Abstract
The effects of varying ionic liquid pretreatment parameters on various sources of lignocellulosic biomass have been studied using X-ray powder diffraction, X-ray fiber diffraction and compositional analysis. Comparative enzymatic hydrolysis and sugar analysis were used to relate the observed changes in cellulose structure to biomass digestibility. In this study the factor most clearly associated with enhanced biomass hydrolysis is the conversion of cellulose fibers from the cellulose I to the cellulose II crystal phase.
Introduction
Terrestrial lignocellulosic biomass is composed of three major components; cellulose, a linear polymer of the hexose sugar, glucose; hemicellulose, a complex branched polymer of the pentose sugar, xylose, and other sugar derivatives; and lignin, a polyphenyl propanoid macromolecular assembly that is covalently cross-linked to hemicellulose. The cellulosic component has a crystalline and fibrillar structure, which is partially oriented in the thick secondary cell wall, whereas the hemicellulosic and lignin components have less ordered structures that encrust the cellulose fibers. These components can serve as a source of carbon based feedstock for fuel and chemical production in much the same way that crude oil serves as the carbon feedstock in petrochemical refineries 1. In particular, the sugars derived from the cellulosic and hemicellulosic portions of biomass can be converted to platform chemicals and products through fermentation or chemical conversion of hexose and pentose sugars. Lignocellulosic biomass is a non-food based renewable resource that includes agricultural and forestry residues (e.g. wheat straw, corn cobs and stover), municipal waste (paper products), herbaceous (e.g. switchgrass), hardwood (e.g. poplar trees) and softwood (e.g. pine) crops 2. Growth of biomass crops, such as wild grasses in mixed species plots, can provide a haven for wildlife, promoting biodiversity exceeding the monoculture plots needed for grain production 3.
The deconstruction of lignocellulosic biomass into simple sugars constitutes a core barrier for producing value added products from the sugar platform 4. Several approaches are being developed to pretreat lignocellulosic biomass so that this deconstruction can be achieved more efficiently, including dilute acid, steam explosion, hydrothermal processes, organic solvents in aqueous media, biological and enzymatic processes, ammonia fiber 5–7 explosion (AFEX), strong alkali processes, and highly-concentrated acid treatment. The choice of pretreatment technology has a major impact on the physiochemical properties of biomass. These properties in turn profoundly affect downstream processing including enzyme selection for saccharification, ease of microbial or chemical processing, sequencing of conversion and separation steps and by-product and waste formation.
Pretreatment can affect hemicellulose hydrolysis, lignin dissolution or redistribution and increase porosity of constituent polysaccharides (cellulose and hemicellulose) providing access for hydrolytic enzymes. The crystalline structure of native cellulose fibers, called cellulose I (and referring collectively to two naturally occurring allomorphs, Iα and Iβ) 8,9 can be a major impediment to its hydrolysis to monomeric sugars 4. Conversion of cellulose fibers to other crystal forms, such as cellulose II 10,11, cellulose IIIII 12 and cellulose IIII 13, or amorphous forms can greatly improve their susceptibility to hydrolysis14
Ionic liquid (IL) pretreatment is an emerging technology in which biomass is incubated in an IL, followed by IL displacement with anti-solvent, forming what is thought to be a largely amorphous cellulose substrate that is rapidly hydrolyzed into its glucose and xylose subunits 15–17. On drying, amorphous cellulose can partially recrystallize into various allomorphs. The improvement in hydrolysis is hypothesized to be primarily due to structural changes in the cellulose constituent 18. In the studies presented here, X-ray powder diffraction (XRD) and X-ray fiber diffraction on biomass samples with unoriented and partially (fiber) orientated cellulosic components, respectively, is used to investigate the effects of varying IL pretreatment parameters (incubation time, temperature and substrate) on the cellulosic structure. The fiber samples corresponded to intact fragments of naturally occurring biomass which have intrinsic texture, whereas the unoriented samples were ground and sieved to remove any naturally occurring texture. The advantage of collecting fiber diffraction data in addition to XRD data is that the diffraction is more resolved and; therefore, weaker features can be more easily detected. After collection of powder and fiber data, comparative enzymatic hydrolysis and sugar analysis were used to relate the observed changes in cellulose structure to biomass digestibility.
Materials and methods
Materials
The IL 1-ethyl 3-methyl imidazolium acetate (EMIM-Ac) was purchased from Sigma Aldrich, (St. Louis, MO, USA). Three different biomass sources were investigated: poplar, switchgrass and corn stover. Poplar samples were provided by the National Renewable Energy Laboratory (Golden, CO, USA) and corn stover and switchgrass samples were provided by the United States Department of Agriculture (USDA, NCUAR Laboratory, Peoria, IL, USA). Two model cellulose samples were also examined to help with interpretation of the X-ray data collected from the biomass samples; a highly crystalline, oriented, fiber sample of almost pure cellulose prepared from dewaxed ramie fibers, purchased from a textile dealer; and a highly crystalline, unoriented, polycrystalline powder of almost pure cellulose, Avicel, (PH-101, from Fluka chemicals, MO, USA). Commercial enzymes, Spezyme CP and Novozyme 188 were obtained from Genencor, (Rochester, NY, USA) and Sigma Aldrich, (St. Louis, MO, USA), respectively.
