Abstract
New neurons are produced and integrated into circuits in the adult brains of many organisms, including crustaceans. In some crustacean species, the 1st- generation neuronal precursors reside in a niche exhibiting characteristics analogous to mammalian neurogenic niches. However, unlike mammalian niches where several generations of neuronal precursors coexist, the lineage of precursor cells in crayfish is spatially separated allowing the influence of environmental and endogenous regulators on specific generations in the neuronal precursor lineage to be defined. Experiments also demonstrate that the 1st-generation neuronal precursors in the crayfish Procambarus clarkii are not self-renewing. A source external to the neurogenic niche must therefore provide cells that replenish the 1st-generation precursor pool, because although these cells divide and produce a continuous efflux of 2nd-generation cells from the niche, the population of 1st-generation niche precursors is not diminished with growth and aging. In vitro studies show that cells extracted from the hemolymph, but not other tissues, are attracted to and incorporated into the neurogenic niche, a phenomenon that appears to involve serotonergic mechanisms. We propose that in crayfish, the hematopoietic system may be a source of cells that replenish the niche cell pool. These and other studies reviewed here establish decapod crustaceans as model systems in which the processes underlying adult neurogenesis, such as stem cell origins and transformation, can be readily explored. Studies in diverse species where adult neurogenesis occurs will result in a broader understanding of fundamental mechanisms and how evolutionary processes may have shaped the vertebrate/mammalian condition.
Keywords: neurogenic niche, serotonin, bromodeoxyuridine, hematopoietic system, Procambarus clarkii
I. A stem cell niche in the crustacean brain
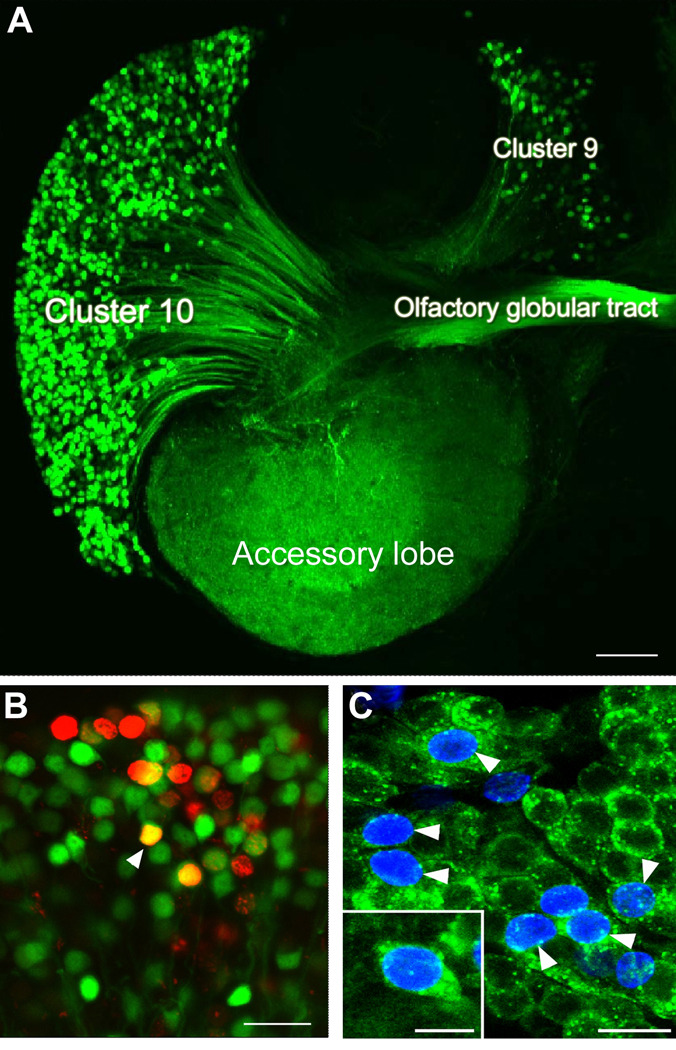
Neuronal proliferation in most regions of the decapod crustacean brain ceases in the period around hatching when the embryonic precursor cells (neuroblasts) die (Harzsch, 2003). The exception to this is in the central olfactory pathway where mitotic activity continues throughout life (Schmidt, 1997; Schmidt and Harzsch, 1999; Harzsch et al., 1999). Adult neurogenesis also occurs in the visual pathway (Sullivan and Beltz, 2005b), though this has been studied in much less detail. In the olfactory pathway, life-long neurogenesis is found among the sensory (Steullet et al., 2000), local (Cluster 9) and projection (Cluster 10) neurons (Figure 1) (Schmidt and Harzsch, 1999). Each Cluster 9 neuron innervates the primary olfactory processing areas, the olfactory lobes (OLs), and higher order processing areas, the accessory lobes (ALs). The Cluster 10 projection neurons innervate either the OL or AL and olfactory globular tract neuropil (Sullivan et al., 2000), and their axons project via the olfactory globular tract (OGT) to neuropil regions in the lateral protocerebrum (Sullivan and Beltz, 2001). The AL is involved in higher-order integration of olfactory, visual and mechanosensory information (Sandeman et al., 1995; Sullivan and Beltz, 2005b).
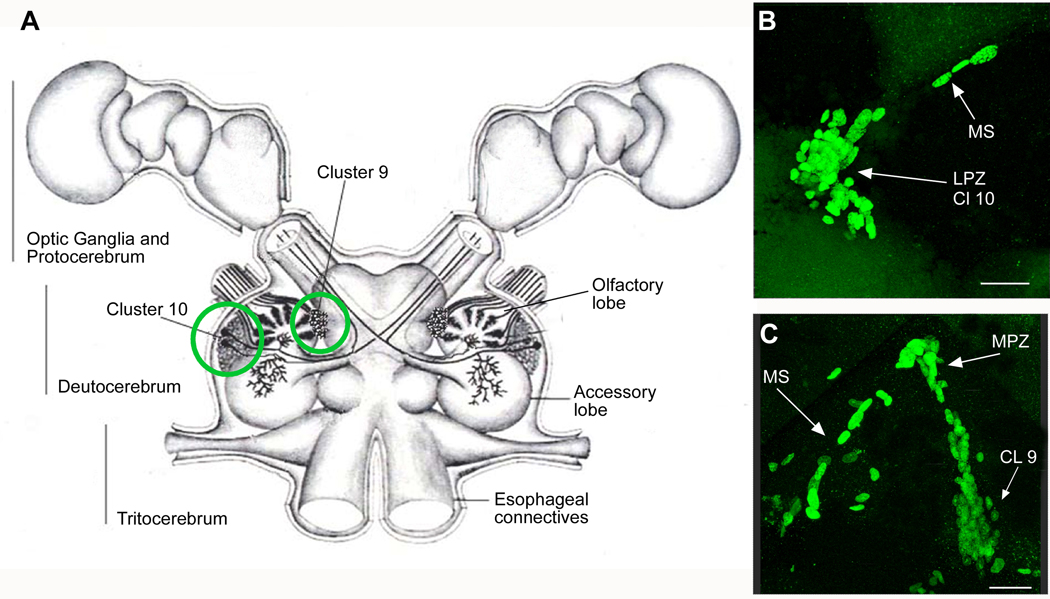
Figure 1.
A. Diagram of the eureptantian (crayfish/lobster) brain including the optic ganglia, and showing the locations of the proto-, trito- and deutocerebral neuropils. The soma clusters 9 and 10 (circled), locations of neurogenesis in the adult brain, flank two prominent neuropil regions of the deutocerebrum, the olfactory (OL) and accessory (AL) lobes. (Names of brain areas are according to Sandeman et al., 1992). B. Bromodeoxyuridine (BrdU)-labeled nuclei in the migratory stream (MS), the lateral proliferation zone (LPZ) and the adjacent cluster 10 (CL10). C. BrdU-labeled cells in the migratory stream, the medial proliferation zone (MPZ) and cluster 9 (CL9) which is separated from the MPZ by a stream of migrating cells. Scale bars: B, C, 20µm. (Figure from Sullivan et al., 2007b).
For many years after the discovery of adult neurogenesis in the crustacean brain, the source of the adult-born neurons was a mystery. This situation changed, however, when antibodies against glutamine synthetase (GS), which labels astrocytes and some neuronal stem cells in vertebrates, revealed a structure on the ventral surface of the crayfish (Procambarus clarkii) brain that extended between the lateral (LPZ) and medial (MPZ) proliferation zones in Clusters 9 and 10 (Figure 2A) (Sullivan et al., 2005; 2007a). In roughly the center of this structure was a swollen region enclosing what appeared to be a hollow cavity. Short-survival time studies using the S-phase marker bromodeoxyuridine (BrdU), in conjunction with immunocytochemistry for GS, labeled a discontinuous stream of BrdU labeled cells lying along the GS-labeled structure between Clusters 9 and 10 (Figure 2B). Some of these features in P. clarkii have been confirmed in studies by others (Song et al., 2007; 2009). In addition, similar structures have been found in the Australian crayfish (Cherax destructor), clawed lobster (Homarus americanus) and green crab (Carcinus maenus) (Sullivan et al., 2007b). The same structure had in fact been discovered and described in much earlier studies in separate members of all the decapod crustaceans (Bazin and Demeusy 1968; Bazin 1970a, 1970b; Sandeman et al., 2011) but without any indication of its function.
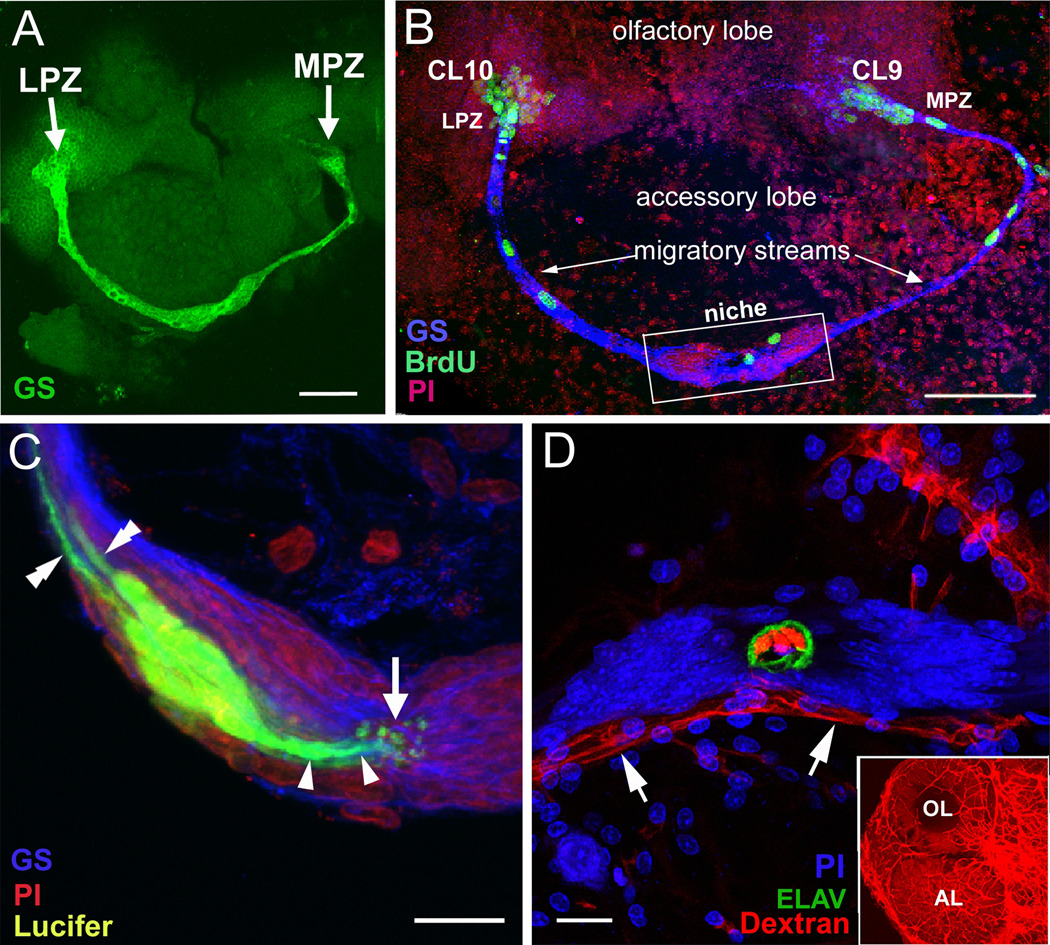
Figure 2.
