Abstract
Primary afferent microstimulation has been proposed as a method for activating cutaneous and muscle afferent fibers to restore tactile and proprioceptive feedback after limb loss or peripheral neuropathy. Large populations of primary afferent fibers can be accessed directly by implanting microelectrode arrays in the dorsal root ganglia (DRG), which provide a compact and stable target for stimulating a diverse group of sensory fibers. To gain insight into factors affecting the number and types of primary afferents activated, we developed a computational model that simulates recruitment of fibers in the feline L7 DRG. The model comprises two parts. The first part is a single-fiber model used to describe the current-distance relation and was based on the McIntyre-Richardson-Grill (MRG) model for excitability. The second part uses the results of the singe-fiber model and published data on fiber size distributions, to predict the probability of recruiting a given number of fibers as a function of stimulus intensity. The range of intensities over which exactly one fiber was recruited was approximately 0.5 – 5 μA (0.1 – 1 nC per phase); the stimulus intensity at which the probability of recruiting exactly one fiber was maximized was 2.3 μA. However, at 2.3 μA, it was also possible to recruit up to three fibers, albeit with a lower probability. Stimulation amplitudes up to 6 μA were tested with the population model, which showed that as the amplitude increased, the number of fibers recruited increased exponentially. The distribution of threshold amplitudes predicted by the model was similar to that previously reported by in vivo experimentation. Finally, the model suggested that medium-diameter fibers (7.3 – 11.5 μm) may be recruited with much greater probability than large-diameter fibers (12.8 – 16 μm). This model may be used to efficiently test a range of stimulation parameters and nerve morphologies to complement results from electrophysiology experiments and to aid in the design of microelectrode arrays for neural interfaces.
1. Introduction
Electrical stimulation of peripheral nerve fibers holds great potential for developing motor and sensory neural prostheses; for review see (Navarro et al., 2005). Extraneural peripheral nerve interfaces (i.e., positioned outside the epineurium) are currently being used in a variety of clinical applications, including cochlear implants (House and Urban, 1973, Clark et al., 1975) and for functional electrical stimulation of paralyzed muscles (McNeal et al., 1977, Peckham et al., 1980, Creasey et al., 1996). However, electrical stimulation via extraneural electrodes tends to preferentially activate larger myelinated fibers (Veltink et al., 1989, Micera and Navarro, 2009), making extraneural stimulation unsuitable for applications requiring selective activation of smaller fibers. By comparison, intraneural interfaces (i.e., electrodes that penetrate the epineurium) offer greater selectivity and can be arranged in high-density arrays to provide a large number of independent sites for activating motor (McDonnall et al., 2004b) or sensory (Middlebrooks and Snyder, 2010) nerve fibers. Investigation of the recruitment of sensory fibers from intraneural stimulation in the dorsal root ganglia (DRG) is what drove the development of this model, although the approach generalizes to microstimulation in other areas of the peripheral nervous system.
Selective activation of different sensory fiber types is important for the development of a somatosensory neural interface for restoring tactile and proprioceptive sensations for neuroprosthetic limbs. Specifically, microstimulation of primary afferent fibers in the DRG is being explored as a means of providing tactile and proprioceptive feedback. A sensory neural interface comprising electrodes in a few DRGs could provide access to the full range of somatosensory fibers covering large portions of a limb in a compact and mechanically stable structure. Previously, we performed primary afferent microstimulation studies with penetrating microelectrodes in the DRG to determine the electrical current thresholds for activating primary afferent fibers. The conduction velocities of fibers that were recruited at the lowest thresholds varied from 38–118 m-s−1, indicating that a wide range of fiber diameters were recruited in isolation. The thresholds for all of these fibers were similar, typically 1–3 μA (Gaunt et al., 2009). This pattern of neutral recruitment order has also been observed with intrafascicular electrodes in the peripheral nerve (Yoshida and Horch, 1993), and suggests that at low intensities, fibers located nearest the electrode can be recruited selectively, independent of fiber diameter. Such selective recruitment would be particularly advantageous for creating a somatosensory neural interface, where it is desirable to activate fibers of various types to transmit specific modes (e.g. tactile and proprioceptive) of somatosensory feedback. However, these electrophysiology experiments were limited in their ability to answer questions regarding the recruitment patterns of fibers by size in response to microstimulation.
Computational models have provided valuable insight into the effects of electrical stimulation on fiber recruitment in peripheral nerves. Veltink and colleagues (Veltink et al., 1989) developed a model that simulated peripheral nerve geometries and conductivities, and then applied electric fields corresponding to stimulation. They used the McNeal model (McNeal, 1976) for nerve fiber excitation to determine the neural response to stimulation and concluded that intraneural or even intrafascicular stimulation was necessary to selectively stimulate fascicles below the surface of a peripheral nerve. Subsequent work by (Veraart et al., 1993, Tyler and Durand, 2002, Schiefer et al., 2008), however, has shown that stimulus current steering methods and nerve reshaping can be used to increase selectivity for even deep fascicles, though there still exist limitations in activating smaller diameter fibers. Meier and colleagues (Meier et al., 1992) modeled electric field distributions in a nerve bundle and the response of single nerve fibers to arbitrary electric fields. They used these models to predict the response of bundles of peripheral nerve fibers to electric fields created from either monopolar or tripolar intrafascicular electrode configurations and found that tripolar stimulation achieved better spatial selectivity and yielded a more physiologically appropriate recruitment order. Recently, Butson and colleagues (Butson et al., 2011) used nerve slices to build a model for simulating intraneural stimulation of a sciatic nerve fascicle. The focus of that work was to examine recruitment as a function of electrode position and stimulation paradigm. They explored such factors as the current density within axons of different sizes, myelination as a barrier to fiber activation, and the spacing between electrode sites of a microelectrode array.
The models developed in these previous studies were based on specific geometries for the fiber bundles and do not generalize easily to other structures. In particular, the irregular and variable arrangement of fibers and cell bodies in the DRG makes it impractical to define a specific geometry to model the various electrode-fiber configurations that are possible. An alternative approach, which has been used for modeling the recruitment of fibers by deep brain stimulation, is to estimate the volume of tissue activated (VTA) by a given stimulus current (McIntyre et al., 2004, Butson and McIntyre, 2005). Those investigators determined the VTA for a stimulus based on which neuronal fibers around an electrode were activated in response to that stimulus. A larger VTA meant that neurons further away from the electrode had been activated.
We used a similar approach to predict the activation of primary afferent fibers of various sizes as a function of stimulus intensity. We started by estimating the current-distance relationship, which determines the activation threshold as a function of the distance between the current source and nearest node of Ranvier. This distance then defines a volume around the electrode, which we term the volume of influence (VoI). Our model simplifies the electric field calculation by assuming a homogeneous, isotropic extracellular medium in a local volume surrounding the electrode, similar to the approach taken in (McIntyre and Grill, 2000). We adapted the multi-compartment neuron model in (McIntyre and Grill, 2000) to determine the current-distance relationship for primary afferent neurons comprising a range of fiber diameters.