Fiber diffraction
Partial (fiber) orientation of cellulose occurs naturally in lignocellulosic biomass. Thin fragments of intact poplar, switchgrass and corn stover biomass suitable for fiber diffraction were incubated in IL at 50°C for 12 to 14 hours; and 120°C for one hour (by immersing a glass vial in a temperature controlled oil bath). After incubation, samples were rinsed with water to displace IL and dried at 50°C. Fiber diffraction data were collected from the pretreated samples at room temperature using an in-house Rigaku FRE with R-Axis IV++ detector. In order to help with interpretation of the X-ray fiber data collected from the biomass samples, fiber diffraction data were collected from the model ramie cellulose fibers in their naturally occurring cellulose I phase, and after mercerization with NaOH in their cellulose II phase. Some of these data were collected at the Advanced Photon Source at Argonne National Laboratory, BioCARS BM-14-C, with an ADSC Q316 Detector. The ramie fibers were mounted tautly around a frame in order to preserve their orientation during mercerization and data collection using previously described methods 10.
X-ray powder diffraction
Poplar, switchgrass, and corn stover biomass samples were ground and sieved (−20/+80 sieve cut) and then incubated with IL at 5 % (w/w) for 12–14 hours at 50°C, and for an hour at 120°C, followed by washing with water to displace IL and drying at 50°C. The purpose of the grinding and sieving process is to remove natural texture and, therefore, partial orientation from the samples. X-ray powder diffraction (XRD) data were collected at 25°C with an X’PERT PRO powder diffractometer PAN 188 analytical with X’celerator detector using Nickel filtered Cu Kα radiation. Samples were scanned over the range of 5–35° (2θ), with a step size of 0.05 and step time of 10 seconds. Crystallinity indices, CrI, were calculated using previously described methods 19.
In order to help with interpretation of the XRD data from the biomass samples, XRD data were collected from the model Avicel cellulose powder in its naturally occurring cellulose I phase, and after mercerization with sodium hydroxide (NaOH) in its cellulose II phase. Avicel was mercerized by incubation in 4.25N NaOH solution at 65°C for 24 hours. The solution was neutralized with hydrochloric acid, and then thoroughly washed with water.
Enzymatic hydrolysis and compositional analysis
Enzymatic hydrolysis followed NREL standard LAP 009 protocol20 with enzyme loadings of 15 FPU/ g glucan of Spezyme CP and 30 CBU/g glucan of Novozyme 188. The hydrolysis was run at 1% (w/v) solid loadings at 50°C in 50mM sodium citrate buffer for 24 hours in a New Brunswick Scientific rotary water bath shaker (Thermo Fischer Scientific, PA, USA) at 200rpm. The compositional analysis for the biomass samples before and after the pretreatment was carried out using NREL standard LAP 002 protocol21. The released sugars were analyzed by HPLC with refractive index detection on a Bio-Rad (Richmond, CA) Aminex HPX-87P carbohydrate analysis column. The mobile phase was HPLC grade water at a flow rate of 0.6 ml/min with a column temperature of 80–85°C. Mixed sugar standards of known concentrations were used to generate standard curves in order to calculate the concentration of released sugars. Glucose and xylose released from glucan and xylan, respectively, were reported as a percentage of theoretical yield of monomeric sugars based on glucan and xylan analysis of untreated substrates.
Results
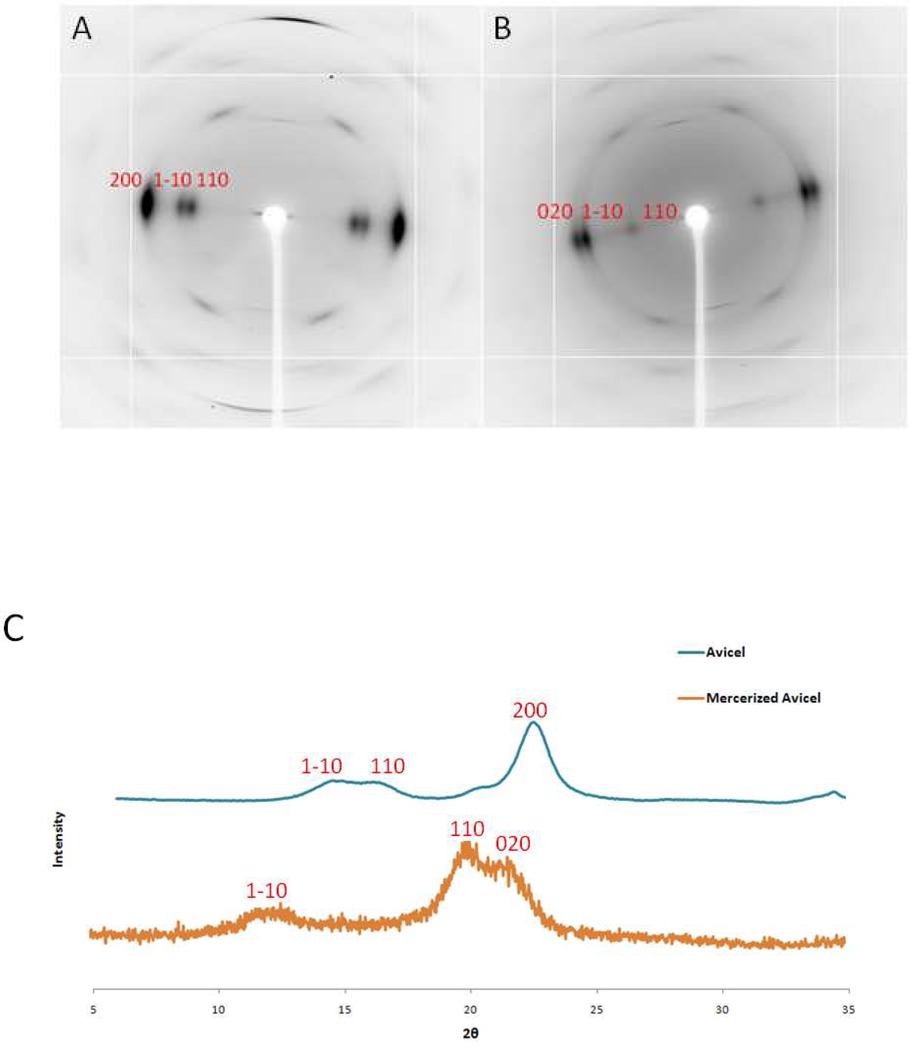
The X-ray fiber diffraction and XRD data collected from the cellulose I and cellulose II phases of the model cellulose fiber (ramie) and powder (Avicel) samples provide a key to interpret the data collected from biomass samples, Figure 1. One of the largest differences between diffraction from the two phases can be seen on the equators (Miller index l = 0) of the fiber diffraction images. In diffraction from cellulose I, there is a doublet of reflections close to the beam center and a single dominant reflection at higher angle. This pattern is reversed in cellulose II, consisting of a single reflection on the equator closer to the beam center and a doublet at higher resolution. This arrangement can also be seen in the XRD scans from the two phases, although the equatorial diffraction is complicated by the superposition of diffraction features from higher layer-lines. Representative Miller indices for these reflections ((1–10), (110) and (200) for cellulose I and (1–10), (110) and (020) for cellulose II) are labeled in Figure 1, although there are overlapping contributions from others. The arrangement of these equatorial reflections serves to identify the presence of the two phases in both powder and fiber diffraction data.