The proliferative system maintaining adult neurogenesis in the crayfish (Procambarus clarkii) brainA. Both the LPZ and MPZ are contacted by the processes of a population of glial cells immunoreactive to glutamine synthetase (green). The somata of these cells form a cluster, the neurogenic niche, on the ventral surface of the brain. B. Left side of the brain of P. clarkii labeled immunocytochemically for the S-phase marker BrdU (green). Labeled cells are found in the lateral proliferation zone (LPZ) contiguous with cluster 10 and in the medial proliferation zone (MPZ) near cluster 9. The two zones are linked by a chain of labeled cells in a migratory stream that originates in the oval region labeled “niche”. Labeling for Drosophila synapsin (blue) and propidium iodide (red) is shown. C. Niche cells (green), labeled by intracellular injection of Lucifer yellow, have short processes (arrowheads) projecting to the vascular cavity (arrow) and longer fibers (double arrowheads) that fasciculate together to form the tracts projecting to the LPZ and MPZ. (blue: glutamine synthetase; red: propidium iodide). D. The vascular connection of the cavity in the centre of the glial soma cluster was demonstrated by injecting a dextran dye into the cerebral artery. The cavity, outlined in green by its reactivity to an antibody to Elav, contains the dextran dye (red) which is also contained within a larger blood vessel that runs along beneath the niche. Propidium iodide (blue) labeling of the glial cell nuclei is also shown. The inset shows the dextran-filled vasculature in the olfactory (OL) and accessory (AL) lobes on the left side of the brain. Scale bars: A, 100 µm; B, 75 µm; C, D, 20 µm. (Images A, C and D from Sullivan et al., 2007a; B from Benton et al., 2011).
Lucifer yellow dye was injected into the swollen region in Procambarus clarkii, revealing a structure filled with bipolar cells (Figure 2C). Studies using the cell cycle marker MCM2–7 (Cvetic and Walter, 2006) indicated that these are in G1 of the cell cycle except when they are progressing through S to M phase (Sullivan et al., 2007a), suggesting that these cells are not terminally differentiated. Injection of dextran-ruby dye (MW 3000) into the dorsal artery that supplies the brain (Figure 2D) or into the pericardial cavity (Benton et al., 2010; 2011) demonstrated that the cavity in the center of this cluster of cells is confluent with the vasculature. Studies using double nucleoside labeling further showed that proliferating (BrdU-labeled) cells migrate from the cluster of cells towards the proliferation zones in Clusters 9 and 10 (Sullivan et al., 2007a), where they divide at least once more before differentiating (Sullivan et al., 2005a). As in the higher vertebrates, the precursor cells residing in the crayfish niche have glial properties and act as both precursor and support cells, the processes of the niche cells guiding the migration of the daughter cells towards Clusters 9 and 10 (see model, Figure 3). Based on these and other features, we proposed that the swollen region constitutes a neurogenic niche containing primary neuronal precursors, with a connection to the blood vascular system via the central cavity (Sullivan et al., 2005; 2007a). Many of these features but with a somewhat different overall organization, have also been described in the spiny lobster, Panulirus argus, where a neurogenic niche containing uniquely-endowed, persistent neuroblasts appears to support adult neurogenesis (Schmidt, 2007).
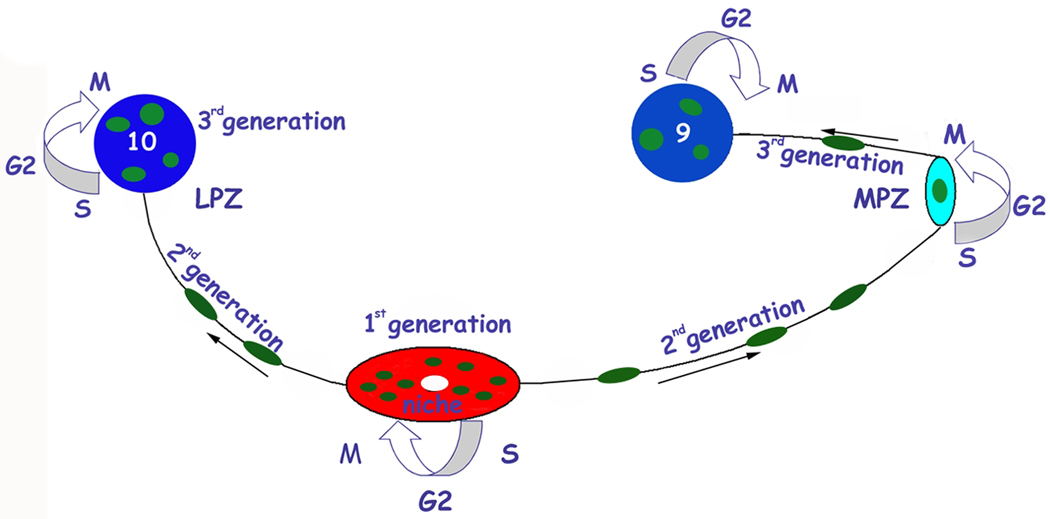
Figure 3.
Model summarizing a view of events leading to the production of new olfactory interneurons in adult crayfish (from Sullivan et al., 2007b). Neuronal precursor (1st generation) cells reside within a neurogenic niche where they undergo mitosis. Their daughters (2nd generation precursors) migrate along tracts created by the fibers of the niche cells, towards either the LPZ or the MPZ. At least one more division will occur in the LPZ and MPZ before the progeny (3rd and subsequent generations) differentiate into neurons. (Image from Zhang et al., 2011).
Within two weeks of the final cell divisions in Clusters 9 and 10, cell counts in BrdU cell survival studies reveal a period of cell death; the surviving cells differentiate into neurons over the next 2–4 weeks (Panulirus argus: Schmidt, 2001; Cherax destructor: Kim and Beltz, 2008; Kim, 2009). The resulting neurons express transmitters that are appropriate for Cluster 9 (allatostatin, orkokinin) and 10 (FMRFamide) neurons (Figure 4) (Sullivan and Beltz, 2007a), and innervate the olfactory and accessory lobes as do the mature local and projection interneurons in these regions (Figure 5) (Sullivan and Beltz, 2005b).
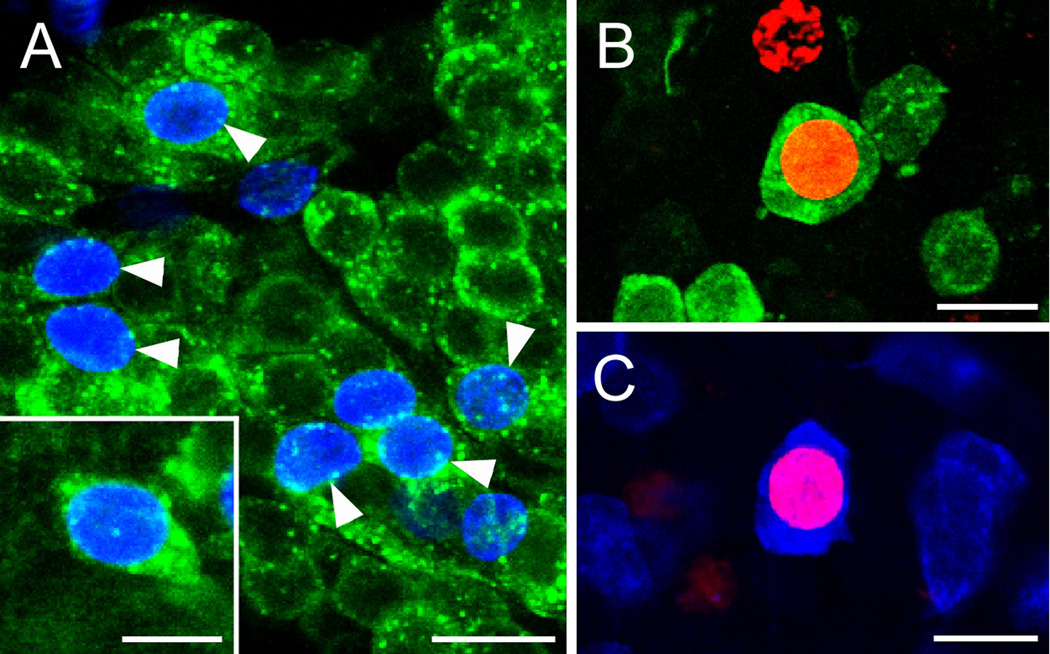
Figure 4.
A. BrdU (blue) and SIFamide (green) immunolabeling in cluster 10 six months after the exposure of P. clarkii to BrdU. Double-labeled cells are indicated by the arrow- heads. The inset shows a higher magnification image of the soma of a double-labeled neuron. B. Double labeling of a soma for BrdU (red) and orcokinin (green) in cluster 9 of an animal exposed 6 months previously to BrdU. C. Double labeling of a cluster 9 soma for BrdU (red) and allatostatin-like peptide (blue). Scale bars: A, 100 µm; B, 20 µm; inset in B, 10 µm; C, D, 15 µm. (Images from Sullivan et al., 2007a).
Figure 5.