Given the assumption that the node of Ranvier is the site of activation, all fibers having a node within the VoI will be activated. Thus, a given stimulus will activate all fibers having at least one node within the boundary of the VoI. We used an analytical approach to determine the probability of finding a node of Ranvier inside the VoI. This probability depends on the internodal distance; smaller fibers have shorter internodal distances and therefore more nodes per unit length. It is not necessary to assume a specific electrode-node geometry because we integrate the probabilities over all possible configurations within the VoI (see appendix for details). We implemented this “likelihood of activation” approach to estimate the recruitment of primary afferent fibers by microstimulation in the DRG. This approach assumes that the distribution of fibers follows published data for the number and distribution of fibers of various diameters in the DRG. Given the current-distance relationship for each fiber diameter, the model provides a likelihood estimate of the number and types of fibers recruited based on the density and distribution of fibers by size. These features lead to a flexible model that can simulate various stimulation scenarios and electrode-fiber geometries, including the inhomogeneous distribution of fibers in the DRG.
2. Methods
A computational model has been developed in two parts. In the first part we simulated activation of single axons by a point source current using NEURON (Hines and Carnevale, 2001). This single-fiber model was used to determine the current-distance relationships of primary afferent fibers having diameters in the range 7.3 – 16 μm. These current-distance relationships predict, for a given stimulus intensity and fiber size, the maximum distance an electrode can be from a node of Ranvier and still activate that fiber. In the second part of the model, we used the predictions from the current-distance relationships to estimate the likelihood of recruiting specific numbers of fibers of a given size within a normative population of DRG neurons. The population model was based on published data for the distribution of fiber sizes in feline L7 DRG (Risling et al., 1983).
2.1. Single-fiber model
Electrical excitability for a single fiber was represented with the double cable model published by McIntyre, Richardson and Grill, referred to as the MRG model and built in the NEURON environment (McIntyre et al., 2002). Although cell bodies are present in the DRG tissue, we assumed that they were not directly excitable in the range of low amplitude (0 – 6 μA) stimulation currents that were simulated and so did not include them in our single-fiber model (see discussion). This single-fiber model uses
| (1) |
taken from (McIntyre and Grill, 2000) to describe the extracellular potential (Vx) acting on the fiber. Equation (1) is evaluated at each discrete compartmental location (x) along the length of the multi-compartmental fiber model to determine if, taken over the entire length of the fiber, the stimulus was sufficient to evoke an action potential in the simulated fiber. This expression is a function of the distance between the stimulating electrode and the segment of the fiber (dx) as well as the current amplitude (I) applied through the resistivity of the extracellular medium (ρext). This current-distance relation was computed for fibers of various discrete diameters (see table 1). These fiber sizes were then included in simulations of heterogeneous populations of fibers.
Table 1.
Model parameters. For each fiber diameter included in the model, the table lists the number of fibers (NfD) found in the feline L7 DRG based on (Risling, Aldskogius et al. 1983), as well as the fractional area of the fibers of interest occupied by each fiber size (RfD), and the intermodal lengths (Lint ) based on (Nilsson and Berthold 1988).
| fD (μm) | 7.3 | 8.7 | 10 | 11.5 | 12.8 | 14 | 15 | 16+ |
|---|---|---|---|---|---|---|---|---|
| NfD | 1780 | 1730 | 1160 | 1920 | 1270 | 1370 | 630 | 990 |
| RfD | 0.06 | 0.09 | 0.08 | 0.17 | 0.14 | 0.18 | 0.10 | 0.17 |
| Lint (μm) | 750 | 1000 | 1150 | 1250 | 1350 | 1400 | 1450 | 1500 |
While the MRG model was validated originally for peripheral motor axons, we used the model to predict activation of sensory fibers in this paper. Although studies have demonstrated that there are differences in the excitability of motor fibers and sensory fibers as a function of stimulus pulsewidth (Veale et al., 1973, Panizza et al., 1998, Panizza et al., 1994), Erlanger and Blair (Erlanger and Blair, 1938) showed that the difference was insignificant at pulsewidths near 200 μs. For these simulations, we used biphasic, charge-balanced stimulus waveforms (200 μs cathodal, 400 μs anodal phases).
We used the single-fiber model to determine the maximum distance a monopolar, point source electrode could be from a fiber’s node of Ranvier and still elicit an action potential from that neuron, for a given stimulus current amplitude and fiber diameter. In addition, we assumed an isotropic homogeneous extracellular medium with ρext equal to 500 Ω-cm (McIntyre and Grill, 2000). This assumption leads to a spherical VoI, with the radius defined as that maximum distance of activation determined from the current distance relationship (figure 1a). Note that this assumption of isotropy may have significantly affected our results. However, we ran additional simulations assuming anisotropy, with resistivity values published for spinal cord (Ranck and Bement, 1965). Using a longitudinal resistivity of 300 Ω-cm and a transverse resistivity of 1200 Ω-cm, we found similar results as when assuming isotropy (see Discussion).
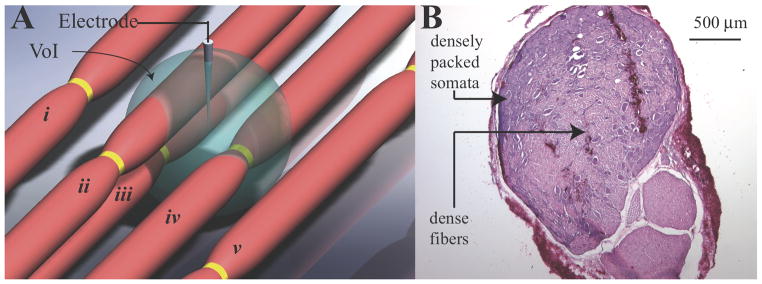
Figure 1.
Sphere representing the volume of influence (VoI) created by a point-source current stimulus delivered by a microelectrode. (A) The radius of the sphere increases with stimulus amplitude and also varies with fiber diameter. The radius is determined by the current-distance relationship calculated with the single-fiber model for neuronal excitation. Fibers iii and iv, having a node of Ranvier within the VoI, will be activated. Fiber ii, though it passes through the VoI, does not have a node of Ranvier within the sphere and thus will not be activated. Fibers i and v, likewise, will not be activated. (B) Transverse section of feline L7 DRG (top) and ventral root (bottom), hematoxylin and eosin (H&E) stained. Cell bodies are predominantly located along the perimeter of the DRG, but are also sparsely distributed in the center among fibers of passage creating a heterogeneous tissue structure.
We used the MRG model to simulate eight discrete fiber sizes (see Table 1). For each fiber size, we used the single-fiber model to determine the extent of the VoI (i.e. the radius) given variations in the stimulus amplitude. The current-distance relationships for the different fibers were then used as inputs to a population model to examine the probability of recruitment in a normative population of axons in the DRG.
