Abstract
B cells play important roles in autoimmune diseases ranging from multiple sclerosis to rheumatoid arthritis. B cells have long been considered central players in systemic lupus erythematosus. However, anti-CD20 mediated B cell depletion was not effective in two clinical lupus studies, while anti-BLyS, which inhibits B cell survival, was effective. Others and we previously found that anti-CD20 based depletion was surprisingly ineffective in tissues of lupus-prone mice, but that persistent high doses eventually led to depletion and ameliorated lupus. Lupus patients might also have incomplete depletion, as suggested in several studies, and which could have led to therapeutic failure. Here we investigated the mechanism of resistance to Ab-mediated cellular depletion in murine lupus. B cells from lupus-prone mice were easily depleted when transferred into normal environments or in lupus-prone mice that lacked serum Ig. Serum from lupus-prone mice transferred depletion resistance, with the active component being IgG. Because depletion is FcγR-dependent, we assayed macrophages and neutrophils exposed to lupus mouse serum, showing they are impaired in IgG-mediated phagocytosis. We conclude that depletion resistance is an acquired, reversible phagocytic defect depending on exposure to lupus serum IgG. These results have implications for optimizing and monitoring cellular depletion therapy.
Introduction
B cells play a critical role in a variety of autoimmune diseases (1, 2). The requirement for B cells was originally demonstrated in animal models lacking B cells from birth (3, 4). The concept that B cells promote autoimmunity was later extended to humans when therapeutic B cell depletion became possible. B cell targeting has shown promise in a variety of diseases (1, 2). However, it is neither effective in all patients nor necessarily in all autoimmune diseases (5). Surprisingly, treatment with an anti-human CD20 (hCD20)1 antibody (Ab), rituximab, did not show efficacy in systemic lupus erythematosus (SLE) in two controlled studies (6, 7), although it appeared effective in anecdotal studies (5, 7). These results in patients are unexpected, because genetic deletion of B cells in lupus-prone MRL mice eliminates disease and is more effective than any other genetic intervention reported in this strain (3, 4, 8). Notably, two trials using an anti-B Lymphocyte Stimulator (BLyS) Ab (belimumab), which targets B cells, did show efficacy in SLE (7). The reasons why rituximab has not proved effective in SLE, while belimumab has worked, are unclear.
Animal models would enable insight into these issues. For this purpose two groups developed similar strains of mice in which hCD20 was expressed via a transgenic bacterial artificial chromosome (9, 10). B cells can be depleted in these animals using anti-hCD20. Two groups have also developed murine CD20 mAbs that can deplete B cells (10, 11). These models provided insight into the mechanisms by which B cell depletion ameliorates autoimmune disease. They also elucidated the mechanisms of in vivo depletion, which mainly depend on Fcγ receptor (FcγR)-mediated phagocytosis of opsonized B cells (9, 11).
A number of groups have used these approaches to demonstrate efficacy of B cell depletion in murine models of lupus (10, 12, 13). Although these studies showed definite effects of B cell depletion, others and we were surprised to find that it was relatively difficult to deplete B cells in lupus-prone mice (10, 13), including MRL/MpJ-Faslpr (MRL.Faslpr), MRL/MpJ.Faswt, and NZB/W. Mild defects in B cell depletion were also seen in NOD mice, another spontaneous model of autoimmunity (14). In the case of MRL.Faslpr mice, even high doses of anti-CD20 did not reverse the defect acutely; strikingly, B cells that were fully coated with Ab were not cleared in these animals. However, persistent administration of high doses of Ab did eventually lead to depletion, which became apparent between 7 and 10 weeks of sustained treatment. At these time points, a progressive therapeutic effect was also observed, demonstrating that B cells can be a therapeutic target in ongoing disease (10). These observations suggest that there is a kinetic, but not absolute block in the clearance of B cells in lupus mice. The block to B cell depletion is age-dependent, like disease itself, although defects are also observed in young lupus-prone mice. Lupus-prone strains that have less severe disease also show a more mild deficiency in B cell depletion. Taken together, these results suggest that the disease process itself could be responsible for ineffective therapeutic B cell depletion (10, 13).
Given the difficulty in depleting B cells in murine lupus models, it seems possible that similar issues could be at play in lupus patients. There are anecdotal reports of poor B cell depletion in peripheral blood (PB) of treated patients, and that poor depletion correlated with poor clinical response (15-17). One caution is that very limited information is available about B cell depletion in secondary lymphoid tissues of patients (16). In this regard, mouse studies suggest that assessment of PB overestimates B cell depletion in tissues in the context of lupus (10).
If the mechanism that obstructs B cell depletion were understood, this would be useful for devising strategies to enhance the therapeutic effect of B cell depletion. Elucidation of the mechanism might also provide insight into the disease itself, as inability to deplete B cells likely relates to generalized defects in cellular clearance, a pathway that is involved in lupus pathogenesis (18). Here, we have used variations on the MRL.Faslpr model, including in vivo and in vitro studies, to investigate the reasons for defective B cell depletion in lupus. Our findings have implications for cellular depletion therapy as well as for understanding the immune dysregulation underlying lupus.