A. The left side of a brain of the crayfish Cherax destructor in which dextran was applied to the accessory lobe. The dextran (green) enters neurons that have their terminals in the accessory lobe and labels the corresponding cell bodies and axons. From this it is clear that both projection neurons in cluster 10, and local interneurons in cluster 9, have their terminals in the accessory lobes and the axons from the projection neurons lie in the olfactory globular tract. B. Cluster 10 cell bodies from an animal that was exposed to BrdU for 12 days and then maintained in fresh pond water for 4 months. At this stage the animal was killed and dextran fluorescein 3000 MW was applied to the accessory lobe. Cells labeled red indicate that they passed through the cell cycle in the presence of BrdU. Cells labeled green indicate that they have terminals in the accessory lobe but did not pass through a cell cycle in the presence of BrdU. Double-labeled cells (orange) are cells that passed through a cell cycle in the presence of BrdU and have differentiated into neurons with their terminals in the accessory lobe. C. Cluster 10 cell bodies with BrdU (blue) and crustacean-SIFamide (green) label six months after being exposed to BrdU. Double-labeled cells, green and blue (arrowheads). Crustacean-SIFamide immunoreactivity is known to be expressed in olfactory interneurons in P. clarkii (Yasuda-Kamatani and Yasuda, 2006) and the presence of double labeling indicates that these cells were born in the adult animal and have differentiated into olfactory interneurons. Scale bars: A, 10µm; B, C, 20µm; C inset, 10µm. (Images from Sullivan and Beltz, 2005a).
II. The neurogenic niche: common strategies in diverse organisms
1. Neurogenic niches: organizational parallels and distinctions
While neuronal stem cell niches in different organisms do have some characteristic properties (see below), there are many parallels across a broad phyletic range. Among these are the glial characteristics of the primary neuronal precursor (stem) cells (but see the exception in zebrafish, Kaslin et al., 2009), a close association between the niche and the vasculature, the presence of a specialized extracellular matrix and basal lamina, directed migration of progeny, and regulation by environmental and endogenous cues (Doetsch et al., 1999; Sullivan et al., 2007a;b; Tavazoie et al., 2008; Kriegstein and Alvarez-Buylla, 2009; Pathania et al., 2010). In addition, the presence of a period of cell death following neuronal proliferation and the time required for the new neurons to begin differentiation (~2–4 weeks) are features of adult neurogenesis that are shared between vertebrate (e.g., van Praag et al., 2002) and crustacean (Panulirus argus, Schmidt, 2001; Cherax destructor, Kim et al., 2009) systems. Many of the organizational properties of the cellular machinery maintaining adult neurogenesis appear, therefore, to be shared by widely disparate taxa. These parallels suggest common strategies for the generation of new neurons in adult brains that may have resulted from shared ancestral origins or evolutionary convergence.
The 1st-generation neuronal precursors
In the mammalian brain, adult-born neurons are incorporated into the olfactory bulb and hippocampus; the neurogenic niches containing the neuronal precursor lineages are located in the subventricular and subgranular zones, respectively. The 1st- generation neuronal precursor cells (neural stem cells) display typical astroglial markers such as GFAP and glutamine synthetase, in addition to stem cell markers such as nestin. As a group, however, the astroglia are a heterogeneous population, with some serving as neurogenic cells and others as quiescent stem cells (Ma et al., 2008; Riquelme et al., 2008; Kriegstein and Alvarez-Buylla, 2009). It has been proposed that the quiescent astroglia may interact, and may also retain the capacity to become neurogenic in the presence of appropriate signals (Nyfeler et al., 2005). Likewise, it is not known whether all niche cells in the crayfish brain have the capacity to become neuronal precursors, although this possibility is implicit in some proposed models (Zhang et al., 2009; Ayub et al, 2011) and is supported by four lines of evidence: Firstly, all niche cells label with the G1-phase marker MCM2–7, except when they are progressing through S to M phase (Sullivan et al., 2007a). This indicates that, although resting in G1, the niche cells are actively in the cell cycle and not terminally differentiated cells. Secondly, all niche cells, including those in M phase, label immunocytochemically for the glial marker glutamine synthetase, indicating that all the niche cells share this molecular property (Zhang et al., 2009). Thirdly, examination of semi-thin sections shows that, with very few exceptions, the niche cells have identical cellular characteristics (Zhang et al., 2009). Finally, the studies of Ayub et al. (2011) demonstrate that the number of S-phase (BrdU-labeled) precursors in the niche is regulated by environmental enrichment, suggesting the existence of a quiescent pool of precursor cells whose status in the cell cycle can be modulated by local conditions.
Vascularization of neurogenic niches
The vascular system has been receiving increasing attention as a common feature of all niches, including those in the nervous system (Tavazoie et al., 2008). In both the subventricular (SVZ) and subgranular (SGZ) zones that support adult neurogenesis of olfactory bulb and hippocampal neurons, respectively, the stem cells lie in close proximity to a rich plexus of blood vessels. Endothelial cells, which are known to regulate stem cell self-renewal and neurogenesis via a cadre of secreted factors, are emerging as critical components of neural stem cell niches (Shen et al., 2004, 2008; Riquelme et al., 2008). Blood vessels also are conduits for circulating hormones and cytokines released from distant sources (Lennington et al., 2003). In addition, the associated extracellular matrix appears to provide a means of cell anchoring in the niche (Shen et al., 2008), as well as creating a supportive microenvironment and architecture (Riquelme et al., 2008).
While details of how the blood vasculature interacts with the crustacean neurogenic niche are not known, some form of communication via the vascular cavity has been demonstrated in two different crayfish species using injection of labeled dextrans directly into the dorsal artery that vascularizes the brain (Figure 2D) (Procambarus clarkii, Sullivan et al., 2005; 2007a; Cherax destructor, Sandeman et al., 2009), or into the pericardial cavity (Benton et al., 2010; 2011). The blood system also is closely associated with the niche in Panulirus argus (Schmidt, 2007). As reviewed below, a variety of cues regulate the numbers of niche cells and the rate of neurogenesis in crustaceans, and ongoing studies are defining how the vascular connections with the niche may support these actions.
Directed migration of neuronal precursors
In the mammalian brain, 3rd generation precursors (called neuroblasts) migrate away from the subventricular zone towards the olfactory bulb along the rostral migratory stream (RMS) by means of chain migration; tens of thousands of cells travel this path to the olfactory bulb daily (Lois et al., 1996), following a route created by astrocytes that then communicate with the migrating cells and regulate their migration (Bolteus and Bordey, 2004). The RMS is deeply embedded in the brain, and therefore it has been difficult to observe the migratory behavior of these cells in living organisms. Recently, magnetic resonance imaging (MRI) techniques in rats (Shapiro et al., 2006; Vreys et al., 2010) and two-photon time-lapse imaging in acute slices of mouse brain (Nam et al., 2007) have been used to examine the dynamic behavior of cells migrating along this pathway; these studies have shown that cell motility in the RMS is more complex than previously thought, involving multiple cell types, behaviors, speeds and directions. Neuronal precursors in the hippocampus also migrate, although over very short distances compared with the RMS, which can be 5 mm in length in the adult mouse. In addition, adult neurogenesis is prevalent in a number of brain regions in fish, and these cells are often guided by radial glial fibers (Zupanc et al., 2005; Zupanc, 2006). In the crustacean brain, the primary neuronal precursor cells residing in the niche appear to serve as both procursor and support cells, with their long glutamine synthetase- and lissencephaly 1 (LIS1)-labeled processes forming a tract along which the 2nd generation precursors travel. Like the rostral migratory stream in mammals, the migratory streams in the crayfish brain are relatively long ---up to 1 mm in adult crayfish, and presumably even longer in larger species. Unlike the RMS, however, the cells traversing the streams are few in number (<15 cells) (Zhang et al., 2009).
Distinctive characteristics of the crayfish neurogenic niche
Potentially important differences in the properties of neurogenic niches and neuronal precursor lineages emerge from comparisons among diverse species. For example, neuronal stem cells in mammals produce both glia and neurons (Gage, 2002), a feature that is distinct from the niche precursors in the crustacean brain, which appear to produce only neurons (Harzsch et al., 1999; Sullivan and Beltz, 2005a). Second, the crustacean niche contains fewer cells and is organizationally less complex than vertebrate neurogenic niches, where multiple precursor generations coexist and relationships among precursor cell types have not been directly demonstrated (Zhao et al., 2008). In the crayfish, neuronal precursor cell generations are spatially segregated from each other; only 1st-generation progenitors are found in the niche, 2nd-generation precursors along the migratory streams; 3rd- and later generations are located in the proliferation zones in Clusters 9 and 10 (see Figure 3). This spatial compartmentalization of the neuronal precursor lineage allows easy assessment of quantitative changes in precursor cell generations. Finally, and perhaps most surprising, is the lack of evidence for asymmetric divisions of the 1st-generation neuronal precursors in the crustacean niche (Zhang et al., 2009; Benton et al., 2011). While the niche precursor cells are, functionally speaking, neuronal stem cells, they appear not to adhere to the basic definition of a stem cell, i.e. possessing the capacity for self-renewal. The corollary of this finding, given that the crustacean neurogenic niche is not exhausted over time (and, in fact, the niche cell population expands as animals grow and age; Zhang et al., 2009), is that these primary neuronal precursors must be replenished from a source extrinsic to the niche. (The issues of self-renewal and replenishment are discussed further in sections 4 and 5).
Studies of adult neurogenesis in diverse species therefore highlight both similarities and differences in the neurogenic niches and underlying processes that produce adult-born neurons. The use of a variety of organisms to examine mechanisms of adult neurogenesis will provide a broader view and a deeper understanding of the basic principles underlying these phenomena, and could also reveal important factors that may have shaped the vertebrate/mammalian condition.
2. Molecular conservation
Glutamine synthetase antibodies label the 1st generation neuronal precursor cells
Glutamine synthetase (GS) is an enzyme that converts glutamate to glutamine and is found predominantly in the nervous system, kidneys and liver in vertebrates (Lemieux et al., 1976; Gebhardt et al., 2007). In the nervous system, GS is a marker of astrocytes and stem cells (Wen et al., 2007; 2009). In the brain of the crayfish P. clarkii, GS immunoreactivity is found in two types of cells: the neuronal precursor cells in the niche and migratory streams, and glia in other parts of the brain. The somata of GS-immunopositive cells in the neurogenic niche are tightly packed. Each has a short process extending to the vascular cavity in the center of the niche (Figure 2C), and a long process extending toward interneuronal clusters 9 and 10 and along which proliferating neuronal precursor cells migrate (Figure 2B). In contrast, GS-immunopositive glia outside the niche bear short processes and are separate from each other. These distinct labeling patterns led us to ask whether the GS antibody labels the same protein in the neurogenic niche as in the rest of the crayfish brain; additionally, we wanted to determine if the GS is enzymatically active and to define the GS-sequence(s).