Population model
A computational model was developed in MATLAB (The Mathworks, Inc., Natick, MA) to determine the probability of recruiting a given number of fibers of a specified diameter, based on the likelihood of capturing a node of Ranvier within the VoI. This probability depends on two factors: 1) the density of axons packed in the VoI (i.e., the number of axons of a given diameter per cross-sectional area of the VoI), and 2) the inter-nodal distance of a given fiber diameter. In the simplest case, we could assume a uniform distribution of fibers in the DRG and compute the number of fibers of a given diameter packed in the VoI (NVoI) to get
| (2) |
In (2), NfD is the total number of fibers of a particular fiber diameter (fD) present in the DRG and is based on published data for fiber size distributions in the L7 DRG of cat (see table 1). AVoI and ADRG are the cross-sectional areas of the VoI and DRG tissue, respectively. For each fiber size, the radius of the VoI increases with the intensity of the stimulus current, and is taken from the current-distance relationship obtained with the single-fiber model. Note that in this work, we focus on the set of fiber sizes ({fD}) corresponding to medium and larger diameter fibers, representing the cutaneous and muscle afferent neurons that are the primary targets of microstimulation in our studies. Equation (2) assumes that the fibers are distributed uniformly throughout the tissue. However, to account for the non-uniform distribution of fibers, cell bodies, and other tissue in the DRG (figure 1b), we modified (2) by
| (3) |
In (3), the number of fibers having diameter fD passing through the VoI includes two additional scaling factors to account for the non-uniform distribution of fibers in the DRG. The first term, RDRG represents the fraction of the VoI cross-section that is occupied by the fibers of interest, and we refer to this term as the packing ratio. The second term, RfD, is the fractional fiber area and it represents the fraction of the fibers of interest comprising fibers of a specific diameter. A conceptual illustration of these parameters is provided in figure 2. As shown in figure 2b, only a portion of the VoI contains fibers of interest, due to the presence of other tissues (e.g. cell bodies, blood vessels, and smaller fibers).
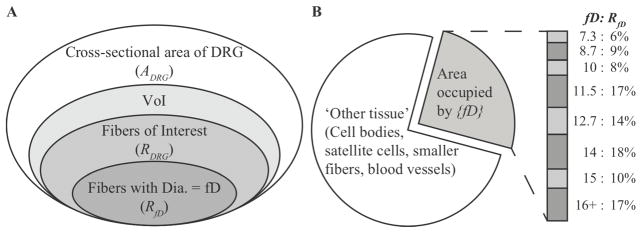
Figure 2.
Conceptual illustration (not drawn to scale) of model parameters associated with the distribution of fibers in the VoI. (A) The VoI comprises a small portion of the total area of the DRG, a fraction of which is in turn composed of the fibers of interest (RDRG). The area associated with fibers of interest is further divided into portions associated with specific fiber diameters (RfD). (B) Pie chart illustrating fractional areas of DRG composed of ‘other tissue’ and the fibers of interest. The packing ratio comprises fiber diameters 7.3 μm and larger, each having a fractional fiber area (RfD) based on the number of fibers of each diameter found in the DRG. The percent area associated with each fiber diameter is shown by the stacked bar plot at the right of the pie chart.
An average value for the packing ratio (RDRG) can be calculated based on fiber counts and cross sectional area for each of the fibers of interest, expressed as
| (4) |
However, the actual value may vary substantially throughout the DRG, being higher in areas densely packed with fibers and lower in areas containing more cell bodies (see figure 1b). To examine the effects of this variation on the model, we performed simulations to predict the probability of recruiting at least one fiber, varying the stimulus intensity from 1 – 6 μA and the packing ratio from 0.1 to 1.0. The simulation results were compared with recruitment threshold data from (Gaunt et al., 2009) to determine an appropriate range of values for RDRG (see discussion).
Published data for the distribution of fibers of various sizes in the L7 dorsal roots of cats is provided in (Risling, Aldskogius et al. 1983). These data were used in
| (5) |
to calculate the fractional area of the fibers of interest that is occupied by fibers of a specific diameter (RfD; see table 1 and figure 2b). Note that the data reported in (Risling et al., 1983) is for fibers in the dorsal roots. To account for the ~30% decrease in fiber diameter that occurs between the DRG and dorsal roots (Suh et al., 1984), we scaled the fiber diameters listed in the Risling data by a factor of 1.4 to represent fiber diameters in the DRG. For example, Risling found that there are approximately 1780 fibers in the dorsal roots with diameter equal to 5.1 μm, corresponding to the group of 7.3 μm fibers in the DRG (see table 1). Lastly, we grouped all fibers having diameters equal to or greater than 16 μm into one group of “16+” μm fibers.
In (3), we estimated the number of fibers of a given diameter that pass through the VoI (NVoI). This equation was evaluated for each specified fiber size, for a given stimulus amplitude. To determine which of these fibers would become activated, we must evaluate the probability of each fiber having a node of Ranvier within the boundary defined by the radius of the VoI (rVoI). The probability that a fiber of a given diameter has a node of Ranvier within the VoI depends on the ratio of the length of fiber captured by the VoI (Lf) over the internodal length of the fiber (Lint). If a fiber passes through the VoI at a known radial distance (rf) from the center, the probability that a node is captured within the VoI is given by
| (6) |
In cases in which the VoI is sufficiently large such that the length of the fiber encapsulated in the VoI is greater than the internodal length of the fiber, this equation would evaluate to 1. For example, a fiber passing through the center of a large VoI (i.e., Lint < 2rVoI) will be guaranteed to have a node of Ranvier within the VoI. However, for stimulation intensities in the range 1 – 6 μA, the diameter of the VoI is ~40 – 240 μm. The range of internodal lengths for the fibers we examined was from 750 – 1500 μm, and thus (6) evaluates to much less than 1 at all values of rf for this range of low intensity of stimuli.
Since the location of the fiber (rf) is not known a priori, we computed an average value of the probability by integrating over all possible locations of the fiber within the VoI and normalizing by the cross sectional area of the VoI as shown in
| (7) |
Equation (7) applies only when the diameter of the VoI is less than the internodal length of the specified fiber (i.e., at low current amplitudes), as is the case for the 1–6 μA range of stimulation current examined here. However, for a higher current amplitude that results in a VoI whose diameter is larger than the internodal length, (7) is insufficient and an alternate equation is required (see appendix).
Next, the probability mass function for a binomial distribution is used to compute the probability of recruiting a given number of fibers of a particular diameter (Nact) from the number of fibers packed in the VoI (NVoI) to get
| (8) |
This calculation was performed for each fiber size and depends on the current amplitude and fiber size, which determine the size of the VoI and hence the number of fibers that can be captured in the VoI as shown in (3).
Finally, we combined the probabilities for activating different fiber sizes to determine the probability of recruiting a particular number of fibers at specific current amplitudes. To get this total probability, we needed to account for all possible combinations of fiber recruitment that yielded the specified total number of fibers (Nrec) as in
| (9) |
We began by defining a matrix of possible combinations (C) that has j columns corresponding to the number of different fiber diameters. Each of the i rows of C is a possible combination of fiber sizes from the set {fD}, such that the sum across the row is equal to Nrec. The set of fiber diameters {fD} to include may be specified as any subset of the fiber diameters listed in table 1. The maximum possible value of Nrec is equal to the sum of NVoI values for the fiber diameters in the set {fD}; that is, one cannot recruit more fibers than could be packed into the VoI. Using (9) we were able to estimate the probability of recruiting specific combinations of fibers (e.g. one medium fiber and one large fiber or two medium fibers and one large fiber) (see appendix for further details).