Figure 1.
Panels A and B are X-ray fiber diffraction images of ramie (cellulose I) and mercerized ramie (cellulose II) controls. Panel C is X-ray powder diffraction of Avicel (cellulose I) and mercerized Avicel (cellulose II) controls. The reflections have been labeled with their Miller indices to highlight distinct differences between cellulose I and cellulose II.
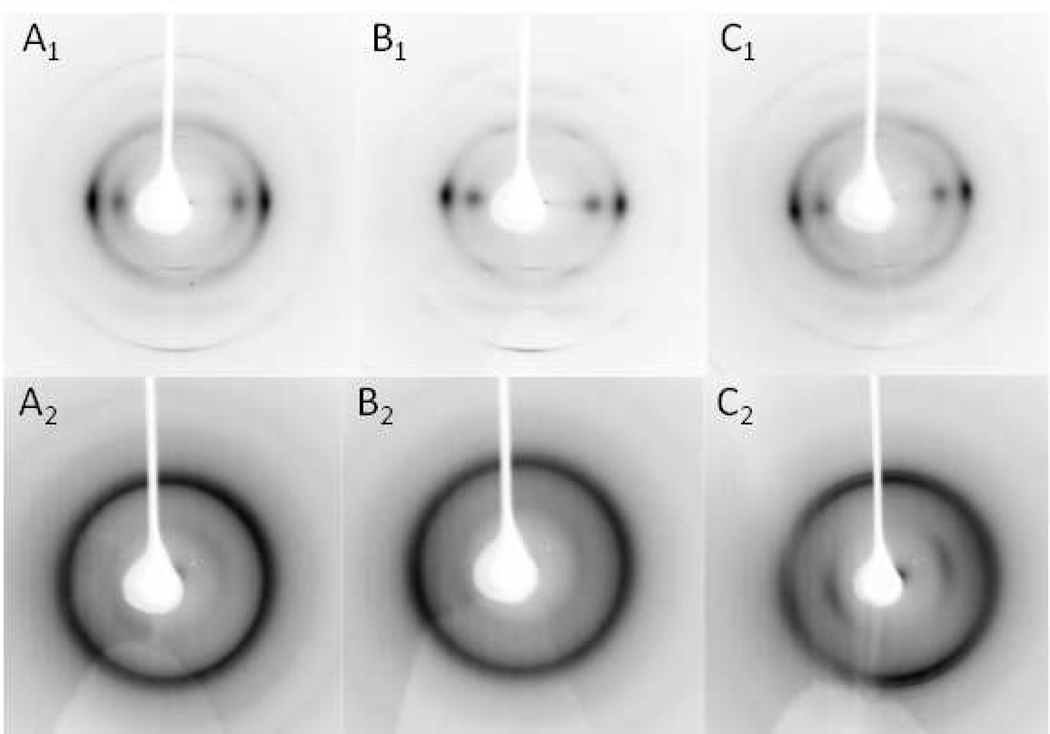
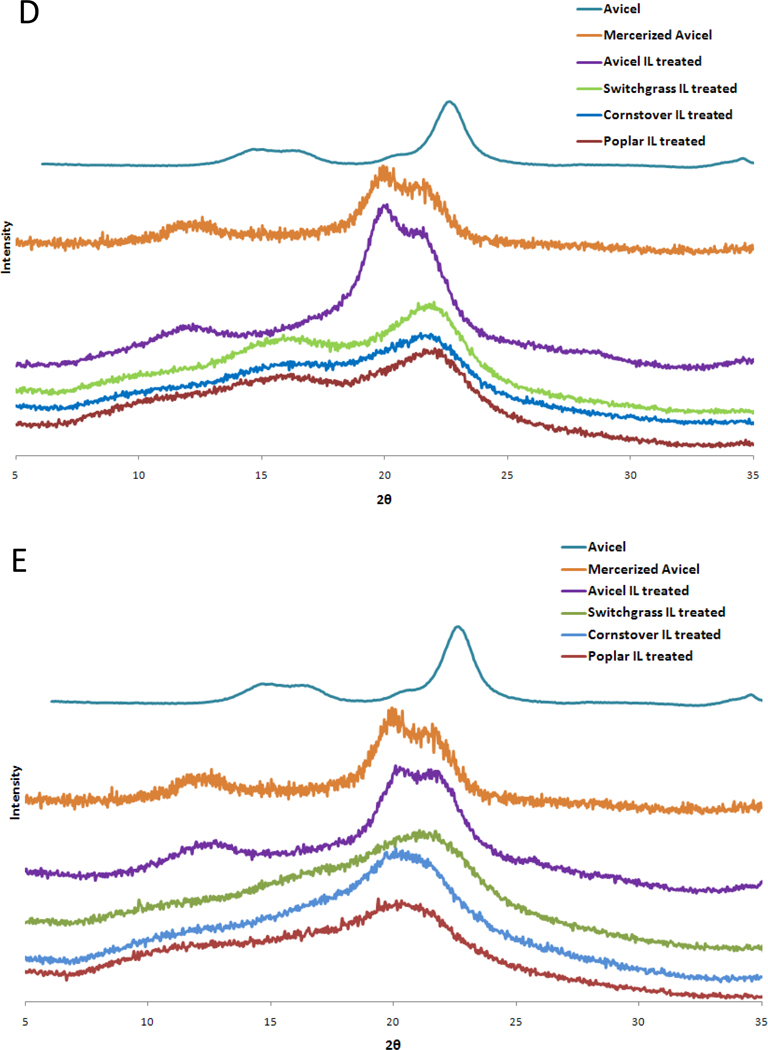
X-ray fiber diffraction and XRD data collected from fiber and powdered samples of all three types of biomass after IL pretreatment at 50°C and 120°C are shown in Figure 2. After pretreatment at 50°C, all biomass samples have recrystallized in the cellulose I phase with the fiber samples retaining a significant amount of cellulose fiber orientation. On the other hand, all biomass samples pretreated at 120°C recrystallized in a form that most closely resembled cellulose II, with only the switchgrass fiber sample retaining a significant amount of cellulose fiber orientation and evidence of residual cellulose I.