GS activity in homogenates of the crayfish brain has been tested by a glutamyl-transfer assay. Accumulation of glutamyl-hydroxamate, the product of the assay, was observed over time and could be blocked with an inhibitor of GS activity, L-methionine-S-sulfoximine (Zhang and Beltz, 2010). A complete nucleotide sequence of GS mRNA from total brain tissue was determined using primers based on sequence homology to lobster (Linser et al., 1997) and other species. This analysis confirmed that GS in the crayfish brain is highly homologous to the GS sequence found in other species. In situ hybridization with DIG–labeled riboprobes from this sequence was performed on crayfish brains. The antisense probe recognized glial cells in several brain regions, but not the cells comprising the neurogenic niche and migratory stream. However, Western blots with anti-GS antibody reveal two bands in the crayfish brain, a 45 kDa dominant band that is similar in size with the known GS in other species, and a novel 37 kDa band (Zhang and Beltz, 2010). It is possible that these two bands represent GS splicing variants, an issue currently being investigated, as is the molecular identity of the GS immunoreactivity in the niche and streams.
LIS1 labels the niche and migratory streams
Lissencephaly1 (LIS1) is a dynein-binding protein that is involved in neuronal migration in vertebrate systems (Morris et al., 1998; Tsai et al., 2005, 2007). In the crayfish brain, an antibody against the LIS1 protein labels the cytoplasm of all cells in the niche and streams (Figure 6) (Zhang et al., 2009). Punctate staining was observed in the cytoplasm of the niche precursor cells and in their processes composing the stream; LIS1 co-labels with GS in these areas and is particularly intense near the vascular cavity (Figure 6C, D). No labeling was seen after LIS1 antibody was pre-absorbed with LIS1 blocking peptide (Santa Cruz, sc-7577p). Western blots revealed an immunopositive band (between 37–50 kDa) in brain preparations from crayfish of three different sizes/ages (lanes 1–3; Figure 6A); this molecular weight is close to the size of the mammalian LIS1 protein (lane 4, mouse brain tissue, ~45 kDa). The presence of LIS1 immunoreactivity in the migratory streams and niche in the crayfish brain implicates this protein in the translocation of the neuronal precursor cells. We have explored the mode of migration by considering shape changes of cells in the streams and observed that the nuclei of migrating cells elongate and undergo deformation consistent with nucleokinetic movement: a leading process is extended in the direction of migration, followed by the saltatory forward movement of the nucleus (Zhang et al., 2009). This motion strategy is employed by newborn cells migrating along radial glial processes in the cerebellum and by migrating cortical neurons (Samuels and Tsai, 2004; Martini and Valdeolmillos, 2010). The hypothesis that 2nd-generation neuronal precursors in the crayfish brain migrate using nucleokinesis is being directly investigated with live imaging of ventral brain slices and isolated niche-stream preparations.
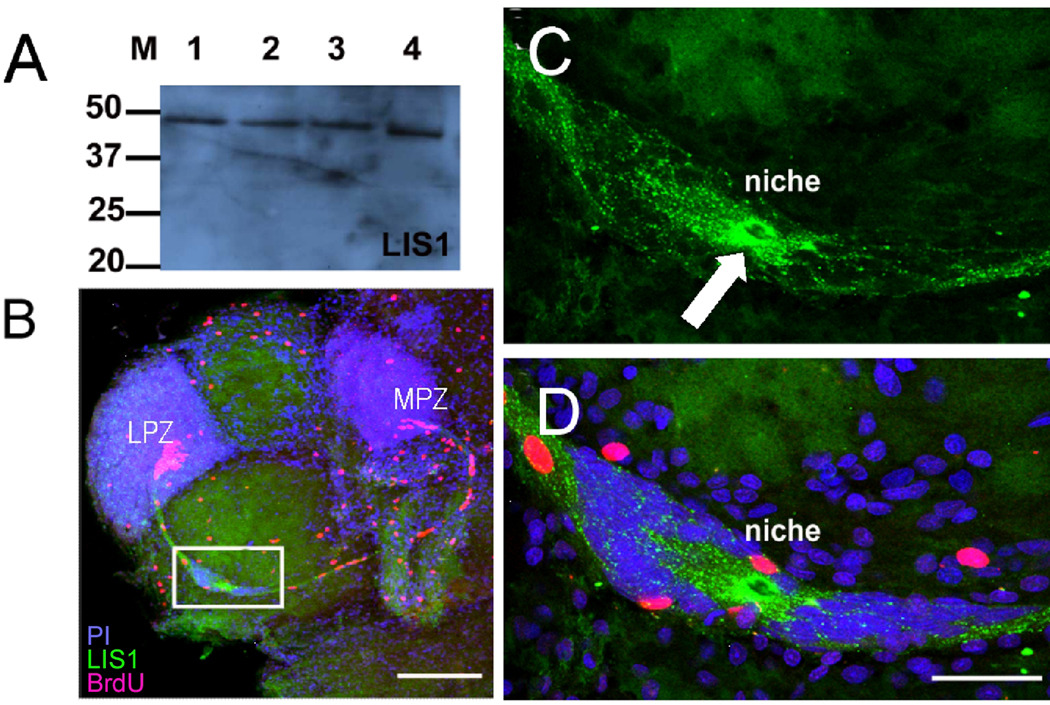
Figure 6.
Precursor cells in the niche express LIS1 protein. A. Western blot for LIS1 on protein preparations from the P. clarkii brain (lane 1, 35mm carapace length (CL); lane 2, 16mm CL; lane 3, 8mm CL and adult mouse brain (lane 4). B. Whole mount brains were double-labeled for BrdU (red) and LIS1 (green) and counterstained with propidium iodide (blue), and optically sectioned with the confocal microscope. The white box outlines the niche, which is magnified in C and D. C. LIS1 staining is found throughout the niche cells and their fibers that form the stream, but is particularly strong immediately around the vascular cavity (white arrow). D. The same magnification as in C, but combining all three fluorescence channels in a single image. Scare bars: B, 200 µm; C, D, 50 µm. (Images from Zhang et al., 2009).
3. Regulation of adult neurogenesis by environmental and endogenous factors
The production of adult-born neurons in the crustacean brain, as in vertebrates, is a highly regulated process. Modulatory factors include the quality of the living conditions (Kempermann et al., 1997; Sandeman and Sandeman, 2000; Scotto-Lomassese et al., 2000), hormonal cycles (Rasika et al., 1994; Harrison et al., 2001), diet (Lindqvist et al., 2006; Beltz et al., 2007; Stangl and Thuret, 2009), physical activity (van Praag et al., 2005; van Praag, 2009), seasonality (Barnea and Nottebohm, 1994; Hansen and Schmidt, 2001), the day-night cycle (Huang et al., 1998; Goergen et al., 2002; Jacobs, 2002), serotonin (Gould, 1999; Brezun and Daszuta, 2000; Beltz et al., 2001; Benton et al., 2008), and nitric oxide (Moreno-Lopez et al., 2004; Matarredona et al., 2005; Benton et al., 2007; Torroglosa et al., 2007). These factors influence the rate and timing of neuronal proliferation and survival in a variety of organisms, and the up- or down-regulation of these processes is generally consistent between crustacean and mammalian species. Hence, factors that regulate adult neurogenesis in the crustacean brain are essentially the same as those discussed in mammals (for reviews see Beltz and Sandeman, 2003; Kempermann, 2006; Sullivan et al., 2007b; Pathania et al., 2010), indicating that not only the process of adult neurogenesis but also mechanisms related to its regulation, are shared across a broad phyletic range.
III. Exploiting the unique advantages of the neurogenic system in adult crustaceans
In the brains of crayfish and clawed lobsters, the system generating adult-born neurons provides a particularly attractive experimental model for exploring fundamental mechanisms underlying adult neurogenesis. First, the neurogenic niche and migratory streams are located superficially on the ventral surface of the brain, just beneath the sheath that encloses the brain. Therefore, these are highly accessible, unlike the niches of vertebrates that are buried deep within the brain. Second, the neurogenic niche and migratory streams continue to function when brains are dissected and placed in organ culture, a situation that we have exploited to examine local influences of serotonin and other factors, in the absence of possible indirect actions of distant tissues (Benton et al., 2008; Zhang et al., 2011). Third, the 1st-, 2nd- and 3rd- generation precursors in the lineage producing adult-born neurons are spatially separated (see Figure 3; Sullivan et al., 2007b), and each of these generations in the neuronal lineage can be readily assessed separately from others; this situation is very different from the subgranular and subventricular zone niches in the mammalian brain where several generations of precursor cells coexist and their lineage relationships are complex (Zhao et al., 2008). Adult neurogenesis in the crustacean brain therefore provides a remarkably convenient model system in which to explore the sequence of cellular and molecular events leading to the production of new neurons in adult brains. Below we review five areas where studies of adult neurogenesis in the crustacean brain have provided unique insights.
1. Does the genesis of olfactory sensory neurons drive central neurogenesis in the adult brain?
It has been proposed in vertebrate and non-vertebrate organisms that the life-long addition of olfactory interneurons to the central olfactory pathway and the turnover of olfactory receptors neurons (ORNs) in the periphery may be functionally related, with new olfactory interneurons added to accommodate the ingrowing axons of the new ORNs (Lledo and Saghatelyan, 2005; Lindsey and Tropepe, 2006). This hypothesis was tested by examining neuronal proliferation in the olfactory system of spider crabs, Libinia emarginata, which stop molting after a terminal, maturational molt (Carlisle, 1957; Hinsch, 1972). The addition of new cuticular sensillae such as aesthetascs, as well as ORNs to the olfactory organ, is dependent on molting. If, as proposed, the continuous addition of olfactory interneurons is a mechanism by which the central olfactory pathway accommodates the addition of ORNs, it would follow that the proliferation of olfactory interneurons in L. emarginata would not continue beyond the terminal molt. A comparison of neuronal proliferation in immature and mature L. emarginata therefore enabled an examination of the interdependence of central and peripheral neurogenesis in the olfactory pathway. These studies showed that the continuous addition of ORNs in L. emarginata ceases at the terminal molt, but that the proliferation and differentiation of olfactory interneurons continues in mature animals (Sullivan and Beltz, 2005c), demonstrating that continuous proliferation of ORNs is not a requirement for life-long neurogenesis among interneurons in the central olfactory pathway in this organism. A test of this hypothesis was only possible because of special features in the life cycle of this crustacean species, but the conclusions may nevertheless be relevant to phylogenetically more advanced species where the contributions of ORNs to the production of central olfactory neurons cannot be readily dissociated.
