Abstract
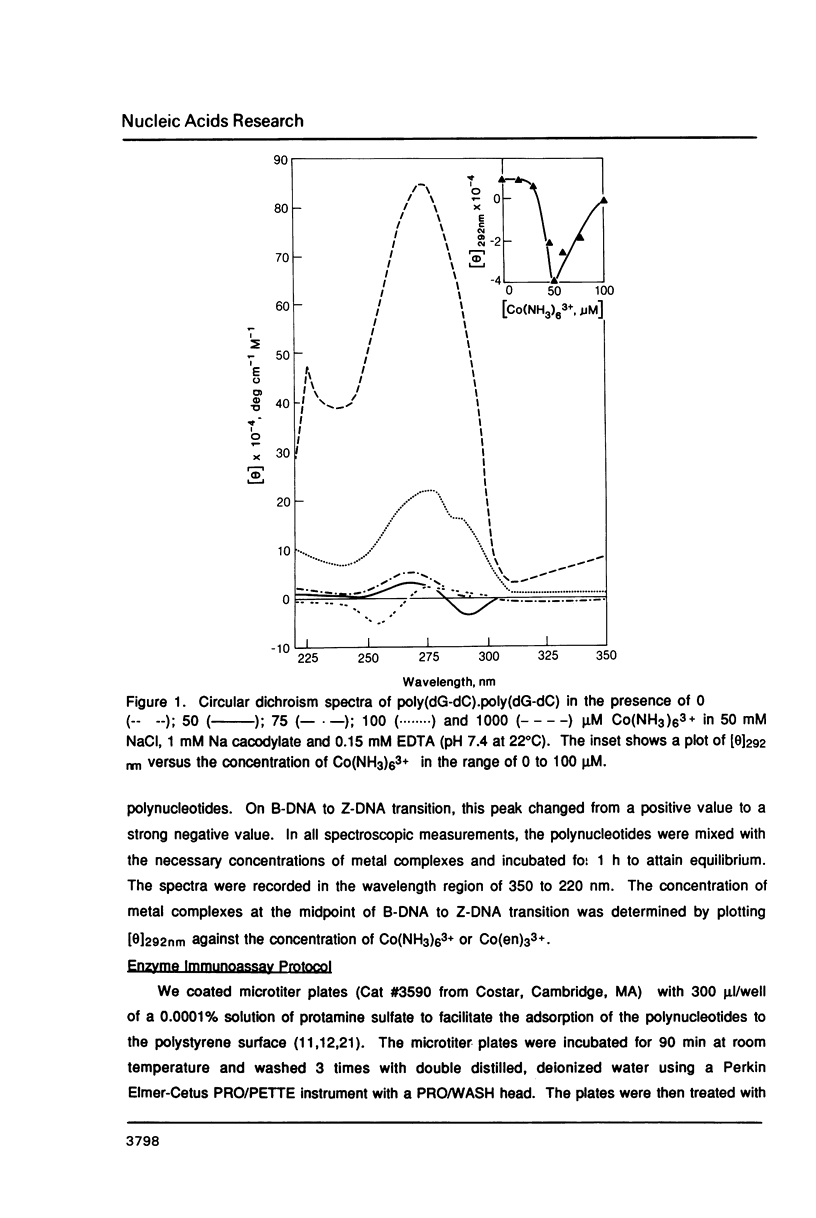
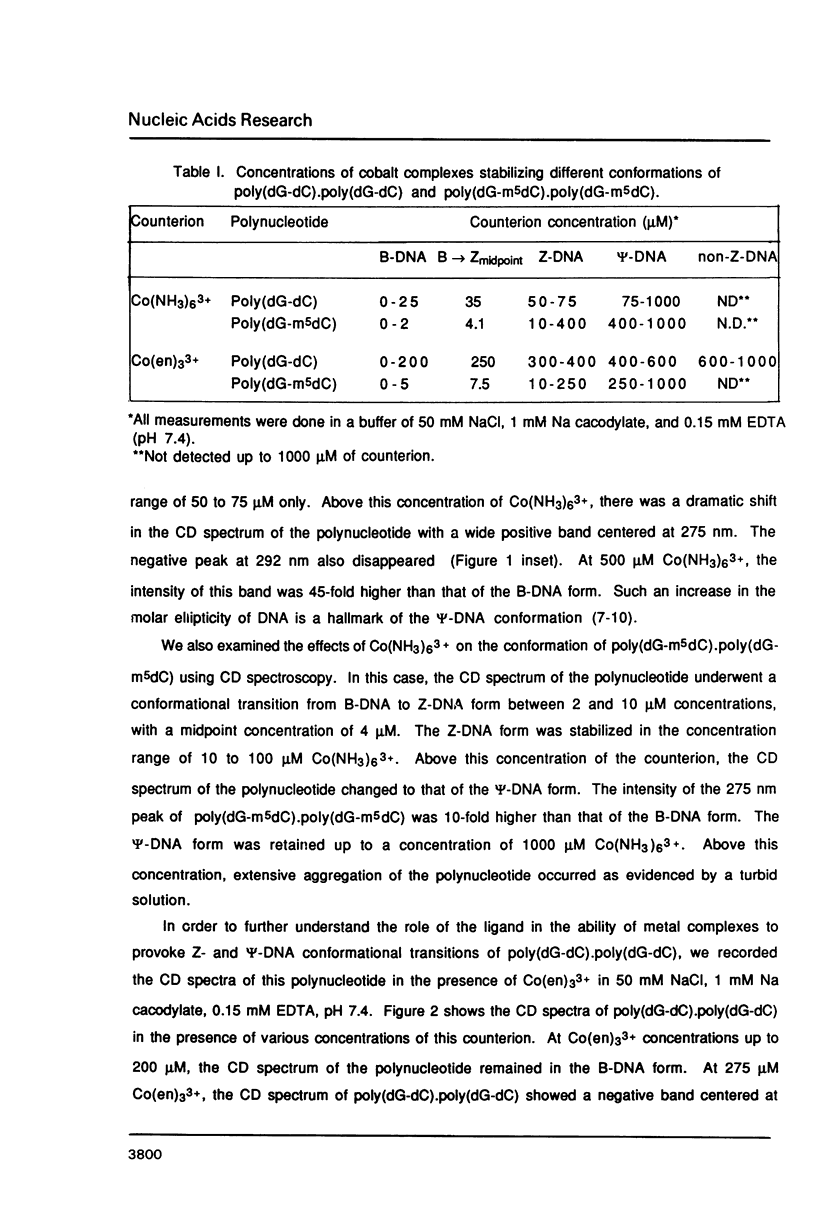
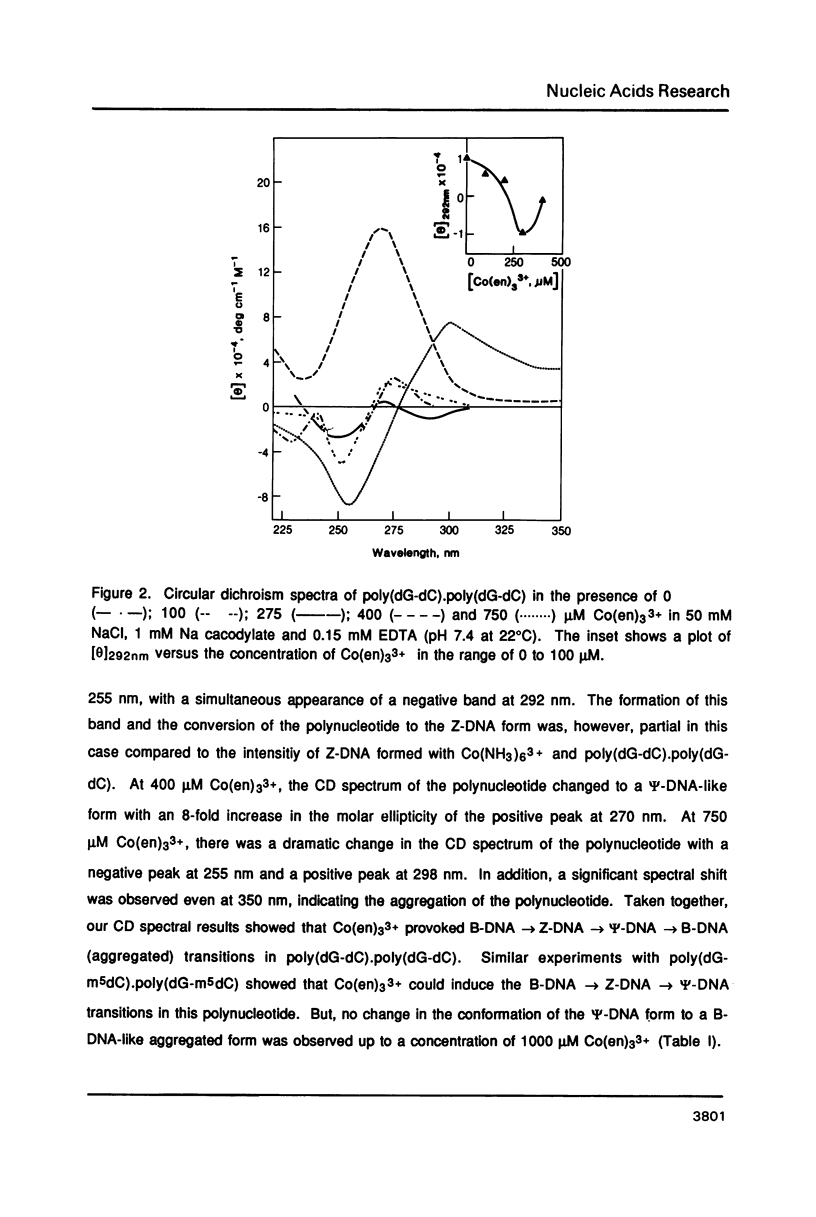
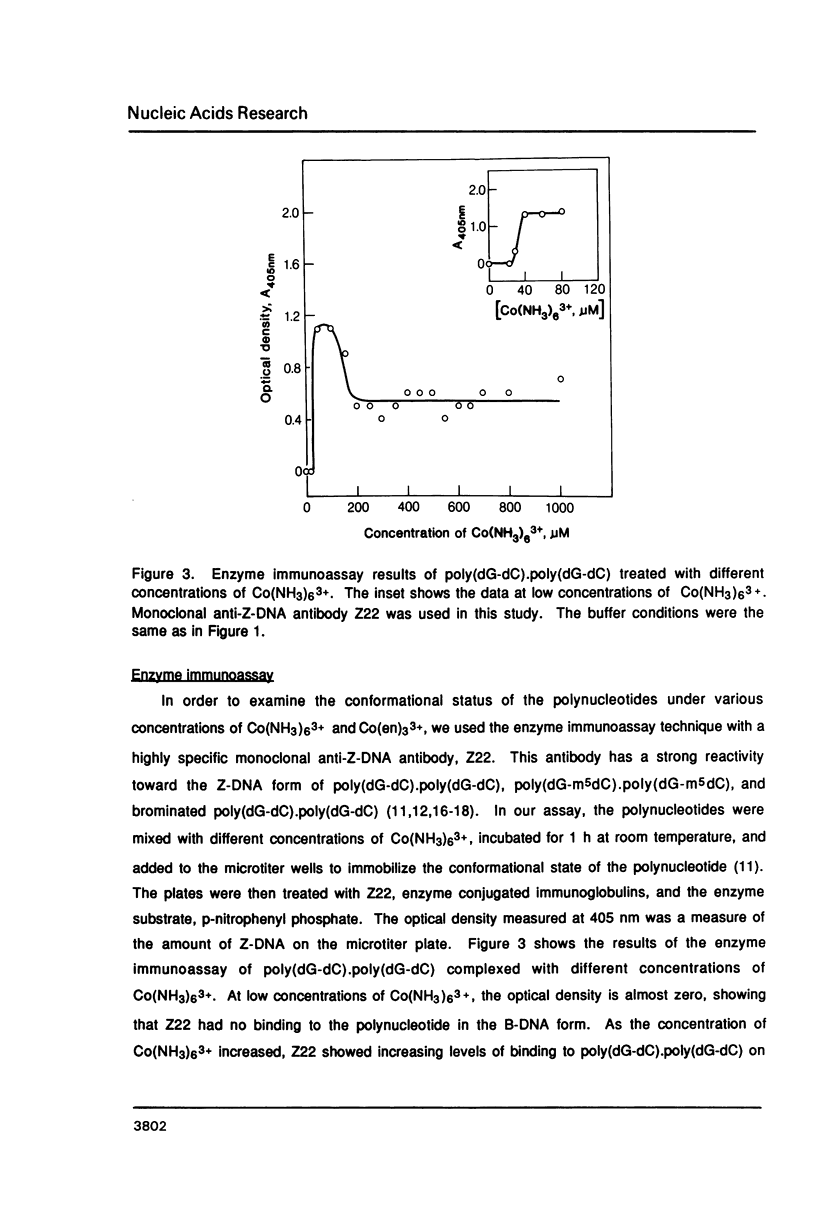
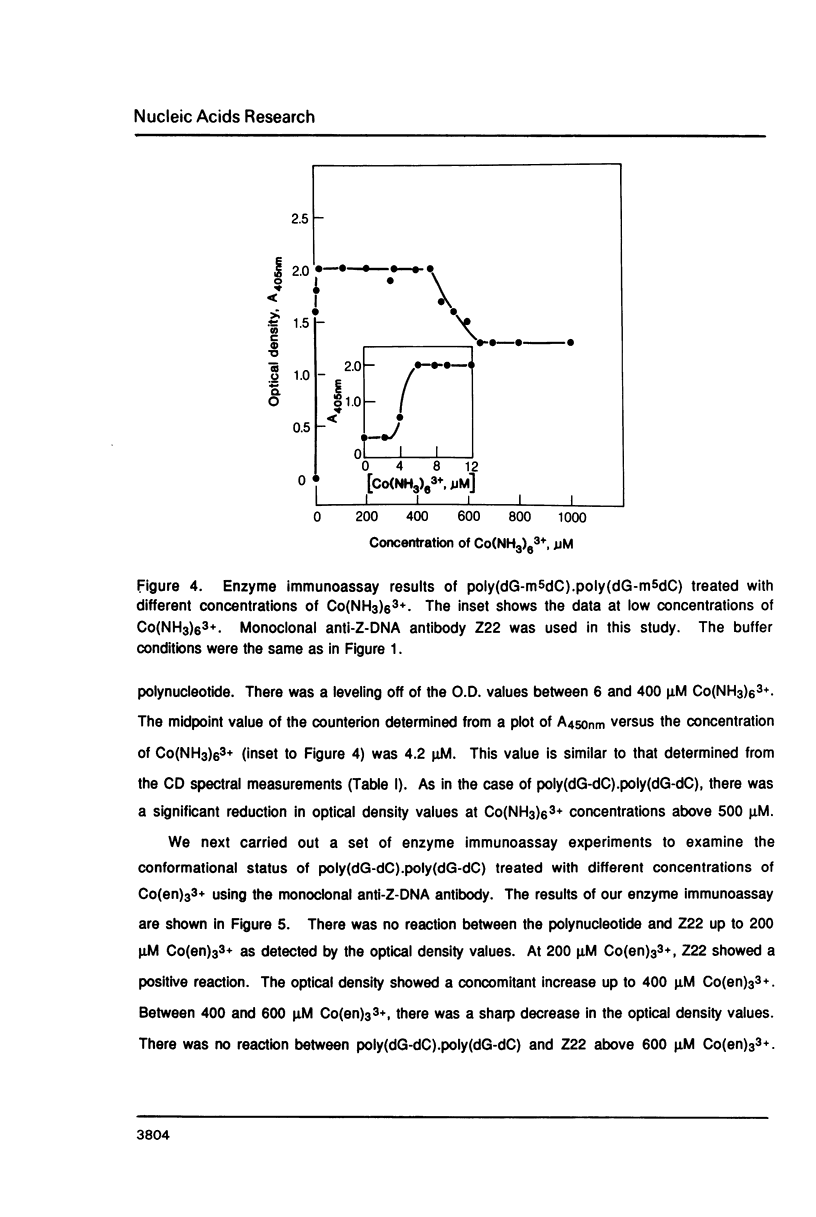
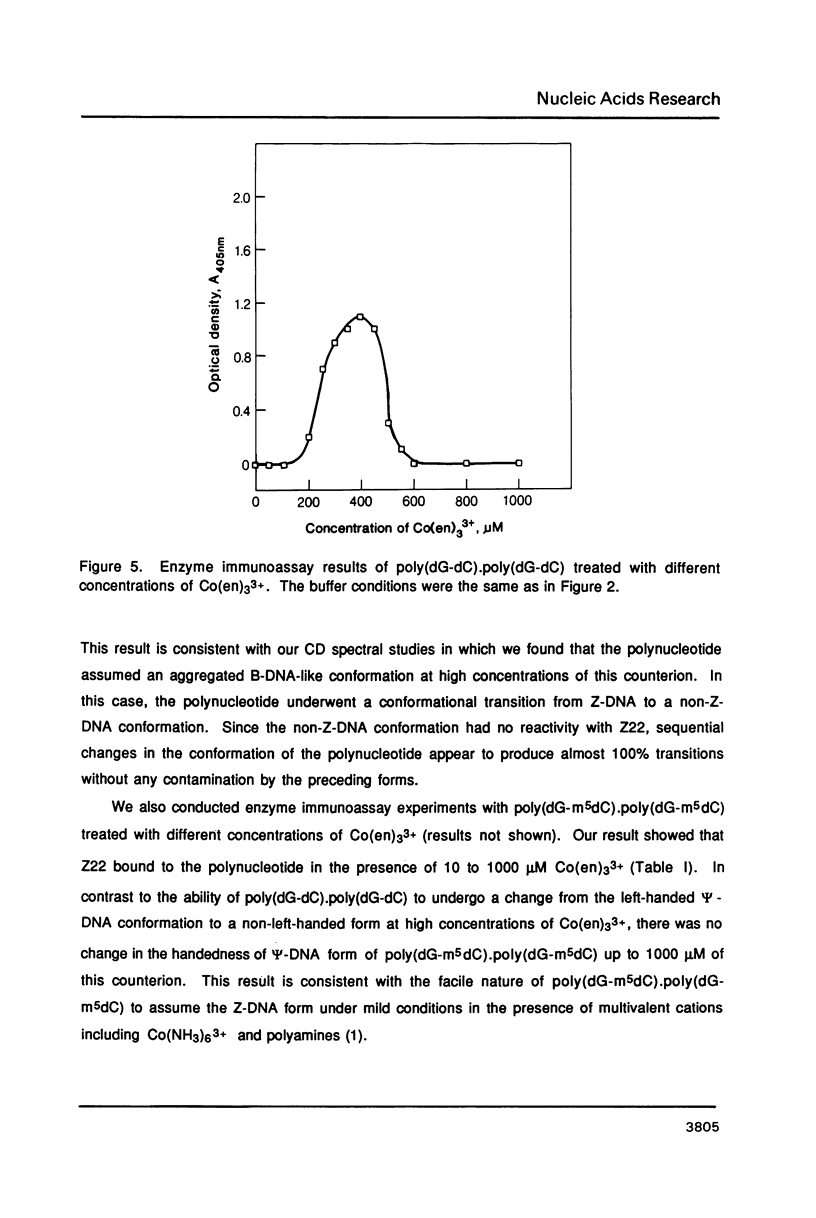
Hexammine cobalt(III) chloride (Co(NH3)6(3+) provokes a B-DNA----Z-DNA----psi-DNA conformational transition in poly(dG-dC).poly(dG-dC) and poly(dG-m5dC).poly(dG-m5dC). The circular dichroism spectrum of psi-DNA is characterized by a manyfold increase of positive ellipticity in the range of 300-225 nm and the complete absence of a negative peak. In order to ascertain the helical handedness of psi-DNA, we used a recently developed enzyme immunoassay technique. This method consisted of treating the polynucleotides with Co(NH3)6(3+) to convert them to the Z- or psi-DNA forms and immobilizing these conformations on a microtiter plate. The plates were subsequently treated with a monoclonal anti-Z-DNA antibody Z22, alkaline phosphatase conjugated, affinity purified immunoglobulins, and the phosphatase substrate. The enzyme-substrate reaction was monitored by reading the absorbance at 405 nm with a microplate autoreader. The