Figure 2.
Panels A, B and C are X-ray fiber diffraction images of recrystallized corn stover, poplar and switchgrass, respectively, treated with IL at 50°C (subscript 1) and 120°C (subscript 2). The biomass samples were washed with water and dried at 50°C following the IL treatment. Panels D and E are the corresponding X-ray powder diffraction patterns of biomass substrates treated with IL at 50°C and 120°C, respectively.
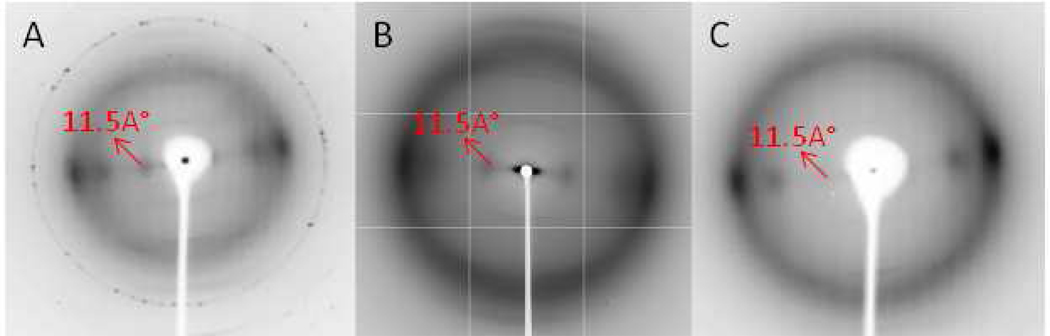
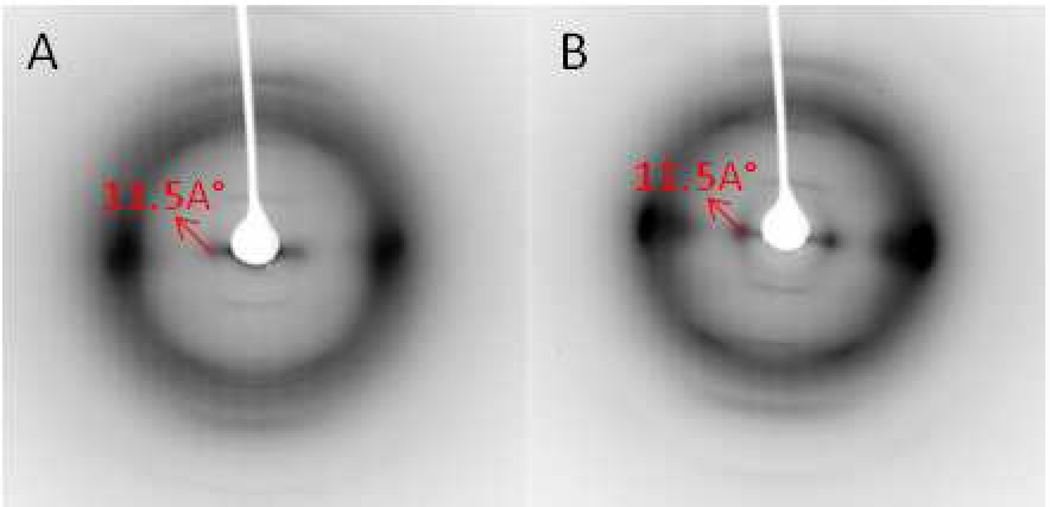
Weak fiber diffraction features were observed during the recrystallization process with biomass samples after pretreatment at 50°C and washing with anti-solvent, but before the samples had completely dried. In particular, a low angle equatorial reflection corresponding to a real-space dimension of ~11.5 Å was clearly observed and is indicated by arrows in Figure 3. This feature was not detected in the XRD data from biomass samples. In order to investigate the possibility that these unexpected diffraction features correspond to an unknown intermediate ordered complex of cellulose and IL, further X-ray powder and fiber diffraction data were collected from the model ramie and Avicel cellulose samples after pretreatment with IL.
Figure 3.