2. Environmental enrichment alters the functionality of the entire neuronal precursor cell lineage
Sandeman and Sandeman (2000) assessed the numbers of BrdU-labeled cells in Clusters 9 and 10 in sexually undifferentiated crayfish (Cherax destructor) maintained in enriched (e.g., large spaces, communal living, plants) and deprived (e.g., small spaces, isolation) living conditions, and demonstrated that the quality of the living environment influences neurogenesis in the crustacean brain. Recent studies have built on this foundation by exploiting the spatial separation of the 1st, 2nd and 3rd generations of neuronal precursor cells (Sullivan et al., 2007a, b) in the crayfish brain, to determine which of these may be altered by environmental conditions (Ayub et al., 2011). In addition we used two sizes of crayfish to examine the size/age dependency of environmental influence. Neurogenesis in the brains of both sexually undifferentiated (carapace length 4 mm, CL4) and sexually differentiated (but not yet sexually active) crayfish (CL10) is enhanced by environmental enrichment as previously demonstrated (Sandeman and Sandeman, 2000). However, environmental enrichment also increases the rate of BrdU incorporation into 1st-generation neuronal precursors residing in the neurogenic niche, indicating that the cell cycle time of these stem cells decreases (Ayub et al., 2011). Further, the number of BrdU-labeled (2nd-generation) cells in the migratory streams was greater in the CL10 enriched crayfish relative to those maintained in a deprived environment, but this effect was not observed in the CL4 crayfish. Overall, these data demonstrate consistent influences of environmental conditions on the cell cycle rate of the 1st-generation neuronal precursors, and revealed effects on 2nd generation migratory precursors that may be age dependent. The fact that animals living in enriched conditions have significantly higher numbers of primary neuronal precursor cells actively in S-phase (BrdU-labeled) in the niche relative to animals reared in deprived conditions, also demonstrates that the neuronal precursor cells are not single uniquely endowed progenitors, but rather comprise a population of cells that can be recruited into the cell cycle in response to local conditions (Ayub et al., 2011).
3. The influence of serotonin on adult neurogenesis is lineage-dependent
Serotonin (5-HT) is a potent regulator of adult neurogenesis in both the crustacean brain (Benton et al., 2008), and in the mammalian brain (Brezun and Dazuta, 1999; 2000). Here we could exploit the spatial separation of the neuronal precursor lineage of adult-born neurons in the crayfish (Procambarus clarkii) to determine which generation(s) is influenced by serotonin, and to identify and localize serotonin receptor subtypes underlying these effects (Zhang et al., 2011). RT-PCR shows that mRNAs of serotonin receptors homologous to mammalian subtypes 1A and 2B are expressed in P. clarkii brain (referred to here as 5-HT1α and 5-HT2β). In situ hybridization with antisense riboprobes reveals strong expression of these mRNAs in several brain regions, including cell clusters 9 and 10 where adult-born neurons reside. Antibodies generated against the crustacean forms of these receptors (Sosa et al., 2004; Clark et al., 2004) do not bind to the 1st-generation neuronal precursors in the neurogenic niche or their daughters as they migrate, but do label these 2nd generation precursors as they approach the Clusters 9 and 10 proliferation zones. Like serotonin, administration of the P. clarkii 5-HT1α-specific agonist quipazine maleate salt (QMS) increases the number of BrdU-labeled cells in Cluster 10; the P. clarkii 5-HT2β-specific antagonist methiothepin mesylate salt (MMS) suppresses neurogenesis in this region. However, serotonin, QMS and MMS do not alter BrdU incorporation into the 1st-generation niche precursor cells or their migratory daughters. Our results demonstrate that the influences of serotonin on neurogenesis are confined to the late 2nd-generation precursors and their descendants. Further, the distribution of 5-HT1α and 5-HT2β mRNAs and protein indicate that these serotonergic effects are exerted directly on specific generations of neuronal precursors. Taken together, these results suggest that the influence of serotonin on adult neurogenesis in the crustacean brain is lineage dependent, and that 5-HT1α and 5-HT2β receptors underlie these effects (Zhang et al., 2011).
Therefore, in contrast to the effect of environmental enrichment, which can alter cell cycle activity in all precursor cell generations (Ayub et al., 2011), serotonin influences BrdU incorporation only into the late 2nd and later generations of cells in the proliferation zones of Clusters 9 and 10. These effects are correlated with the expression of serotonin receptors in these precursor generations (Zhang et al., 2011). The more extensive influence of environment on the neuronal precursor cell lineage likely reflects the many physiological changes that occur in response to living conditions, where many variables are introduced.
4. 1st-generation neuronal precursors: cell divisions and self-renewal capacity
Studies exploring the mode of division of the niche precursors in crayfish revealed that these cells undergo geometrically symmetrical divisions (Zhang et al., 2009). These divisions often occur where the streams emerge from the niche, or in the initial segments of the streams (Figure 7). The cytoplasm of the dividing/migratory cells is GS-labeled (Figs. 2A, B and 7, inset), a characteristic feature of the niche cells, thus confirming the ancestry of these cells, although it is not known if all niche cells are competent to become neuronal precursors. However, as elaborated above, the common cytological features of the niche cells (Zhang et al., 2009), their labeling with antibodies against the G1-phase marker MCM2–7 (Sullivan et al., 2007a) and the fact that the size of the pool of S-phase cells can be regulated (Ayub et al., 2011), suggest that many (if not all) niche cells are competent to become neuronal precursor cells.
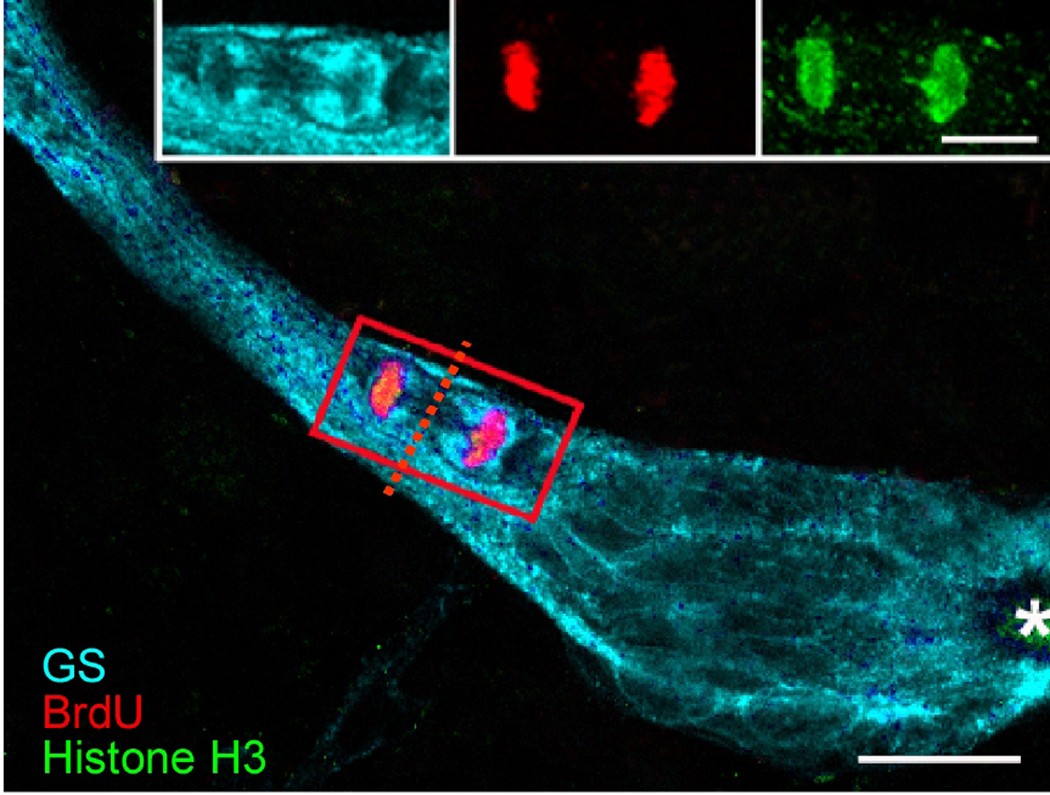
Figure 7.
This image shows a triple-labeled M-phase cell near the emergence of the stream from the niche, immunolabeled with glutamine synthetase (cyan), phosphohistone-H3 (green) and BrdU (red). Each fluorescent channel is shown separately in the inset. The cleavage planes of niche cells are always oriented perpendicular to the track of the stream. Cytoplasmic labeling of the dividing cell for GS confirms that the GS-labeled niche cells are the precursors of the neuronal lineage. The outlines of other niche cell nuclei are GS-labeled. The asterisk marks the vascular cavity. Scale bar 20 µm. (Images from Zhang et al., 2009).
Bromodeoxyuridine (BrdU; S phase) and phosphohistone-H3 (M phase) labeling demonstrates that the G2 phase of the cell cycle in the 1st generation niche precursor cells is short, as these cells label with both markers during a 6-hr span (Figure 7, insets). However, the time to complete the entire cell cycle is relatively long (>48 hrs) in the niche precursor cells (Benton et al., 2011). This cycle time was calculated based on the fact that the streams, which are the only egress from the niche, have never been seen to contain more than 10–12 1abeled cells and that these cells require 5–7 days to complete their migration to the proliferation zones (Sullivan et al., 2007a). Further, the numbers of cells in the niche remain stable for each size/age of crayfish (Zhang et al., 2010). Therefore, it is possible to deduce the rate of 1st generation precursors emerging from the niche using long (5-day, based on the minimum migration time) BrdU incubations, to reveal all cells that have emerged from the niche (the source) and entered the streams (the sink) over the minimum migration period. A second approach comparing the numbers of labeled cells in the streams following 1, 3 and 5-day BrdU incubations confirms that the 1st generation niche precursors require a minimum of 48 hours to complete a full cell cycle. Because each side of the niche generally contains a single active precursor cell, this means that the maximum output of the niche is 1 cell per day, except in the case of specific regulators (e.g., enriched environment; Ayub et al., 2011) that increase the activity of these precursors cells beyond “control” levels.
The geometrically symmetrical niche cell divisions generally occur near the emergence of the migratory streams and pairs of cells are frequently observed along the migratory route, indicating that both daughter cells exit from the niche (Zhang et al., 2011). These data therefore suggest that the outcome of these mitoses is unlike the self-renewing divisions that are generally associated with stem cells where one daughter remains at the site of division to replenish the stem cell pool, and the other pursues a different fate. Several approaches have been used to directly test the self-renewal capacity of the 1st generation neuronal precursor cells residing in the niche. Among these, pulse-chase BrdU-EdU (5-ethynyl-2’-deoxyuridine) studies were conducted in P. clarkii. In planning these experiments, the long (>36 hours) clearing time following a 24-hour BrdU exposure (Benton et al., 2011) and the cycle time (>48 hours) of the 1st-generation niche precursor cells were taken into account. Animals were maintained in pond water for 3.5 or 7 days between the 24-hour BrdU and 6-hour EdU exposures, and killed immediately after the EdU exposure. Such experiments result in only EdU-labeled cells in the niche; all BrdU labeling is confined to the streams (3.5 days) and/or proliferation zones (3.5 and 7 days) (Figure 8; Benton et al., 2011). These data demonstrate that the earlier-labeled (BrdU, red label) group of cells has moved away from the niche, and that none are left behind to divide and maintain the niche population, confirming our earlier suspicions (Zhang et al., 2009).