Materials and Methods
Mice
Human CD20 (hCD20) BAC transgenic mice (10) were backcrossed >20 generations either on MRL.Faslpr or BALB/c backgrounds. The Jh-/- (19) mice were backcrossed >20 generations with MRL.Faslpr as described (20). The mIgM mice (20) lack circulating antibodies, by virtue of expression of a BCR transgene that lacks the secreted exon of IgM, were also maintained on a Jh-/- background, and were fully backcrossed to MRL.Faslpr. hCD20.MRL.Faslpr mice were crossed with mIgM mice to produce hCD20.mIgM mice, which were maintained as Jh-/- on the MRL.Faslpr background. MRL.Faslpr mice were purchased from Jackson Laboratories, BALB/c from Charles River Laboratories and C.C3H-H2k (BALB.K) from Harlan, UK. F1 progeny of BALB/c x BALB.K mice were used as recipients. All mouse experiments were approved by the Yale Institutional Care and Use Committee.
Immunodepletion
Mice were injected with either 2H7 (IgG2b) or 18B12 (IgG1) monoclonal Abs (10, 21) in sterile PBS as described in figure legends. Age-matched, control mice were treated simultaneously with same amount of total mouse IgG (Rockland) dialyzed into PBS.
Serum Transfer
Serum from BALB/c (>6 wk old) or old MRL.Faslpr (>14 wk old) mice was collected either by retroorbital or cardiac puncture and was filter-sterilized. Pooled BALB/c and MRL.Faslpr sera were analyzed for Ig isotypes, anti-Smith (Sm) Abs, and anti-nucleosome Abs as described (22). Presence of anti-nuclear and anti-DNA Ab in MRL.Faslpr serum was confirmed using indirect immunofluorescence of fixed HEp-2 cells (Antibodies, Inc.) on coded samples, as described (22). 0.5 ml of BALB/c or MRL.Faslpr serum was injected i.v. into hCD20.BALB/c mice on d0-d6, once/day. In a similar experiment, mIgM mice received 1 ml of donor serum (BALB/c or MRL.Faslpr) on d0, followed by 0.5 ml on d2-d6, once/day.
Separation of IgG and non-IgG components of serum followed by transfer and immunodepletion
Sera from 4 month and older MRL.Faslpr mice were pooled and passed through a 0.45μ filter to remove particulates and run over a Protein G column (Bio-Rad) equilibrated in binding buffer (20mM Na Phosphate pH 7.0). The flow-through was collected as the non-IgG fraction. After washing, the column was eluted with 0.1 M Glycine-HCl buffer at pH 2.7 and eluates immediately neutralized with 0.5M Tris, pH 8.0 (IgG fraction). Both fractions were spin dialyzed into PBS, and the volume of each fraction was adjusted to equal the volume of the serum that had been applied to the column. The purity of the fractions was checked by ELISA for IgG and SDS-PAGE gel. ELISA results showed that, compared to intact serum, >90% of the IgG was recovered in IgG fraction and 1% IgG was in the non-IgG fraction (not shown). Protein gel results demonstrated a very high degree of purity of the IgG fraction and a high level of depletion from the non-IgG fraction (Supplemental Fig. 1).
6-10 wk old hCD20 Tg BALB/c mice, or Tg-negative controls, were randomly divided into four groups, to receive: a) PBS, b) pooled, unfractionated serum, c) IgG fraction or d) non-IgG fraction. 0.5 ml was administered i.p. every day from d0 to d6. On d7, 0.5 mg 2H7 was given, i.v. to each mouse. On d11, mice were sacrificed and splenocytes, LN cells and PB were analyzed by FACS. For comparison purposes, untreated mice were also analyzed at same time.
Cell Transfer
B cells were purified to >90% purity from the spleens of hCD20.BALB/c and hCD20.MRL.Faslpr mice with the EasySep Negative Selection Mouse B Cell Enrichment Kit (StemCell Technologies), followed by labeling with either carboxyfluorescein succinimide ester (CFSE, Invitrogen) or CellTracker Orange (5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine, (CMTMR, Invitrogen), according to manufacturer's instructions. Approximately equal numbers of CFSE and CMTMR labeled cells were injected into the recipients on d0. Mice were treated with a single i.v. dose of 100 μg of 2H7 the following morning and sacrificed on d3.
Phagocytosis assay
Fresh SRBCs (MP Biomedicals) were labeled with PKH26 (Sigma-Aldrich) according to the manufacturer's instructions. The labeling reaction was stopped with RPMI1640 containing 1% bovine serum albumin. SRBCs were then opsonized with rabbit anti-SRBC IgG (Fitzgerald Industries) or used non-opsonized. CD11b+ splenocytes were isolated from mice as indicated using streptavidin microbeads (Miltenyi) after labeling with biotinylated anti-CD11b (M1/70). CD11b+ cells were then incubated together with SRBCs at a ratio of 1:2 in serum free RPMI 1640 for 60 min at 37 °C. Phagocytosis was stopped with ice-cold 2 mM EDTA in PBS. SRBCs that had not been internalized were lysed with ammonium chloride buffer.
FACS analysis
Abs to CD4 (GK1.5), CD5 (53.7), CD8 (TIB105), CD21 (7G6), CD22 (2D6), IgMa (RS3.1), CD11b (M1/70), and FcγR (2.4G2) were prepared and labeled in our lab, as described (23). The mAb against mCD20 (18B12, mouse IgG1) and hCD20 (2H7, Mouse IgG2b) were generated and purified as described (10, 21). The 2H7 hybridoma was a gift from Dr. E. Clark, University of Washington, Seattle, WA. Abs against CD3e (145-2C11), CD19 (1D3.2), CD23 (B3B4), F4/80 and Gr-1 were purchased from BD Pharmingen. Anti-mouse IgD (11-26c.2a), anti-mouse CD11c (N418) and streptavidin PE/Cy7 were from BioLegend and streptavidin Alexa 647 was from eBiosciences. Samples were analyzed on FACSCalibur or LSR II (BD Biosciences) and the data were analyzed using FlowJo software (TreeStar).