2.2. Electrophysiology
Data reported from a previous in vivo study were used for comparison with model predictions. For details on the methods used to collect these data refer to (Gaunt et al., 2009). The University of Pittsburgh Institutional Animal Care and Use Committee approved all procedures. To summarize, adult cats were implanted with penetrating microelectrode arrays in the L7 DRG for stimulation and a 5-pole nerve cuff electrode around the sciatic nerve for recording elicited antidromic action potentials. Single channel microstimulation in the dorsal root ganglia was performed to determine the threshold stimulus amplitude at which a response could be recorded in the nerve cuff using stimulus-triggered averaging. A charge-balanced biphasic, cathodic-leading 200 μs pulse followed by a 400 μs anodic pulse was used for stimulation. Thresholds were typically between 1 and 3 μA and approximately 97% of the observed thresholds were less than or equal to 6 μA (Gaunt et al., 2009). We therefore chose this value as an upper limit on stimulus amplitude for simulations in the present paper.
The nerve cuff had two recording sites at a fixed separation distance (8 mm). The propagation delay was measured within the nerve cuff as the time it took for an action potential to propagate between the two recording sites. The electrode separation distance divided by this propagation delay yielded the conduction velocity for an activated unit. An estimate of the fiber diameter was obtained by dividing the conduction velocity by 5.66 based on (Boyd and Kalu, 1979). Fiber diameters detected in the in vivo study ranged from 7.3 – 16 μm, which drove the selection of the fiber sizes tested in our model.
3. Results
In this study, we used the single-fiber model to compute current-distance relationships for each of 8 different fiber sizes (see Table 1). We used these relationships to compare the probabilities of recruiting each of these fiber sizes as a function of the number of fibers that could fit within the resulting VoI and their internodal lengths. Finally, we used the population model to predict recruitment in a heterogeneous population of fibers in the DRG. Simulations were run to determine the number of fibers, by size, that would be activated in response to a given stimulus intensity. We verified these results against primary afferent microstimulation recruitment data reported previously (Gaunt et al., 2009).
3.1. Current-distance relationship
Activation of a neural fiber depends on several factors, including stimulus waveform and amplitude, fiber size, and distance from the stimulating electrode. For example, the inverse relationship between the extracellular voltage and the distance between the electrode and a node of Ranvier drives the nonlinear behavior of the current-distance relation as expressed in (1). Figure 3a illustrates the effect of increasing fiber diameter on the current-distance relation, as predicted by the single-fiber model. In general, the electrode-to-node distance increases with the stimulus amplitude. At higher intensities, the larger diameter fibers can be activated at much greater distances. This gives rise to the so-called ‘reverse recruitment’ phenomena that has been described for muscle activation with epineural electrodes (Veltink et al., 1989, Micera and Navarro, 2009). However, for amplitudes below 10 μA (see inset in figure 3a), there is little difference in the current-distance relationship for fibers of different diameters. Thus, at these low intensities, the radius of the VoI is effectively the same for all myelinated fibers within the range tested. Although the VoI is the same for these fibers, this does not mean that these different sized fibers have equal likelihood of being recruited in the 0–10 μA range. In order for a fiber to become activated, one of its nodes of Ranvier must be captured within the VoI.
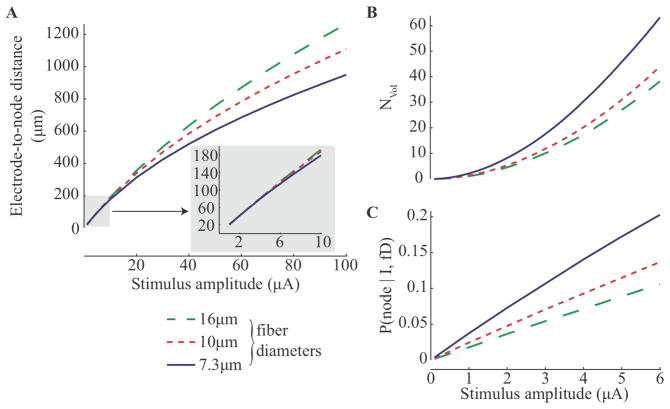
Figure 3.
Effects of fiber diameter on current-distance relation, fiber packing and probability of having a node in the VoI. (A) Current-distance relationships from single-fiber model. Electrode-to-node distance corresponds to the radius of a spherical VoI centered about a stimulating electrode. (B) The number of fibers that can be packed into the VoI assuming a packing ratio equal to 1. (C) Probability of capturing a node of Ranvier in the VoI as predicted by (7). Fiber sizes of 7.3 μm, 10 μm and 16 μm were simulated.
To compare the likelihood of recruiting different fiber sizes, we simulated populations of neurons assuming a packing ratio (RDRG) of 1 in (3) to estimate the number of fibers passing through the VoI. Figure 3b shows the number of fibers penetrating the VoI, which increases in size with the stimulation intensity (1–6 μA). Note that there are considerably more 7.3 μm diameter fibers penetrating the VoI. This is because there are nearly twice as many 7.3 μm diameter fibers as there are 10 and 16+ μm fibers in the DRG (see table 1). In addition, there is a higher probability of capturing a node within the VoI (see figure 3c) for the 7.3 μm diameter fibers because the internodal length (Lint) is shorter (table 1), as shown in (7). Thus, figures 3b and 3c suggest a bias towards recruitment of smaller diameter fibers, due to the relatively greater number of 7.3 μm fibers and the higher number of nodes per unit length as compared to the larger fibers.
3.2. Thresholds for single-fiber recruitment in a population
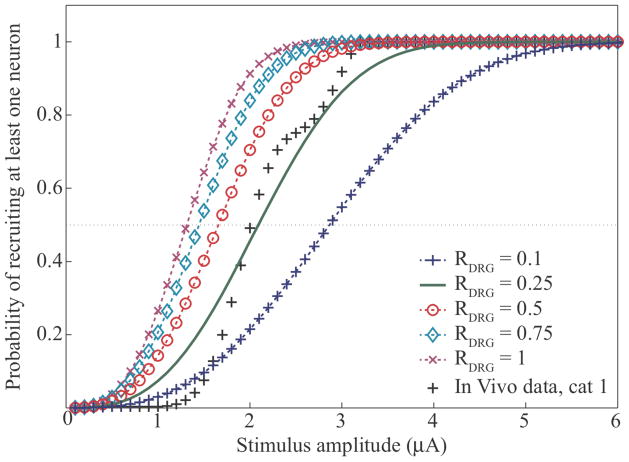
Using the population model, we first explored the effects of the packing ratio on recruitment predictions based on (9). We ran simulations to predict the probability of recruiting at least one fiber, varying the stimulus intensity from 1–6 μA and the packing ratio from 0.1 to 1.0 in (3). The model predictions are shown in figure 4. In general, the probability of recruiting at least one fiber is very low for stimulation amplitudes < 1 μA and increases with the stimulus amplitude. Note that the rate of increase is much faster when the packing ratio is high. We defined the recruitment ‘threshold’ as the stimulation amplitude that yields a 0.5 probability of recruiting at least one fiber, indicated by the dotted line in figure 4. The recruitment threshold is ~1 μA when the packing ratio is 1, but increases to nearly 3 μA when the packing ratio is only 0.1. The series of ‘+’ symbols indicate the cumulative probability of recruitment from subject 1 in our previous in vivo experiment (Gaunt et al., 2009). We found that for a packing ratio of 0.26, the population model provided an excellent fit (R2 > 0.9) to those in vivo data. We also used the population model to fit recruitment data for subject 4 from the same published data and found a packing ratio of 0.11 yielded the best fit (R2 > 0.9). The variability in the packing ratios for these two experiments is likely due to differences in the electrode placement between the two experiments; the density of fibers around the electrodes was apparently higher in subject 1 than in subject 4 as suggested by the higher packing ratio found for subject 1. Using the model to fit the electrophysiology data across all four subjects, the best packing ratio was found to be 0.2 (R2 > 0.9). Thus, despite the potential for large differences in fiber density throughout the DRG, the range of values for the packing ratio is fairly narrow (RDRG = ~0.1 – 0.3) for the data obtained in our in vivo study.
