011). There are several proposed comparisons between different layers of avian and mammalian hippocampi. Atoji & Wild (2004) proposed that the V-shaped region of the avian hippocampus is homologous to the mammalian dentate gyrus (Fig. 3). The dorsomedial region of the avian hippocampus has been proposed to be homologous to the mammalian Ammon’s horn or CA (Atoji & Wild, 2004). Kahn et al. (2003), on the other hand, suggested that the V-shaped region of the avian hippocampus is homologous to the mammalian Ammon’s horn, while the dorsal area of the avian dorsomedial regions is homologous to the mammalian dentate gyrus. See Rattenborg et al. (2011) for a detailed description of both mammalian and avian hippocampus.
Figure 2.
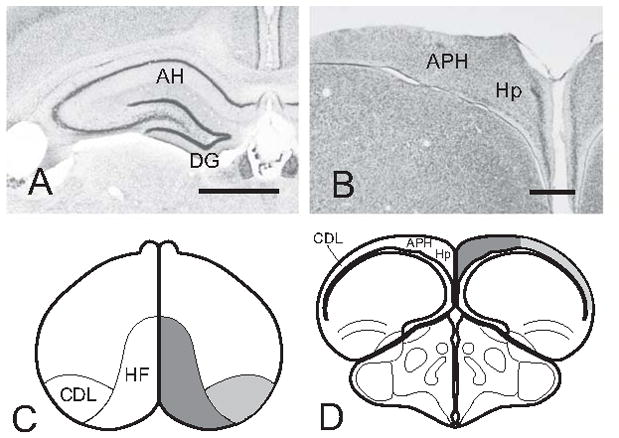
(A, B) Histological comparison of the hippocampal formation between the rat and the pigeon. (A) Layers of the dentate gyrus (DG) and cornu ammonis (CA) are conspicuous in the rat. (B) The V-shaped layer is the only readily apparent structure in the pigeon hippocampus (Hp). APH - area parahippocampalis. (C,D) Location and extent of the pigeon hippocampal formation (HF, dark gray) and dorsolateral corticoid area (CDL, light gray). (C) Dorsal view. (D) Transverse section. Scale bars in A,B = 1 mm. Reproduced with permission from Atoji & Wild (2007) following Rattenborg et al. (2010).
Figure 3.
Hypothesized homology between mammalian and avian sub-regions of the hippocampus. Mammalian dentate gyrus is hypothesized to be homologous to avian medioventral V-shaped layer (light grey), mammalian cornu ammonis (CA) and subiculum to avian dorsomedial region (DM), mammalian entorhinal cortex to avian dorsolateral region (DL). Ma - magnocellular region, Pa - parvocellular gerion, Po -cell-poor region, CDL - dorsolateral corticoid area. Reproduced with permission from Atoji & Wild (2007) following Rattenborg et al. (2010).
Another attribute regarding the anatomy of neuronal recruitment, which might be worth noting, is the spatial distribution of new neurons within a specific region. For example, in the HC of black-capped chickadees, Barnea & Nottebohm (1994) observed a non-random distribution of new neurons: six weeks after the neurons were born, 95% of them were found within a narrow band of 350μm from the VZ. Since this pattern was the same at longer intervals, Barnea & Nottebohm (1994) inferred that it represents the final distribution of new neurons within this brain region. Hoshooley et al. (2007) found that this pattern already exists six days after the new neurons are born and traveled from the VZ in the same species and in the same brain region. A very similar spatial distribution of new HC neurons is currently being revealed in several other songbird species (Gabaly, Terkel & Barnea, unpublished observations). Such general pattern might indicate that the HC is not a single functional unit, but rather consists of several sub-units, which differ in their degree of plasticity. This notion gets further support from other studies with different species and a different brain region. Barnea et al. (2006) and Adar et al. (2008a) found that one of the factors that influence the survival of new NC neurons in adult zebra finches is their position within this brain region. Taken together, such findings indicate that questions about neuronal recruitment and turnover may have to take into account several variables, including spatial distribution of the newly recruited neurons within the investigated brain region. If a study samples a large area and averages the results from all locations sampled, actual differences might go unnoticed, thus rendering the strategy that the brain uses to replace its neurons incomprehensive.
As to the amount of neuron recruitment in the adult avian brain, quantitative data are only available for HVC and the HC. It appears that incorporation of new neurons in HVC shows species differences: 0.1–0.7% new neurons per day are reported for canaries, 0.1–0.2% for zebra finches, and about 0.4% for Bengalese finches. In the HC 0.15–0.37% of all neurons are newly recruited ones (reviewed by Gahr et al 2002).
Survival, neuronal death and replacement
The number of HVC neurons does not increase with age; thus, it was suggested that the continual addition of new neurons represents the replacement of old ones that are lost (Alvarez-Buylla et al. 1992; Kirn & Nottebohm 1993). Additionally, it was found that the life-span of neurons born after hatching may vary from days to months, and some cells die even while migrating. New HVC neurons can have substantial lifespans of 4–8 months or longer and then they disappear (review in Pytte et al. 2008). Survival of new neurons might depend on the time of the year they are born. For example, new HVC neurons that are born at the end of the summer and early fall when birds are acquiring a new repertoire are still present eight months later, when the repertoire is used during the following breeding season (Kirn et al. 1991; Nottebohm et al. 1994). Therefore, the life of many of the HVC neurons seems commensurate with the life of memories that underlie the behavior controlled by that nucleus.
Accordingly, the interpretation was that the neurons remain functional for a limited period of time and are then replaced by the new ones. Hence, neuron death is necessary for subsequent neuronal incorporation in circuits that undergo constant neuronal turnover. Earlier studies provided only correlational data for the idea that cell death plays a pivotal role in the regulation of neuronal replacement. For example, in canaries, seasonal peaks in HVC neuron recruitment are preceded by peaks in cell death (Kirn et al. 1994). The evidence for neuronal replacement was only numerical, since we do not know if a new neuron becomes functional in the same position and capacity as the one it replaces. However, more recent studies provided more detailed information showing, for example, that selective killing of HVC neurons leads to a subsequent increase in the incorporation of new neurons of the same kind (Scharff et al. 2000). In addition, when mature neuron degeneration was experimentally suppressed, new neuronal recruitment into the system decreased significantly (Thomson and Brenowitz, 2009). Thus it appears that for the replaceable neuron types, the number of healthy neurons present can regulate and even constrain the number of new cells that are added. Periods of cell death may make room for a wave of new neuron addition, which continues until available circuit space is occupied once again (Kirn et al. 1994; Scharff et al. 2000; Nottebohm 2008).
Data from another brain region – NC - suggest that neuronal survival can be determined by additional factors. As already mentioned above, in adult zebra finches the location of a neuron within the NC, combined with other factors, can determine how long it will survive (Barnea et al. 2006). Since NC processes auditory and somatosensory information (Vates et al. 1996), and since zebra finches recognize each other by vocalization (Zann 1996), Barnea et al. (2006) proposed that different parts of the brain may upgrade memories at different time intervals, yielding an anatomical representation of time in the brain. In a more recent study with the same species and in the same brain region, Adar et al. (2008a) found that neuron’s age at the time that the bird is exposed to environmental change can also determine its survival. If the bird was introduced into novel complex social settings, neurons that were 1-month old at this time survived better than neurons that were 3-months old at the same time, and these effects were position dependent within the NC. Taken together, these results suggest that brains ‘use’ exquisite calculations in their ‘decision’ of which cells to replace and which to keep, under what circumstances, and for how long. This idea can lay out a hypothetical choreography and rationale for neuronal replacement: while older replaceable neurons must be eliminated as the animal grapples with a surge of new information, the same surge serves as a positive stimulus for survival of the younger new neurons. This response remains the brain’s most radical way to respond to acute changes in the amount and novelty of information it must process and perhaps, store.
FUNCTIONAL SIGIFICANCE OF ADULT NEUROGENESIS
One of the most important questions about adult neurogenesis is its function and whether it can directly cause changes in memory (Lindsey & Tropepe 2006; Scharff et al., 2000; Deng et al. 2010; Aimone et al. 2010; Leuner et al. 2006; Nottebohm 2002; Kempermann 2002, 2008; Kempermann et al. 2004). There is good evidence that new neurons are indeed incorporated into the existing neural circuits and therefore seems fully functional (Paton & Nottebohm 1984; Kirn & Nottebohm 1993; Scharff 2000; van Praag et al. 2002; Song et al. 2005), yet it is still not clear whether these new neurons function in the same way as the old neurons. While it initially seemed logical that new neurons should be causally related to enhanced learning, the evidence for this causal relationship is still equivocal despite extremely intensive research trying to establish such link (Leuner et al. 2006; Deng et al. 2010; Kempermann 2008). Even though multiple studies showed that an increase in learning results in increased adult neurogenesis, learning is not always affected when neurogenesis levels are reduced, whether experimentally or naturally (Leuner et al. 2006). Most work on birds so far has been correlational (e.g. Pytte et al. 2007; Kirn 2010) but even such correlational studies casted some doubts that addition of new neurons to the song control system is necessary for new memories (Tramontin and Brenowitz 1999; Alvarez-Borda and Nottebohm 2002). One exception was a study by Cheng et al. (2004), which documented neurogenesis-dependent restoration of some hypothalamus function in ring doves (Streptopelia risoria) following lesions. In these birds, the hypothalamus is involved in response to specific acoustic vocalizations and lesions to the hypothalamus disrupt such response while also causing an increase in neuronal recruitment. Such lesion-induced neuronal recruitment then appears to restore hypothalamus responsiveness to vocalizations suggesting a functional role of newly added neurons (Cheng et al. 2004). In adult songbirds, induced death of neurons that are regularly replaced (‘replaceable’) resulted in song deterioration and, at the same time, in increased recruitment of the same type of neurons, suggesting that neuronal recruitment is necessary for restoring a learned behavior, but this study again provides only indirect evidence for potential functional significance of adult neurogenesis (Scharff et al. 2000). All in all, however, there appears to be no unambiguous evidence for any specific functional role of neurogenesis in birds (Bolhuis & Macphail 2001; Gahr et al. 2002).
More recently, many experiments on rodents focused on blocking adult neurogenesis in the hippocampus by using chemical, irradiation or genetic ablation of neurogenesis and testing effects of such ablation on memory (Deng et al. 2010; Leuner et al. 2006). Results of such experiments have been mixed and provided no unambiguous support for the hypothesis that neurogenesis is causally linked to learning and memory; some studies showed learning impairments in animals with reduced neurogenesis while other studies detected no such negative effects. For example, cyclin D2 knockout mice have no adult neurogenesis, yet they can learn new tasks (Jaholkowski et al. 2010). Several species of bats that are likely to rely heavily on spatial memory for foraging have been reported to have no adult neurogenesis (Amrein et al. 2007), suggesting that hippocampal dependent learning may function without neuronal replacement. Other studies, on the other hand, showed that blocking adult hippocampal neurogenesis leads to impairments in hippocampal-dependent learning and memory function (Clelland et al. 2009; Jessberger et al. 2009; Winocur et al. 2006; Imayoshi et al. 2008; Snyder et al. 2005; Goodman et al. 2010). It is possible that inconsistency of many experiments using chemical and irradiation oblation results from potential side effects of such treatments as it is difficult to ensure that they affect only production of new neurons.
There have been multiple hypotheses about neurogenesis function. One hypothesis suggests that new neurons are recruited into the existing neural circuits and are directly involved in all stages of memory processing (Schinder & Gage 2004). Another hypothesis suggests that new neurons are necessary to avoid catastrophic interference when learning new information (Wiskott et al. 2006; Deng et al. 2010). Wiskott et al. (2006) assumed that when animals exhibit stable environment, old mature neurons are stable and provide reliable service, but when animals are introduced into a new environment, they need to learn new information without interfering with old, previously learned information and neurogenesis may be necessary to provide this interference avoidance. To avoid interference between old and new memories, animals may need to separate events by using different neurons for different memories – pattern separation (Aimone et al. 2010; Deng et al. 2010). At the same time, a related set of information may need to be connected via pattern integration so that all of this information may be retrieved at the same time (Aimone et al. 2010). New neurons may play important role in these processes. For example, Clelland et al. (2009) found that blocking neurogenesis resulted in impaired spatial performance when cues had little spatial separation, but not when cues were more spatially separated. Such finding provides support to the hypothesis that new neurons may be needed for spatial pattern separation. Even though research on pattern separation has been mainly conducted in rodents and in the laboratory, its implications are clearly relevant to natural systems such as food-caching birds that rely on spatial memory and on the hippocampus for food caching and cache retrieval. Many food-caching birds cache food, retrieve caches as well as re-cache previously made food caches on a daily basis (especially in the winter) and so they need to keep track of old and new caches (Pravosudov & Smulders 2010). Neurogenesis may be an important adaptive process that prevents interference of old and new memories and allows better management of food caches. This notion is in line with previous studies (Barnea et al. 2006; Adar et al. 2008a), with a different species (zebra finch) and a different brain region (NC), which propose that different parts of the brain upgrade memories at different time intervals, yielding an anatomical representation of time in the brain (see above, “Survival, neuronal death and replacement”).
Kempermann (2008) proposed the neurogenic reserve hypothesis that suggests that adult neurogenesis provides a ‘neurogenic reserve’ allowing the brain to remain flexible in learning and recruiting new neurons from this reserve when there is new information to be learned. Since many of the new neurons do not get incorporated into the existing neural circuits and simply die, the neurogenic reserve hypothesis predicts that these new neurons should get incorporated only when there is a need for new learning; otherwise new neurons simply cycle through (Kempermann 2008). This hypothesis may potentially explain the mixed results obtained with neurogenesis ablation as learning deficits should be observed only when the reserve of ‘ready to be incorporated’ neurons is depleted. So if memory experiments following the ablation are performed prior to these new neurons reaching recruitment age, no negative effects on learning may be observed. This hypothesis also explains the positive effects of physical activity on neuron production. So far, however, it appears that no experimental studies provided direct and unambiguous support to the neurogenic reserve hypothesis.