Panels A, B and C are the X-ray fiber diffraction images of an intermediate structure observed in recrystallizing corn stover, poplar and switchgrass, respectively, after IL dissolution at room temperature followed by a water wash.
XRD scans of Avicel powders pretreated with IL at 50°C (Figure 2) indicated that the cellulose recrystallized as cellulose II with no indication of the intermediate diffraction features. In contrast, fiber diffraction from ramie cellulose fibers treated at 23°C and 50°C reproduced the intermediate diffraction features, as shown in Figure 4. If these ramie fibers were kept under tension during the entire pretreatment process, the cellulose recrystallized in the cellulose I phase. However, if the fibers lost tension during addition of the IL, they appeared to completely dissolve, resulting in a gelatinous mass following addition of an anti-solvent. When dried, this material showed no fiber orientation and resulted in a powder diffraction pattern (Figure 5) that most closely resembles cellulose II.
Figure 4.
Panels A and B are the X-ray fiber diffraction images of intermediate structures in recrystallizing ramie samples after IL dissolution at room temperature and 50°C, respectively, followed by a water wash.
Figure 5.
X-ray fiber diffraction image of recrystallized ramie sample after IL dissolution at room temperature for 24 hours, followed by a water wash.
To evaluate the role of cellulose structure on substrate digestibility, the enzymatic hydrolysis of cellulose and biomass to monomeric sugars was assessed. The hydrolysis was more complete for Avicel samples of cellulose II (mercerized with NaOH) than for cellulose I as reported by others 22 (Table 1). The cellulose II sample from mercerized Avicel had a lower crystallinity index compared to cellulose I. Powdered biomass samples treated with IL at 50 or 120 °C were also hydrolyzed. Differences in digestibility of substrates for wet samples and those dried post IL expulsion were assessed (Table 2). Biomass samples treated with IL at 50°C (cellulose I) exhibited lower 24 hour hydrolysis yields of monomeric sugars compared to samples treated at 120°C. There appears to be no large differences in digestibility of wet and dry substrates. However, wet samples produced consistently higher monomeric sugar yields than dried samples. The 24 hour yields of monomeric sugars for IL pretreated biomass increased by a factor of about three or more at a treatment temperature of 50°C and by a factor of more than six at 120°C over that of untreated substrate. The yields of glucose and xylose after 24 hour hydrolysis were: both 6% for untreated poplar; 18% and 8% respectively for untreated corn stover; and 13% and 3%, respectively, for untreated switchgrass. Increasing the incubation temperature of IL pretreatment resulted in nearly complete conversion of polysaccharides to monomeric sugars with negligible differences in lignin content (Table 3).
Table 1.
‘Native’ Avicel, cellulose I, and cellulose II (mercerized with NaOH) were enzymatically hydrolyzed with 15 FPU/ g glucan Spezyme CP and 30 CBU/g glucan Novozyme 188 at 1%(w/v) solid loading. All the measurements were an average of triplicates.
| Substrate | % Glucose Yield | % CrI | |
|---|---|---|---|
| 5 hr | 24 hr | ||
| Cellulose I | 7±5 | 35±11 | 78 |
| Cellulose II | 39±4 | 87±9 | 54 |
Table 2.
Biomass pretreated with IL at 50 or 120°C and enzymatically hydrolyzed by 15 FPU/ g glucan Spezyme CP and 30 CBU/g glucan Novozyme 188 at 1 % (w/v) solid loading. Wet: Hydrolysis on wet substrate after IL treatment and displacement with water. Dry: Hydrolysis of substrates dried after IL treatment and displacement with water. Results are an average of duplicates with less than 8% deviation between samples.
|
Substrate |
Wet | Dry | ||
|---|---|---|---|---|
| % Glucose 24h |
% Xylose 24h |
% Glucose 24h |
% Xylose 24h |
|
| Poplar 50°C | 45 | 24 | 37 | 20 |
| Poplar 120°C | 95 | 81 | 87 | 73 |
| Corn stover 50°C | 51 | 30 | 45 | 27 |
| Corn stover 120C | 105 | 72 | 76 | 56 |
| Switchgrass 50°C | 29 | 12 | 28 | 10 |
| Switchgrass 120°C | 86 | 48 | 80 | 42 |
Table 3.
The weight percent of glucose, xylose and lignin in native and IL-treated (at 50°C and 120°C) poplar, switchgrass and corn stover are given below. Lignin is the sum of acid soluble and acid insoluble lignin. All the measurements are an average of triplicate samples except for switchgrass and corn stover measurements at 50°C. These are an average of duplicate samples with less than 6% deviation. The crystallinity indices were calculated on the dry substrates.
| Substrate | % Glucose | % Xylose | % Lignin | CrI |
|---|---|---|---|---|
| Poplar | 37±1.4 | 11±0.6 | 24±0.4 | 38 |
| Poplar 50°C | 30±3 | 11±1 | 23±6 | 19 |
| Poplar 120°C | 29±8 | 10±2 | 25±3 | 22 |
| Corn stover | 28±4 | 14±2 | 20±5 | 23 |
| Corn stover 50°C | 32 | 16 | 20 | 19 |
| Corn stover 120°C | 25±1 | 11±1 | 19±3 | 29 |
| Switchgrass | 28±3 | 15±3 | 21±6 | 21 |
| Switchgrass 50°C | 19 | 10 | 17 | 22 |
| Switchgrass 120°C | 20±2 | 12±1 | 22±1 | 26 |
Discussion
Both X-ray powder and fiber diffraction data show that, following IL displacement with water and drying, lignocellulosic biomass pretreated with IL recrystallizes to cellulose I under mild conditions (50°C or less) and to cellulose II under harsh conditions (120°C). Increasing the incubation temperature during pretreatment does not result in any major differences in the crystallinity indices of the cellulose I or cellulose II components or in the lignin content of biomass samples (Table 3). The hydrolysis yields are significantly greater (by a factor of about 2) for the pretreated biomass samples that contain cellulose II compared to those containing cellulose I. This observation is true regardless of whether the samples are wet or dry (Table 2). Therefore, one of the clearest factors associated with enhanced hydrolysis of the IL pretreated biomass observed in this study is the transformation of cellulose I to cellulose II.