Figure 4.
Sensitivity of population model to the packing ratio. The black markers represent published electrophysiology data for a single subject (Gaunt, Hokanson et al. 2009). The other traces represent model simulation results of recruiting at least one fiber for different packing ratios. The model best predicts the electrophysiology data for a packing ratio of approximately 0.26 (R2>0.9) for cat 1, and 0.2 (R2>0.9) across subjects.
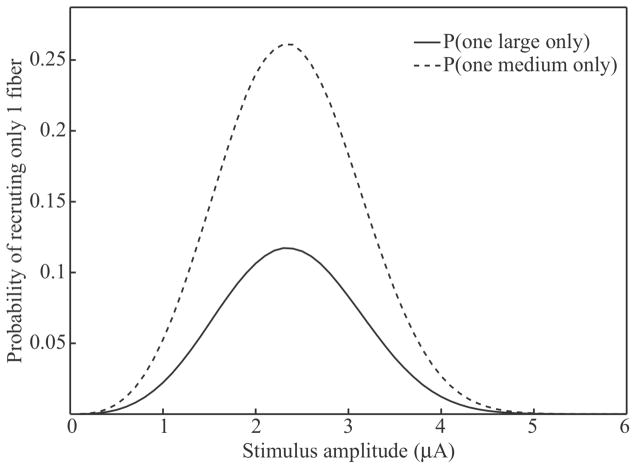
Gaunt and colleagues also observed that in 53% of electrodes tested in 4 animals, medium-diameter fibers were recruited at lower current amplitudes than the larger fibers recruited with the same stimulating electrode (Gaunt et al., 2009). This result suggests a small bias favoring recruitment of medium-diameter fibers when stimulating at the lowest-intensity that yielded an identifiable response (i.e. a so-called threshold response). We examined this bias with the population model by calculating the probability of recruiting only one large (i.e., {fD}large = 12.8 – 16+ μm) or one medium (i.e. {fD}medium = 7.3 – 11.5 μm) diameter fiber in isolation across a range of stimulation amplitudes. Recruitment of exactly one fiber in isolation corresponds to the threshold responses that were recorded in the previous in vivo study. For this analysis, we used a packing ratio of 0.26 to allow direct comparison of the model results to the data set for subject 1 in the in vivo study (see figure 4).
Figure 5 shows the probability of recruiting only one large or medium fiber across stimulation amplitudes (0–6 μA). Both probability curves have a maximum at 2.3 μA and span a range of stimulation amplitudes from approximately 1–4 μA. In the in vivo study, 100% of the threshold responses were found at stimulation amplitudes between 1.5 and 3 μA. Furthermore, 13 of the 24 (54%) threshold responses were from medium diameter fibers. However, the probability curves in Figure 5 suggest an even stronger bias, with the probability of recruiting a medium diameter fiber at threshold more than twice that for large diameter fibers across nearly the entire range of stimulation amplitudes. The nature of this bias will be discussed later (see Discussion).
Figure 5.
Model predictions for the probability of recruiting only 1 large or medium diameter fiber in isolation.
3.3. Multi-fiber recruitment in the DRG
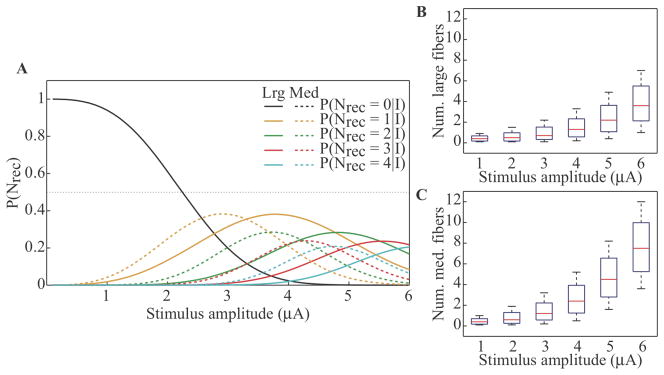
The population model was used further to examine the probability of recruiting different numbers of fibers ranging in size from 7.3 – 16 μm, over a range of stimulus intensities (figure 6a). We chose a packing ratio of 0.2 to represent the average packing ratio determined from fitting the model to in vivo data across all subjects reported by Gaunt and colleagues (Gaunt et al., 2009). Solid and dashed traces in figure 6a show separate distributions for the recruitment of large and medium fibers, respectively. For these simulations, we determined the probability of getting Nrec larger fibers irrespective of the number of medium fibers recruited (solid traces), or vice versa (dashed traces). This plot demonstrates that medium fibers can be recruited at lower intensities than larger fibers even when multiple fibers are being recruited. Furthermore, this difference increases as the number of fibers being recruited increases. The maximum probability for these curves decreases nonlinearly as the number of fibers recruited increases. In addition, the current at which the maximum probability is reached increases at a decaying rate as the number of fibers recruited increases. That is, as more fibers are recruited, there is a trend for the distribution to be flatter and shift less along the abscissa.
Figure 6.
Recruitment of multiple fibers in a heterogeneous population across stimulation amplitudes in the range 1–6 μA. (A) Traces represent the probabilities of recruiting exactly zero, one, two, three, or four fibers. Solid traces represent recruitment of large fibers (12.8 to 16+ μm) while dashed traces represent recruitment of medium fibers (7.3 to 11.5 μm). (B) and (C) Range of numbers of large and medium fibers recruited, respectively, given stimulus amplitude.
Finally, we examined the number of fibers recruited in a typical population of medium and larger DRG fibers (≥ 7.3 μm). The boxplots in figures 6b and 6c show the range for the number of fibers recruited in the large and medium diameter fiber sets, respectively. The boxplots show the 5th, 25th, 50th, 75th and 95th percentiles of the distributions of numbers of fibers recruited. As the current is increased, the median increases exponentially, with growth rates of 0.47 μA−1 and 0.53 μA−1 for large and medium fibers, respectively. Thus, the rate at which additional fibers are recruited depends on the fiber size; as the VoI grows with stimulation intensity, the number of medium fibers having nodes of Ranvier captured within the VoI increases more quickly.
4. Discussion
In developing this computational model, we sought to address questions about the number and sizes of primary afferent fibers recruited by primary afferent microstimulation in the DRG. Previously published computational models were designed to address recruitment in specific peripheral nerve structures using a deterministic approach. Here we specified the stimulus intensity and fiber sizes of interest to define a volume of tissue activated. The model uses a probabilistic approach to estimate recruitment of fibers in the DRG, but it can be adapted readily to model different tissue morphologies given information on the distribution of fiber sizes in the tissue. Finally, we compared model predictions to data collected from in vivo experiments and explored the factors accounting for the pattern of recruitment observed.