Wilbrecht & Kirn (2004) proposed several hypotheses for adult neurogenesis in avian song control brain areas: (1) adult neurogenesis is an epiphenomenon remaining from developmental neurogenesis and may serve no particular function. This hypothesis suggests that adult neurogenesis may simply be a ‘hold-over’ process following developmental neurogenesis that is critical to generate necessary brain neurons. Since adult neurogenesis is much less intense than developmental neurogenesis, it may serve no particular function. (2) adult neurogenesis is involved in song learning and new neurons are directly involved in learning. This hypothesis builds on a premise that existing neurons become less plastic with age and as a result may not be useful for processing continuously coming new information in long-lived species. Hence, the solution may be simply to discard old neurons that may contain no longer useful information and replace them with new ones that would allow acquiring new information. This hypothesis predicts most intense neuron replacement at times when learning new information is especially intense – for example during song learning seasons. (3) adult neurogenesis may provide motor flexibility for both song learning and maintenance. This hypothesis is a version of the previous one and suggests that songbirds need to accumulate an access of new neurons so that it can train and maintain ‘error’ free neurons that can function without errors in producing a correct song. In other words, this hypothesis predicts that some neurons may make errors and result in incorrect song production. Generating new neurons would allow birds to discard these neurons and build a collection of neurons that do not make errors. This hypothesis assumes that the number of error-free neurons would increase with age and neuron recruitment rates should decrease with age. (4) adult neurogenesis is necessary for replacing old neurons that became damaged after intense use. This hypothesis suggests that neurogenesis is unrelated to learning and that new neurons simply replace old neurons that became damaged after extensive use.
While all of these hypotheses seem plausible for both mammals and birds, there is no unambiguous support for any one of them and it remains unclear whether adult neurogenesis is causally linked to learning and memory. More research is needed to figure out the role of adult neurogenesis.
REGULATION OF NEUROGENESIS AND NEURONAL RECRUITMENT
Adult neurogenesis is a dynamic process and extensive studies have shown that its various stages and final outcome are regulated by a wide range of internal and external factors in both mammals and birds. However, despite these studies, the mechanisms that control adult neurogenesis are still not fully understood. Over the years, a few excellent reviews have summarized the current knowledge about these aspects, mostly in mammals (e.g. Fuchs & Gould 2000; Gould & Gross 2002; Ming & Song 2005; Rakic 2002; Aimone et al. 2010). In this section we will focus on some factors that are known to affect neurogenesis, neuronal recruitment and survival in the adult avian brain.
Internal factors
Much work has been done on the complex interactions between hormones and neurogenesis, both in mammals (e.g. see review by Galea 2008) and in birds. In the latter group, the song control system provided an excellent model for such research because of the specialized nature of this system that consists of clearly defined nuclei that control various aspects of singing behavior (such as song learning, production and perception). Such specialization of the song control nuclei made the study of brain/behavior relationships in birds much easier than in mammals in which hormone target tissues like the limbic system are involved in regulating many different physiological processes and behaviors.
In birds, sex steroids (testosterone, estradiol) have pronounced effects on song learning and production and on the juvenile development and adult plasticity of the song circuits (reviewed in Ball et al. 2008; Harding 2008; Gahr et al. 2002). However, these sex steroids do not appear to regulate the rate of cell division in the VZ (Brown et al 1993), a finding further supported by Rasika et al. (1994). This suggests that seasonal differences in the incorporation of labeled neurons into HVC (see below) may be due to regulation of some other mechanisms, such as differential neuronal migration or survival.
Testosterone appears to increase recruitment and survival of new HVC neurons (Rasika et al. 1994) and later studies also demonstrated that steroids regulate the trophic factor BDNF, which, in turn, increases HVC neuron survival (Louissaint et al. 2002; Nottebohm 2004; Rasika et al. 1999). However, this positive effect of testosterone on neuronal recruitment was found only during a restricted time window, 14–20 days after the new cells are born (Alvarez-Borda et al. 2004). Although testosterone has a pronounced and rapid effect on HVC, its efferent targets, RA and Area X, respond more slowly. Several studies revealed that this is due to the fact that testosterone and its metabolites act directly on HVC, which in turn provides some permissive or trophic support of growth to its efferent targets, RA and Area X (reviewed in Brenowitz 2008).
Estrogens also influence neuronal differentiation and survival in the avian brain, but do not seem to directly influence cell proliferation (see review in Lee et al. 2007). Williams et al. (1999) demonstrated that estrogens also promote the initial migration of new neurons from SVZ explants in vitro. The convergence of evidence suggests that estrogens act as neurotrophic agents and supply migratory support for the newly-formed neurons in reaching their end destinations (Lee et al. 2007).
As can be seen in the song control system, some effects are androgen dependent and some are estrogen dependent. One question is why singing and the song control nuclei should be activated by multiple metabolites rather than by testosterone itself. In other avian species (pigeons, quail and chicken) vocalization during interactions with females appears to be purely androgen dependent. The answer could be that in songbirds, involving multiple metabolites allows finer grained control of singing under different social context (see Harding 2008).
Studies on hormones, behavior, and brain nuclei typically focus on one or two hormones, and often we do not know enough on the effects of other hormones. In addition, most research has focused on the effects of gonadal steroids on the song system. But we know that other hormones also play a role. For example, many bird species sing outside the breeding season, and it has been found that this singing appears to be independent of gonadal androgens and rely, for example, on extragonadal synthesis of DHEA in several avian species (Soma & Wingfield 2001). In addition, a variety of other hormones appears to be involved in modulating the vocal control system and singing, such as vasotocin, thyroid hormones, and other peptide hormones including vasoactive intestinal peptide (VIP; see review in Harding 2008). VIP was found to stimulate prolactin release in songbirds during the breeding season and may be involved in decreasing of singing by males that exhibit parental care (Maney et al. 1999).
Another hormone that may be important in this respect is prolactin. Barkan et al. (2007) found an increase in new neuron recruitment in the NC, a region that is involved in sound processing and therefore is likely to play a role in auditory parent-offspring recognition in brains of breeding zebra finches. This increase coincides with the need to memorize vocalizations of nestlings before they fledge and therefore it was suggested that it may enable parents-offspring recognition and hence selective parental care. The follow-up study (Barnea & Pnini, unpublished data) found that prolactin levels in the blood of both parents were highest at hatching, and that this peak preceded the increase in NC neuronal recruitment by 3–4 weeks. In mammals, there is evidence that prolactin mediates an increase in neurogenesis in SVZ of pregnant female mice and is likely to be important for maternal behavior (Shingo et al. 2003; Larsen et al. 2008). It is also known that three weeks are required for neuronal migration from the birthplace to a target region in the avian brain (Kirn et al. 1999). Taken together, it was suggested (Barkan et al. 2007) that a temporal and functional positive correlation exists between prolactin levels and neuronal recruitment in NC. An ongoing study (Weizman & Barnea) currently tests this possibility by manipulating prolactin levels in the blood of adult zebra finches and observing neuronal recruitment in various parts of their brains.
Age is another internal factor that is known to affect neurogenesis. Age-related decline in the production of new neurons has been widely recorded in mammals (e.g. Rao et al. 2006), although it varies significantly among species, including wild-living ones (Amrein et al. 2004; 2009), and is not necessarily predictive of cognitive status (Bizon & Gallagher 2003). In birds, neuron addition and loss occur throughout post-hatching life, but during the juvenile growth phase cell proliferation in the VZ is higher than in adults (DeWulf & Bottjer 2002). At an early age, neuron addition surpasses neuron loss and once adult neuron numbers have been attained these two processes are more closely matched (Wilbrecht & Kirn 2004). In addition to its effect on neurogenesis, age also affects neuronal recruitment and in adulthood neuron recruitment seems to continue to decrease with age, also shown in a non songbird species, the ring dove (Streptopelia risoria; Ling et al., 1997). For example, in canaries neurogenesis decreases between 1 and 4 years, but not evenly throughout the telencephalon. LPO, hyperstriatum accessorium (HA), and ventral hyperstriatum showed a marked decrease in new neuronal recruitment, whereas neurogenesis in neostriatum and HC decreased to a lesser degree (Alvarez-Buylla et al, 1994). Differential decrease in age-related neuronal recruitment was observed in zebra finches, where this decrease was significant in HVC (a region necessary for song production), but not in Area X or the HC (regions not essential for singing; Pytte et al., 2007). Moreover, the rate of this decrease varies between species, and seems to depend on the stability of the behaviors they serve. For example, in canaries, where adult song changes from year to year, HVC neurons that project to RA are replaced at a rather stable yearly turnover (Alvarez-Borda, 2002). However, in zebra finches, whose adult song changes little from year to year, the recruitment of new HVC neurons is dramatically reduced with age (Wang et al. 2002). From the comparison of these two species, and also from the evidence that in canaries neuron turnover rates differ between times of the year (Nottebohm et al. 1994), one can also conclude that cell age is not likely the primary reason for neuronal replacement.
Since virtually all biological processes manifest at least some degree of circadian variation, it is reasonable to assume that this would also be the case with adult neurogenesis. However, not much work has been done on the question whether neuronal proliferation is diurnally regulated. One of the few studies was done in lobsters, where circadian control of neurogenesis was found in olfactory projection neurons with a peak in cell proliferation at dusk (Goergen et al. 2002). This question was also tested in mammals, but results are not always consistent. Some studies found no circadian variation in HC cell proliferation (Ambrogini et al., 2002; Holmes et al., 2004; Van der Borght et al., 2006) and others (Kochman et al., 2006) report a circadian variation in the hillus but not in the granule cell layer, suggesting a possible circadian influence on gliogenesis rather than neurogenesis. However, a recent study (Gerstner et al., 2008) supports the hypothesis of a diurnal cycle in neurogenesis by showing that Fabp7, downstream of notch and expressed in neuronal precursors, is diurnally regulated in HC neuron precursors in adult rodents. To the best of our knowledge, this question has not yet been studied in birds, and an ongoing study investigates whether neurogenesis in brains of adult zebra finches follows a diurnal cycle (Hornfeld, Terkel & Barnea, unpublished).
External factors
The song control system provides the most pronounced example of seasonal plasticity in an adult vertebrate brain and currently serves as one of the leading models for the study of brain plasticity. Seasonal changes in song behavior are accompanied by changes in the song nuclei in essentially every seasonally breeding songbird species that has been examined (reviewed in Brenowitz 2008).
As described above, sex steroid hormones influence song behavior and the song control circuits in the brain. Since secretion of gonadal steroids varies seasonally, these hormonal seasonal changes, in turn, modulate song production and correlate with various seasonal changes in the song control system. Androgens have been shown to play an important role in the seasonal plasticity of the song system in adult males of some species (reviewed by Kirn 2010). In male canaries, which are open-ended song learners (they learn new songs during their entire life) and seasonal breeders, decreases in testosterone levels in males coincide with periods of unstable song and with cell death in the HVC (Kirn et al. 1994; Nottebohm et al. 1987). Subsequent increases in testosterone levels that precede the breeding season promote the replacement of lost neurons and re-growth of HVC. When males have high steroid levels and are in full breeding condition, HVC volume and total neuron number are highest and neuronal replacement is low (see Review by Kirn 2010). Accordingly, the suggested hypothesis is that the seasonal neuronal turnover in HVC provides a neural substrate for plasticity of song production. Seasonal dynamics in HVC were also observed in other species, including a species that does not modify its adult song; we refer to these issues and discuss their potential interpretations below, see “the song system model”.
Seasonal differences in new neuronal recruitment have also been reported in the avian hippocampus. In free-ranging black-capped chickadees, the amount of adult-generated hippocampal neurons correlated with changes in food storage and retrieval, behaviors that involve spatial learning (Barnea & Nottebohm 1994). However, a later study with the same species (Hoshooley & Sherry 2007) failed to find such seasonal differences, and this may be due to the fact that the examined birds were kept in captivity for the period between capture and sacrifice.
The biological relevance of adult neurogenesis is suggested by the observation that it can be modulated by experience. In mammals, learning may increase, decrease or not significantly affect the survival of newly born neurons, and this varying effect can be due to the existence of a critical period in the development of new neurons, during which time their survival can be altered (see refs in Epp et al. 2007). In any case, collectively, data from various species support the view that the number of newly generated neurons in relevant brain regions may be enhanced under conditions of increased learning opportunities (see refs in Fuchs & Gould 2000).
In birds, effect of learning on production of new neurons has been found mostly at an early age (Patel et al. 1997; but see Pravosudov & Omanska 2005) and therefore the general assumption is that in adults, neurogenesis occurs at a constant rate. Only later stages, such as new neuronal survival and recruitment, seem to be affected by various factors related to experience and environment. The question whether neuronal replacement is necessary for learning is still somewhat open. For example, if that was the case, then one would expect that blocking song imitation during the sensitive period for song learning in juvenile zebra finches would disrupt the recruitment of new neurons in HVC. However, when this was done, neuron addition occurred at normal rates although the birds were unable to imitate their tutor’s song. Nevertheless, manipulations that do alter neuron recruitment also affect song learning. This suggests that new neuron addition to HVC may be permissive for song plasticity, while the process of song imitation has conditional effects on neuronal replacement even though these effects are not yet fully understood. It could be that neuronal replacement in necessary but not sufficient for song learning. In any case, the relationship between new neurons and (song) learning is probably not simple, and the observations that new neurons continue to be added in the brains of zebra finches after song crystallization adds to these doubts (review in Pytte et al. 2008).
It could be that high metabolic demands associated with repeated use of neurons result in short neuronal life span, and therefore a possible function for neuronal replacement may be to replace neurons that become damaged by use. If so, then one would expect a correlation between rates of neuronal incorporation and behavior performance, such as signing. Indeed, there is evidence that singing, and as a result functional activity of the circuitry that incorporates new neurons, contributes to the survival of incoming neurons and promotes neuron addition in adult canaries. Survival of new HVC neurons is greater in singing than in non-singing birds, and a positive causal link exists between pathway use, neurotrophin expression (BDNF), and new neuron survival (Li et al. 2000). Similarly, there is a positive correlation between natural variation in the amount of singing and new HVC neuron addition (Alvarez-Borda & Nottebohm 2002). Recently, Pytte et al. (2010) further tested the relationship between use of a specific brain region and the survival of new neurons within that brain region, and showed that use-dependent neuronal survival also occurs in the higher auditory processing region of the songbird caudomedial nidopallium (NCM).
The effects of other external factors - environmental enrichment and physical activity – on neurogenesis has also been extensively examined. In mammals, both factors seem to increase neurogenesis in the HC (see refs in Fuchs & Gould 2000), however it has been suggested that this increase is via dissociable pathways, and should therefore be considered distinct interventions with regard to plasticity (Olson et al. 2006). In birds, environmental complexity may also enhance the survival of newly born neurons in the adult brain. This possibility was initially suggested by Barnea & Nottebohm (1994) that found more new hippocampal neurons incorporated in the brains of adult black-capped chickadees living in the wild, compared with those living in captivity. However, a recent study with the same species (Tarr et al. 2009) reported no differences in the number and density of new hippocampal neurons (but reduction in volume in the captive birds), but their different methods of counting new neurons may have resulted in negative results. LaDage et al. (2010), on the other hand showed dramatic differences in the number of new neurons between wild and captive mountain chickadees (Fig. 4), in addition to showing a strong effect of learning on hippocampal neurogenesis. The captivity effect might be caused by several factors (such as stress, social isolation, lack of exercise, or reduced opportunity to cache in the case of a food storing species), and therefore it is difficult to discern individual effects of each of these factors on the HC.