Cellulose I in Avicel, which is lignin-free, is easily converted to cellulose II by mild IL pretreatment at lower temperature. Cellulose in ramie fibers (also having low lignin content (~1%) 23) will recrystallize to cellulose I if the fibers are kept under mechanical tension. In comparison, this same substrate will be converted to cellulose II if the fibers lose tension and are allowed to fully dissolve during mild IL pretreatment at low temperature conditions. The biomass samples which contain relatively large amounts of lignin (~20%, Table 3) require higher temperature IL pretreatment in order to drive the transformation of cellulose I to cellulose II.
The dependence on lignification and mechanical tension of the IL induced conversion of cellulose I to cellulose II in biomass is remarkably similar to the behavior of cellulose in biomass during mercerization with NaOH. In the X-ray fiber diffraction studies of Revol & Goring 24 it was found that cellulose I in spruce wood (containing ~20% lignin)25 treated with NaOH only partially converted to cellulose II, whereas complete conversion occurred in Kraft pulp, in which much of the lignin had been removed. Two possible contributing factors to this behavior were identified.
First, the presence of lignin may restrict the NaOH swelling of cellulose in wood so that it does not completely dissolve. The residual presence of some association of parallel oriented chains similar to that found in cellulose I, may promote nucleation of cellulose I rather than cellulose II when the alkali is washed out. Electron and X-ray fiber diffraction studies of cellulose mercerization with NaOH have also provided evidence for recrystallization to cellulose I rather than cellulose II in laterally compressed regions of ramie samples26. In their X-ray fiber diffraction studies of the conversion of cellulose I to cellulose II via the formation of Na-cellulose I, Nishiyama et al, noted that cellulose I could be regenerated from Na-cellulose I when swelling of cellulose in ramie fibers is restricted 27.
Secondly, in the model of the cell wall of wood proposed by Kerr & Goring (1975)28, lignin encrusts and separates cellulose fibers that have opposite polarity. Within each cellulose I fibril the cellulose chains have parallel orientations, and therefore the fibrils themselves have an overall polarity.9 However within crystallites of cellulose II the chains are antiparallel with no overall polarity.10 Blackwell et al. proposed that conversion of cellulose I to cellulose II requires that chains migrate between fibers that have opposite polarities29. The presence of lignin in between fibers of opposite polarities prevents this from occurring.
If the above explanations for the behavior of cellulose during NaOH mercerization are also be relevant to the behavior of cellulose in biomass during treatment with IL at different temperatures then it seems clear that some type of change in lignin structure or distribution must occur at elevated temperatures. The experimental results presented here indicate that there is no loss of lignin during IL treatment (Table 3) but they provide no direct evidence for the nature of any structural change to lignin. Electron microscopy of biomass pretreated with dilute acid at high temperatures revealed the presence of droplets on the biomass surface attributed to a redistribution of lignin30.It was proposed that lignin coalesces and moves through the cell wall matrix and forming surface droplets at elevated temperatures. In recent imaging studies of biomass AFEX pretreatment it was shown that cell wall decomposition products, such as lignin-based phenolics, are redistributed to the outer cell wall surfaces, thus creating highly porous structures which may enhance enzyme access to cellulose31. However, neither AFEX nor dilute acid pretreatment promotes a conversion of cellulose I to cellulose II.
Although the same type of pore forming process may be occurring during IL treatment at elevated temperatures, it is also possible that more dramatic changes are taking place that allow complete and unrestricted cellulose swelling, including the melting of lignin. The glass transition temperature, Tg, of lignin has been found to vary between 100 to 160°C depending on the monolignol chemical composition and biomass source30,32–34. In the fiber diffraction studies reported here, it was observed that the cellulose in switchgrass pretreated at higher temperature (120°C) retained a significant amount of orientation during recrystallization whereas the cellulose in corn stover and poplar did not. This difference may be due to a higher Tg in switchgrass. Arora et al. report that switchgrass treated with IL under a range of temperatures from 100 to 160°C yielded the highest initial hydrolysis rates when treated with IL at 160°C pointing to a high Tg of lignin in switchgrass 32.
The explanation that lignin is restricting the swelling of cellulose during IL pretreatment at low temperature, is in agreement with previous imaging studies, 35 and makes the observation of a low-temperature, intermediate ordered structure of cellulose and IL particularly interesting, because it suggests that IL mediates the recrystallization of the native cellulose to cellulose I or cellulose II. Possible identification of the process of intermediate formation can be seen in the ramie recrystallization diffraction data shown in Figure 4. In these diffraction images, there is a strong equatorial diffraction feature near the position of (200) reflection in cellulose I. This feature corresponds to the distance between hydrophobically stacked pyranose rings (~4.5 Å). Furthermore, there is a pronounced reduction or absence of diffraction at the positions of the (110) and (1–10) cellulose I reflections. There is also the appearance of strong diffraction corresponding to a spacing of ~11.5Å. This spacing is much larger than the maximum equatorial unit cell parameters of cellulose I (cellulose Iβ has space group P21 with a reduced unit cell of a = 7.784 Å, b = 8.201 Å, c = 10.38 Å, and γ = 96.5°) or cellulose II (space group P21 with a reduced unit cell of a = 8.10 Å, b = 9.03 Å, c = 10.31 Å, and γ = 117.10°) 9,10.