4.1. Recruitment order
We began by running simulations to explore the recruitment of fibers based on size, which suggested that smaller fibers were as likely or more likely to be recruited than larger fibers over the range of fiber sizes and stimulus intensities tested. At large stimulus intensities (i.e., I > ~30 μA), the electrode can activate larger fibers at a greater distance compared to smaller fibers, as demonstrated by the current-distance relationship (Li and Bak, 1976). However, at the stimulus intensities that we tested (less than 10 μA) the current-distance relationship was effectively the same for the different fiber diameters (figure 3a).
The difference in probabilities of recruiting different-sized fibers was due to a combination of two main factors: 1) the number of fibers of a given size that are likely to be present in the VoI and 2) the likelihood of a fiber having a node of Ranvier in that VoI. Equation (3) and figure 3b showed that as fiber diameter decreased, the number of fibers that were likely to be packed into a VoI increased. This behavior was influenced by the distribution of fibers by size (NfD). In the case of feline L7 DRG, the distribution of fiber sizes is skewed toward medium size fibers over larger fibers. Equation (7) and figure 3c showed that as fiber size decreased, and accordingly as internodal length decreased, the probability of those fibers having a node of Ranvier in the VoI increased. Because of these two factors, there was a greater likelihood of recruiting a smaller fiber before a larger fiber in our model.
4.2. Recruitment in a population
After examining recruitment order, we explored the contribution of the packing ratio to recruitment in a population. The impact of the packing ratio on predictions of recruitment with the population model is important because of the direct physiological implication of this parameter. We found that the model best fit the recruitment data observed in vivo for a packing ratio of 0.2 (R2 > 0.9). To validate this parameter, we used (4) to calculate a value for the packing ratio based on measurements of ADRG, which are approximately 4.9 – 7.1 mm2 for DRG diameters measured to be 2.5 – 3 mm. Using the fiber diameter distribution data from Risling and colleagues (Risling et al., 1983), the area occupied by fibers larger than or equal to 7.3 μm is approximately 1.15 mm2; this is the value of the numerator in (4). Dividing this area by the cross-sectional area of feline DRG yields a packing ratio of 0.16 – 0.23. This packing ratio range corresponds closely to the range found in our simulations (0.11 – 0.26). Furthermore, using the ‘best-fit’ values for packing ratio and solving (4) for ADRG yields DRG diameters in the range 2.4 – 3.6 mm, which again agrees well with our measured values.
The packing ratio was varied to evaluate the sensitivity of the model to this parameter. As the packing ratio increased, the likelihood of recruiting neurons at lower amplitudes increased because of the greater number of fibers passing through the VoI (figure 4). DRG are heterogeneous structures and the packing ratio represents only an average value; the packing ratio may be higher or lower in different sections of the tissue (see Fig. 1b). For example, the local volume around a stimulating electrode may have a high packing ratio if the electrode is surrounded by a bundle of fibers, or it may have a small packing ratio if there are cell bodies occupying a large portion of the VoI.
To explore the recruitment of different fiber sizes, we simulated a heterogeneous population of neurons, varying the fiber sizes included in the population. Figure 5 demonstrates the different contributions of medium fibers (7.3 μm – 11.5 μm) versus large fibers (12.8 μm – 16+ μm) to the probability of recruiting a fiber in this population. This figure shows that when stimulating at low intensities, the chance of recruiting a single medium fiber is more than twice that of recruiting a single large fiber. Thus, the population model suggests a much stronger bias favoring recruitment of medium fibers than was observed in vivo. One likely source of this discrepancy stems from the differences in the ability to record propagating action potentials from large and medium fibers in a nerve cuff, as was used in our in vivo study. The recorded voltage of an extracellular signal increases in a power law fashion with increasing conduction velocity, which is closely related to fiber size (Milner et al., 1981). Thus, action potentials from smaller fibers are more difficult to detect and it is possible that some active medium fibers may have gone undetected in the nerve cuff recordings used to identify thresholds in the in vivo study.
4.3. Assumptions and limitations
In this paper, we assumed that the extracellular medium was infinite, homogeneous and isotropic (McIntyre and Grill, 2000). Ranck and Bement showed that the extracellular medium of the spinal cord dorsal columns in cat is anisotropic, with a longitudinal resistivity of approximately 300 Ω-cm and a transverse resistivity of approximately 1200 Ω-cm (Ranck and Bement, 1965). This anisotropy would change the shape of the VoI from spherical to ellipsoidal by altering the resistivity parameter (ρext) to have a longitudinal and a transverse component, rather than being direction-insensitive. We altered the model to assume an ellipsoidal VoI with these extracellular resistivity parameters and found no significant difference in the probability of recruiting one or a few fibers (data not shown). For the simulation conditions that we tested and presented in this paper, we felt that this simplifying assumption using a spherical VoI was justified.
We did not include cell bodies in the model, assuming that the site of activation would always occur at the nodes of Ranvier rather than at the soma. Amir and Devor (Amir and Devor, 2003) developed a model for frog DRG neurons to explore the soma’s role in propagating an action potential. We used their model to test the DRG neuron’s response to extracellular stimulation as a function of current amplitude and electrode placement (data not shown). We found that, regardless of current amplitude or electrode position, we could not activate the neuron by stimulating the cell body. In addition, previous work has shown that the site of action potential generation is always at a node of Ranvier and not the cell body (Ranck, 1975, Gustafsson and Jankowska, 1976, Lu et al., 2008). This work led us to assume that only nodes of Ranvier were potential sites for activation in response to extracellular stimulation within the ranges of stimulus parameters tested.
Only stimulus amplitudes less than 100 μA were tested with the single-fiber model and less than 10 μA were tested for the population model. The larger amplitude range (0 – 100 μA) was tested with the single-fiber model to explore the current-distance relationship over a wide range of currents. The smaller current range, less than 10 μA, was of particular interest here because intraneural microstimulation experiments have demonstrated that this lower current range is sufficient to elicit neuronal responses (McDonnall et al., 2004a, Gaunt et al., 2009). In addition, stimulation currents less than 6 μA accounted for 97% of the threshold responses reported for in vivo experiments (Gaunt et al., 2009). Of equal relevance, the single-fiber model predicts that as the current intensity is increased above 10 μA, the distance an electrode can be from the fiber to achieve recruitment increases beyond 200 μm (figure 3a). Commercially available electrode arrays have inter-electrode spacing ranging from 200–400 μm (Mushahwar et al., 2007, Gaunt et al., 2009). This spacing appears to be appropriate based on the model’s results for recruiting small numbers of neurons at stimulus amplitudes below 10 μA, though different recruitment circumstances may require that this spacing be altered.
5. Conclusions
We have developed a model that predicts recruitment of sensory fibers in the DRG in response to extracellular microstimulation. The model offers some insights into the factors governing recruitment in a mixed population of fibers. Our results indicate that at low intensities (< 10 μA), smaller fibers are more likely to be recruited as compared to larger fibers over the ranges of fiber sizes and distributions considered here. Furthermore, the results from these simulations suggest that previous in vivo studies may have underestimated the chance of recruiting medium diameter fibers with primary afferent microstimulation in the DRG. The model was also able to simulate the recruitment of multiple fibers, which can be used to predict, for a given stimulus condition, the most likely number of fibers, by size, that will be recruited.