Results
Reduced efficacy of Ab-mediated depletion in MRL.Faslpr mice could stem from defects in elements of the clearance mechanisms, such as macrophages or FcγRs; or cell-intrinsic resistance by lymphocytes to depletion. We designed a series of experiments to distinguish among these possibilities. Our central hypothesis was that, in lupus, an excess of immune complexes (ICs) disables FcγR function on macrophages (and other phagocytic cells), limiting the ability of macrophages to deplete even fully opsonized cells.
Depletion is restored in MRL.Faslpr mice that have B cells but lack Ab
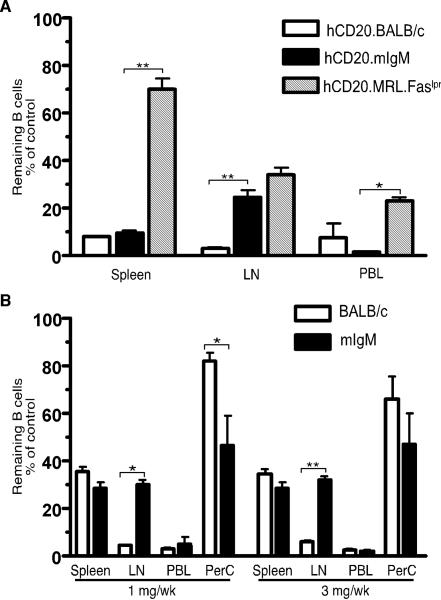
If our hypothesis were correct, mIgM.MRL.Faslpr mice that have B cells and systemic disease but lack secreted Ab (20), would be susceptible to B cell depletion (Fig. 1). To test this we crossed the hCD20 Tg onto this background and used anti-hCD20 (2H7) to deplete B cells. Treatment of 6-8 wk old hCD20.mIgM.MRL.Faslpr and hCD20.BALB/c mice with 4 mg/wk of 2H7 for 2 wks resulted in >90% B cell depletion in spleen and PB for both strains while hCD20.MRL.Faslpr mice were significantly more refractory to depletion (spleen, 30%; PB, 47%, Fig. 1A). B cells in LN of hCD20.mIgM mice had an intermediate susceptibility to depletion (76%) compared to the hCD20.BALB/c mice (97%) and hCD20.MRL.Faslpr mice (66%). Similar results were obtained in a second set of experiments in which we treated 6-8 wk old mIgM.MRL.Faslpr mice with either 1 mg/wk or 3 mg/wk of anti-mCD20, 18B12, twice weekly for 2 wks (depletion was: spleen, 70%; LN, 70%; PB, >95%; peritoneal cavity >50%, Fig. 1B). B cell depletion in BALB/c from a previously reported similar experiment (Fig. 3c of (10)) is shown for comparison. Again, depletion in the spleen, peritoneal cavity and PB was comparable in the mIgM.MRL.Faslpr and BALB/c strains. Depletion in the LN was less effective in mIgM.MRL.Faslpr mice, a difference that could relate to the accumulation of DN T cells in the enlarged LN that occurs in lpr mice, which might prevent macrophages from coming in contact with B cells (9).
Figure 1.
Efficient depletion of B cells in MRL.Faslpr mice lacking circulating Ab. (A) Six-to-eight wk old hCD20.BALB/c (n=2), hCD20.MRL.Faslpr (n=5) and hCD20.mIgM.MRL.Faslpr (n=6 for treated, n=4 for control, referred to as hCD20.mIgM on the figure label) mice were treated with 4mg/wk of 2H7 Ab i.p., given in divided doses twice/wk for 2 wks. Residual CD22+ B cells in spleen, LN and PB, as percentage of the average B cells in IgG treated, age-matched control mice are shown. The hCD20.BALB/c and hCD20.MRL.Faslpr data are derived from ref. (10) Fig. 2c and Fig. 3b, respectively, and are shown for comparison. (B) Six-to-eight wk old mIgM (n>6) and BALB/c (n>3) mice were treated with either 1 mg/wk or 3 mg/wk of 18B12 mAb i.p., twice/wk for 2 wks. Residual CD19+ B cells are plotted as in panel A for all sites including peritoneal cavity (PerC). The BALB/c data are derived from ref. (10) Fig. 3c, and are shown for comparison. Data are from 1 experiment for hCD20.mIgM and 2 for mIgM mice. Error bars represent SEM. *, p < 0.05; **, p < 0.01 by Mann-Whitney Test.
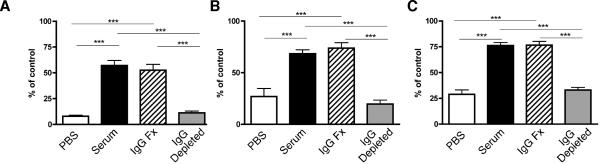
Figure 3.
The active component in serum contains IgG. Serum from older MRL.Faslpr mice was collected and separated into IgG and non-IgG containing fractions. Unfractionated serum (black bars), IgG fraction (hatched bars), non-IgG fraction (gray bars) or PBS (open bars) was then infused into hCD20.BALB/c mice as described in Methods. Mice were then treated with 2H7 and the fraction of B cells in the indicated tissues was measured by FACS as previously reported (10) and is plotted as a percent of the average of the value of untreated control mice analyzed simultaneously. (A) PB, (B) LN, and (C) Spleen. Bars show the mean and error bars the SEM. Data combined from two independent experiments with n=7-8 mice per group. ***, p <0.0001 by Student's unpaired t test.