Figure 4.
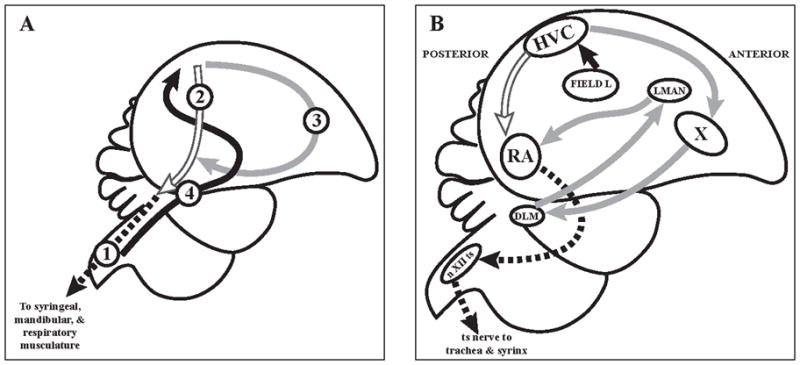
The song control system in birds. (A) The song control system can be imagined as consisting of four modules. Module #1 is in the brain stem and shared by vocal learners as well as by non-vocal learners. Module #2 is a telencephalic module that tells module #1 what to do. Module #3 starts from module #2 and then returns to it; it is necessary for vocal learning but not for production of learned song. Modules #2 and #3 are very well developed in vocal learners, less so or absent in non-learners. Module #4 is the ascending auditory pathway that conveys information about the sounds to be imitated and auditory feedback about the sounds produced. (B) Schematic diagram of the nuclei and connections of modules #2 and #3 and their relation to modules #1 and #4. All the connections shown are ipsilateral and each right and left brain half duplicates the anatomy of the other side. Abbreviations: RA - robust nucleus of archipallium; X - area X of basal ganglia; IMAN - lateral part of the magnocellular nucleus of the anterior nidopallium; DLM - medial portion of dorsolateral thalamic nuclus; nXII - tracheosyringeal part of the hypoglossal nucleus. Field L - auditory nidopallium. Adopted with permission from Nottebohm & Liu (2010).
Complex social setting is one of the factors associated with environmental enrichment and it was found to have a stimulus effect on neuron proliferation, and in some cases in a site-specific manner, in mammals (e.g. Fowler et al. 2002). In addition, it has been shown that social rearing conditions can modify neurogenesis, but this effect could be reversed by subsequent group rearing (Lu et al. 2003). The positive interplay between enriched sensory and social conditions and neurogenesis occurs not only in mammals but also in other taxa. For example, it has been reported in insects, where it is direct and not mediated via hormonal control (Scotto-Lomassese et al. 2002).
In birds, it is likely that adult neuronal replacement is influenced by the actions and attributes of conspecifics since social enrichment has been found to enhance survival of adult-formed neurons in several avian brain regions (Barnea et al. 2006; Lipkind et al. 2002). In one study (Lipkind et al. 2002), zebra finches that were introduced to large, mixed-sex groups of unfamiliar birds had more new neurons in HVC, Area X and NC, compared with birds that were housed singly or with an unfamiliar mate that did not differ from each other. It is unlikely that the amount of singing could account for these results (Adar et al. 2008b) and therefore we suggested that increased demands on systems underlying new auditory memory formation may enhance neuronal survival. Moreover, subsequent work on the NC (Adar et al. 2008a) showed that the timing and degree of change in social complexity relative to a new neuron’s age impacted that neuron’s chances of survival. Social stability seems to favor survival of pre-existing neurons over newly-formed ones, whereas social change seems to favor survival of new neurons over older ones.
It is tempting to speculate that the richness and variability of the environment stimulate neurogenesis and that in turn the newly generated neurons improve the capabilities of adult animals to exploit their habitat. However, there are numerous potentially relevant variables in the environment (whether physical or social), and therefore it is difficult to determine the relative importance of a specific stimulus to such brain plasticity. Very few studies have dealt with this complicated issue, although it might be important, especially when investigating complex behaviors such as social interactions or spatial learning. An interesting example is a study in electric fish, which showed that stimuli are sufficient to increase neurogenesis in adult brains through a single modality (Dunlap et al. 2008).
Stress is another external factor that has been shown to inhibit neurogenesis and its effect appears to be common across species and life stages in mammals. Most effects of stress are frequently mediated by elevated levels of glucocorticoid hormones, which in many cases serve as a direct cause in affecting neurogenesis (Mirescu & Gould 2006). For example, stressful experiences are known to decrease the number of new neurons in the dentate gyrus of the mammalian brain (Fuchs & Gould 2000; Gould & Gross 2002) and fear learning transiently impairs hippocampal cell proliferation (Pham et al. 2005). So far, not much work in this respect has been done in birds, and we still do not know enough about if and how stress may affect different stages of avian adult neurogenesis and neuronal recruitment. From the few available studies, corticosterone has been shown to decrease neurogenesis in the avian song control system (Newman et al. 2010). On the other hand, moderate elevation of glucocorticoid hormones had no effect on hippocampal cell proliferation rates in mountain chickadees (Pravosudov & Omanska 2005). It is more then likely, however, that stress and glucocorticoid hormones have the same effect on neurogenesis in both birds and mammals.
In summary, a whole battery of factors, which might be regulated differentially by internal and external environments appear to influence neuron survival and recruitment. The relationships between the various factors might be complex. Experimentally, it is easier to study a correlation between a specific factor (internal or external) and brain plasticity (neuronal recruitment/survival, or size of brain regions). More difficult and challenging is to test the interactions between such factors and to understand the direction of causation between them. For example, in the relationship between singing behaviors, seasonality, testosterone, and song nuclei - do changes in testosterone levels cause changes in singing behavior, which, in turn, cause changes in the size of song nuclei and in the number of neurons in these nuclei, or does causation work in the opposite direction? In other words, one may ask whether an increase in neuronal numbers in song nuclei in breeding birds is a consequence of high rates of singing at this time of the year, or that the behavioral changes follow changes in the song nuclei. This complex question is discussed in detail by Brenowitz (2008) who presents evidence for the latter suggestion. Brenowitz (2008) argues that at least in most species examined, seasonal changes in the song nuclei are predominantly regulated by hormonal changes, and that the subsequent changes in song behavior play a secondary role in reinforcing neuronal changes by mechanisms such as song-induced expression of BDNF.
THE GENETIC BASIS OF NEUROGENESIS
Most of the research on adult neurogenesis has been focused on its plasticity and how various environmental features and behavior may affect neurogenesis (Deng et al. 2010; Nottebohm 2002; Kempermann 2002, 2008; Leuner et al. 2006). However, it remains important to understand whether and how much neurogenesis might be under genetic control because it is fairly clear that learning and many brain processes including adult neurogenesis are at least partially controlled genetically (Kirn 2010; Lindsey & Tropepe 2006). At this point, we know that there are significant differences in adult neurogenesis between different bird species – for example food-caching chickadees appear to have more intense hippocampal neurogenesis than non-caching passerine species (e.g. Hoosholey and Sherry; data in Chancellor et al., 2011; LaDage et al. 2010, 2011). It is unlikely that these differences simply reflect behavioral differences and experiences between these species and it is more plausible to hypothesize that different species may have evolved different adult neurogenesis rates due to different selection pressures (e.g. Pravosudov & Smulders 2010).
It would be important to understand how much variance in neurogenesis within the same species can be explained by genetic basis vs plastic responses. For example, Hurly et al. (2008) showed that individual variance in neuron incorporation rates into the HVC of zebra finches can be explained by the nest of origin, which suggests that either genetics or early developmental conditions predetermine neurogenesis in the song control system. Large differences in hippocampal neurogenesis in black-capped chickadees between different populations (Chancellor et al. 2011) also appear to be affected by either genetics, maternal effects or early developmental conditions while being largely unaffected by environmental conditions/experiences in adult life (Roth, LaDage, Freas, Pravosudov, unpublished). Similarly, analyses of different lines in mice also suggest strong genetic component defining hippocampal neurogenesis (Kempermann et al. 1997; Pozniak and Pleasure 2006).
All of these data suggest that it is crucial to understand the magnitude of genetic control of neurogenesis in animals, and birds may be an excellent system to investigate the relative contributions of genetics and plasticity into neurogenesis function, as birds show large, naturally occurring variation in neurogenesis rates (e.g. Chancellor et al. 2011). Recently sequenced genome of the zebra finch should provide new opportunities to identify specific genome regions that may be involved in regulation of neurogenesis (Clayton 2009). Finally, recently demonstrated ability to produce transgenic birds will also provide an important model of genetic regulation of neurogenesis (Agate et al. 2009). If neurogenesis is at least partially under genetic control, it can be affected by natural selection, which may produce drastically different patterns depending on the strength of selection pressures (e.g. more selection pressure for enhanced spatial learning and, potentially, for increased hippocampal neurogenesis in food-caching species) and the costs of maintaining neurogenesis (e.g. when there is no costs and no obvious benefits, neurogenesis may be greatly reduced in some species). Comparing multiple species with known phylogenies and life history traits, in particular those related to cognitive demands, may allow better understanding of the evolution of the brain and adult neurogenesis as well as the factors that may have contributed to evolutionary changes in the brain and brain processes (e.g. Jarvis et al. 2005; Amrein & Lipp 2009).
MODELS TO STUDY NEUROGENESIS
Song system
Song control system in the brain of songbirds presents one of the most amazing models to investigate adult neurogenesis because of its extreme season-related plasticity in song learning (Goldman 1998; Tramontin & Brenowitz 2000; Brenowitz 2004; Nottebohm 2004; Kirn 2010; Nottebohm & Liu 2010). Many songbirds learn their songs from tutors during developmental sensitive phase and then use auditory feedback during song practice so that the final version of the song matches that of its tutor. Many bird species also learn new songs during their entire lives. Brain song control system is critical for song learning and lesions to the song control regions result in song learning and production impairments (Nottebohm & Liu 2010). According to Nottebohm & Liu (2010), the song control system consists of four main functional modules (Fig. 4). Brainstem nuclei and pathways controlling respiration and unlearned sound production comprise Module 1. The pre-motor telencephalic nuclei HVC and RA comprise Module 2, which provides information to Module 1. Module 3 connects HVC (which is shared with Module 2) to the basal ganglia (Area X, thalamus (DLM), which is connected to the anterior cortex (LMAN), which, in turn connects to RA. This vocal learning pathway is similar to mammalian cortical-basal ganglia-thalamic-cortical loops (Jarvis et al., 2005). Ascending auditory pathway, which connects to HVC comprises Module 4. HVC plays a central role in the song control system as it is connected with all four Modules and both receives information and controls the outputs. Neuronal addition to HVC continues during song learning and these new neurons project to RA suggesting important role of these new neurons in song learning.
In addition to seasonal plasticity of song learning, there are species-specific differences in song control area size, which appear to be related to the differences in song repertoire (Szekely et al. 1996). In his pioneering work, Nottebohm (1981) reported that some song control nuclei (HVC) increase by almost 100% in spring in the canary, while in spotted towhee (Pipilio maculates) HVC volume has been reported to triple between fall and spring (Tramontin & Brenowitz 2000). Nottebohm (1981) suggested that this increase is to support production of stable song during spring. Following this work, Goldman & Nottebohm (1983) reported constant neuronal production into the song control system in adult canaries, and then Kirn et al. (1994) reported that neuronal recruitment into the HVC in adult male canaries shows two distinct peaks – one in fall and one in spring. The interpretation of the latter result connected these two peaks in neurogenesis with the two peaks in song instability and in new song syllables emergence in canaries (Kirn et al. 1994). Finally, Paton & Nottebohm (1984) showed that new neurons are recruited into the functional circuits in the song control system. So altogether, Nottebohm and colleagues clearly demonstrated that new neurons are being produced in adult bird brains year round, some of these neurons get incorporated into the existing functional circuits, at least in the song control system, and seasonal peaks in neuron production rates coincide with the peaks in song learning. Even though Altman & Das (1965) were the first to report adult neurogenesis in rodents, the early work of Nottebohm and colleagues on neurogenesis in song control system in birds served as an impetus to the following explosion of research on adult neurogenesis in both birds and mammals.
After providing clear evidence that new neurons are being constantly produced in adult brains, the focus of research shifted to identifying causes and consequences of variation in adult neurogenesis. Again, song control system in birds provided a great model for these studies because it shows tremendous neural plasticity (Brenowitz 2004; Tramontin & Brenowitz 2000). For example, in white-crowned sparrows (Zonotrichia leucophrys), experimental withdrawal of testosterone, which may naturally occur after the breeding season, resulted in 22% reduction in HVC volume within 12 h and in 26% reduction in HVC neuron numbers in 4 days (Thompson et al. 2007). On the other hand, increase in day length and exposure to testosterone resulted in 69% increase in HVC volume and in addition of 50,000 new neurons within seven days (Tramontin & Brenowitz 2000; Tramontin et al. 2000). In wild song sparrows (Melospiza melodia) the number of HVC neurons increased by 67% from late autumn to early spring (Smith et al. 1997). What makes the song control system especially attractive for investigation of adult neurogenesis is that all of that enormous variation in the brains of songbirds occurs regularly and naturally and does not represent pathological cases frequently observed in laboratory rodent models.
One of the most intriguing questions, however, remains about what is the role of new neurons in song control nuclei. Initial studies of seasonal variation suggested that neurogenesis supports song learning because neurogenesis peaks at specific times of new song learning (Brenowitz 2004). On the other hand, Tramontin & Brenowitz (1999) also reported seasonal dynamics of HVC neurogenesis in adult age-limited western song sparrows (Melospiza melodia morphna), which learn their song during the first year and then retain stable song repertoire. Such finding suggests that reported seasonality may be unrelated to song learning. A study by Alvarez-Borda and Nottebohm (2002) separated the effects of testosterone and song learning on neurogenesis and found that castrated canaries (‘testosterone free’) had significantly reduced HVC neurogenesis, but there were no differences between intact and castrated canaries in the amount of singing, syllable diversity or song stability. Even though this study was not designed to test the causal relationship between neurogenesis and song learning, it indirectly suggests that neurogenesis does not cause changes in syllable diversity or song stability, as both of these parameters did not differ between castrated animals with reduced neurogenesis and controls. Interestingly, the amount of singing in ‘testosterone free’ birds correlated with the number of new HVC neurons, suggesting that the amount of singing may cause more neurogenesis. Wilbrecht et al. (2006) experimentally prolonged sensitive period for song learning by rearing zebra finches in isolation and showed that such prolongation also resulted in prolonged period of neurogenesis. The number of newly added HVC neurons also correlated with syllable variability, and Wilbrecht et al. (2006) interpreted this finding as supporting the idea that neurogenesis facilitates song change. However, combined with the Alvarez-Borda & Nottebohm (2002) study, data from Wilbrecht et al. (2006) seem to rather support the idea that increase in neurogenesis is a consequence of changes in song learning rather then the cause. On the other hand, Brenowitz (2008) provided other data that seem to support the opposite and suggest that behavioral changes follow changes in song-related brain areas.
