Abstract
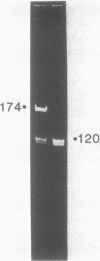
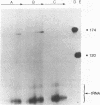
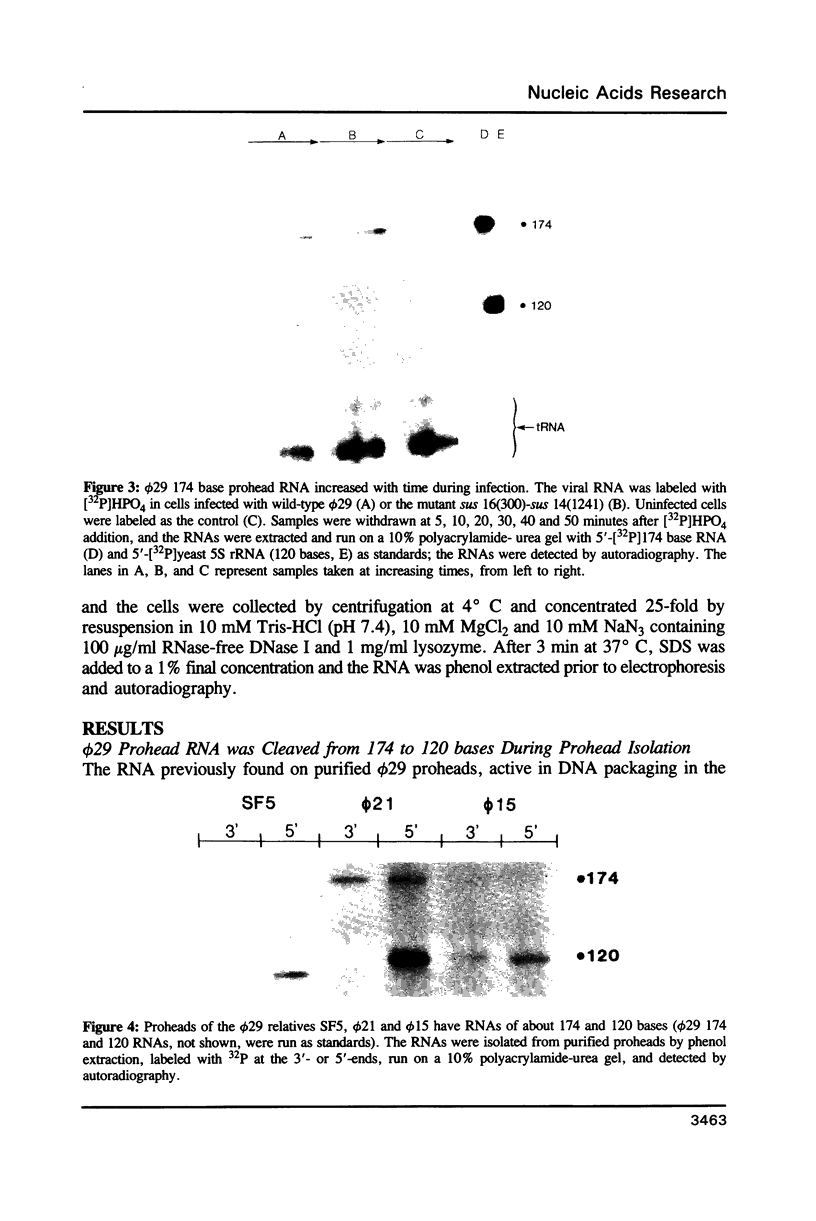
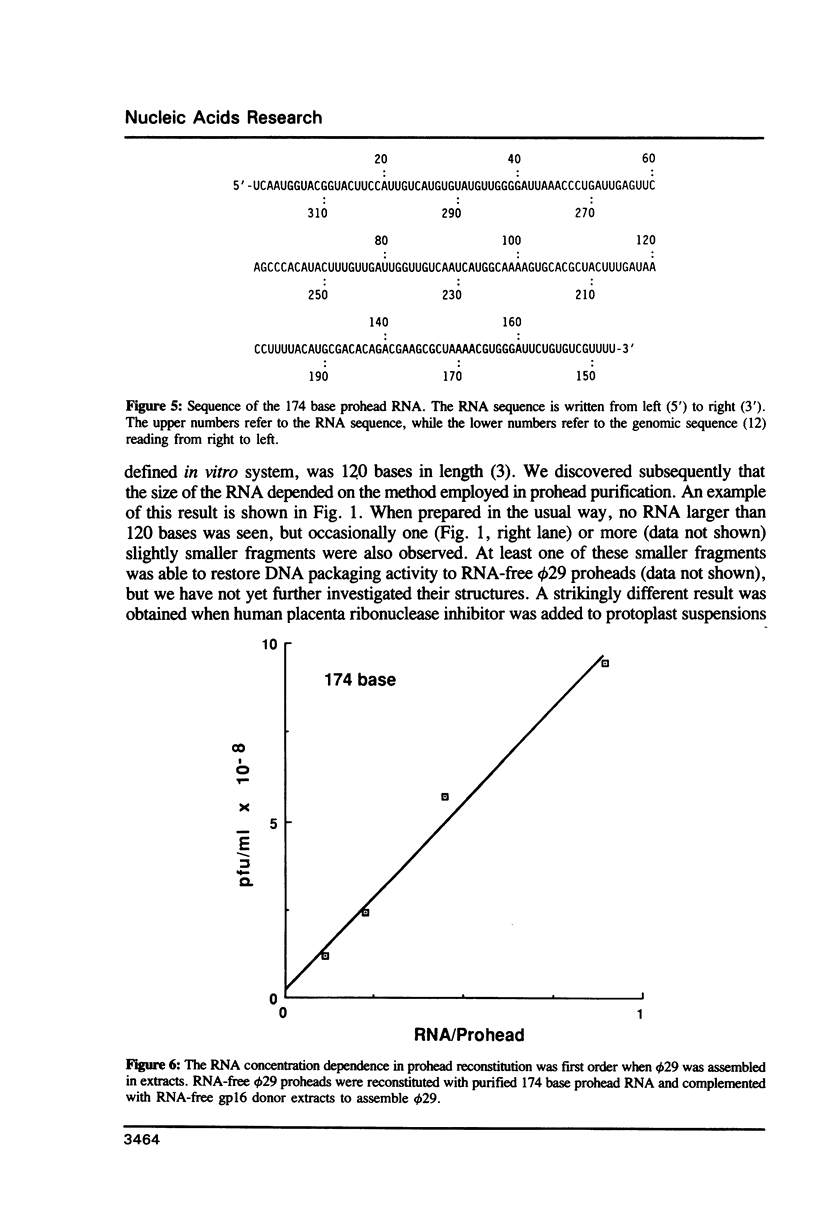
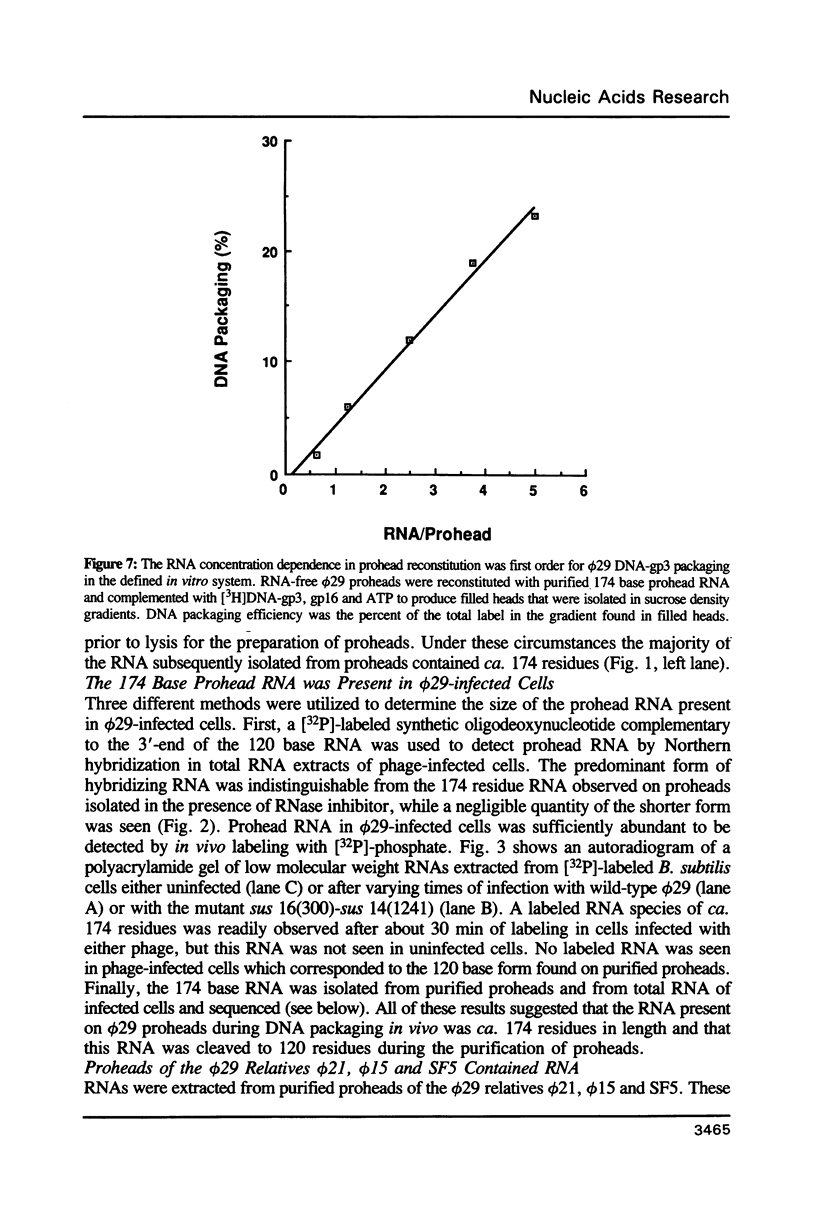
We previously demonstrated (Guo et al., 1987. Nucl. Acids Res. 15, 7081-7090) that purified proheads of bacteriophage phi 29 contain an RNA of 120 bases which is essential for DNA packaging. Here we report that this RNA exists primarily as a polymer of ca. 174 residues in phage-infected cells and that ca. 54 bases are cleaved from its 3'-terminus by adventitious nucleases during the purification of proheads. The long and short forms of the RNA had similar activity in in vitro DNA packaging and phage assembly. We report the sequence of the long form of the RNA and show that similar long and short forms can be isolated from the proheads of the phi 29 relatives phi 21, phi 15 and SF5. The concentration dependence in the reconstitution of RNA-free proheads suggests that one copy of the RNA is sufficient to restore DNA packaging activity to RNA-free proheads. However, quantitative measurements indicate that 5 to 6 copies of the