One interpretation of these new diffraction features is that the recrystallizing cellulose orders with initial assembly of chains that are stacked with their glucose monomers face to face. These stacks of chains would appear to associate with each other, side by side, with a spacing of ~11.5Å, as shown in Figure 6. In between the stacks of chains, it is possible that IL solvent molecules are present and interact with the hydroxyl groups displayed at the edges of the stacks. This presence of IL solvent and interaction with hydroxyl groups is similar to the incorporation of solvent molecules between stacks of cellulose chains in cellulose swollen by liquid ammonia 36,37 or EDA 38. The low resolution reflection corresponding to the spacing of ~11.5 Å tends to be smeared towards the beam center, indicating a large range of side by side distances between the hydrophobic stacks of chains possibly caused by IL solvent. It is interesting to note that diffraction with low resolution features can also be found during the mercerization of cellulose as it adopts various complexes with Na and water, as most recently reported by Kobayashi et al (2011)39.
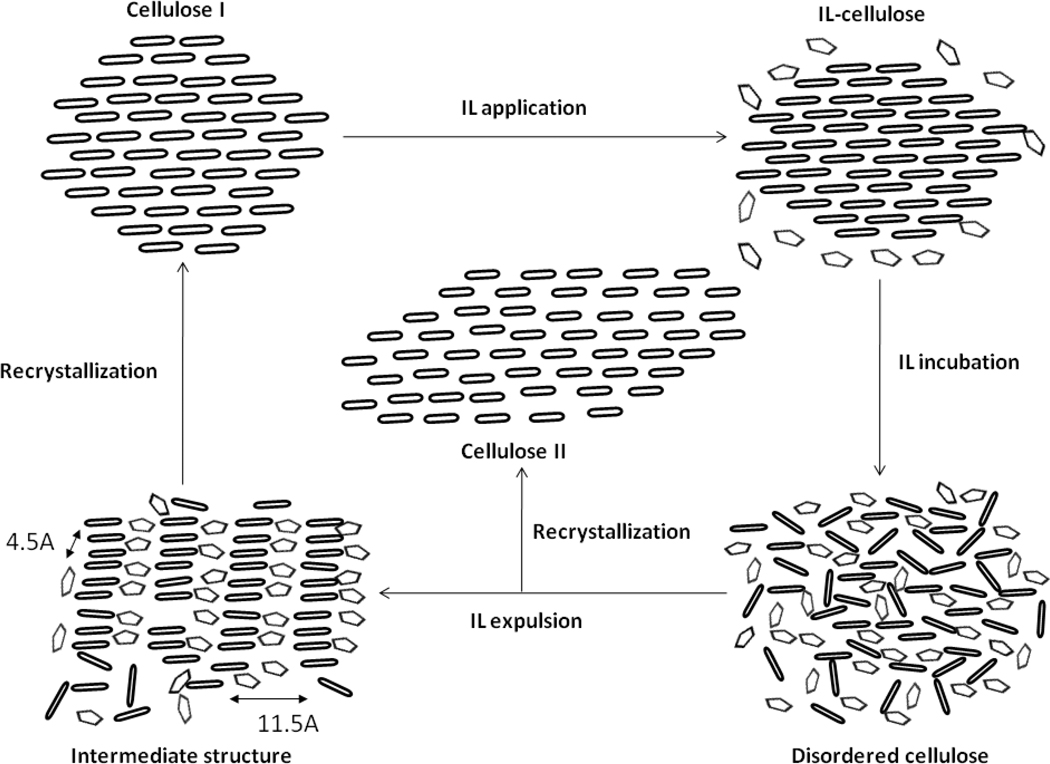
Figure 6.
Schematic representation of IL penetration, IL displacement with water and recrystallization of cellulose fibers. The ellipses represent pyranose rings and pentagons represent the IL. The figure depicts the formation of IL-cellulose complex upon application of IL to the cellulose fibers and the formation of disordered cellulose upon further incubation in IL. After cellulose incubation in IL for a desired period of time and expulsion with water, an intermediate structure is formed with expansion of cellulose fibrils and IL intercalation between the cellulose fibrils. Then upon further washing and drying, the cellulose recrystallizes to either cellulose Iβ or cellulose II depending on the severity of the IL treatment.
Conclusion
In summary, conversion of cellulose I to cellulose II is an attribute that increases biomass digestibility. This conversion is enhanced during IL pretreatments at higher temperatures. One explanation for this behavior is that lignin is restricting the swelling of cellulose during IL pretreatment at low temperature. At higher temperatures, there would appear to be a change in lignin that allows for complete cellulose dissolution. These results suggest that selection of biomass feedstocks with lower lignin glass transition temperatures and may promote the conversion of cellulose fibers to cellulose II and improve pretreatment efficiency and biomass digestibility.
Acknowledgements
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract No. DE-AC02-06CH11357. Use of the BioCARS Sector 14 was supported by the National Institutes of Health, National Center for Research Resources, under grant number RR007707. PL was supported by a Program Development grant from Oak Ridge National Laboratory. The CSMB is supported by the Office of Biological and Environmental Research of the US Department of Energy. CS and IS were partially supported by the Ohio Third Frontier Advanced Energy Program 2009 grant #09-053 with SuGanit Systems and National Science Foundation, CBET 0933250.