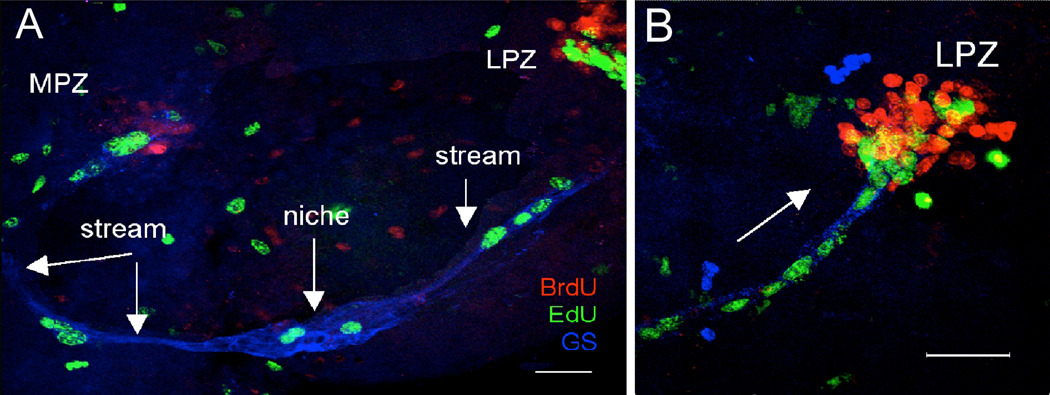
Figure 8.
A. Live crayfish were incubated in BrdU for 24 hours and then maintained in fresh pond water for 7 days. Just before sacrifice, they were treated with EdU (green) for 6 hours. Fixed brains also were labeled immunocytochemically for glutamine synthetase (blue) to reveal the niche and streams. S phase cells in the niche and streams are labeled only with EdU, demonstrating that migration is uni-directional (away from the niche) and that niche cell divisions are not self-renewing, as no BrdU-labeled cells remain in the niche. B. Higher magnification image of the LPZ. The BrdU-labeled cells which were labeled first in the sequence are found only in the proliferation zones (MPZ and LPZ) and not in the niche. Arrow indicates direction of migration. Scale bars: A, 100 µm; B, 50 µm. (Images from Benton et al., 2011).
A second line of evidence pertinent to self-renewal capacity comes from the incubation of animals in 10−9 M serotonin, which is known to stimulate neurogenesis in these animals (Beltz et al., 2001; Benton and Beltz, 2001; Benton et al., 2008; Zhang et al., 2011). This treatment increases the number of BrdU-labeled cells in the Cluster 10 proliferation zones but does not alter the number of S phase (BrdU-labeled) cells in the niche or streams (Zhang et al., 2011). However, the total number of cells in the niche in serotonin-treated animals increases by >20% in 24 hrs (Benton et al., 2010; 2011). This highly significant increase (ANOVA, p < 0.0001) in the total number of cells in the absence of additional cell divisions among the niche cells, suggests that the additional cells are being recruited to the niche from an outside source. Whether or not all of these recruited cells are competent to become neuronal precursors, the potential for an important relationship between the hematopoietic and neurogenic systems is clear from these findings, which are discussed further below.
A crucial question to address is why the number of 1st-generation niche precursor cells does not diminish over time, as these divide and migrate away. The data above suggest that neuronal precursors must be recruited to the niche from a source extrinsic to the niche because the niche precursor cells do not undergo self-renewing divisions. That the number of niche cells can increase without additional mitoses following exposure of animals to serotonin is an intriguing finding that may relate directly to the attraction of specific cell types to the niche (Benton et al., 2010; 2011).
5. Neuronal precursors from a non-neuronal source?
To explore possible relationships between the niche and other tissues, several cell types were isolated from their respective tissues and labeled with the fluorescent marker CellTracker™ Green CMFDA (CTG; Invitrogen). Labeled cells were then introduced into culture dishes containing freshly dissected, desheathed crayfish brains, followed by a 6-hr incubation period at 18°C. The distribution of labeled cells in each culture dish was subsequently visualized to determine whether cells showed any affinity for the brains and/or associated niches.
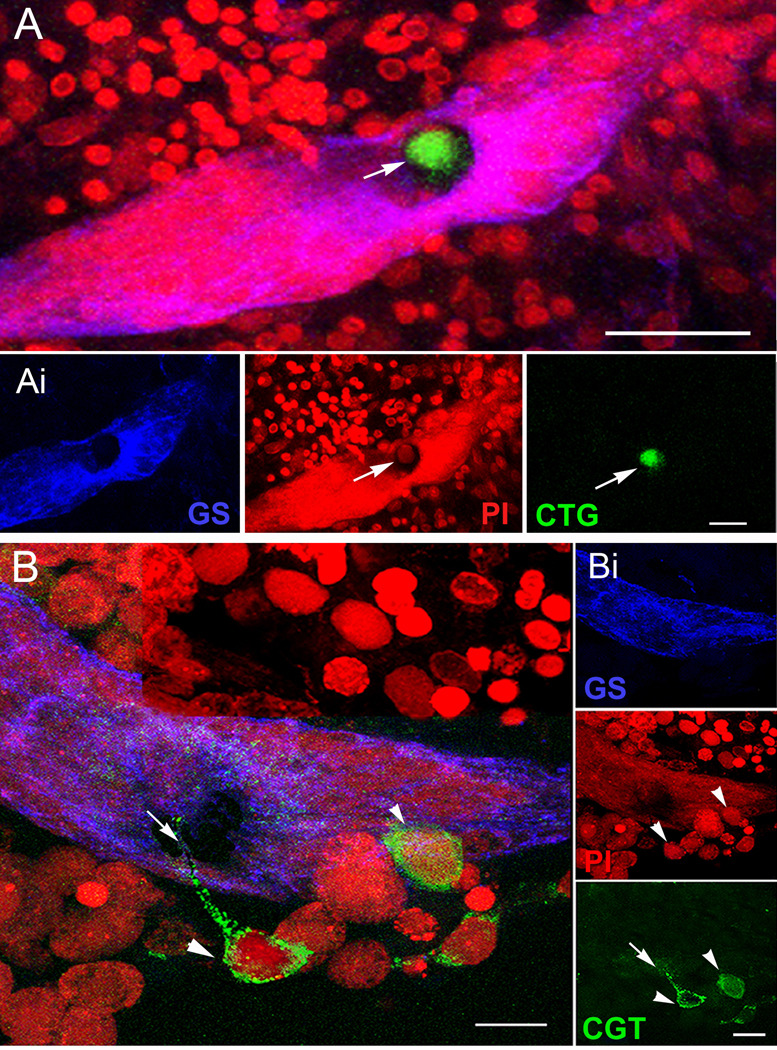
Of the various cell types tested, most remained evenly distributed in the culture dishes and showed no particular affinity for the brains or niches (Table 1). For instance, in brains incubated with CTG-labeled cells from hepatopancreas, 10 out of 11 niches were devoid of labeled cells in the vascular cavity and niche. In contrast, cells extracted from the hemolymph showed a remarkable affinity for the niche. CTG-labeled circulating hemocytes were found in the vascular cavities in 77% of niches with which they were co-cultured (Figure 9A). They were also found among the precursor cells in the niches (Figure 9B), and some of these cells labeled for glutamine synthetase (Benton et al., 2010; submitted), a marker of the 1st generation niche precursor cells in these crayfish (see Figures 2A, B and 7).
TABLE 1.
The relationship between the neurogenic niche and dissociated, CellTracker™ Green (CTG)-labeled cells from four crayfish tissues (green gland, hepatopancreas, hematopoietic tissue and hemolymph) was examined in co-cultured, desheathed brains. Complete exposure to the niche/ventral brain surface appears to be essential for the niche region to attract CGT-labeled hemocytes in vitro, because no cells are attracted to the niche when sheathed brains are placed in co-culture with CTG-labeled hemocytes. The number and the percentage of total niches containing CTG+ cells appear in the second and third columns. The Chi-square test of independence was used to test the significance of these findings; the result is chi square (7, N=111) =34.8885, p < 0.001, indicating that the number of niches containing CTG+ cells was significantly dependent on the cell type. The attraction of hemocytes to the niche relative to other cell types is therefore of statistical significance.
|
1. Dissociated cells from crayfish tissues labeled with CTG. |
Total number of niches |
Percent of niches with CTG labeled cells |
| Green Gland | 10 | 0% |
| Hepatopancreas | 11 | 0% |
| Hematopoietic Tissue | 12 | 9% |
| Hemocytes | 30 | 77% |
|
2. Changes in cell culture medium with CTG labeled hemocytes |
||
| Hemocytes + 5-HT×10−9 M | 12 | 17% |
| Hemocytes + MMS ×10−8 M | 16 | 31% |
|
3. Treatment prior to co-culture, culture + CTG labeled hemocytes |
||
| 5-day PCPA pretreatment | 8 | 12.5% |
| Sheath left on dissected brain | 12 | 0% |
Figure 9.
CellTracker™-labeled cells (CTG-labeled cells) are found in the vascular cavity and in, and on, the neurogenic niche itself. A. Projections of confocal stacked images of the neurogenic niche. Position of a CTG-labeled cell in the vascular cavity with all fluorescence channels merged. Ai. (Left, center, right panels) Glutamine synthetase (GS, left) immunoreactivity in the niche; propidium iodide (PI, center) labeling all cell nuclei, with arrow pointing to the nucleus of the CTG-labeled cell; CTG-labeled cell (right). B. Other CTG-labeled cells have interesting morphological characteristics, two of which can be seen just outside the niche (arrowheads); one has a long process extending into the niche (arrow), and another CTG-labeled cell inserts on the outer margin of the niche. Bi. Top, middle, and bottom panels represent the separate channels: GS outlining the niche (top); PI revealing cell nuclei, arrowheads pointing to the respective CTG-labeled cells in B (middle); and CTG-labeled cells with arrow pointing to the fine process coming from the CTG-labeled cell (bottom). Scale bars: A, 50 µm; Ai, 20 µm; B, 20 µm; Bi, 10 µm. (Images from Benton et al., 2011).
Serotonin and the neurogenic niche
Our studies described above suggested that serotonin may serve as a signal to attract cells to the niche. Immunocytochemical techniques were used to explore this possibility and to determine whether the niche contains serotonin. Serotonin immunoreactivity is indeed present as a discontinuous rim around the vascular cavity (Benton et al., 2010; 2011). We do not know the cellular origins of this structure, nor how it may be associated with the niche precursor cells because none of the niche cells were themselves labeled.