RNA are present on proheads isolated from phage-infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornsti M. A., Reilly B. E., Anderson D. L. Bacteriophage phi 29 proteins required for in vitro DNA-gp3 packaging. J Virol. 1984 Jun;50(3):766–772. doi: 10.1128/jvi.50.3.766-772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti M. A., Reilly B. E., Anderson D. L. In vitro assembly of the Bacillus subtilis bacteriophage phi 29. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5861–5865. doi: 10.1073/pnas.78.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Viñuela E., García N., Santisteban A. Structure of the head-tail connector of bacteriophage phi 29. J Mol Biol. 1982 Jan 15;154(2):311–324. doi: 10.1016/0022-2836(82)90066-3. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P. X., Bailey S., Bodley J. W., Anderson D. Characterization of the small RNA of the bacteriophage phi 29 DNA packaging machine. Nucleic Acids Res. 1987 Sep 11;15(17):7081–7090. doi: 10.1093/nar/15.17.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P. X., Erickson S., Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNA. Science. 1987 May 8;236(4802):690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- Guo P., Grimes S., Anderson D. A defined system for in vitro packaging of DNA-gp3 of the Bacillus subtilis bacteriophage phi 29. Proc Natl Acad Sci U S A. 1986 May;83(10):3505–3509. doi: 10.1073/pnas.83.10.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P., Peterson C., Anderson D. Prohead and DNA-gp3-dependent ATPase activity of the DNA packaging protein gp16 of bacteriophage phi 29. J Mol Biol. 1987 Sep 20;197(2):229–236. doi: 10.1016/0022-2836(87)90121-5. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Ito J. Transcription of the genome of bacteriophage phi 29: isolation and mapping of the major early mRNA synthesized in vivo and in vitro. J Virol. 1977 Sep;23(3):562–577. doi: 10.1128/jvi.23.3.562-577.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Moreno F., Viñuela E., Salas M., Reilly B. E., Anderson D. L. Genetic analysis of bacteriophage phi 29 of Bacillus subtilis: integration and mapping of reference mutants of two collections. J Virol. 1976 Aug;19(2):495–500. doi: 10.1128/jvi.19.2.495-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]