More work is necessary to better understand role of neurogenesis in the song control system and, more specifically, to uncover the function of new neurons. Song control system provides excellent opportunities to continue to untangle the cause-effect relationship between neurogenesis, song learning and production, and the environment (Kirn 2010; Nottebohm & Liu 2010).
Food hoarding
Food-caching birds have been another useful model to investigate adult neurogenesis in the hippocampal formation. There are two types of food hoarding: larder-hoarding -where the animal creates a few large caches which it often defends, and scatter-hoarding - where the animal creates multiple caches, often with each individual food item stored in a unique place. Scatter-hoarding species such as jays, chickadees and nuthatches store only one or at most a few food items in a single location and some of these species store tens and hundreds of thousands of food items scattered in thousands of different spatial locations over these birds’ home ranges, which could be quite large (Pravosudov & Smulders 2010). These birds then use their food caches during the winter weeks and months after making them and rely, at least in part, on spatial memory to recover these caches (Shettleworth 1995; Pravosudov & Smulders 2010).
Scatter-hoarding species have been at the center of investigations relating memory and the hippocampus because these species are well known to use spatial memory during food caching (Smulders & DeVoogd 2000) and for cache recovery (Shettleworth 1995; Shettleworth 2003; Pravosudov & Smulders 2010). Experimental lesion studies also convincingly showed that the hippocampus is necessary for memory-based cache recovery (Sherry & Vaccarino 1989; Hampton & Shettleworth 1996). There are two main aspects of food-caching birds that make them especially a good model for investigations relating memory and the brain. First, different species and even different populations of the same species rely on cached food to a different degree depending on the environmental conditions and, as a result, these birds show species differences in both spatial memory and hippocampal morphology (Krebs et al. 1989; Sherry et al., 1989; Pravosudov & Clayton 2002; Roth et al. 2009; Pravosudov & Smulders 2010). Second, food caching is a highly seasonal behavior (e.g. Pravosudov 2006) and therefore there may be seasonal variation in demands on memory and the brain related to either food caching itself or to cache retrieval and consequently season-related plasticity in the brain (Pravosudov & Smulders 2010; Sherry & Hoshooley 2010).
Krebs et al. (1989) and Sherry et al. (1989) were the first to hypothesize that food-caching birds should have enhanced memory and an enlarged hippocampus as a result of intense selection pressure for better memory needed to recover previously cached food. Most of the work that ensued focused largely on the hippocampal volume and memory and the results, while not always consistent, still provided fairly solid support that scatter-hoarding birds do have relatively larger hippocampi than non-caching species (Shettleworth 2003; Pravosudov & Smulders 2010). Comparison of multiple populations of a single species with a wide distribution range (black-capped chickadee, Poecile atricapillus) also showed that populations living in harsher environments that provide higher demands for cached food and, therefore, for enhanced mechanisms of successful cache recovery (e.g. memory) have better spatial memory, larger hippocampal volumes and more hippocampal neurons (Pravosudov & Clayton 2002; Roth & Pravosudov 2009; Roth et al. 2011).
If neurogenesis is involved in memory function, it is plausible to predict that food-caching birds should also have more intensive hippocampal neurogenesis compared to non-caching species. Since scatter-hoarding species constantly need to form new memories encoding information about newly made caches, it is plausible to predict that neurogenesis is highly adaptive by providing new neurons for new memories. At the same time, caches made during previous years may be either used or lost and so the old neurons containing these old memories may not be needed any more and they may be purged to avoid interference. So far, only a single study compared one food-caching species with one non-caching species and reported higher hippocampal neurogenesis in the former (Hoshooley & Sherry 2007). While this study indeed supports the idea of higher neurogenesis in food-caching birds, more species need to be compared in order to establish whether this is a general pattern. One step towards establishing a general trend relating more intensive neurogenesis and food-caching related memory demands was provided in a study by Chancellor et al. (2011) who reported higher neurogenesis in black-capped chickadees from harsher environments with more reliance on cached food. Interestingly, chickadees from harsh environments had more intensive hippocampal neurogenesis both in absolute terms and relative to the larger total number of hippocampal neurons compared to birds from milder environments suggesting higher turnover rates in more northern birds (Chancellor et al. 2011). It remains unclear whether such differences are a result of plastic response to varying memory demands and use or a product of natural selection, but some preliminary data suggest genetic or early developmental effects (Roth, LaDage, Freas and Pravosudov, in prep.). Nonetheless, it has been reported that spatial learning soon after fledging results in increased hippocampal cell proliferation rates in food-caching marsh tits, Parus palustris (Patel et al. 1997) and that experimental reduction in memory use via restriction of food caching and retrieval results in lowered hippocampal neurogenesis in fully developed mountain chickadees, Poecile gambeli (LaDage et al. 2010). These results strongly suggest that memory use is indeed correlated with intensity of hippocampal neurogenesis, but it remains unclear whether increased neurogenesis is simply a consequence of higher memory use or whether increased neurogenesis causally enhances memory function.
Seasonal variation in food caching behavior presents another great opportunity to investigate whether hippocampal neurogenesis may be related to potentially seasonally changing demands on spatial memory (e.g. Pravosudov 2006). The first study of seasonal variation in hippocampal neuron recruitment rates in food-caching black-capped chickadees reported higher neuron recruitment rates in birds injected with cell division marker during potential peak of autumnal food caching (Barnea & Nottebohm 1994). While many interpreted such results as supporting the hypothesis that higher neuron incorporation rates coincided with the peak of caching and thus these new neurons may be important for higher memory demands during caching, others suggested that such interpretation may not be fully justified (Pravosudov & Smulders, 2010).
The main issue here is that Barnea & Nottebohm (1994) measured neuron incorporation 6 weeks after injecting the birds with a cell division marker. Hence, when treating birds in October, they measured incorporation several weeks after the potential peak of food caching. As neuron production rates were not measured, it remains unclear whether higher neuron incorporation rates measured after the caching peak was a result of higher neuron production rates occurring specifically at the peak of caching, or a result of higher new neuron survival rates that occurred after the peak of food caching and was more likely influenced by memory-based cache retrieval (Pravosudov & Smulders 2010).
It is also possible that seasonal variation in hippocampal neuronal recruitment may be related to large scale seasonal changes in the environment, such as defoliation of trees, changes in social environment related to flock formation during early autumn, use of enlarged home ranges during non-breeding season (Barnea & Nottebohm 1994). Nonetheless, most of these changes occur earlier and prior to the observed peak in hippocampal neuron incorporation in birds injected with cell division marker in October and sampled 6 weeks later (Barnea & Nottebohm 1994). Therefore, it remains unclear what specifically may affect neuronal survival and incorporation between October and November-early December in chickadees.
Further studies of seasonal variation in neuron production in black-capped chickadees found no seasonal differences and concluded that the results of Barnea & Nottebohm (1994) may only be explained by differences in new neuron survival (Hoshooley & Sherry 2004). In the follow up study, Hoshooley et al. (2007) did find seasonal variation in hippocampal neuron recruitment rates following 1 week after the injections, but the peak was observed in January rather than in October or soon after the October as in Barnea & Nottebohm (1994). So it is possible that seasonal variation in hippocampal neurogenesis may be unrelated to variation in memory-based food caching and it still remains possible that Hoshooley et al. (2007) did not replicate the Barnea & Nottebohm (1994) findings because they only measured 1 week rather than 6-week neuronal survival. Such differences in the age of new neurons make the comparison between the Hoshooley et al. (2007) and Barnea & Nottebohm (1994) studies quite weak as they investigated seasonal variation in two different populations of new neurons – young, recently born neurons and mature neurons. It is possible that neuron production and early survival are not affected by seasonally changing environments while survival of older new neurons just prior to their incorporation into the existing neural circuits may be susceptible to changes in the environment. More studies are clearly needed to better understand the nature of seasonal variation in hippocampal neurogenesis and the factors directly involved in affecting neuron production or survival and/or recruitment.
Interestingly, Smulders et al. (1995, 2000) reported seasonal variation in both hippocampal volume and the number of hippocampal neurons and suggested that chickadees have larger hippocampi with more neurons during the peak of food caching in order to process more spatial information about cache locations during caching. However, all attempts to replicate these findings in the same species have failed and reported no seasonal variation in either hippocampal volume or neuron numbers (Hoshooley & Sherry 2004; Hoshooley et al. 2007; Sherry & Hoshooley 2009). It is also important to note that experimental changes in photoperiod had no effect on hippocampal neurogenesis in black-capped chickadees suggesting that any potential seasonal effects in hippocampal neurogenesis are not driven by seasonally changing photoperiod (Hoshooley et al. 2005).
Interestingly, captivity has been reported to have a negative effect on hippocampal neurogenesis in wild-caught birds (Barnea & Nottebohm 1994; LaDage et al. 2010; but see Tarr et al. 2009), but not in hand-reared birds (Roth et al. in prep.). It is likely that neurogenesis suppression occurs as a result of stress in wild-caught birds placed in captivity, while such stress may not be present in hand-reared birds. It is also interesting that juvenile birds appear to have higher hippocampal neurogenesis rate than adults (Barnea & Nottebohm 1996) suggesting a prolonged maturation effect similar to that observed in song control system (Wang et al. 2002).
Overall, food-caching birds present a great model to investigate neurogenesis in both natural conditions and in controlled laboratory environment because they naturally express memory-based behaviors, which in turn could be linked to both the environment and to neural mechanisms such as hippocampal neurogenesis.
Bird migration
Many migratory bird species regularly migrate large distances from their breeding to wintering grounds returning with remarkable accuracy to the same locations and reusing the same stopover sites along the migration route (e.g. Bingman & Cheng 2005). Birds have been reported to use a variety of navigational mechanisms including geomagnetic, celestial, olfactory and spatial cues (Bingman & Cheng 2005). Spatial, hippocampal-dependent learning appears to be one of these mechanisms that may guide migratory birds over familiar terrain and it appears to also be involved in creating and maintaining large-scale cognitive navigational maps (Bingman & Cheng 2005; Bingman et al. 2003, 2005; Frost & Mouritsen 2006). Hippocampal lesions disrupt landmark-based navigation and prevent formation of cognitive navigational maps in homing pigeons (Bingman et al. 2003, 2005). In addition, displacement studies showing that only adult, but not juvenile migratory birds may compensate for displacement (Thorup et al. 2007) suggest that learning is involved in formation of large scale navigational maps.
Several studies showed that migratory species and subspecies have better spatial memory and enlarged hippocampus with more neurons compared to non-migratory species and/or subspecies (Healy et al. 1996; Cristol et al. 2003; Mettke-Hoffman & Gwinner 2003; Pravosudov et al. 2006). In addition, a most recent study showed that migratory adult birds have more intense hippocampal neurogenesis compared to non-migratory adults (LaDage et al. 2011). All of these data suggest that migratory birds indeed rely, at least in part, on hippocampal-dependent spatial learning for migration and therefore present another good model for investigation of learning and hippocampus processes such as neurogenesis within the natural paradigm of migration. It still remains unknown whether migratory birds maintain increased hippocampal neurogenesis throughout the year or only during migration. It is also possible that neurogenesis may increase prior to migration in order to prepare the hippocampus for heavier memory loads during migration. Birds may have to re-fresh their memory during every migration and new neurons may be essential for this process. Finally, it is also possible that increased hippocampal neurogenesis during migration may be a function of intense physical exercise (flight) during migration as it has been reported that increased exercise may cause an increase in neurogenesis in mammals (e.g. van Praag 2008). An ongoing study (Barkan, Yom-Tov, & Barnea, in preparation) which compares HC neuronal recruitment in migratory birds versus resident ones in several closely related species might provide some answers. To sum, migratory birds present a good model to address multiple questions about neurogenesis control starting from learning and motor stimulation and ending with hormones as it has been reported that migratory birds have elevated levels of plasma corticosterone specifically during migration (Landys et al. 2004; Nilsson & Sandell 2009).
Sociality
Social environment
Many animal species including birds live in complex social environments and such environment may not always be stable as individuals move from group to group and get exposed to new individuals. If adult neurogenesis is providing new neurons for learning new information (e.g. Nottebohm 2002), it may be hypothesized that different social environments may present different challenges for learning – very stable small groups may provide the least demands on learning about social settings and larger, unstable groups may present more information to learn and as a result higher demands on the brain. Birds are a good model to investigate the relationships between social settings, learning and adult neurogenesis as they naturally exist in groups of various complexity and dynamics.
Lipkind et al. (2002) placed adult zebra finches in three social environments, solitary, pairs and groups, and found that the number of newly added neurons in three brain regions (NC, HVC and area X) was significantly larger in birds placed in large groups. All three of these brain regions are involved in vocal communication and Lipkind et al. (2002) suggested that more communications with more group members in larger groups contributed to increased survival of new neurons. In a different study, Barnea et al. (2006) also found that zebra finches maintained in larger groups had more new neurons both in NC, and also, surprisingly, in the HC. Interestingly, this study reported different survival times for new neurons in these two brain areas: neuronal turnover was more intense in NC than in the HC suggesting that neurogenesis can be regulated differently in different brain areas possibly depending on different types of learning. In contrast to Barnea et al. (2006), a different study with another species found no differences in hippocampal neurogenesis between mountain chickadees (Poecile gambeli) maintained either solitary, in pairs or in small groups of four individuals (Fox et al. 2010). It is possible that groups of chickadees were too small to detect significant differences in neurogenesis as zebra finches in Barnea et al. (2006) were maintained in much larger groups (40––45 individuals). Fox et al. (2010) also used doublecortin to measure neurogenesis, which allows tracking new neurons only until they are 3 to 4 weeks old and provides a combined estimate of neuron production and survival within that period. Barnea et al. (2006) measured specifically neuron survival using longer time intervals, from 40 to 150 days. It is also possible that different species may respond differently to changes in social environment. For example, zebra finches occur in large colonies of up to 300 individuals in the wild, while chickadees form much smaller groups.