monoclonal anti-Z-DNA antibody had no reactivity to the B-DNA form, but bound strongly to both the Z- and psi-DNA forms, showing that Co(NH3)6(3+)-induced psi-DNA form of the polynucleotides exists in the left-handed Z-DNA conformation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu H. S., Marton L. J. The interaction of spermine and pentamines with DNA. Biochem J. 1987 May 15;244(1):243–246. doi: 10.1042/bj2440243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaschun H., Damaschun G., Becker M., Buder E., Misselwitz R., Zirwer D. Study of DNA-spermine interactions by use of small-angle and wide-angle X-ray scattering and circular dichroism. Nucleic Acids Res. 1978 Oct;5(10):3801–3809. doi: 10.1093/nar/5.10.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. B., Schnneider G., Schur P. H. Enzyme immunoassay for antibodies to native DNA. Specificity and quality of antibodies. Arthritis Rheum. 1983 Jan;26(1):52–62. doi: 10.1002/art.1780260109. [DOI] [PubMed] [Google Scholar]
- Eichhorn G. L., Shin Y. A., Butzow J. J. Transitions induced by metal complexes among several forms of DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):125–127. doi: 10.1101/sqb.1983.047.01.016. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Gessner R. V., Quigley G. J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Structural basis for stabilization of Z-DNA by cobalt hexaammine and magnesium cations. Biochemistry. 1985 Jan 15;24(2):237–240. doi: 10.1021/bi00323a001. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. Compact form of DNA induced by spermidine. Nature. 1976 Jan 29;259(5541):333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hingerty B. E., Brown R. S., Klug A. Stabilization of the tertiary structure of yeast phenylalanine tRNA by [Co(NH3)6]3+. X-ray evidence for hydrogen bonding to pairs of guanine bases in the major groove. Biochim Biophys Acta. 1982 Apr 26;697(1):78–82. doi: 10.1016/0167-4781(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Jordan C. F., Lerman L. S., Venable J. H. Structure and circular dichroism of DNA in concentrated polymer solutions. Nat New Biol. 1972 Mar 22;236(64):67–70. doi: 10.1038/newbio236067a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre M. F., Reich C. Contribution of light scattering to the circular dichroism of deoxyribonucleic acid films, deoxyribonucleic acid-polylysine complexes, and deoxyribonucleic acid particles in ethanolic buffers. Biochemistry. 1980 Nov 11;19(23):5214–5223. doi: 10.1021/bi00564a010. [DOI] [PubMed] [Google Scholar]