The model has a number of potential applications. This model could be used to aid the design of microelectrode arrays, taking advantage of the model’s ability to predict the number and size of axons recruited as a function of stimulus intensity. The model is able to provide information on the size of the VoI and thus the geometry of an array could be designed to minimize overlap between adjacent VoIs while maximizing specificity for target fibers. Besides stimulus intensity, other stimulus parameters may be tested, such as pulsewidth or polarity, on the effects of recruiting neurons in a population. In addition, other fiber sizes (less than 7.3 μm) may be incorporated into the model to represent other sensory modalities, such as nociceptors, thermal receptors, or other primary afferent fibers. For example, it is currently difficult to ascertain the degree to which we are recruiting pain fibers in these primary afferent microstimulation paradigms. Given the distribution of nociceptive fibers, the population model could offer some insight into the probability of activating these other fiber types.
Acknowledgments
Funding for this project was provided by NIH NS056136, NIH EB007749, TATRC W81XH-07-1-0716 and through an IGERT training fellowship under NSF grant DGE-0549352. The authors would like to thank Dr. G. Bard Ermentrout for advice on the development of the model; Dr. Robert Gaunt for addressing questions relating to the electrophysiological recruitment experiments; Dr. Tim Bruns for providing constructive feedback in writing this paper; and Erika Rost for tissue processing and histology.
Appendix
The appendix provides additional information on the derivation of equations used to estimate the probability of capturing a node in the VoI. Additionally, we provide an example to illustrate how we estimated the probability of recruiting a given number of fibers from a set of fiber sizes as a function of stimulus amplitude.
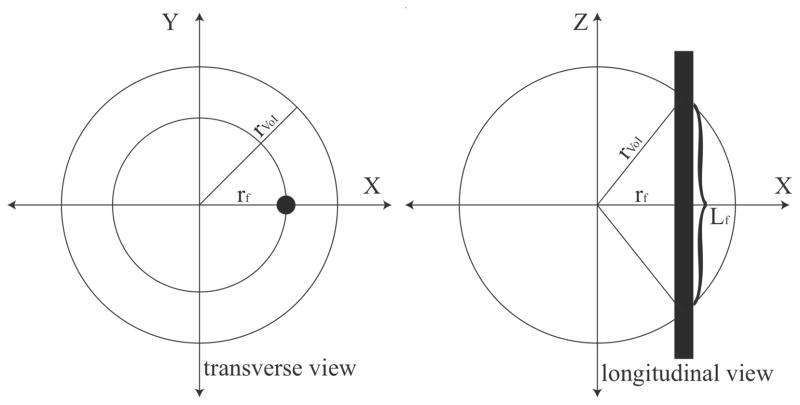
To describe the probability of a fiber of given size having a node of Ranvier in the spherical VoI at a certain radial distance from its center, P(node|I,fD,rf), we divided the length of fiber encapsulated within the VoI (Lf) by the internodal length of the fiber (Lint) as in
| (A.1) |
We expanded on the definition of Lf
| (A.2) |
using the Pythagorean Theorem with the radial position of the fiber in the transverse plane (rf) and the radius of the spherical VoI (rVoI) as the hypotenuse (see figure A.1). We then multiplied the probability of finding a node of Ranvier by the circumference of the circle with radius rf, then integrated with respect to rf over the range [0, rVoI]
Figure A.1.
Schematic depiction of fiber passing through spherical volume of tissue activated (VoI). The outer circle represents the boundary of the VoI, while the filled circle (transverse view) and the thick line (longitudinal view) represent the fiber of passage. The probability of a fiber having a node of Ranvier in the VoI is the ratio of the length of fiber contained within the VoI (Lf) to the internodal length of the fiber (Lint). This probability must be calculated for all possible locations of the fiber in the VoI to get a total average probability.
| (A.3) |
to determine an average value of this probability. The integral was evaluated, using (A.2), to
| (A.4) |
and then simplified to
| (A.5) |
However, if the diameter of the spherical VoI was larger than the internodal length of the fiber, then the probability of finding a node might exceed 1. We capped this probability at 1 by first setting (A.1) equal to 1 as in
| (A.6) |
and then solving for rf
| (A.7) |
The radial position (rf) at which the length of fiber encapsulated within the spherical VoI is equal to the internodal length is denoted as rL. The integral from (A.3) was separated into two integrals to get
| (A.8) |
In the first integral, the length of fiber encapsulated within the sphere was set to the internodal length and the integral was taken over the range [0, rL]. The second integral determined the average probability as in (A.3), but over the range [rL, rVoI]. Equation (A.8) was evaluated and then simplified to
| (A.9) |
In the population model, (A.9) was used to describe the probability of a fiber having a node of Ranvier within the VoI in the case where the diameter of the VoI was larger than the internodal length of the specified fiber. Note that (A.3–5) are only valid for Lint greater than 2rVoI, whereas (A.8) and (A.9) are valid for Lint less than or equal to 2rVoI. In fact, (A.9) simplifies to (A.5) if rL is set to zero; rL approaches zero as the interval of rf values over which the entire internodal length is captured by the VoI approaches zero.
Finally, using probabilities from (A.9), we estimated the probability of recruiting a given number of fibers from a set of fiber sizes as a function of stimulus amplitude (see 9). As an example, let us assume that there are three fiber sizes in a population, termed A, B and C. To determine the probability of recruiting two fibers among A and B, but no C fibers, we begin by finding that there are three ways to get two fibers: one A and one B; two A’s and zero B’s; zero A’s and two B’s. For each combination, the probabilities for each neuron’s state are multiplied; these probabilities are then summed across combinations. The equation to get the probability of recruiting two fibers among A or B, without getting any C fibers is expressed as
| (A.10) |
This example illustrates the general means by which we determined the probability of recruiting a given number of fibers from a group of specified fiber sizes, given the probabilities of recruiting various numbers of fibers for all fibers of interest at the specified current level.
Contributor Information
D J Bourbeau, Email: dennisbourbeau@gmail.com.
J A Hokanson, Email: jim.hokanson@gmail.com.
J E Rubin, Email: rubin@math.pitt.edu.
D J Weber, Email: djw50@pitt.edu.