Because increased serotonin levels lead to an increase in the niche precursor cell population in vivo and because serotonin is localized around the vascular cavity, we further examined the relationship between cells circulating in the vasculature and the niche. This was done by introducing serotonin or methiothepin mesylate salt (MMS, a P. clarkii-specific serotonin 2β receptor antagonist; Spitzer et al., 2008), into brain-hemocyte co-cultures. Our hypothesis was that if serotonergic mechanisms are involved in the attraction between hemocytes and the niche in our co-cultures, then serotonin or molecules that would interfere with serotonergic mechanisms might alter the behavior of the cell type responsible for increases in the number of niche cells. In practice we found labeled cells in only 17% of the niches/vascular cavities in brain-hemocyte co-cultures to which 10−9 M serotonin was added. This is a significantly lower rate of cell incorporation into the niche than brain-niche co-cultures incubated in normal medium without serotonin (Table 1). Similarly, in brain-hemocyte co-cultures to which 10−8 M MMS (10−8 M) was added to the normal culture medium, CTG-labeled cells were found in only 5 of the 16 vascular cavities, and no CTG-labeled cells were found in the niche cell clusters (Table 1). These results demonstrate a change in cell behavior as a result of exposure to serotonin or MMS, suggesting that serotonergic signaling mechanisms may at least partly explain the affinity between the CTG-labeled hemocytes and the niche. This conclusion is reinforced by the finding that the attraction of CTG-labeled cells for the niche is severely reduced in brains of crayfish pretreated with parachlorophenylalanine (PCPA), an inhibitor of serotonin synthesis (Table 1).
It follows that if serotonin is acting directly to attract cells to the niche, then some circulating hemocytes should contain serotonin receptors. RT-PCR indeed shows that mRNAs of serotonin receptor subtypes 1α and 2β are present not only in the nervous system, but also in hematopoietic tissue (HPT) and hemocytes (Benton et al., 2010; 2011). In Western blots, antibodies against crustacean 5-HT1α and 5-HT2β receptors label proteins around 37kDa and 80kDa, respectively.
Glutamine Synthetase Immunoreactivity: A Marker of Neuronal Precursor Cells and a Subtype of Hemocytes
Some of the CTG-labeled cells that were attracted to the niche were immunoreactive for glutamine synthetase (Benton et al., 2011), a marker of the 1st generation neuronal precursors in the procambarid neurogenic niche. We therefore asked whether any cells circulating specifically in the hemolymph label for this marker. Indeed, one type of circulating cell labels immunocytochemically with GS antibodies (Ayub et al., 2011; Benton et al., 2010; 2011) a finding that has been confirmed by Western blot analysis (Zhang and Beltz, 2010). The GS-immunoreactive cells have fine cytoplasmic granules and often have pseudopodia and/or processes, consistent with the phenotype of semi-granular hemocytes, which have been proposed by others to be the crustacean hematopoietic stem cell (Chaga et al., 1995; Johansson et al., 2000). Further studies of specific types of hemocytes and their behavior relative to the niche will be greatly facilitated by work done in the laboratories of Irene and Kenneth Söderhäll, where hemocyte production and maturation have been examined in a closely related crayfish, Pacifastacus leniusculus. They have developed methods for isolating hematopoietic stem cells from P. leniusculus (Söderhäll et al., 2003), separated specific classes of hemocytes in viable condition (Söderhäll and Smith, 1983; Lin et al., 2008), identified lineage marker proteins for hematopoietic, semi-granular and granular cells (Wu et al., 2008), and demonstrated that semi-granular and granular hemocytes emerge from separate hematopoietic lineages (Wu et al., 2008). Further, their studies show that injection of crayfish with microbial polysaccharides (Wu et al., 2008) or a crustacean cytokine in the prokineticin family (Söderhäll et al., 2005; Lin et al., 2010) causes rapid release of hemocytes from hematopoietic tissues, phenomena that make possible in vivo tests of several aspects of our current model.
IV. A new synthesis and a novel hypothesis
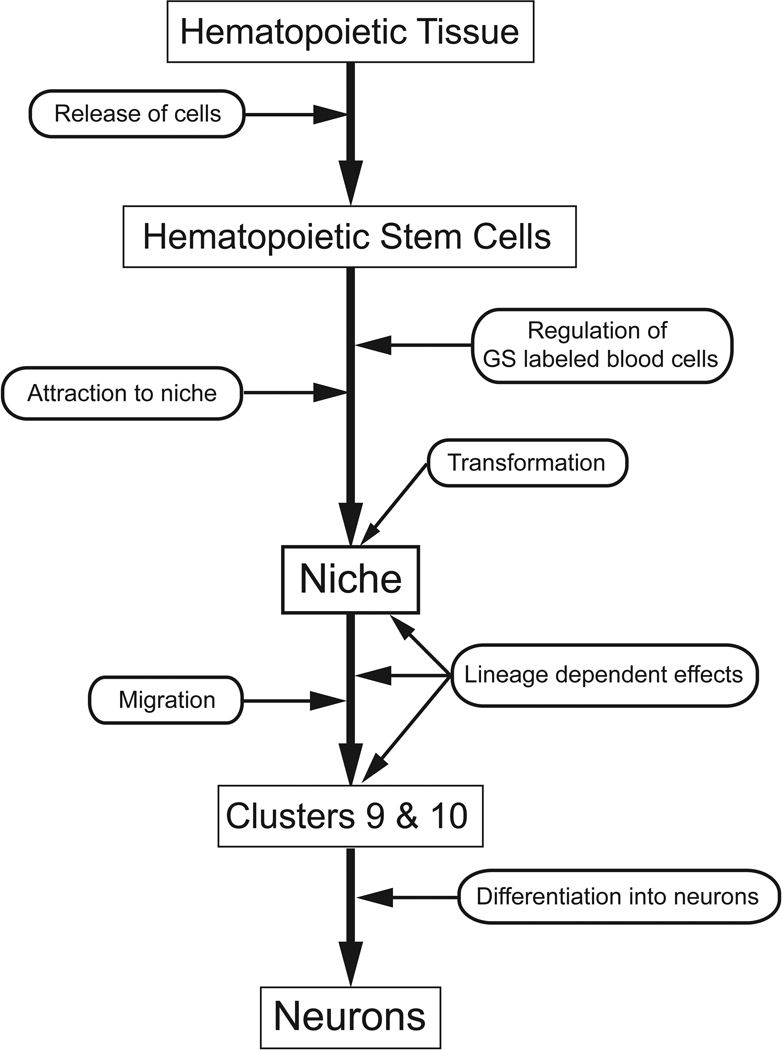
The data described above have led to a revision and expansion of our original model of adult neurogenesis in crayfish (Figure 3), which assumed that the primary precursors residing in the niche are self-renewing (Sullivan et al., 2007b), and we are now testing the alternative hypothesis that hematopoietic stem cells may be replenishing the niche cell pool (Benton et al., 2010; 2011). In our current model (Figure 10) of adult neurogenesis we propose that stem cells released from hematopoietic tissues are attracted to the niche where they are transformed into 1st-generation neuronal precursor cells. These aspects of our model are presently hypothetical.
Figure 10.
Our current model of adult neurogenesis in the crayfish P. clarkii, beginning with hematopoietic tissue, the release of hematopoietic stem cells, their attraction to the niche and transformation into niche cells. These aspects of our model are hypothetical. The niche cells (1st generation neuronal precursors), which label for glutamine synthetase, divide symmetrically to produce daughters that migrate along processes of the niche cells towards cell cluster 9 or 10. These 2nd generation neuronal precursors divide at least once more in the proliferation zones in the cell clusters, before differentiating into neurons. These aspects of the model are supported by several types of data, reviewed in this paper.
Further, we propose that the 1st generation neuronal precursor cells in the niche that label for glutamine synthetase divide symmetrically to produce daughters, both of which migrate along processes of the niche cells towards cell cluster 9 or 10 (Sullivan et al., 2007a; Zhang et al, 2009). In these cell clusters, the 2nd generation neuronal precursors divide at least once more before differentiating into neurons (Sullivan et al., 2005b; 2007b). These aspects of the model are supported by several types of data that have been peer-reviewed, published and are described above.
There are several important implications of the proposed model. Perhaps most critical is the underlying suggestion that the neurogenic niche, rather than providing an environment for nurturing long-lived stem cells, may instead be providing an environment where pluripotent stem cells may transform into 1st-generation neuronal precursors; the resulting niche cells are functionally equivalent to neuronal stem cells in mammals in terms of their position in the neuronal precursor lineage, but they do not meet the criterion of self-renewal. Instead, our model suggests that “pre-stem” cells (that, before their transformation, are not "neural" cells at all) circulating in the vasculature will be renewed by hematopoietic tissues. Also implicit in this model is the idea that the transformation from circulating hemocyte to 1st-generation neuronal precursor would involve molecular changes typical of a mesenchymal-epithelial transition, a process that would be supported by the niche. The inclusion of the present data into current theoretical frameworks may therefore require the revision and expansion of our definitions for “neuronal stem cells” and “stem cell niche”, terms that were proposed well before direct tests of many of the proposed features. For example, the first demonstration of self-renewal of mammalian neuronal stem cells in vivo was obtained relatively recently (Suh et al., 2007) and long after the stem cell definition was proposed based primarily on in vitro and non-vertebrate studies (Zhao et al., 2008). We therefore suggest that the crayfish neurogenic niche may function as a "transformational niche" by changing mesenchymal cells derived from hematopoietic tissues into epithelial cells that function as 1st-generation neuronal precursors.
There are additional reasons to suspect a close relationship between the hematopoietic system and the niche in crayfish: (1) The crayfish niche is a discrete organ. Although contained within the brain sheath, it is physically isolated from the nearby brain tissues. However, the niche apparently does communicate with the vascular system via the vascular cavity (e.g., Figure 2D) and the extracellular milieu, as the niche is not bounded by a membrane. The migratory streams connecting the niche with the proliferation zones have been shown to support migration only away from the niche (Sullivan et al., 2007a; Benton et al., 2011), and it is therefore unlikely that they participate in the maintenance or renewal of the niche cell population. By means of the vascular connection or the extracellular milieu surrounding the niche, the hematopoietic system therefore has the opportunity to interact with the niche via its production of circulating cells. (2) A small population of hemocytes labels immunocytochemically for glutamine synthetase (GS), a marker of the niche stem cells (Benton et al., 2010; 2011). Further, the proportion of GS-immunoreactive hemocytes increases after 2–3 weeks of environmental enrichment, a treatment that also promotes neurogenesis in these animals (Ayub et al., 2011). (3) Hemocytes express 5-HT1α and 5-HT2β receptors (Western blot and PCR analysis) (Benton et al., 2011). Cells that expand the niche pool in response to serotonin would be expected to express these receptors, if the serotonergic effect is a direct one. The finding that addition of serotonin or MMS to the culture alters the affinity of vascular cells for the niche is also consistent with the presence of these receptors on hemocytes. (4) Niche precursor cells have the same large dimensions and unusual chromatin pattern that are seen in some circulating and perivascular cells that are thought to be of hematopoietic origin, and indeed semi-thin sections show one cell type (Type III) that has a stalk connecting the cell body to the niche (Zhang et al., 2009). This feature is highly reminiscent of CTG-labeled hemocytes that insert into the niche, although the semi-thin sections in our previous studies were prepared from freshly dissected and fixed brains from untreated crayfish.