Adar et al. (2008a) reported that large changes in social complexity (45 individuals in a group) enhanced survival of younger new neurons (one month old) in NC of zebra finches, whereas smaller complexity (one male and one female) enhanced survival of older new neurons (three months old). Adar et al. (2008a) suggested that younger neurons (1 month old) are not yet committed to a specific function and as a result complex social environment may direct them to a new specific task resulting in longer survival. Three-months neurons, on the other hand, have already been engaged and new learning demands may render them useless.
Interestingly and in contrast to Barnea et al. (2006), Adar et al. (2008a) found no differences in new neuron survival either in the HVC or the HC between both complex and simple social environments and between survival of young (one month) and older (3 months) new neurons. The main difference between the Barnea et al. (2006) and the Adar et al. (2008) studies was between the timing of neuronal birth in relation to social changes. Barnea et al. (2006) investigated survival of neurons born immediately prior to social changes, while Adar et al. (2008a) investigated survival of neurons born either 1 or 3 months prior to social changes. These differences suggest that hippocampal neurons that have already matured may be more resistant to social changes than younger neurons. More studies are needed to better understand the relationship between social complexity and neurogenesis in different brain areas.
Social dominance
Many socially living animals including birds form dominance hierarchy in which socially dominant individuals control most of the resources. In such systems, subordinate individuals may be socially stressed because of aggression by dominants, and, in addition, subordinates may have limited access to many important resources such as good feeding sites and predator-safe areas. At the same time, in some species, most notably in mammals, it is dominants that are more stressed because of the constant need to maintain their dominance (Creel et al. 1996). Interestingly, it appears that there are no known avian systems in which dominants may be more stressed than subordinates. Social stress has been suggested to have a negative effect on neurogenesis (Gould & Tanapat 1999; Kozorovitskiy & Gould 2004; Gheusi et al. 2009), but many mammalian studies used models that do not naturally form social dominance hierarchy. Many bird species, on the other hand, naturally form social dominance hierarchy within complex social groups and thus present a good model to investigate the effects of naturally occurring dominance interactions on the brain and adult neurogenesis. So far, there has been only a single study that showed negative effects of social subordination on hippocampal cell proliferation in food-caching mountain chickadees and such negative effects were also associated with decreased memory performance (Pravosudov & Omanska 2005). These results are somewhat similar to those reported for rats (Kozorovitsky & Gould 2004), except that in rats only neuronal survival, but not neuron production rates was affected by dominance status. Pravosudov & Omanska (2005) did not investigate the effects of dominance on new neuron survival rates and it is quite possible that it would be affected as well. More experimental studies are needed to better understand whether and how social dominance may affect both neuron production and neuron survival in animals, but in order to separate the effects of social dominance from innate individual properties, it is imperative to manipulate social status experimentally. It would also be important to investigate the effect of social dominance on neurogenesis in brain areas other than the hippocampus (Gheusi et al. 2009).
Methodological considerations
Neurogenesis is a process that can be quantified, and during the years there has been a continued improvement in detection of newly generated cells and for resolving stages of neuronal lineage commitment. Here we focus only on a few labeling methods that are commonly used in birds, and discuss advantages and drawbacks of these labeling techniques when applied to the avian brain.
DNA replication of stem cells during the S phase can be used to birth-date proliferating cells by supplying exogenous markers that are incorporated into the DNA of the cells. Since these markers are retained in the postmitotic cell, they enable a subsequent detection of a cohort of dividing cells that were labeled at a known time, and provide a tool to asses the existence of neurogenesis, possible changes in neurogenesis under different conditions, and the fate of the new neurons at a later date. The traditional approach to detect proliferation is by incorporation of [3H]thymidine using autoradiography (Sidman et al. 1959). This technique was invaluable for the study of the time of origin of neurons in a variety of species, including rodents (Angevine 1965) and non-humans primates (Rakic 1973, 1985). More recently, thymidine analogs have been introduced as an alternative method for [3H]thymidine, and Bromodeoxyuridine (BrdU) is the most commonly used one (Miller & Nowakowski 1988). Both markers label cells for about 2 h after they are injected systemically (Cameron & Mckay 2001) – perhaps for even shorter time in birds (Alvarez-Buylla et al. 1990) – and so the time at which cell division occurred can be determined with some precision. When determining the time of cell division is not an objective, multiple injections are often given over a period of several hours or several days in order to label a greater number of dividing cells.
The use of [3H]thymidine and BrdU have some advantages and drawbacks, and hence these methods are preferable for some applications and limited in others: 1) BrdU can be quickly detected by immunocytochemistry, while [3H]thymidine autoradiography requires at least 2 wk of exposure. 2) When using [3H]thymidine, it is difficult to detect the deposition of silver grains in combination with immunoperoxidase labeling for phenotypic markers due to their overlap in the cell obscuring visualization. On the other hand, BrdU permits single or multiple labeling with such markers, which are then easily detected by bright-field, fluorescence and confocal microscopy to determine co-localization (e.g. Magavi & Macklis 2008). 3) Unlike the thin sections needed for autoradiography, which limits the detection of labeled cells to the most superficial 3–5 μm, the thicker sections (40–50 μm) that can be used for BrdU make it suitable for design-based stereological quantification. 4) On the other hand, the use of [3H]Thymidine is advantageous since its incorporation is stoichiometric, meaning silver grains correlate with percentage of DNA labeled. Therefore, [3H]Thymidine can be used as a quantitative marker: counting grains allows tracing of the number of divisions a cell has gone through since treatment (e.g Hornfeld et al. 2010). 5) BrdU requires DNA denaturing step usually using hydrochloric acid, which results in tissue damage and may produce variation in staining quality (Gould 2007). 6) Another disadvantage of BrdU is that its molecular structure is inherently different from the natural structure of thymidine, which causes steric hindrance. Therefore, at high doses the abnormalities it causes in DNA transcription and protein translation might lead to mutation and cell toxicity, compromising the overall health and behavior of the organism (e.g. Kolb et al. 1999). Furthermore, BrdU causes abnormal proliferation in vivo (Goldworthy 1992; however see Hancock et al. 2009). Hence, BrdU might affect the very population under examination and therefore one has to be cautious with doses and frequency used (e.g. Cameron & Mckay 2001; Burns and Kuan 2005, Sekerkova et al. 2004, and see review by Taupin 2007).
Newly generated cells in the adult brain progress through a series of lineage commitment stages prior to maturation (Kempermann et al. 2004). Therefore, endogenous markers that are transiently expressed only in the neuronally committed cell population could potentially replace [3H]thymidine or BrdU labeling for detecting neurogenesis. This might be especially important when studying neuronal survival in wild populations, because injected individuals would not need to be released and then recaptured after the required time interval (such as in Barnea & Nottebohm 1994). In many bird species recapture would be extremely difficult if not impossible. Therefore in such cases, endogenous markers for the detection of neurogenic activity might provide a good alternative. Several such markers have been described and the most widely used is doublecortin, which is expressed only in immature neurons (Brown et al. 2003; Rao and Shetty 2004; Couillard-Despres et al. 2005). The doublecortin antibody has been validated for both mammals and birds and it has been established that it is specific to the doublecortin protein (e.g. Hannan et al. 1999; Francis et al. 1999; Brown et al. 2003; Boseret et al. 2007). Numerous studies confirmed that doublecortin labels only immature neurons and not astrocytes, microglia or oligodendrocytes (Yang et al. 2004; Rao & Shetty 2004; Brown et al. 2003). In rodents, it has been shown that new neurons cease expressing doublecortin at about two weeks (e.g. Brown et al. 2003), while in birds it is expressed in new neurons for about 30 days (Balthazart et al. 2008). Most importantly, a few studies confirmed that doublecortin provides qualitatively the same results as the ones produced by using BrdU (Brown et al. 2003; Couillard-Despres et al. 2005). Doublecortin has also been validated in birds within an ecologically relevant paradigm of memory use, and food-caching mountain chickadees that were deprived of food caching and cache retrieval has significantly fewer doublecortin-labeled hippocampal neurons compared with the birds that freely cached food and retrieved caches in the laboratory environment (LaDage et al. 2010). In addition, wild chickadees had significantly more doublecortin-labeled hippocampal neurons than captive birds (Fig. 5), mirroring the results of Barnea & Nottebohm (1994) who used [3H]Thymidine. The main drawback of doublecortin is that it does not enable a separation between production and survival of new neurons. Nonetheless, doublecortin presents a good alternative to BrdU for investigations of adult neurogenesis in wild birds.
Figure 5.
Examples of doublecortin staining in the hippocampus of free-ranging birds (A) and of captive birds deprived of memory-based experiences (B). (C) A doublecortin stained neuron.
Endogenous markers for mature neurons, as NeuN or HU, together with a birth-dating marker, allow quantification of new neurons. In the adult avian brain HU was found to be expressed by all neurons, but not glia. HU is also expressed by neuronal daughter cells still within the SZ, and therefore can be used as a very early indicator of neuronal differentiation (Barami et al. 1995). With such markers, multiple immunofluorescence labeling has become the preferred method for discrimination of labels due to its ability to separately excite fluorophores and discriminate their emission based on spectral properties (Fig. 6).
Figure 6.
Z-stack images from the HC of European reed warbler (Acrocephalus scirpaceus), under a confocal microscope. Neurons represent only green cytoplasm (labeled by the endogenous marker HU; e.g. cell #1) while new neurons also represent red nuclei (labeled by the birth-date exogenous marker BrdU; cell # 2), these two markers have to co-localize within the same cell along several Z positions. Images were collected at 1.0 μm interval. (A) red 543 nm wavelength frame; (B) green 488nm wavelength frame; (C) combined red and green wavelengths. (Photographed by Shay Barkan).
COMPARISON WITH OTHER GROUPS AND EVOLUTIONARY ASPECTS
In the last few decades, adult neurogenesis has become one of the most research-intensive fields in the neurosciences and several reviews (e.g. Cayre et al 2002; Garcia-Verdugo et al. 2002; Lindsey & Tropepe 2006; Gould 2007) give a comparative picture of this phenomenon in various taxonomic groups. However, despite much progress, we still know very little about the anatomical organization, species diversity, functional significance and evolutionary history of this trait. The basic cell and molecular biology of adult neurogenesis is of paramount importance, but without considering how the natural environment regulates neurogenesis and how this trait has evolved, our understanding remains incomplete. The current knowledge rests on studies of only several dozen species, of which only a few have undergone detailed anatomical mapping for the presence of this trait (Lindsey & Tropepe 2006). Considering that the animal kingdom consists of ~ 1.5 million species, this represents a very tiny sampling of the potential diversity of adult neurogenesis.
Comparison with invertebrates
Invertebrates received little attention with respect to adult neurogenesis and all available knowledge stems primarily from insects and crustaceans. In several insects, neurogenesis was found in the mushroom bodies, the dominant integrative centre for multimodal inputs, and which might also have a role in learning and memory (Strausfeld et al. 1998). Hence, neurogenesis in insects might play a similar adaptive role as in birds and mammals. Another possible parallel between invertebrates and birds and mammals can be suggested from evidence that in several crustacean species, adult neurogenesis occurs in the central olfactory pathway (reviewed in Lindsey & Tropepe 2006; Schmidt & Harzsch 1999). This might indicate a similar role as to that in mammals, in which new neuronal recruitment to the OB is pronounced, and also in birds, in which neuronal recruitment in the OB was reported, although to a lesser degree than in other brain regions (Barkan et al., 2007).
Comparison with other vertebrates
Adult neurogenesis has been identified in all vertebrate species that have been examined so far, and the proliferative birthplace of new neurons in many other vertebrates resides, as in birds, in the subventricular zone. However, despite sharing this common feature, numerous differences in neurogenic compartments between vertebrate classes have been reported distal to this region. Proliferation and neuronal differentiation in vertebrates other than birds and mammals are more pronounced, and neurogenesis has been shown to occur in many more regions than in the mammalian brain. Interestingly, however, the OB and areas thought to be equivalent to the mammalian DG are still among the most neurogenic regions. The greatest number of proliferation zones is found in the teleost fishes (Reviewed by Zupanc 2008) and evidence suggests that the rate of cell proliferation is also much higher in the adult fish brain than in the adult mammalian brain. For example, in the brown ghost knifefish (Sternachella schotti), approximately 10,000 cells are born within 2-hour period, corresponding to approximately 0.2% of the total population (Zupanc and Horschke 1995). In contrast, in the DG of adult rats, 9,000 cells are formed daily (Cameron and McKay 2001), corresponding to 0.003% of the estimated 330 million cells in the whole brain of adult rats (Herculano and Lent 2005). As in birds and mammals, proliferation activity in fishes appears to be decreasing with age (Kranz & Richter 1975) and apoptosis occurs concomitantly to neurogenesis and thus could regulate the rate of birth of newly formed cells (Soutschek & Zupanc 1996).
Similar to mammals and to a lesser extend to birds, adult neurogenesis in the olfactory epithelium has been observed in several amphibians (reviewed in Lindsey & Tropepe 2006). Reptiles, as birds, contain only one neurogenic region during adulthood, which is localized in the walls of the ventricular zone, an area that is believed to be homologous to the dentate gyrus in the mammalian HC, and that has been compared with the ‘hot spots’ in the avian VZ (reviewed in Lindsey & Tropepe 2006).
In addition to songbirds, which have played a pivotal role in the study of adult neurogenesis, rodents provided another traditional model and more recently, mammalian research has been extended to New and Old World primates and postmortem humans. In contrast to the avian brain, in which the VZ is the only neurogenic area, in the mammalian brain new neurons are produced in both the subventricular zone (SVZ; also known as periventricular zone or subependymal zone) lining the lateral ventricle, and the subgranular zone of the DG of the HC (reviewed in Taupin & Gage 2002). There have been extensive analyses of these two neurogenic compartments (e.g. Ming & Song 2005; Alvarez-Buylla & Lim 2004; Doetsch & Hen 2005; Lindsey & Tropepe 2006). Unlike birds, where cells generated in the VZ migrate to most of telencephalic areas, in mammals, the neurons born in the SVZ migrate almost exclusively in the olfactory bulb, where they differentiate into interneurons (Corotto et al 1993). In the HC the proliferative cells give rise to new granular cells and glia cells, and therefore in this case the migration of new neurons is reduced. There is evidence for adult neurogenesis in several additional areas, including the neocortex, amygdale and substantia nigra, but this evidence has been difficult to replicate consistently (reviewed in Gould 2007). As in birds, mammalian neuronal replacement is highly dynamic, can be modified by many internal and external factors (e.g. reviewed in Ming & Song 2005), and may be central for mediating behavioral tasks that are based on learning and memory (Doetsch & Hen 2005).