- Möller A., Gabriels J. E., Lafer E. M., Nordheim A., Rich A., Stollar B. D. Monoclonal antibodies recognize different parts of Z-DNA. J Biol Chem. 1982 Oct 25;257(20):12081–12085. [PubMed] [Google Scholar]
- Nickol J., Behe M., Felsenfeld G. Effect of the B--Z transition in poly(dG-m5dC) . poly(dG-m5dC) on nucleosome formation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1771–1775. doi: 10.1073/pnas.79.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Weiner L. M., Lowenhaupt K., Scholten P., Möller A., Rich A., Stollar B. D. Analysis of Z-DNA in fixed polytene chromosomes with monoclonal antibodies that show base sequence-dependent selectivity in reactions with supercoiled plasmids and polynucleotides. J Biol Chem. 1986 Jan 5;261(1):468–476. [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Revet B., Delain E., Dante R., Niveleau A. Three dimensional association of double-stranded helices are produced in conditions for Z-DNA formation. J Biomol Struct Dyn. 1983 Dec;1(4):857–871. doi: 10.1080/07391102.1983.10507489. [DOI] [PubMed] [Google Scholar]
- Shin Y. A., Butzow J. J., Sinsel L. D., Clark P., Pillai R. P., Johnson W. C., Eichhorn G. L. Metal-induced sequential transitions among DNA conformations. Biopolymers. 1988 Sep;27(9):1415–1432. doi: 10.1002/bip.360270908. [DOI] [PubMed] [Google Scholar]
- Shin Y. A., Eichhorn G. L. Formation of psi (+) and psi (-) DNA. Biopolymers. 1984 Feb;23(2):325–335. doi: 10.1002/bip.360230211. [DOI] [PubMed] [Google Scholar]
- Shin Y. A., Eichhorn G. L. Reversible change in psi structure of DNA-poly(Lys) complexes induced by metal binding. Biopolymers. 1977 Jan;16(1):225–230. doi: 10.1002/bip.1977.360160117. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Baarsch M. J., Messner R. P. Immunological detection of B-DNA to Z-DNA transition of polynucleotides by immobilization of the DNA conformation on a solid support. Anal Biochem. 1988 Feb 1;168(2):358–366. doi: 10.1016/0003-2697(88)90330-2. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A., Canellakis Z. N. Differential effects on the B-to-Z transition of poly(dG-me5dC).poly(dG-me5dC) produced by N1- and N8-acetyl spermidine. Biopolymers. 