Taken together, these experiments demonstrate that in the absence of circulating antibodies B cells are readily depleted even in autoimmune-prone mice. In light of the finding that despite identical genetic backgrounds, depletion efficacy is markedly different in MRL.Faslpr and mIgM.MRL.Faslpr mice, which differ in the presence or absence of serum IgM and not in other disease parameters (20), we conclude that genetic aspects of the MRL.Faslpr strain background alone cannot explain the defects in B cell depletion and that a factor related to secreted Ig must play an important role.
Serum from lupus-prone, but not the normal mice, blocks depletion
The prior results implicate serum IgG in the inhibition of B cell depletion in a lupus-prone background. To obtain direct evidence for this, we transferred serum from lupus-prone or normal mice to a host that is normally capable of depletion and assessed the ability of this serum to inhibit subsequent depletion. We initially chose to use serum rather than purified IgG, as it seemed possible that isolation of the IgG could change its properties (e.g. by dissociating from ICs), or that serum could contain important non-IgG factors.
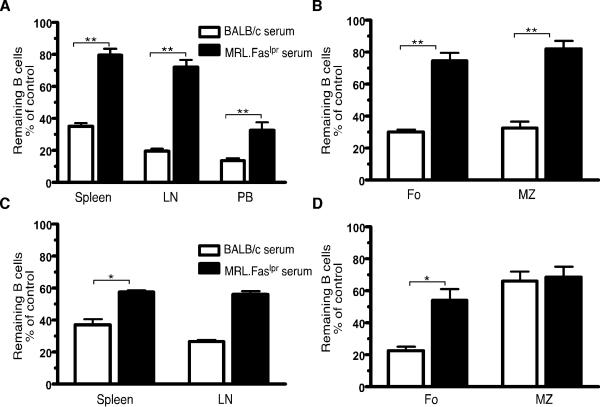
Eight-10 wk old hCD20.BALB/c mice and 9-11 wk old mIgM.MRL.Faslpr mice were infused with serum from BALB/c (>6 wk old) or MRL.Faslpr (>14 wk old) mice (Fig. 2). As expected, the MRL.Faslpr serum had elevated concentrations of total IgG, as well as anti-DNA Ab, anti-chromatin Ab, anti-nucleosome Ab and anti-Sm Ab compared to the BALB/c serum, which was negative for these autoantibodies. On d7 after serum infusion, mice were treated with a single i.v. dose of 0.5 mg of 2H7 (hCD20.BALB/c mice, Fig. 2A, 2B) or 18B12 (mIgM.MRL.Faslpr mice, Fig. 2C, 2D). Enumeration of B cells on d11 showed that infusion of MRL.Faslpr serum significantly and very substantially inhibited B cell depletion in spleen, LN and PB (Fig. 2A and 2C). In the spleen, inhibition of depletion was seen both in follicular and marginal zone subpopulations in hCD20.BALB/c mice (Fig. 2B) but only in follicular B cells in mIgM.MRL.Faslpr mice (Fig. 2D). Therefore, serum transfer can render a permissive recipient substantially less capable of B cell depletion. MRL.Faslpr serum was potent in these experiments compared to that of BALB/c.
Figure 2.
Serum from lupus-prone mice blocks B-cell depletion in BALB/c mice and MRL.Faslpr mice lacking serum Ab. (A, B) Serum from BALB/c or aged MRL.Faslpr mice was transferred into eight-to-ten wk old hCD20.BALB/c (n=5) prior to treatment with a single dose of 0.5 mg 2H7 i.v. on d7. Residual CD22+ B cells in spleen, LN and PB (A), and splenic CD19+ B cell subpopulations (Fo: Follicular cells and MZ: Marginal zone cells, defined as CD23hiCD21lo and CD23loCD21hi respectively) (B) were analyzed on d11. (C, D) Serum from BALB/c or aged MRL.Faslpr mice was transferred into nine-to-eleven wk old mIgM MRL.Faslpr Tg mice (n>3) prior to treatment with a single dose of 0.5 mg 18B12 i.v. on d7. Residual CD22+ B cells in spleen and LN (C), and splenic B cell subpopulations (D) were analyzed on d11. Residual B cells in all cases are calculated as percentage of the average B cells in control-IgG treated, age-matched mice (infused with same serum). Error bars represent SEM. *, p < 0.05; **, p < 0.01 by Mann-Whitney Test.
The active component in serum that blocks B cell depletion is IgG
We hypothesized that the active factor in serum mediating inhibition of depletion contained IgG. Alternatively, as MRL.Faslpr mice have intense inflammation, it was possible that the component was cytokine-derived. To test this, we depleted IgG from pooled serum of older MRL.Faslpr mice using protein G (Supplemental Fig. 1). hCD20.BALB/c mice were then infused with either the depleted serum (non-IgG fraction) or the IgG fraction. Control mice received either unfractionated serum or PBS. Recipients were then given anti-hCD20, i.v., as above (Fig. 2) and assessed 4 days later for depletion. As shown in Fig. 3, the unfractionated serum inhibited depletion, confirming results in Fig. 2. Critically, the IgG-containing eluate inhibited B cell depletion to the same degree as the unfractionated serum. Conversely, serum depleted of IgG had no effect, demonstrating that none of the “non-IgG components” were capable of inhibiting B cell depletion with anti-CD20.