References
- AMIR R, DEVOR M. Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophys J. 2003;84:2181–91. doi: 10.1016/S0006-3495(03)75024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD IA, KALU KU. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol. 1979;289:277–97. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTSON CR, MCINTYRE CC. Tissue and electrode capacitance reduce neural activation volumes during deep brain stimulation. Clin Neurophysiol. 2005;116:2490–500. doi: 10.1016/j.clinph.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTSON CR, MILLER IO, NORMANN RA, CLARK GA. Selective neural activation in a histologically derived model of peripheral nerve. J Neural Eng. 2011;8:036009. doi: 10.1088/1741-2560/8/3/036009. [DOI] [PubMed] [Google Scholar]
- CLARK GM, HALLWORTH RJ, ZDANIUS K. A cochlear implant electrode. J Laryngol Otol. 1975;89:787–92. doi: 10.1017/s0022215100081020. [DOI] [PubMed] [Google Scholar]
- CREASEY G, ELEFTERIADES J, DIMARCO A, TALONEN P, BIJAK M, GIRSCH W, KANTOR C. Electrical stimulation to restore respiration. J Rehabil Res Dev. 1996;33:123–32. [PubMed] [Google Scholar]
- ERLANGER J, BLAIR EA. Comparative observations on motor and sensory fibers with special reference to repetitiousness. American Journal of Physiology. 1938;121:431–453. [Google Scholar]
- GAUNT RA, HOKANSON JA, WEBER DJ. Microstimulation of primary afferent neurons in the L7 dorsal root ganglia using multielectrode arrays in anesthetized cats: thresholds and recruitment properties. J Neural Eng. 2009;6:55009. doi: 10.1088/1741-2560/6/5/055009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON B, JANKOWSKA E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINES ML, CARNEVALE NT. NEURON: a tool for neuroscientists. Neuroscientist. 2001;7:123–35. doi: 10.1177/107385840100700207. [DOI] [PubMed] [Google Scholar]
- HOUSE WF, URBAN J. Long term results of electrode implantation and electronic stimulation of the cochlea in man. Ann Otol Rhinol Laryngol. 1973;82:504–17. doi: 10.1177/000348947308200408. [DOI] [PubMed] [Google Scholar]
- LI CL, BAK A. Excitability characteristics of the A- and C-fibers in a peripheral nerve. Exp Neurol. 1976;50:67–79. doi: 10.1016/0014-4886(76)90236-3. [DOI] [PubMed] [Google Scholar]
- LU H, CHESTEK CA, SHAW KM, CHIEL HJ. Selective extracellular stimulation of individual neurons in ganglia. J Neural Eng. 2008;5:287–309. doi: 10.1088/1741-2560/5/3/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONNALL D, CLARK GA, NORMANN RA. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans Neural Syst Rehabil Eng. 2004a;12:208–15. doi: 10.1109/TNSRE.2004.828425. [DOI] [PubMed] [Google Scholar]
- MCDONNALL D, CLARK GA, NORMANN RA. Selective motor unit recruitment via intrafascicular multielectrode stimulation. Can J Physiol Pharmacol. 2004b;82:599–609. doi: 10.1139/y04-047. [DOI] [PubMed] [Google Scholar]
- MCINTYRE CC, GRILL WM. Selective microstimulation of central nervous system neurons. Ann Biomed Eng. 2000;28:219–33. doi: 10.1114/1.262. [DOI] [PubMed] [Google Scholar]
- MCINTYRE CC, GRILL WM, SHERMAN DL, THAKOR NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–69. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- MCINTYRE CC, RICHARDSON AG, GRILL WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol. 2002;87:995–1006. doi: 10.1152/jn.00353.2001. [DOI] [PubMed] [Google Scholar]
- MCNEAL DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976;23:329–37. doi: 10.1109/tbme.1976.324593. [DOI] [PubMed] [Google Scholar]
- MCNEAL DR, WATERS R, RESWICK J. Experience with implanted electrodes. Neurosurgery. 1977;1:228–9. doi: 10.1097/00006123-197709000-00029. [DOI] [PubMed] [Google Scholar]
- MEIER JH, RUTTEN WL, ZOUTMAN AE, BOOM HB, BERGVELD P. Simulation of multipolar fiber selective neural stimulation using intrafascicular electrodes. IEEE Trans Biomed Eng. 1992;39:122–34. doi: 10.1109/10.121643. [DOI] [PubMed] [Google Scholar]
- MICERA S, NAVARRO X. Bidirectional interfaces with the peripheral nervous system. Int Rev Neurobiol. 2009;86:23–38. doi: 10.1016/S0074-7742(09)86002-9. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOKS JC, SNYDER RL. Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci. 2010;30:1937–46. doi: 10.1523/JNEUROSCI.4949-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER TE, STEIN RB, GILLESPIE J, HANLEY B. Improved estimates of conduction velocity distributions using single unit action potentials. J Neurol Neurosurg Psychiatry. 1981;44:476–84. doi: 10.1136/jnnp.44.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSHAHWAR VK, JACOBS PL, NORMANN RA, TRIOLO RJ, KLEITMAN N. New functional electrical stimulation approaches to standing and walking. J Neural Eng. 2007;4:S181–97. doi: 10.1088/1741-2560/4/3/S05. [DOI] [PubMed] [Google Scholar]
- NAVARRO X, KRUEGER TB, LAGO N, MICERA S, STIEGLITZ T, DARIO P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–58. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- PANIZZA M, NILSSON J, ROTH BJ, GRILL SE, DEMIRCI M, HALLETT M. Differences between the time constant of sensory and motor peripheral nerve fibers: further studies and considerations. Muscle Nerve. 1998;21:48–54. doi: 10.1002/(sici)1097-4598(199801)21:1<48::aid-mus7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- PANIZZA M, NILSSON J, ROTH BJ, ROTHWELL J, HALLETT M. The time constants of motor and sensory peripheral nerve fibers measured with the method of latent addition. Electroencephalogr Clin Neurophysiol. 1994;93:147–54. doi: 10.1016/0168-5597(94)90078-7. [DOI] [PubMed] [Google Scholar]
- PECKHAM PH, MORTIMER JT, MARSOLAIS EB. Controlled prehension and release in the C5 quadriplegic elicited by functional electrical stimulation of the paralyzed forearm musculature. Ann Biomed Eng. 1980;8:369–88. doi: 10.1007/BF02363440. [DOI] [PubMed] [Google Scholar]
- RANCK JB., JR Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- RANCK JB, JR, BEMENT SL. The Specific Impedance of the Dorsal Columns of Cat: An Inisotropic Medium. Exp Neurol. 1965;11:451–63. doi: 10.1016/0014-4886(65)90059-2. [DOI] [PubMed] [Google Scholar]
- RISLING M, ALDSKOGIUS H, HILDEBRAND C, REMAHL S. Effects of sciatic nerve resection on L7 spinal roots and dorsal root ganglia in adult cats. Exp Neurol. 1983;82:568–80. doi: 10.1016/0014-4886(83)90081-x. [DOI] [PubMed] [Google Scholar]
- SCHIEFER MA, TRIOLO RJ, TYLER DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng. 2008;16:195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUH YS, CHUNG K, COGGESHALL RE. A study of axonal diameters and areas in lumbosacral roots and nerves in the rat. J Comp Neurol. 1984;222:473–81. doi: 10.1002/cne.902220402. [DOI] [PubMed] [Google Scholar]
- TYLER DJ, DURAND DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- VEALE JL, MARK RF, REES S. Differential sensitivity of motor and sensory fibres in human ulnar nerve. J Neurol Neurosurg Psychiatry. 1973;36:75–86. doi: 10.1136/jnnp.36.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELTINK PH, VAN VEEN BK, STRUIJK JJ, HOLSHEIMER J, BOOM HB. A modeling study of nerve fascicle stimulation. IEEE Trans Biomed Eng. 1989;36:683–92. doi: 10.1109/10.32100. [DOI] [PubMed] [Google Scholar]
- VERAART C, GRILL WM, MORTIMER JT. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans Biomed Eng. 1993;40:640–53. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]
- YOSHIDA K, HORCH K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans Biomed Eng. 1993;40:492–4. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]