References
- 1.Weislogel A, Tyson S, Johnson D. Handbook on Bioethanol: Production and Utilization. Taylor and Francis; 1996. [Google Scholar]
- 2.Somma D, Lobkowicz H, Deason JP. Clean Technol. Environ. Policy. 2010;12:373–380. [Google Scholar]
- 3.Giles J. New Sci. 2007 Aug.18–24:8–9. [Google Scholar]
- 4.Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 5.Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ. Bioresour. Technol. 2010;101:4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 6.Eggeman T, Elander RT. Bioresour. Technol. 2005;96:2019–2025. doi: 10.1016/j.biortech.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Wyman CE. Biofpr. 2008;2:26–40. [Google Scholar]
- 8.Nishiyama Y, Sugiyama J, Chanzy H, Langan P. J. Am. Chem. Soc. 2003;125:14300–14306. doi: 10.1021/ja037055w. [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama Y, Langan P, Chanzy H. J. Am. Chem. Soc. 2002;124:9074–9082. doi: 10.1021/ja0257319. [DOI] [PubMed] [Google Scholar]
- 10.Langan P, Nishiyama Y, Chanzy H. Biomacromolecules. 2001;2:410–416. doi: 10.1021/bm005612q. [DOI] [PubMed] [Google Scholar]
- 11.Langan P, Nishiyama Y, Chanzy H. J. Am. Chem. Soc. 1999;43:9940–9946. [Google Scholar]
- 12.Wada M, Heux L, Nishiyama Y, Langan P. Biomacromolecules. 2009;10:302–309. doi: 10.1021/bm8010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada M, Chanzy H, Nishiyama Y, Langan P. Macromolecules. 2004;37:8548–8555. [Google Scholar]
- 14.Chundawat S, Bellesia G, Uppugundla N, da Costa Sousa L, Gao D, Cheh A, Agarwal UP, Bianchetti CM, Phillips GN, Jr., Langan P, Balan V, Gnanakaran S, Dale BE. J. Am. Chem. Soc. 2011 doi: 10.1021/ja2011115. accepted. [DOI] [PubMed] [Google Scholar]
- 15.Dadi A, Schall CA, Varanasi S. Appl. Biochem. Biotechnol. 2007;137:407–422. doi: 10.1007/s12010-007-9068-9. [DOI] [PubMed] [Google Scholar]
- 16.Dadi AP, Varanasi S, Schall CA. Biotechnol. Bioeng. 2006;95:904–910. doi: 10.1002/bit.21047. [DOI] [PubMed] [Google Scholar]
- 17.Samayam IP, Schall CA. Bioresour. Technol. 2010;101:3561–3566. doi: 10.1016/j.biortech.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Doherty VT, Robert J, Linhardt RJ, Dordick JS. Biotechnol. Bioeng. 2009;102:1368–1376. doi: 10.1002/bit.22179. [DOI] [PubMed] [Google Scholar]
- 19.Segal L, Creely JJ, Martin AEJ, Conrad CM. Text. Res. J. 1959;29:786–794. [Google Scholar]
- 20.Selig M, Weiss N, Ji Y. Enzymatic saccharification of lignocellulosic biomass.LAP-009 NREL Analytical Procedure. Colorado: National Renewable Energy Laboratory (NREL); 1996. [Google Scholar]
- 21.Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D. Determination of structural carbohydrates and lignin in biomass.LAP-002 NREL Analytical Procedure. Colorado: National Renewable Energy Laboratory (NREL); 1996. [Google Scholar]
- 22.Wada M, Ike M, Tokuyasu K. Polym. Degrad. Stab. 2010;95:543–548. [Google Scholar]
- 23.Norman AG. Biochem. J. 1936;30:831–838. doi: 10.1042/bj0300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revol JF, Goring DAI. J. Appl. Polym. Sci. 1981;26:1275–1282. [Google Scholar]
- 25.Wolfgang W. Holzforschung. 2002;56:395–401. [Google Scholar]
- 26.Kim N-H, Sugiyama J, Okano T. Makuzai Gakkaishi. 1990;36:120–125. [Google Scholar]
- 27.Nishiyama Y, Kuga S, Okano T. J. Wood Sci. 2000;46:452–457. [Google Scholar]
- 28.Kerr AJ, Goring DAI. Cellul. Chem. Technol. 1975;9:563–573. [Google Scholar]
- 29.Blackwell J, Kolpak FJ, Gardner KH. Tappi J. 1978;61:71–72. [Google Scholar]
- 30.Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB. Biotechnol. Progr. 2007;23:1333–1339. doi: 10.1021/bp0702018. [DOI] [PubMed] [Google Scholar]
- 31.Chundawat SPS, Donohoe BS, da Costa Sousa L, Elder T, Agarwal UP, Lu F, Ralph J, Himmel ME, Balan V, Dale BE. Energy Environ. Sci. 2011;4:973–984. [Google Scholar]
- 32.Arora R, Manisseri C, Li C, Ong MD, Scheller HV, Vogel K, Simmons BA, Singh S. Bioenerg. Res. 2010;3:134–145. [Google Scholar]
- 33.Irvine GM. Wood Sci. Technol. 1985;19:139–149. [Google Scholar]
- 34.Hatakeyama T, Nakamura K, Hatakeyama H. Polymer. 1982;23:1801–1804. [Google Scholar]
- 35.Lucas M, Wagner GL, Nishiyama Y, Hanson BL, Samayam IP, Schall CA, Langan P, Rector KD. Bioresour. Technol. 2011;102:4518–4523. doi: 10.1016/j.biortech.2010.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada M, Nishiyama Y, Forsyth VT, Langan P. Cellulose. 2011 doi: 10.1107/S0907444910032397. [DOI] [PubMed] [Google Scholar]
- 37.Wada M, Nishiyama Y, Langan P. Macromolecules. 2006;39:2947–2952. [Google Scholar]
- 38.Nishiyama Y, Wada M, Hanson BL, Langan P. Cellulose. 2010;17:735–745. doi: 10.1007/s10570-010-9415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayoko K, Satoshi K, Eiji T, Masahisa W. Carbohydr. Polym. 2011;83:483–488. [Google Scholar]