Both MRL.Faslpr and BALB/c B cells are efficiently depleted in a permissive host
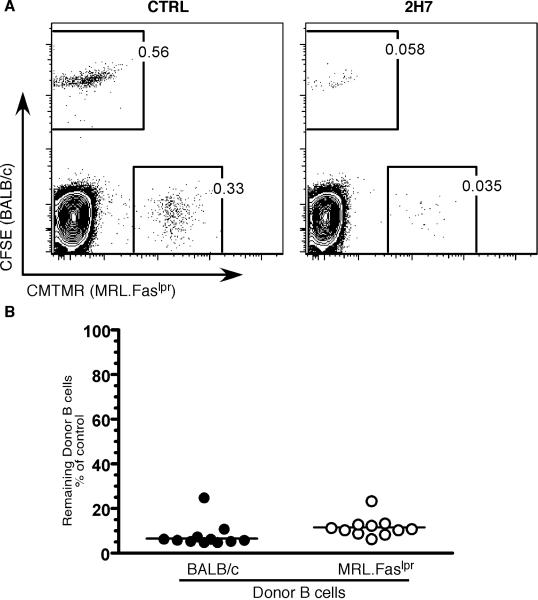
The above experiments did not test whether inherent resistance in the B cells of MRL.Faslpr mice could also contribute to defective B cell depletion. Such B cell resistance could have been genetic, or acquired over time as a result of B cell exposure to the autoimmune-prone environment. To test whether MRL.Faslpr B cells were more resistant to depletion, we compared the ability of MRL.Faslpr or BALB/c B cells to be depleted upon transfer to a permissive environment. To generate a strain that would accept B cells from both MRL.Faslpr and BALB/c mice, we crossed BALB.K mice (BALB/c congenic for the H2k haplotype matching that of MRL.Faslpr ) with BALB/c mice to obtain H2dxk heterozygous BALB/c mice and used these as the recipient strain. Purified B cells from hCD20.BALB/c and hCD20.MRL.Faslpr mice were labeled with CFSE and CMTMR, respectively, prior to co-transfer. The recipient mice were treated on d1 with 100 μg of 2H7 or control IgG Ab i.v. and analyzed for B cell depletion on d3 (Fig. 4). Mice treated with 2H7 showed ~90% depletion of both hCD20.BALB/c and hCD20.MRL.Faslpr donor B cells, compared to the control IgG treated mice (Fig. 4A). It should be noted that the CD19+, CFSE-, CMTMR- cells in these plots are recipient B cells. A summary of B cell depletion from 11 mice is shown in Fig. 4B. The similar degrees of depletion indicate that there is no inherent difference, whether genetic or acquired, in the susceptibility of B cells from either lupus-prone or normal mice. Rather, when transferred to a permissive environment, MRL.Faslpr B cells are readily depleted; our prior studies demonstrated that the same MRL.Faslpr B cells are dramatically resistant to depletion when they remain in situ in their original hosts (10).
Figure 4.
Resistance to depletion is not a B cell intrinsic trait. B cells from six-to-eight wk old hCD20.BALB/c and hCD20.MRL.Faslpr mice were purified; labeled with fluorescent dyes, CFSE and CMTMR respectively; and co-transferred into seven-to-nine wk old H2d x k heterozygous BALB/c mice. On d1, the recipient mice were treated with 100 μg of a single dose of either 2H7 Ab (n=11) or a control IgG Ab (n=8) and the mice were analyzed on d3. (A) Representative FACS plots of CD19+ gated B cells showing residual donor B cells after the CTRL Ab (Left panel) or 2H7 (Right Panel) treatment. (B) Comparison of the residual donor B cells in spleen of recipient mice, as percentage of the average frequency of the donor B cells in IgG treated mice. Each symbol is an individual recipient mouse. hCD20.BALB/c donor, closed circles; hCD20.MRL.Faslpr donor, open circles. Lines represent Median. Data are combined from two independent experiments.
Ab-mediated phagocytosis by macrophages and neutrophils is less efficient in MRL.Faslpr vs. B-cell deficient MRL.Faslpr mice
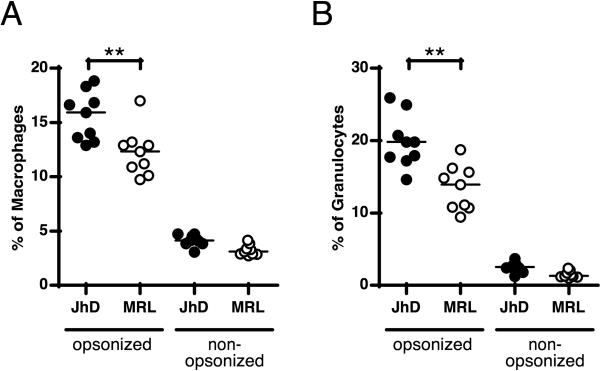
Since it has been shown that depletion is chiefly mediated by macrophages in an FcγR-dependent way (9, 11), we hypothesized that efficacy of macrophage-mediated phagocytosis should depend on whether the macrophages have developed in the presence or absence of serum Ab/ICs. To test this, we compared macrophages from B cell-deficient mice that had never been exposed to serum IgG to those from B cell intact mice, both of the MRL.Faslpr genetic background. We isolated splenic CD11b+ cells and used them in an IgG-dependent phagocytosis assay. The isolation procedure included both macrophages and CD11b+ neutrophils, allowing us to assess the function of each cell type, as distinguished by F4/80 and Gr-1 staining (Supplemental Fig. 2). As predicted, both populations isolated from Ab-deficient mice phagocytosed IgG-opsonized cells significantly better than the comparable populations from B cell intact MRL.Faslpr mice (Fig. 5). Thus, genetically identical macrophages and neutrophils that developed in the presence of Ig are inferior at phagocytosis of IgG-opsonized cells compared to those derived from an Ig-deficient environment.