Neuronal turnover
Another interesting comparative feature of adult neurogenesis is neuronal turnover, which is the death of mature neurons in a local circuit in order to compensate for the insertion of newly recruited neurons. It is worth noting that in all vertebrate models (fish, birds, mammals), neurogenesis and apoptosis occur simultaneously and appear to be tightly linked (reviewed in Cayre et al. 2002). The integral role of apoptosis in adult neurogenesis was also found in lobsters, where the birth and death of neurons in the olfactory pathway appear to be coupled together (Harzsch et al. 1999). However, many questions still remain with respect to the relationship of neuronal turnover and the regulation of circuit function and, ultimately, behavior. For example, there is evidence that maturation periods of newly generated neurons differ between species (Ngwenya et al. 2006). How do such differences compare to the rate of hippocampal-dependent learning and memory acquisition between species? Answers to such questions, from various taxonomic groups and species, will enable a better understanding for the role of neurogenesis in circuit plasticity and behavior.
Regulation by internal and external factors
As in birds, neurogenesis and new neuronal survival appear to be regulated by both internal and environmental cues also in other groups. In invertebrates, mushroom body neurogenesis has been found to be regulated by hormones (reviewed in Cayre et al. 2002). In non-mammalian vertebrates, the neuropeptide somatostatin seems to be an important regulator for neurogenesis in adult brain (Zupanc 1999 a & b). In the mammalian brain, Gould and colleagues have shown that adrenal hormones inhibit both neurogenesis and apoptosis and also regulate migration of newly produced neurons (reviewed in Cayre et al. 2002). Such regulation implies physiological consequences: for example, stress, which increases glucocorticoids levels, inhibits proliferation of granule cells precursors (Gould et al. 1997; 1998). However, unfortunately so far, in birds not much is known about how stress may affect adult neurogenesis and neuronal recruitment, and future studies are needed. External environmental conditions also play a role in the regulation of adult neurogenesis. Like in birds, seasonal variation in neurogenesis and neuronal recruitment also occurs in other groups, such as amphibians (Chetverukhin & Polenov 1993), reptiles (Ramirez et al. 1997) and mammals (Huang et al. 1998). Sensory inputs also influence adult neurogenesis in many other species apart from birds. For instance, enriched environment increases neuron proliferation rates in crickets (Scotto-Lomassese et al. 2000) and in crayfish (Sandeman & Sandeman 2000). Similar observations were reported in mammals and evidence suggests that growth factors may mediate the effect of environment on neurogenesis (reviewed in Cayre et al. 2002).
Neurogenesis as a general phenomenon
Overall, it appears that similar processes are underlying neurogenesis in the adult brain of invertebrates and vertebrates. In both groups this phenomenon occurs in important brain structures that display remarkable analogies, as they receive multiple sensory information and play a central role in learning and memory processes. Hence, adult neurogenesis is of fundamental biological importance and unraveling the origin of this trait and the selection pressures that have given rise to its presence or absence is a great challenge. An initial step to addressing the phylogenetics of adult neurogenesis should start by making structural and functional homologies between simple and more complex animals. Among vertebrates, adult neurogenesis seems to be an evolutionary conserved trait and comparisons across vertebrate taxa suggest that it is likely to have risen in a common ancestor (Zupanc 2001). However, more derived species (i.e. birds, mammals) have undergone reduction in this trait. Moreover, reptiles and birds, which are phylogenetically closely related, share a single neurogenic region in the lateral ventricular zone (Fig. 2B in Lindsey & Tropepe 2006). Thus, cladistic analysis of such trends would be important for understanding this phenomenon.
The precise driving forces and selection pressures contributing to preserve adult neurogenesis will be revealed once we understand the relation between the plasticity of this trait and its function. It has been suggested that adult neurogenesis is a remnant of embryonic development, rather than an adaptation to specific selective pressures in adult life. However, since there are major differences between embryonic and adult neurogenesis, regulatory mechanisms may have been partly co-opted for a specifically adapted adult function. As Lindsely & Tropepe (2006) advocated, a comprehensive analyses of adult neurogenesis in various vertebrate and invertebrate species will lead to a more complete understanding of its origin.
PERSPECTIVES, FUTURE DIRECTIONS AND POSSIBLE IMPLICATIONS
The formation of the nervous system has been widely studied during development in species and models from different evolutionary origins. However, it is now clear that the central nervous system plasticity does not cease at the end of development. Thus, the dogma of the neural fixity in the brain of adult animals is no more a question of the day, especially since neurogenesis has been demonstrated in the brain of various adult invertebrate and vertebrate species. In addition, the discovery of neurogenesis in human brains (Eriksson et al. 1998) aroused additional interest of the neurobiologists. Still, despite much progress, neurogenesis, new neuronal turnover, and their functional significance and evolutionary aspects, are not yet fully understood. In this section we highlight several issues that might be important when considering future directions and studies.
Comparative investigations
As mentioned before, we still know little about the species diversity of adult neurogenesis and our current knowledge rests on studies of only several dozen species from various phyla and classes. Continuing to rely solely upon the current model organisms will prevent our ability to delineate the full understanding of adult neurogenesis, including its regulation and functions. Therefore, comparative investigations should be extended to more species in different taxonomic groups in order to underpin the diversity and plasticity of adult neurogenesis. Such an interclass investigation might yield insights into questions such as why phylogenetically older vertebrates (i.e. fish) display more neurogenic compartments than modern vertebrates (i.e. mammals). Examples of this approach in insects, crustaceans and reptiles are reviewed by Lindesy & Tropepe (2006).
Extending the comparative investigation is worthwhile also within classes or related groups of species, especially when species-specific variation is observed. In birds, the first and classical model to study adult neurogenesis was the relation between singing behavior and the song control system, which is undoubtedly a powerful model because of several reasons. For example, song learning has parallels to speech acquisition: like humans, songbirds must hear the sounds of adults during a sensitive period, and must hear their own voice while learning to vocalize. The capacity of such hearing-dependent vocal learning is not widespread, and apart from humans, no primates have been shown to learn their complex vocalizations. Among the rest of the mammals, only whales and dolphins and some bats show evidence of vocal learning (Brainard & Doupe, 2002). Another reason that makes the song system a powerful model is that it includes discrete brain structures that are required for singing, which is a well-defined and quantifiable behavior. And indeed, this model, which has been tested in several avian species, provided important insights into basic issues in neuroscience, such as perceptual and sensomotor learning, regulation of plasticity, and the control and function of neurogenesis. However, in addition to the song control system, it is important to study the relationships between the brain and behavior, as well as the internal and external factors that influence these inter relationships in other avian models. Such relatively new models (e.g. food-hoarding, breeding, migration, sociality) are being used in recent years, and have already provided interesting insights, as have been discussed in various parts of this review. A broader and deeper understanding of adult neurogenesis including its regulation and functions can be achieved if these models, as well as additional ones, will be further studied in a variety of species that differ in their biology and natural history.
Biological relevance and context
Most studies of adult neurogenesis focus on laboratory experiments in which animals are kept in ‘standard housing’ and which affords little opportunity for sensory, cognitive or motor stimulation. However, a growing body of evidence, which comes from various systematic groups, suggests that enriched environments have significant effects on mechanisms of experience-dependent plasticity, including adult neurogenesis (e.g. reviewed by Gould & Gross 2002; Lindsey & Tropepe 2006; Nithianantharajah & Hannan 2006). These effects raise the question of how representative are laboratory studies when contrasted with those conducted in the species natural environments. No doubt, laboratory experiments are important for our understanding of the basic mechanisms that underlie the complex phenomenon of adult neurogenesis. Nevertheless, it is critical that we approach the results of laboratory-bred or captive animals with caution and not take for granted that the same finding will apply to animals in natural environments. To minimize the problem, we should at the very least try and provide the animals with enriched (physical or social) conditions, to ensure that the question asked is being tested, as much as possible, in the right biological context.
Another direction at which we should aim is to take the neurobiological studies into the field and thereby avoid problems that are being caused by the simplified laboratory setup. For example, it has been shown that some attributes, such as brain volume (e.g. Smulders et al. 2000), hippocampal volume (e.g. LaDage et al., 2009; Tarr et al. 2009), or new neuronal recruitment (Barnea & Nottebohm 1996; LaDage et al. 2009), are affected by captivity. Hence, the importance of studying natural populations is obvious and has already been emphasized before in relation to various systematic groups (Amrein et al 2008; Barnea 2009; 2010; Boonstra et al 2001; Lindsey & Tropepe 2006). Despite the growing awareness to the importance of conducting research in appropriate behavioral and ecological contexts, for obvious reasons, many of the studies are still carried out under laboratory settings. It is crucial to understand that even the best enriched laboratory environment is infinitely poorer than the natural environment for most vertebrate species. It is quite likely that what we see in all laboratory studies is significantly reduced neurogenesis that never reaches the normal natural levels and so the conclusions that environmental enrichment enhances neurogenesis may need to be reconsidered and reformulated to reflect the more likely phenomenon that impoverished environment impairs neurogenesis. It would be important to investigate whether enriching already natural environment has any effect on neurogenesis. Finally, studying neurogenesis in wild animals may provide a better model to better understand all variables that may indeed regulate neurogenesis in natural conditions. Therefore, promoting studies on natural populations should be a major aim in the study of brain plasticity, and should be regarded as an important complement to laboratory experiments. To prove successful, this requires an integrative approach that would necessitate the collaboration between ethologists, evolutionists, anatomists, physiologists, and neurobiologists.
Therapeutic implications
The discovery that new neurons are added to several regions in the adult brain, and the characterization of adult neural stem cells in mammals has generated considerable interest in the scientific community with the goal of developing new cell-based regenerative treatments for neurodegenerative diseases, spinal cord injury, and acute damage due to stroke. Indeed, the number of new neurons and its modulation by a variety of factors indicated that adult neurogenesis is a significant phenomenon, and it has been offered as a possible key to understanding phenomena such as depression, Alzheimer’s disease and schizophrenia. The growing interest in this direction is reflected in several recent reviews. For example, in the book “Adult Neurogenesis” (edited by Gage, Song and Kempermann), a whole section is dedicated to the possible relations between adult neurogenesis and disease, with chapters on hippocampal neurogenesis and depression and anxiety (Sahay et al. 2008), evidence of an involvement of neurogenesis in neurodegenerative diseases (Brundin et al. 2008), neurogenesis and epilepsy (Jessberger and Parent 2008), and effects of stoke on adult neurogenesis (Lindvall and Kokaia 2008).
The growing understanding of the complexity of song and its importance to birds, along with the growing acceptance of the claimed feats of memory and reasoning exhibited by birds, led to a reexamination of the structure and function of the avian brain (Jarvis et al., 2005). The consortium reviewed studies indicating that the avian hyperstiratum, neostriatum, and archistriatum might be homologous to mammalian pallial regions, and presents two working hypotheses about homologies between avian and mammalian pallial subdivisions. The nuclear-to-layered hypothesis suggests that the lateral, ventral and dorsal pallial subdivisions of birds are homologous to the mammalian neocortex. The nuclear-to-claustrum/amygdale hypothesis states that the avian dorsal pallium is homologous to the mammalian neocortex and that the avian lateral-ventral pallium is homologous to the mammalian amygdala and claustrum. These hypotheses are not resolved yet and the consortium concluded that the evidence is still not strong enough for any specific proposed homologies to be incorporated into a new pallial terminology. Therefore, instead of the three traditional striatal subdivisions (hyperstriatum, neostriatum and archistriatum) they renamed four main subdivisions in the avian pallium (hyperpallium, mesopallium, nidopallium, acropallium). Similarly in mammals, in the absence of a universally accepted alternative to neocortex (or isocortex), the consortium proposed the term ‘six-layered cortex’. They further stated that since quite sophisticated cognitive functions are carried out by the six-layered cortex in mammals and by the nuclear pallial areas in birds, the former is not the only neuroarchitectural solution for the generation of complex cognitive behaviors.
These conclusions are consistent with recent paleontological findings which indicate that the paths to mammals and birds digressed millions of years ago when ancestral amphibians gave rise to stem amniotes and subsequently gave rise to parallel paths of evolution: one leading to reptiles and birds and another leading to mammals. These findings are combined with another relatively recent paleontological discovery that birds evolved from reptiles 50–100 million years later than the development of mammals from their precursors (Carroll, 1988; Evans, 2000; reviewed by Gordon 2010; Jarvis et al., 2005). Thus, the organization of pallial domains in the avian brain is a relatively modern development, as opposed to the conventional view, which regarded the avian brain as more primitive than mammalian brain, and therefore discoveries with respect to avian brain are no longer considered irrelevant to questions of human neurophysiology and medicine. Since, for reasons explained above, birdsong is a powerful model to study adult neurogenesis, understanding brain function in birds might provide insights for advancing methods of brain repair. For example, the observation that birds that incorporate new neurons nonetheless have an unchanging adult song is of interest. Even the disruption of adult zebra finch song that is triggered by ablation of RA-projecting neurons is followed by gradual recovery of song to its pre-ablation state (Scharff et al. 2000). Songbirds thus provide an example of a system where learned capacities and memories persist despite neural turnover. The mechanisms underlining such reliance to cell loss and replacement may be particularly relevant and there are strong hopes that future studies will lead to cures for human brain damage and disease.
Obviously, the capability to change the neuronal population in conjunction with the ability to alter the connections between neurons provides the brain with the potential to modify itself – to – learn at timescales ranging from milliseconds to minutes to weeks. Undoubtedly, our understanding of the added possibilities is just beginning, and the full story and potential behind this process are not yet realized. However, in relation to the possibility of using our growing knowledge about neuronal replacement for medical implications, we should end with a word of caution. Several investigators (e.g. Gould & Gross 2002) have already indicated that although the desire that further study on adult neurogenesis would advance methods for brain repair is strong, one has to be careful in hoping that it will provide answers to a wide array of previously intractable problems.
Acknowledgments
We are very much aware of the possibility that we missed important contributions in some sections, while in others we purposely limited the discussion by highlighting only representative examples due to space limitations. We apologize to colleagues for these omissions. We are grateful to two anonymous reviewers whose comments contributed much and improved this review. Thanks are also due to Rachel Aharon-Shriki for her professional help in some of the figures, and to our colleagues who gave their permission to use figures from their publications. Vladimir Pravosudov was supported by grants from NSF (IOB-0615021) and NIH (MH076797). Anat Barnea was supported by the Israel Science Foundation, The National Institute for Psychobiology in Israel, and The Open University Research Fund.