1985 Apr;24(4):725–729. doi: 10.1002/bip.360240411. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Toroidal condensation of Z DNA and identification of an intermediate in the B to Z transition of poly(dG-m5dC) X poly(dG-m5dC). Biochemistry. 1985 Jan 29;24(3):713–719. doi: 10.1021/bi00324a026. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. A left-handed (Z) conformation of poly(dA-dC).poly(dG-dT) induced by polyamines. Nucleic Acids Res. 1986 Aug 26;14(16):6721–6733. doi: 10.1093/nar/14.16.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Hexammineruthenium (III) chloride: a highly efficient promoter of the B-DNA to Z-DNA transition of poly-(dG-m5dC).poly(dG-m5dC) and poly(dG-dC).poly(dG-dC). Biochimie. 1988 Feb;70(2):221–226. doi: 10.1016/0300-9084(88)90064-8. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Structural specificity of polyamines in left-handed Z-DNA formation. Immunological and spectroscopic studies. J Mol Biol. 1988 May 20;201(2):463–467. doi: 10.1016/0022-2836(88)90155-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Bustamante C., Maestre M. F. The optical activity of nucleic acids and their aggregates. Annu Rev Biophys Bioeng. 1980;9:107–141. doi: 10.1146/annurev.bb.09.060180.000543. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Brennan R., Chapman K. A., Goodman T. C., Hart P. A., Hillen W., Kellogg D. R., Kilpatrick M. W., Klein R. D., Klysik J. Left-handed DNA helices, supercoiling, and the B-Z junction. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):77–84. doi: 10.1101/sqb.1983.047.01.010. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Monomolecular condensation of lambda-DNA induced by cobalt hexamine. Biopolymers. 1983 Jun;22(6):1595–1620. doi: 10.1002/bip.360220612. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Martin J. C., Wells R. D. Condensed form of (dG-dC)n X (dG-dC)n as an intermediate between the B- and Z-type conformations induced by sodium acetate. Biochemistry. 1983 May 10;22(10):2398–2405. doi: 10.1021/bi00279a015. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Arndt-Jovin D. J., Robert-Nicoud M., McIntosh L. P., Thomae R., Jovin T. M. Immunoglobulin recognition of synthetic and natural left-handed Z DNA conformations and sequences. J Mol Biol. 1984 Jul 5;176(3):369–415. doi: 10.1016/0022-2836(84)90495-9. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