Figure 5.
Fc receptor-mediated phagocytosis is impaired in macrophages and granulocytes from MRL.Faslpr mice relative to MRL.Faslpr .Jh-/- mice. Splenic CD11b+ cells were incubated with opsonized or non-opsonized, PKH26-labeled SRBCs for 60 min. Engulfment of SRBCs by (A) macrophages (F4/80hi, GR-1int) and (B) granulocytes (GR-1hi, F4/80lo) was measured by flow-cytometry (line represents median) by scoring for positive staining in the red PKH26 channel, as shown in Supplemental Fig. 2. Comparisons are between Jh-/- mice (closed circles, each symbol an individual mouse) and MRL.Faslpr mice (open circles). Data are combined from two independent experiments. **, p<0.01 by Student's t test.
Discussion
Lupus is considered the prototypical Ab-mediated autoimmune disease. Since the first evidence that B cells could be effective therapeutic targets came from lupus models (4, 20), it was particularly surprising that two controlled clinical trials of rituximab in SLE failed to show efficacy. These failed trials even caused the basic tenet that B cells are important in the pathogenesis of human SLE to be questioned (24). The reasons for the failure of these trials are not clear, but are important to understand given the ongoing clinical and translational efforts in this area. The interest is further heightened because anti-BLyS, that likely depends on B cell depletion and inhibition, has shown efficacy in two separate trials, and has recently been FDA-approved for treatment of some SLE patients, suggesting that B cells are indeed a valid target in SLE (7).
When attempting to model B cell depletion using anti-CD20 in murine SLE, others and we were struck by the difficulty in achieving B cell depletion in several different lupus-prone mouse strains (10, 13). In the current report, we have advanced our understanding of the mechanism behind such difficulty in depleting B cells. Using transfer experiments, we showed that serum IgG—but not other serum components—can induce a severe and reversible defect in Ab-mediated depletion in vivo. This was traceable to a defect in macrophage (and neutrophil) IgG-dependent phagocytosis. The mechanism is consistent with data showing that in vivo cellular depletion with anti-CD20 is largely FcγR-mediated (9, 11). Genetic background, aside from its role in inducing the presence of hypergammaglobulinemia and serum ICs, was not a factor since B cells from either MRL.Faslpr or BALB/c were equally depleted in a permissive host; and B cells in MRL.Faslpr mice lacking serum Ig (mIgM) or those in BALB/c mice were similarly sensitive to depletion.
At present it is unclear which forms of serum IgG are most potent in blocking FcγR-mediated clearance of opsonized B cells. Presumably ICs are the key element, as the ligand for the relevant FcγRs is considered to be ICs and not monomeric IgG. In support of this, depletion defects albeit more mild, are seen in younger MRL.Faslpr mice, prior to significant serum hypergammaglobulinemia (10). However, further studies of different types of ICs, including those purified from sera as well as reconstructed using defined sera and/or mAbs, will be required in order to better define this. It is possible that particular types of autoantibodies/ICs will be more potent, in part because of their ability to also stimulate macrophages and neutrophils via TLRs (25, 26). In the same vein, glycosylation status of IgG and/or its ability to ligate inhibitory FcγR2b could also be important (27). We hope in the future to be in a position to perform studies to test these various ideas.
Though we focused our studies on depletion of B cells in the MRL model, it is reasonable to think that the same mechanism extends to other murine SLE models that also show defects in depletion of both B cells and T cells (10, 13, 28), as well as SLE patients. This is because the proposed mechanism depends on ICs and autoantibodies present in the IgG fraction of serum. The production of autoantibodies and thus ICs is a common feature in virtually all lupus patients and mouse models. Therefore, this mechanism is predicted to be common among lupus mice and patients as long as sufficient levels of IgG ICs are present. Indeed, we propose that, since autoantibodies are common in a wide variety of autoimmune syndromes, some degree of resistance to depletion may be present—possibly subclinically—in a range of conditions. Notably this could include rheumatoid arthritis, a disease in which rituximab can be an accepted therapy, where extent of depletion in synovium (29) or in PB (30) can vary and be associated with clinical response. Unfortunately, it is not currently possible to routinely determine the extent of cellular depletion in secondary lymphoid tissue of treated patients. Notably, results in the mouse models underscore the notion that monitoring of PB can lead to an overestimation of the full extent of depletion in tissues.
Supporting the idea that FcγR-mediated phagocytosis is defective in lupus in general is older literature describing difficulty in clearing opsonized RBCs in lupus patients (31). Further, there are reports of an in vitro defect of macrophage IgG-dependent phagocytic function in lupus-prone NZB, NZB/W, and MRL.Faslpr mice (32). The studies presented in Fig. 5 confirm these earlier in vitro data. In this connection, it is notable that even with respect to PBL, there are a number of reports of relative difficulty in depleting B cells in SLE patients (16, 33, 34). Lupus patients also often show faster B cell reconstitution, another measure of reduced efficacy of anti-CD20 Ab in depleting B cells, which could in turn reflect both impaired phagocytic clearance function and reduced therapeutic Ab half-life (16, 33, 34).