ABBREVIATIONS
- DG
dentate gyrus
- HC
hippocampus
- OB
olfactory bulb
- VZ
ventricular zone
- RA
robust nucleus of the archistriatum
- LPO
lobus parolfactorius
- NC
nidopallium caudale
- VIP
vasoactive intestinal peptide
- HA
hyperstriatum accessorium
- NCM
caudomedial nidopallium
- BrdU
Bromodeoxyuridine
References
- Adar E, Nottebohm F, Barnea A. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. J Neurosci. 2008a;28:5394–5400. doi: 10.1523/JNEUROSCI.5706-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar E, Lotem A, Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav Brain Res. 2008b;187:178–184. doi: 10.1016/j.bbr.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Agate RJ, Scott BB, Haripal B, Lois C, Nottebohm F. Transgenic songbirds offer an opportunity to develop a genetic model for vocal learning. Proc Natl Acad Sci USA. 2009;106:17963–17967. doi: 10.1073/pnas.0909139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone J, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Borda B. Thesis dissertation. New York: The Rockefeller University; 2002. On new neurons in canary brains. [Google Scholar]
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc Natl Acad Sci USA. 2004;101:3957–3961. doi: 10.1073/pnas.0308118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264:159–170. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in the adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Mapping of radial glia and of a new cell type in adult canary brain. J Neurosci. 1988;8:2707–2712. doi: 10.1523/JNEUROSCI.08-08-02707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling CY, Nottebohm F. High vocal center growth and its relation to neurogenesis, neuronal replacement and song acquisition in juvenile canaries. J Neurobiol. 1992;23(4):396–406. doi: 10.1002/neu.480230406. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling CY, Yu WS. Contribution of neurons born during embryonic juvenile and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song control nuclei. J Comp Neurol. 1994;347:233–248. doi: 10.1002/cne.903470207. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Gracia-Verdugo JM, Mateo AS, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Barbanti I, Cuppini R. persistently high corticosterone levels but not normal circadian fluctuations of hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology. 2002;76:366–372. doi: 10.1159/000067581. [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Poletaeva, Bologova NV, Lipp HP. marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus. 2004;14(8):1000–1010. doi: 10.1002/hipo.20018. [DOI] [PubMed] [Google Scholar]
- Amrein I, Dechmann DKN, Winter Y, Lipp H-P. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLOS One. 2007;1:e455: 1–8. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Lipp HP, Boonstra R, Wojtowicz JM. Adult hippocampal neurogenesis in natural populations of mammals. In: Gage FH, Kempermann G, Song H, editors. Adult neurogenesis. Vol. 30. Cold Spring Harbor Laboratory Press; 2008. pp. 645–659. [Google Scholar]
- Amrein I, Lipp HP. Adult hippocampal neurogenesis of mammals: evolution and life history. Biol Letters. 2009;5:141–144. doi: 10.1098/rsbl.2008.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB., Jr Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl. 1965;2:1–70. [PubMed] [Google Scholar]
- Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Wild JM. Limbic system in birds: morphological basis. In: Watanabe S, Hofman MA, editors. Integration of Comparative Neuroanatomy and Cognition. Keio University Press; Tokyo: 2007. pp. 97–123. [Google Scholar]
- Ball GF, Riter LV, Macdougall-Shackleton SA, Balthazart J. Sex differences in brain and behavior and the neuroendocrine control of the motivation to sing. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 26. Cambridge University Press; 2008. pp. 240–255. [Google Scholar]
- Balthazart J, Boseret G, Konkle ATM, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci. 2008;27:801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- Barkan S, Ayali A, Nottebohm F, Barnea A. Neuronal recruitment in adult zebra finch brain during a reproductive cycle. Dev Neurobiol. 2007;67:687–701. doi: 10.1002/dneu.20379. [DOI] [PubMed] [Google Scholar]
- Barnea A. Interactions between environmental changes and brain plasticity in birds. Gen Comp Endocrinol. 2009;163(1/2):128–134. doi: 10.1016/j.ygcen.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Barnea A. Wild neurogenesis. Brain Behav Evol. 2010;75:86–87. doi: 10.1159/000306483. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal recruitment. Proc Natl Acad Sci USA. 1996;93:714–718. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Mishal A, Nottebohm F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: an anatomical representation of time? Behav Brain Res. 2006;167:63–74. doi: 10.1016/j.bbr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Bingman VP, Cheng K. Mechanisms of animal global navigation: comparatrive perspectives and enduring challenges. Ethol Ecol Evol. 2005;17:295–318. [Google Scholar]
- Bingman VP, Hough GE, II, Kahn MC, Siegel JJ. The homing pigeon hippocampus and space: in search of adaptive specialization. Brain Behav Evol. 2003;62:117–127. doi: 10.1159/000072442. [DOI] [PubMed] [Google Scholar]
- Bingman VP, Gagliardo A, Hough GE, II, Ioale P, Kahn MC, Siegel JJ. The avian hippocampus, homing in pigeons and the memory representation of large-scale space. Integr Comp Biol. 2005;45:555–564. doi: 10.1093/icb/45.3.555. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of bird song memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Macphail EM. A critique of the neuroecology of learning and memory. Trend Cogn Sci. 2001;5:426–433. doi: 10.1016/s1364-6613(00)01753-8. [DOI] [PubMed] [Google Scholar]
- Boseret G, Ball GF, Balthazart J. The microtubule-associate protein doublecortin is broadly expressed in the telencephalon of adult canaries. J Chem Neuoranat. 2007;33:140–154. doi: 10.1016/j.jchemneu.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS. The anterior forebrain pathway and vocal plasticity. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 20. Cambridge University Press; 2008. pp. 240–255. [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Brenowitz E. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the song control system in adult birds. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 27. Cambridge University Press; 2008. pp. 332–349. [Google Scholar]
- Brenowitz EA, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci USA. 2002;99:12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brown SD, Johnson F, Bottjer SW. Neurogenesis in adult canary telencephalon is independent of gonadal hormone levels. Neurosci. 1993;13(5):2024–2032. doi: 10.1523/JNEUROSCI.13-05-02024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Winkler J, Masliah E. Adult neurogenesis in neurodegenerative diseases. In: Gage FH, Kempermann G, Song H, editors. Adult neurogensis. Vol. 24. Cold Spring Harbor Laboratory Press; 2008. pp. 503–534. [Google Scholar]
- Burd GD, Nottebohm F. Ultrastructural characterization of synaptic terminals formes on newly generated neurons in a song control nucleus of the adult canary forebrain. J Comp Neurol. 1985;240:143–152. doi: 10.1002/cne.902400204. [DOI] [PubMed] [Google Scholar]
- Burns KA, Kuan CY. Low doses of bromo-and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur J Neurosci. 2005;21:803–807. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carroll RL. Vertebrate Paleontology and Evolution. W.H. Freeman; NY: 1988. pp. 1–13. [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Strambi C, Strambi A. The common properties of neurogenesis in the adult brain: from invertebrates to vertebrates. Comp Biochem Physiol B. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Chancellor LV, Roth TC, II, LaDage LD, Pravosudov VV. The effect of environmental harshness on neurogenesis: a large scale comparison. Develop Neurobiol. 2011;71:246–252. doi: 10.1002/dneu.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MF, Peng JP, Chen G, Gardner JP, Bonder EM. Functional restoration of acoustic units and adult-generated neurons after hypothalamic lesions. J Neurobiol. 2004;60:197–213. doi: 10.1002/neu.20014. [DOI] [PubMed] [Google Scholar]
- Clayton DF. Integrating genomes, brain and behavior in the study of songbirds. Curr Biol. 2009;19:R865–R873. doi: 10.1016/j.cub.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Creel S, Creel NM, Monfort SL. Social stress and dominance. Nature. 1996;379:212. [Google Scholar]
- Cristol DA, Reynolds EB, Leclerc JE, Donner AH, Farabaugh CS, Ziegenfus CWC. Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim Behav. 2003;66:317–328. [Google Scholar]
- Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone J, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Age and sex differences in mitotic activity within the zebra finch telencephalon. J Neurosci. 2002;22(10):4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15(1):121–8. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Scharff C. Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav Evol. 2001;58:306–322. doi: 10.1159/000057572. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, McCarthy EA, Jashari D. Electrocommunication signals alone are sufficient to increase neurogenesis in the brain of adult electric fish, Apteronotus leptorhynchus. Develop Neurobiol. 2008;68:1420–1428. doi: 10.1002/dneu.20673. [DOI] [PubMed] [Google Scholar]
- Chetverukhin VK, Polenov AL. Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. I Ventricular zone cell proliferation. Cell Tissue Res. 1993;271(2):341–50. doi: 10.1007/BF00318621. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149(2):273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;11:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Evans SE. In: Evolutionary Developmental Biology of the Verebal Cortex. Bock GR, Cardew G, editors. John Wiley & Sons; Chichester: 2000. pp. 109–113. [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51 (2):115–28. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fox RA, Roth TC, II, LaDage LD, Pravosudov VV. No effect of social group composition of size on hippocampal formation morphology and neurogenesis in mountain chickadees (Poecile gambeli) Develop Neurobiol. 2010;70:538–547. doi: 10.1002/dneu.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Frost BJ, Mouritsen H. The neural mechanisms of long distance animal navigation. Curr Opinion Neurobiol. 2006;16:481–488. doi: 10.1016/j.conb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur J Neurosci. 2000;12(7):2211–4. doi: 10.1046/j.1460-9568.2000.00130.x. [DOI] [PubMed] [Google Scholar]
- Gage F. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M. Delineation of brain nucleus: comparisons of cytochemicals, hodological, and cytoarchitectural views of the song control nucleus HVC of the adult canary. J Comp Neurol. 1990;294:30–36. doi: 10.1002/cne.902940104. [DOI] [PubMed] [Google Scholar]
- Gahr M, Leitner S, Fusani L, Rybak F. What is the adaptive role of neurogenesis in adult birds? In: Hofman MA, Boer GJ, Holtmaat AJGD, van Someren EJW, Verhaagen J, Swaab DF, editors. Progress in Brain Research. Elsevier Science; 2002. pp. 233–254. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:567–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gerstner JA, Bremer QZ, Vander Heyden WM, LaVaute TM, Yin JC, Landry CF. Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PLoS ONE. 2008;3(2):e1631. doi: 10.1371/journal.pone.0001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, lledo PM. A niche for adult neurogenesis in social behavior. Behav Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Galea A. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57(2):332–41. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Goergen EM, Bagay LA, Rehm K, Bentonm JL, Beltz BS. Circadian control of neurogenesis. J Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36:267–286. [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy TL, Butterworth BE, Maronpot RR. Concepts, labeling procedures, and design of cell proliferation studies relating to carcinogenesis. Environ Health Perspect. 1993;101(suppl 5):59–65. doi: 10.1289/ehp.93101s559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JT. The 2006 Benjamin Franklin Medal in Life Science presented to Fernando Nottebohm, Ph. D for demonstration of central neurogenesis in adult avians, with concomitant implications for the theory of memory and for the future of neurological repair in injury and disease. J Franklin Inst. 2010;347:708–718. [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neurosci. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: Some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psych. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17(7):2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95(6):3168–71. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Shettleworth SJ. Hippocampal lesions impair memory for location but not color in passerine birds. Behav Neurosci. 1996;110:831–835. doi: 10.1037//0735-7044.110.4.831. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Henke RC, Seeto GS, Capes-Davis A, Dunn J, Jeffrey PL. Expression of doublecortin correlates with neuronal migration and pattern formation in diverse regions of the developing chick brain. J Neurosci Res. 1999;55:650–657. doi: 10.1002/(SICI)1097-4547(19990301)55:5<650::AID-JNR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Harding CF. Hormonal modulation of singing behavior: methodology and principles of hormone action. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 25. Cambridge University Press; 2008. pp. 240–255. [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. J Neurosci. 1999;19(9):3472–85. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SD, Gwinner E, Krebs JR. Hippocampal volume in migratory and non-migratory warblers: effect of age and experience. Behav Brain Res. 1996;81:61–68. doi: 10.1016/s0166-4328(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Hornfeld SH, Terkel J, Barnea A. Neurons recruited in the nidopallium caudale, following changes in social environment, derive from the same original population. Behav Brain Res. 2010;208:643–645. doi: 10.1016/j.bbr.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Sherry DF. Neuron production, neuron number, and structure are seasonally stable in the hippocampus of the food-storing blackcapped chickadee (Poecile atricapillus) Behav Neurosci. 2004;118:345–355. doi: 10.1037/0735-7044.118.2.345. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Sherry DF. Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Develop Neurobiol. 2007;67:406–414. doi: 10.1002/dneu.20316. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Phillmore LS, MacDougall-Shakleton SA. An examination of avian hippocampal neurogenesis in relation to photoperiod. Mol Neurosci. 2005;16:987–991. doi: 10.1097/00001756-200506210-00021. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Phillmore LS, Sherry DF, MacDougall-Shackeleton SA. Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus. Brain Behav Evol. 2007;69:161–168. doi: 10.1159/000096984. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Phillmore LS, Sherry DF, MacDougall-Shackleton SA. Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus. Brain Behav Evol. 2007;69:161–168. doi: 10.1159/000096984. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Abdallah NMB, Knapsak E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewitcz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Memory. 2010;16:439–451. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Parent JM. Epilepsy and adult neurogenesis. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Vol. 25. Cold Spring Harbor Laboratory Press; 2008. pp. 535–548. [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MC, Hough GE, 2nd, Ten Eyck GR, Bingman VP. Internal connectivity of the homing pigeon (Columba livia) hippocampal formation: an anterograde and retrograde tracer study. J Comp Neurol. 2003;459:127–141. doi: 10.1002/cne.10601. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempremann G, Wiskott L, Gage F. Functional significance of adult neurogenesis. Curr Opinion Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kirn JR. The relationship of neurogenesis and growth of brain regions to song learning. Brain Language. 2010;115:29–44. doi: 10.1016/j.bandl.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Nottebohm F. Direct evidence for loss and replacement of projection neurons in adult canary brain. J Neurosci. 1993;13:1654–1663. doi: 10.1523/JNEUROSCI.13-04-01654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J Neurosci. 1991;11:1756–1762. doi: 10.1523/JNEUROSCI.11-06-01756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, O’Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci USA. 1994;91:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Fishman Y, Sasportas K, Alvarez-Buylla A, Nottebohm F. The fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J Comp Neurol. 1999;411(3):487–494. [PubMed] [Google Scholar]