Prior to embarkation on an expensive course of mAb therapy, it may be cost-effective to determine the likelihood that B cell depletion will be effective. Our studies suggest approaches for developing biomarkers to identify patients at risk for incomplete depletion (35); these could include amounts of circulating ICs or measures of macrophage/monocyte function. Such biomarkers could be used for selecting patients to receive Ab-mediated depletion, tailoring dosing regimens, and for monitoring efficacy. Our findings also provide a rationale to develop non-invasive tests for directly monitoring the degree of B cell depletion in secondary lymphoid or target tissue, which has only rarely been done via biopsy (16, 29).
In our model, defects were acquired and not the result of genetic differences in macrophages or neutrophils. Macrophage defects in lupus mice have been reported by several investigators (36-39); however, some reports studied uptake of apoptotic (40) and not opsonized cells, or described defective IC degradation (41). Although some defects were thought to be genetically determined (42) in part due to FcγR polymorphisms (43), it is likely that some were due to acquired dysfunction. Notwithstanding our evidence of acquired defects in MRL.Faslpr mice, it is possible that in some patients or murine models, primary macrophage defects based on genetic differences could also contribute to impaired ability to deplete B cells. For example, polymorphisms in FcγRIIIA that adversely affect B cell depletion in patients are well-described, for example (33).
The fact that defects in anti-CD20 mediated B cell depletion can be acquired and result from serum IgG or ICs suggests that they could be reversible. In patients, one might postulate that plasmapheresis or column-based IgG depletion could eliminate enough IgG and ICs to potentiate depletion. Similarly, treatments that ameliorate autoantibody production, such as TACI-Ig (44), could be synergistic with anti-CD20. Indeed, there is evidence for synergism in both SLE and RA between nonspecific immunosuppression and anti-CD20 therapy, though the mechanism has not been defined (7). In this sense our results are encouraging and suggest that further investigation could improve the therapeutic approach by enabling enhanced deletion of B cells.
One alternative approach is based on the idea that anti-CD20 based depletion might be effective if maintained over a long period of time. This is because effective B cell depletion will, in turn, reduce autoantibodies (10, 45), many of which derive from short-lived plasmablasts (46) that are replenished by CD20+ precursors. A small amount of initial depletion should lead to a small reduction in serum autoantibodies and ICs. This could set up a positive feedback loop, as the first small decrement in IC levels would enable the next round of depletion to be somewhat more effective until ultimately depletion goes to completion over time.
This feedback mechanism might explain why long-term treatment of MRL.Faslpr mice was effective in our prior study (10). Interestingly, persistent treatment that did lead to effective B cell depletion was accompanied by only small reductions in total IgG levels. It was, however, accompanied by a substantial reduction in serum anti-chromatin, anti-DNA, and anti-IgG; this suggests that the component in serum that inhibits depletion is autoantibody-containing ICs rather than just non-specific IgG. Though rituximab is known to have a long terminal half-life in vivo in lymphoma patients (47), the pharmacokinetics of rituximab are little-studied and highly variable in SLE patients (15).
However, much more rapid clearance of Abs has been reported in the setting of lupus (15). Further, if B cell depletion is not achieved initially, the terminal half-life phase will also not be reached, and new B cells will emerge that presumably will serve as a sink for absorbing anti-CD20. For these reasons, a different dosing strategy for anti-CD20 from that used for lymphoma may be necessary for efficacy in SLE.
Though targeting B cells has already proven to be an effective therapy for a remarkable range of autoimmune diseases patients, much remains to be learned about the mechanisms behind these effects as well as the optimal way to administer therapy. By default, “standard RA dosing” has generally been used in most autoimmune disease studies, yet it is still not clear how best to induce remissions, how long to maintain B cell depletion, and whether to treat only relapses or to maintain depletion prophylactically. It is likely that the answers will be different depending on the disease and possibly even the patient within a specific disease group. The studies reported here elucidate a potential complicating factor in SLE and possibly other diseases, and at the same time suggest methods to measure, monitor and overcome these obstacles to Ab-mediated cellular therapy. They also reveal new insights into macrophage and neutrophil defects in IgG-dependent phagocytosis that are linked to disease and exposure to lupus serum IgG.
Supplementary Material
Acknowledgements
We thank Jaime Cullen and Cuiling Zhang for expert technical assistance and the Yale Animal Resources Center for outstanding animal care.
Supported by NIH AR44077 to MJS. Dr. Ahuja was supported by an Arthritis Foundation Postdoctoral Fellowship. Dr. Teichmann was supported by a DFG Research Fellowship.