- Kochman LJ, Webber ET, Fornal CA, Jacobs BL. Circadian variation in mouse hippocampal cell proliferation. Neurosci Lett. 2006;406:256–259. doi: 10.1016/j.neulet.2006.07.058. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pedersen B, Ballermann M, Gibb R, Whishaw IQ. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neurosci. 1999;19:2337–46. doi: 10.1523/JNEUROSCI.19-06-02337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz VD, Richter W. Neurogenesis and regeneration in the brain of teleosts in relation to age (Autoradiographic studies) Z Alternsforsch. 1975;30(4):371–82. [PubMed] [Google Scholar]
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. Hippocampal specialization of food-storing birds. Proc Natl Acad Sci USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage LD, Roth TC, II, Fox RA, Pravosudov VV. Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proc R Soc B. 2010;365:859–867. doi: 10.1098/rspb.2009.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDage LD, Roth TC, Pravosudov VV. Hippocampal neurogenesis is associated with migratory behavior in adult but not juvenile white-crowned sparrows (Zonotrichia leucophrys ssp) . Proc R Soc B. 2011;278:138–143. doi: 10.1098/rspb.2010.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landys MM, Wingfield JC, Ramenofsky M. Plasma corticosterone increases during migratory restlessness in the captive white-crowned sparrow Zonotrichia leucophrys gambelii. Horm Behav. 2004;46:574–581. doi: 10.1016/j.yhbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Lee DA, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferativ regions of the adult zebra finch brain. Dev Neurobiol. 2007;67(8):1107–1117. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relation between behavior, neurotrophin expression and new neuron survival. Proc Natl Acad Sci. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Progr Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Neurogenesis following stroke affecting the adult brain. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Vol. 26. Cold Spring Harbor Laboratory Press; 2008. pp. 549–570. [Google Scholar]
- Ling C, Zuo M, Alvarez-Buylla A, Cheng MF. Neurogenesis in juvenile and adult ring doves. J Comp Neurol. 1997;379(2):300–12. [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult song brain. Neuron. 2002;34 (6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183(2):600–9. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Macklis JD. Identification of newborn cells by BrdU labeling and immunocytochemistry in vivo. Methods Mol Biol. 2008;438:335–43. doi: 10.1007/978-1-59745-133-8_25. [DOI] [PubMed] [Google Scholar]
- Maney DL, Schoech SJ, Sharp PJ, Wingfield JC. Effecs of vasoactive intestinal peptide on plasma prolactin in passerines. Gen Comp Endocrinol. 1999;113:323–330. doi: 10.1006/gcen.1998.7220. [DOI] [PubMed] [Google Scholar]
- Mello C, Jarvis ED. behavior-dependent expression of inducible genes in vocal learning birds. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 31. Cambridge University Press; 2008. pp. 398–406. [Google Scholar]
- Mettke-Hofmann C, Gwinner E. Long-term memory for a life on the move. Proc Natl Acad Sci USA. 2003;100:5863–5866. doi: 10.1073/pnas.1037505100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hipppcampus. 2006;16(3):233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Peters A, Rosene DL. Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. J Comp Neurol. 2006;498(2):204–16. doi: 10.1002/cne.21045. [DOI] [PubMed] [Google Scholar]
- Newman AEM, MacDougall-Shackleton SA, An YS, Kriengwatana B, Soma KK. Corticosterone and dehydroepiandrosterone have opposing effects on adult neuroplasticity in the avian song control system. J Comp Neurol. 2010;518:3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- Nilsson ALK, Sandell MI. Stress hormone dynamics: an adaptation to migration? Biol Lett. 2009;5:4809–483. doi: 10.1098/rsbl.2009.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;9:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adulthood. Hope for a New Neurology. In: Nottebohm F, editor. Ann NY Acad Sci. Vol. 457. 1985. pp. 143–161. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J Neurosci. 2002;22:624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F. The road we traveled – discovery, choreography, and significance of brain replaceable neurons. Ann NY Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The discovery of replaceable neurons. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 36. Cambridge University Press; UK: 2008. pp. 405–448. [Google Scholar]
- Nottebohm F, Stokes TM, Leonrad CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC. Seasonal changes in gonadal hormone levels of adult male canaries and their relation to song. Behav Neural Biol. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Alvarez-Buylla A. Neurogenesis and neuronal replacement in adult birds. In: Cuello AC, editor. Neuronal Cell Death and Repair. Amsterdam: Elsevier Science Publishers; 1993. pp. 227–236. [Google Scholar]
- Nottebohm F, O’Loughlin B, Gould K, Yohay K, Alvarez-Buylla A. The life span of new neurons in a song control nucleus of the adult canary brain depends on time of the year when these cells are born. Proc Natl Acad Sci USA. 1994;91:7849–7853. doi: 10.1073/pnas.91.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Liu WC. The origins of vocal learning: new sounds, new circuits, new cells. Brain Language. 2010;115:3–17. doi: 10.1016/j.bandl.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Patel SN, Clayton NS, Krebs JR. Spatial learning induces neurogenesis in the avian brain. Behav Brain Res. 1997;89:115–128. doi: 10.1016/s0166-4328(97)00051-x. [DOI] [PubMed] [Google Scholar]
- Paton JA, Nottebohm F. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Pham K, McEwen BS, Ledoux JE, Nader K. Fear learning transiently impairs hippocampal cell proliferation. Neurosci. 2005;130(1):17–24. doi: 10.1016/j.neuroscience.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniak C, Pleasure SJ. Genetic control of hippocampal neurogenesis. Genome Biol. 2006;7:207. doi: 10.1186/gb-2006-7-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV. On seasonality in food storing behavior in parids: do we know the whole story? Anim Behav. 2006;71:1455–1460. [Google Scholar]
- Pravosudov VV, Calyton NS. A test of the adaptive specialization hypothesis: population differences in caching, memory and the hippocampus in black-capped chickadees (Poecile atricapilla) Behav Neurosc. 2002;116:515–522. [PubMed] [Google Scholar]
- Pravosudov VV, Omanska A. Dominance-related changes in spatial memory are associated with changes in hippocampal cell proliferation rates in mountain chickadees. J Neurobiol. 2005;62:31–41. doi: 10.1002/neu.20065. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Smulders TV. Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Phil Trans R Soc B. 2010;365:859–867. doi: 10.1098/rstb.2009.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV, Kitaysky AS, Omanska A. The relationship between migratory behavior, memory and the hippocampus – an intraspecific comparison. Proc R Soc B. 2006;273:2641–2649. doi: 10.1098/rspb.2006.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytte CL, Gerson M, Miller J, Kirn JR. Increased stereotypy in adult zebra finch song correlated with a declining rate of adult neurogenesis. Develo Neurobiol. 2007;67:1699–1720. doi: 10.1002/dneu.20520. [DOI] [PubMed] [Google Scholar]
- Pytte CL, Wibrecht L, Kirn JR. Regulation and function of neuronal replacement in the avian song system. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Vol. 28. Cambridge University Press; 2008. pp. 350–366. [Google Scholar]
- Pytte CL, Parent C, Wildstein S, Varghese C, Oberlander S. Deafening decreases neuronal incorporation in the zebra finch caudomedial nidopallium (NCM) Beahv Brain Res. 2010;211:141–147. doi: 10.1016/j.bbr.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Kinetics of proliferation and latency between final cell division and onset of differentiation of cerebellar stellate and basket neurons. J Comp Neurol. 1973;147:523–546. doi: 10.1002/cne.901470407. [DOI] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Rakic P. neurogenesis in adult primates. Prog Brain Res. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- Ramirez C, Nacher J, Molowny A, Sanchez-Sanchez F, Irurzun A, Lopez-Garcia C. Photoperiod-temperature and neuroblast proliferation-migration in the adult lizard cortex. Neuroreport. 1997;8(9–10):2337–2342. doi: 10.1097/00001756-199707070-00047. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc Natl Acad Sci USA. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Martinez-Gonzalez D, Roth TC, II, Pravosudov VV. Hippocampal memory consolidation during sleep: a comparison of mammals and birds. Biol Rev. 2011 doi: 10.1111/j.1469-185X.2010.00165.x. Early Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TC, Pravosudov VV. Hippocampal volume and neuron numbers increase along a gradient of environmental harshness – a large-scale comparison. Proc Royal Soc B. 2009;276:401–405. doi: 10.1098/rspb.2008.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TC, LaDage L, Pravosudov VV. Variation in hippocampal morphology along an environmental gradient: controlling for the effect of day length. Proc Royal Soc B. 2011 doi: 10.1098/rspb.2010.2585. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R, Duman RS. Hippocampal neurogenesis: depression and antidepressant responses. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Vol. 23. Cold Spring Harbor Laboratory Press; 2008. pp. 483–502. [Google Scholar]
- Sandeman R, Sandeman D. “Impoverished” and “enriched” living conditions influence the proliferation and survival of neurons in crayfish brain. J Neurobiol. 2000;45(4):215–226. [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Mackils JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Gage F. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology. 2004;19:253–261. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Strambi A, Charpin P, Augier R, Aouane A, Cayre M. Influence of environmental stimulation on neurogenesis in the adult insect brain. J Neurobiol. 2000;45(3):162–171. [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Aouane A, Strambi A, Cayre A. Sensory inputs stimulate progenitor cell proliferation in an adult insect brain. Curr Biol. 2002;12:1001–1005. doi: 10.1016/s0960-9822(02)00889-8. [DOI] [PubMed] [Google Scholar]
- Sekerkova G, Ilijic E, Mugnaini E. Bromodeoxyuridine administered during neurogenesis of the projection neurons causes cerebellar defects in rat. J Comp Neurol. 2004;470:221–39. doi: 10.1002/cne.11016. [DOI] [PubMed] [Google Scholar]
- Sherry DE, Hoshooley JS. The seasonal hippocampus of food-storing birds. Behav Processes. 2009;80:334–338. doi: 10.1016/j.beproc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Sherry DE, Hoshooley JS. Seasonal plasticity in food-storing birds. Phil Trans R Soc B. 2010;365:933–943. doi: 10.1098/rstb.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DE, Vaccarino AL. Hippocampus and memory for food caches in the black-capped chickadee. Behav Neurosci. 1989;103:308–318. [Google Scholar]
- Shettleworth SJ. Memory and hippocampal specialization in food-storing birds: challenges for research on comparative cognition. Brain Behav Evol. 2003;62:108–116. doi: 10.1159/000072441. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. memory in food storing birds: from the field to the skinner box. In: Alleva E, Fasolo A, Lipp H-P, Nadel L, editors. Behavioral Brain Research in Naturalistic and Semi-naturalistic Settings; Proc. NATO Advanced Study Institute Series Maratea, Italy, Kluwer Academic Publishers; The Hague. 1995. pp. 158–179. [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299(5603):117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Miale IL, Fedr N. Cell proliferation and migration in the primitive ependymal zone: An autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1995;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Harzsch S. Comparative analysis of neurogenesis in the central olfactory pathway of adult decapos crustaceans by in vivo BrdU labeling. Biol Bull. 1999;196:127–136. doi: 10.2307/1542558. [DOI] [PubMed] [Google Scholar]
- Smulders TV, DeVoogd TJ. Expression of immediate early genes in the hippocampal formation of the black-capped chickadee (Poecile atricapillus) during a food-hoarding task. Behav Brain Res. 2000;114:39–49. doi: 10.1016/s0166-4328(00)00189-3. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Sasson AD, DeVoogd TJ. Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. J Neurobiol. 1995;27:15–25. doi: 10.1002/neu.480270103. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Shiflett MW, Sperling AJ, DeVoogd TJ. Seasonal changes in neuron numbers in the hippocampal formation of a food-hoarding bird, the black-capped chickadee. J Neurobiol. 2000;44:414–422. doi: 10.1002/1097-4695(20000915)44:4<414::aid-neu4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Song H, Kempermann G, Wadiche LO, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J Neurosci. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek J, Zupanc GK. Apoptosis in the cerebellum of adult teleost fish, Apteronotus leptorhynchus. Brain Res Dev Brain Res. 1996;97(2):279–86. doi: 10.1016/s0165-3806(96)00145-9. [DOI] [PubMed] [Google Scholar]
- Strausfeld JJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- Szekely T, Catchpole CK, DeVoogd A, Marchl Z, Devoogd TJ. Evolutionary changes in a song control area of the brain (HVC) are associated with evolutionary changes in repertoire size among European warblers (Sylviiday) Proc R Soc B. 1996;263:607–610. [Google Scholar]
- Tarr BA, Rabinowitz JS, Imtiaz MA, DeVoogd TJ. Captivity reduces hippocampal volume but not survival of new cells in a food-storing bird. Develop Neurobiol. 2009;69:972–981. doi: 10.1002/dneu.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69(6):745–9. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci USA. 2007;104:15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Neurogenesis is an adult avian song nucleus is reduced by decreasing caspase-mediated apoptosis. J Neurosci. 2009;29:4586–4591. doi: 10.1523/JNEUROSCI.5423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup K, Bisson IA, Bowlin MS, Holland RA, Wingfield JC, Ramenofsky M, Wikelski M. Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proc Natl Acad Sci USA. 2007;104:18115–18119. doi: 10.1073/pnas.0704734104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol. 1999;40:316–326. doi: 10.1002/(sici)1097-4695(19990905)40:3<316::aid-neu4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. TINS. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. J Neurosci. 2000;20:854–861. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Borght K, Ferrari F, Klauke K, Roman V, Havekes R, Sgoifo A, Van der Zee EA, Meerlo P. Hippocampal cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav Brain Res. 2006;167:36–41. doi: 10.1016/j.bbr.2005.08.012. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromol Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telenchephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wang N, Hurley P, Pytte C, Kirn JR. Vocal control neuron incorporation decreases with age in the adult zebra finch. J Neurosci. 2002;22:10864–10870. doi: 10.1523/JNEUROSCI.22-24-10864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrecht L, Kirn J. Neuron addition and loss in the song system regulation and function. Ann N Y Acad Sci. 2004;1016:659–683. doi: 10.1196/annals.1298.024. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Williams H, Gangadhar N, Nottebohm F. High levels of new neuron addition persist when the sensitive period for song learning is experimentally prolonged. J Neurosci. 2006;26:9135–9141. doi: 10.1523/JNEUROSCI.4869-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Yang HKC, Sundholm-Peters NL, Goings GE, Walker AS, Hyland K, Szele FG. Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neuorsci Res. 2004;76:282–295. doi: 10.1002/jnr.20071. [DOI] [PubMed] [Google Scholar]
- Zann R. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- Zupanc GKH. Neurogenesis, cell death and regeneration in the gymnotiform brain. J Exp Biol. 1999a;202:1435–1446. doi: 10.1242/jeb.202.10.1435. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. Up-regulation of somatostatin after lesions in the cerebellum of teleost fish Apteronotus leptorhynchus. Neurosci Lett. 1999b;268:135–138. doi: 10.1016/s0304-3940(99)00299-2. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. A comparative approach towards the understanding of neurogenesis. Brain Behav Evol. 2001;58:246–249. doi: 10.1159/000057568. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. Adult neurogenesis in Teleost fish. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Vol. 27. Cold Spring Harbor Laboratory Press; 2008. pp. 571–592. [Google Scholar]
- Zupanc GKH, Horschke I. Proliferation zones in the brains of gymnotiform fish: time course, origin, and type of new cells produced. Neurol. 1995;160:78–87. [Google Scholar]