Abbreviations used
- hCD20
human CD20
- BLyS
B Lymphocyte Stimulator
- SLE
Systemic Lupus Erythematosus
- MRL.Faslpr
MRL/MpJ-Faslpr
- PB
peripheral blood
- FcγR
Fc receptor gamma chain
- IC
immune complex
- LN
lymph node
- BM
bone marrow
- TACI
transmembrane and CAML interactor
References
- 1.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Ann. Rev. Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 2.Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5:564–576. doi: 10.1038/nrd2085. [DOI] [PubMed] [Google Scholar]
- 3.Chan OT, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999;163:3592–3596. [PubMed] [Google Scholar]
- 4.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looney RJ. B cells as a therapeutic target in autoimmune diseases other than rheumatoid arthritis. Rheumatology (Oxford) 2005;44(Suppl 2):ii13–ii17. doi: 10.1093/rheumatology/keh618. [DOI] [PubMed] [Google Scholar]
- 6.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh H-J, Zhang D, Brunetta PG. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase ii/iii systemic lupus erythematosus evaluation of rituximab trial. Arthritis & Rheumatism. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan OTM, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol. Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 9.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 10.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 11.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The Innate Mononuclear Phagocyte Network Depletes B Lymphocytes through Fc Receptor-dependent Mechanisms during Anti-CD20 Antibody Immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Chen F, Putt M, Koo YK, Madaio M, Cambier JC, Cohen PL, Eisenberg RA. B cell depletion with anti-CD79 mAbs ameliorates autoimmune disease in MRL/lpr mice. J Immunol. 2008;181:2961–2972. doi: 10.4049/jimmunol.181.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B Lymphocyte Depletion by CD20 Monoclonal Antibody Prevents Diabetes in Nonobese Diabetic Mice despite Isotype-Specific Differences in Fc{gamma}R Effector Functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 15.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 16.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 17.Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol. 2005;17:550–557. doi: 10.1097/01.bor.0000172798.26249.fc. [DOI] [PubMed] [Google Scholar]
- 18.Walport MJ. Lupus, DNase and defective disposal of cellular debris. Nat Genet. 2000;25:135–136. doi: 10.1038/75963. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 20.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark EA, Shu G, Ledbetter JA. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985;82:1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlomchik MJ, Zharhary D, Saunders T, Camper S, Weigert M. A Rheumatoid factor transgenic mouse model of autoantibody regulation. Int. Immunol. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 24.Merrill JT, Buyon JP. Rituximab: wanted dead or alive. Arthritis Rheum. 2010;62:2188–2191. doi: 10.1002/art.27544. [DOI] [PubMed] [Google Scholar]
- 25.Lovgren T, Eloranta ML, BÂve U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis & Rheumatism. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 26.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 28.Wofsy D, Seaman WE. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987;138:3247–3253. [PubMed] [Google Scholar]
- 29.Koen V, Rogier MT, Carla AW, Dirkjan van S, DaniÎlle MG, Paul PT. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis & Rheumatism. 2007;56:772–778. doi: 10.1002/art.22400. [DOI] [PubMed] [Google Scholar]
- 30.Dass S, Rawstron AC, Vital EM, Henshaw K, McGonagle D, Emery P. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis & Rheumatism. 2008;58:2993–2999. doi: 10.1002/art.23902. [DOI] [PubMed] [Google Scholar]
- 31.Frank MM, Hamburger MI, Lawley TJ, Kimberly RP, Plotz PH. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979;300:518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- 32.Russell PJ, Steinberg AD. Studies of peritoneal macrophage function in mice with systemic lupus erythematosus: Depressed phagocytosis of opsonized sheep erythrocytes in vitro. Clin. Immunol. Immunopathol. 1983;27:387–402. doi: 10.1016/0090-1229(83)90091-0. [DOI] [PubMed] [Google Scholar]
- 33.Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, Looney RJ. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48:455–459. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 34.Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, Pullman-Mooar S, Barnack F, Striebich C, Looney RJ, Prak ETL, Kimberly R, Zhang Y, Eisenberg R. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Annals of the Rheumatic Diseases. 2008;67:1724–1731. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 35.Sutter JA, Kwan-Morley J, Dunham J, Du Y-Z, Kamoun M, Albert D, Eisenberg RA, Luning Prak ET. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): Factors associated with B lymphocyte recovery. Clinical Immunology. 2008;126:282–290. doi: 10.1016/j.clim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis & Rheumatism. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Tas SW, Quartier P, Botto M, Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Annals of the Rheumatic Diseases. 2006;65:216–221. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J, Kern P, Herrmann M. Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Steinbach F, Henke F, Krause B, Thiele B, Burmester GR, Hiepe F. Monocytes from systemic lupus erythematous patients are severely altered in phenotype and lineage flexibility. Ann Rheum Dis. 2000;59:283–288. doi: 10.1136/ard.59.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-Prone Mice Have an Abnormal Response to Thioglycolate and an Impaired Clearance of Apoptotic Cells. J Immunol. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 41.Kanno H, Tachiwaki O, Nose M, Kyogoku M. Immune complex-degradation ability of macrophages in MRL/Mp-lpr/lpr lupus mice and its regulation by cytokines. Clinical & Experimental Immunology. 1994;95:115–121. doi: 10.1111/j.1365-2249.1994.tb06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dang-Vu AP, Pisetsky DS, Weinberg JB. Functional alterations of macrophages in autoimmune MRL-lpr/lpr mice. J Immunol. 1987;138:1757–1761. [PubMed] [Google Scholar]
- 43.Pritchard NR, Cutler AJ, Uribe S, Chadban SJ, Morley BJ, Smith KG. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–230. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 44.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 45.Ferraro AJ, Drayson MT, Savage CO, MacLennan IC. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 46.Jacobi AM, Mei H, Hoyer BF, Mumtaz IM, Thiele K, Radbruch A, Burmester GR, Hiepe F, Dorner T. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:305–308. doi: 10.1136/ard.2008.096495. [DOI] [PubMed] [Google Scholar]
- 47.Maloney DG. Preclinical and phase I and II trials of rituximab. Semin Oncol. 1999;26:74–78. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.