V. Significance and Conclusions
The details that have emerged from our study of the crayfish neurogenic niche are of interest for several reasons. First, they provide an example of 1st-generation neuronal precursor cells that apparently do not undergo self-renewing divisions, indicating that these primary neuronal precursors in adult crayfish must be replenished from a source external to the neurogenic niche. We have demonstrated that in co-cultures, hemocytes have an affinity for the niche, and that some of these cells extend processes and express glutamine synthetase, as do the 1st generation neuronal precursors that comprise the niche. A small percentage of cells extracted from the hemolymph of crayfish also label with the niche cell marker glutamine synthetase. The findings that serotonin treatment increases the niche cell population, that serotonin is localized to the rim of the vascular cavity, and that serotonin receptors are found in hemocytes, are consistent with the behavior of these cells in niche co-cultures, where flooding the culture dish with serotonin or the 5-HT2β receptor antagonist MMS interferes with their affinity for the niche. Given these findings, the hematopoietic system appears to be a very likely source of primary neuronal precursor cells in crayfish. Should this be the case, we still do not know whether cells recruited into the niche are transformed into resident bipolar niche cells that will eventually become primary neuronal precursors (an "assembly line" or “inventory” model), or whether the recruited cells become transformed immediately into 1st generation neuronal precursors (a "conveyor belt" or “just-in-time” model). The first scenario would suggest that the niche cells represent progressive stages in the transformation and activation as 1st-generation precursors. The second, that newly recruited cells may be fast-tracked through the niche, escorted by the niche cells, as they progress through the cell cycle and divide. In either case, however, the studies of Ayub et al. (2011) show that the numbers of niche cells actively engaged in the cell cycle can be modulated, presumably in response to local signals that are, in turn, influenced by the animal's living conditions. Therefore, the 1st generation neuronal precursors cannot be single (or a few) specially-endowed cells, but rather are a dynamic population whose proliferative potential is regulated. Experiments designed to differentiate between the “inventory” and “just-in-time” models are currently underway. In addition, our studies will focus on the apparent transformation of hemocytes from mesenchymal to epithelial niche cells, and on in vivo studies to track labeled hemocytes over periods of several days or weeks, which will allow us to observe and test their transdifferentiation potential.
The proposal that hematopoietic stem cells may play a central role in adult neurogenesis is not new. Mammalian bone marrow cells have a proclivity to migrate to the brain when infused into a host animal (Eglitis and Mezey, 1997; Kopen et al., 1999; Brazelton et al., 2000). In studies where bone marrow cells were grafted into the lateral ventricle they migrated throughout the brain, including areas undergoing active postnatal neurogenesis. The descendants of these cells expressed a variety of glial and neuronal markers (Egletis and Mezey, 1997; Mezey et al., 2000; Mahmood et al., 2001; Chen et al., 2001), and in some studies these cells developed the characteristics of astrocytes (Kopen et al., 1999). In vitro, bone marrow cells have been induced by various means to form neurons (Sanchez-Ramos, 2002; Sanchez-Ramos et al., 1998, 2000, 2001; Kohyama et al., 2001) and in one study the bone marrow-derived neurons responded to depolarizing stimuli, showing a rapid and reversible calcium increase in response to acetylcholine, a response characteristic of neurons (Kohyama et al., 2001). While the identities of specific types of bone marrow cells that are the source of neurons and glia in these studies is not always clear, other experiments specifically used hematopoietic cells, and found that in the brain these could acquire neural features and express neural genes (Goolbsy et al., 2003; Gottschling et al., 2007). However, other studies suggest that cell fusion may account for the acquisition of such broad properties by stem cells (Moorshead et al., 2002; Wagers et al., 2002; Wells, 2002; Coyne et al., 2006). Nevertheless, the idea that cells derived from bone marrow can transdifferentiate into neuronal and glial precursors in response to signals in the brain persists in the literature.
Our current results show that the 1st generation neuronal precursor cells comprising a neurogenic niche in the adult crayfish brain are not self-renewing and that their numbers are not depleted as their daughters migrate away. The corollary of these findings is that these neuronal precursor cells must be replenished from a source extrinsic to the niche. Our data point to the hematopoietic system as one possible source of neuronal precursor cells, a finding that, if confirmed in further studies, has profound mechanistic, developmental and evolutionary implications.
Acknowledgements
The authors thank P. Carey and V. LePage for care of the animals used in these studies. The work was supported by NIH R01 MH67157, NSF IBN 0344448 and 0091092, NSF IOS 0818259, NSF DBI 0922895 and a Brachman Hoffman Fellowship from Wellesley College.
Abbreviations
- 5-HT
5-hydroxytryptamine; serotonin
- AL
accessory lobe
- BrdU
bromodeoxyuridine
- CL
carapace length
- CTG
CellTracker™ Green CMFDA
- EdU
5-ethynyl-2’-deoxyuridine
- GS
glutamine synthetase
- LIS1
lissencephaly1
- LPZ
lateral proliferation zone
- MMS
methiothepin mesylate salt
- MPZ
medial proliferation zone
- MRI
magnetic resonance imaging
- OL
olfactory lobe
- OGT
olfactory globular tract
- ORN
olfactory receptor neuron
- PCPA
parachlorophenylalanine
- QMS
quipazine maleate salt
- RMS
rostral migratory stream
- SGZ
subgranular zone
- SVZ
subventricular zone
References Cited
- Ayub N, Benton JL, Zhang Y, Beltz BS. Environmental enrichment influences neuronal stem cells in the adult crayfish brain. Dev. Neurobiol. 2011 doi: 10.1002/dneu.20864. epub ahead of print, PMID 21192037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl. Acad. Sci. USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin F. Étude comparée de l’organe deutocérébral des Macroures Reptantia et des Anomoures (Crustacés Décapodes) Arch. Zool. Exp. Gen. 1970a;111:245–264. [Google Scholar]
- Bazin F. Les organs deutocérébraux chez deux Crustacés Décapodes Macroures Reptantia: Panulirus regius de Brito Capello, Scyllarus arctus (L.) B. Soc. Zool. Fr. 1970b;96:87–92. [Google Scholar]
- Bazin F, Demeusy N. Existance d’organes intracérébraux énigmatiques chez le Crustacé Décapode Carcinus maenas (L.) C. R. Acad. Sci. 1968;267:356–358. [Google Scholar]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Struct. Dev. 2003;32:39–60. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transiaent uptake of serotonin by newborn olfactory projection neurons. Proc. Natl. Acad. Sci. USA. 2001;98:12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci. Lett. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton J, Beltz BS. Effects of embryonic serotonin depletion in olfactory interneurons in lobsters. J. Neurobiol. 2001;46:193–205. doi: 10.1002/1097-4695(20010215)46:3<193::aid-neu1002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Benton JL, Goergen EM, Rogan SC, Beltz BS. Hormonal and synaptic influences of serotonin on adult neurogenesis. Gen. Comp. Endocrin. 2008;158:183–190. doi: 10.1016/j.ygcen.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Sandeman DC, Beltz BS. Nitric oxide in the crustacean brain: regulation of neurogenesis and morphogenesis in the developing olfactory pathway. Dev. Dyn. 2007;236:3047–3060. doi: 10.1002/dvdy.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Zhang Y, Kirkhart CR, Sandeman DC, Beltz BS. Primary neuronal precursors in the crayfish brain: self-renewal or replenishment? Soc. Neurosci. Abstr. 2010;36 doi: 10.1186/1471-2202-12-53. 233.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Zhang Y, Kirkhart CR, Sandeman DC, Beltz BS. Primary neuronal precursors in adult crayfish brain: replenishment from a non-neuronal source. BMC Neuroscience. 2011 doi: 10.1186/1471-2202-12-53. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J. Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur. J. Neurosci. 2000;12:391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- Carlisle DB. On the hormonal inhibition of molting in decapod Crustacea. II. Terminal anecdysis in crabs. J. Marine Biol. Ass. UK. 1957;36:291–307. [Google Scholar]
- Chaga O, Lignell M, Söderhäll K. The haematopoietic cells of the freshwater crayfish Pacifasticus leniusculus. Anim Biol. 1995;4:59–70. [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Clark MC, Dever TE, Dever JJ, Xu P, Rehder V, Sosa MA, Baro DJ. Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J. Neurosci. 2004;24:3421–3435. doi: 10.1523/JNEUROSCI.0062-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- Cvetic CA, Walter JC. Getting a grip on licensing: mechanism of stable Mcm2–7 loading onto replication origins. Mol. Cell. 2006;21:143–144. doi: 10.1016/j.molcel.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J. Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R, Baldysiak-Figiel A, Krugel V, Ueberham E, Gaunitz F. Hepatocellular expression of glutamine synthetase: an indicator of morphogen actions as master regulators of zonation in adult liver. Prog. Histochem. Cytochem. 2007;41:201–266. doi: 10.1016/j.proghi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J. Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Goolsby J, Marty MC, Heletz D, Chiappelli J, Tashko G, Yarnell D, Fishman PS, Dhib-Jalbut S, Bever CT, Jr, Pessac B. Hematopoietic progenitors express neural genes. Proc. Natl. Acad. Sci. USA. 2003;100:14926–14931. doi: 10.1073/pnas.2434383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling S, Eckstein V, Saffrich R, Jonas A, Uhrig M, Krause U, Seckinger A, Miesala K, Horsch K, Straub BK, Ho AD. Primitive and committed human hematopoietic progenitor cells interact with primary murine neural cells and are induced to undergo self-renewing cell divisions. Exp. Hematol. 2007;35:1858–1871. doi: 10.1016/j.exphem.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. Neurogenesis in the central olfactory pathway of the adult shore crab Carcinus maenas is controlled by sensory afferents. J. Comp. Neurol. 2001;441:223–233. doi: 10.1002/cne.1408. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Cate HS, Swanson ES, Derby CD. Postembryonic proliferation in the spiny lobster antennular epithelium: rate of genesis of olfactory receptor neurons is dependent on molt stage. J Neurobiol. 2001;47:51–66. doi: 10.1002/neu.1015. [DOI] [PubMed] [Google Scholar]
- Harzsch S. Ontogeny of the ventral nerve cord in malacostracan crustaceans: a common plan for neuronal development in Crustacea, Hexapoda and other Arthropoda? Arthropod Struct. Dev. 2003;32:17–37. doi: 10.1016/S1467-8039(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. J. Neurosci. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsch GW. Some factors controlling reproduction in the spider crab, Libinia emarginata. Biol. Bull. 1972;143:358–366. doi: 10.2307/1540059. [DOI] [PubMed] [Google Scholar]
- Huang S, Sato S. Progenitor cells in the adult zebrafish nervous system express a Brn-1-related POU gene, tai-ji. Mech. Dev. 1998;71:23–35. doi: 10.1016/s0925-4773(97)00199-8. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Adult brain neurogenesis and depression. Brain Behav. Immun. 2002;16:602–609. doi: 10.1016/s0889-1591(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Johansson MW, Keyser P, Sritunyalucksana K, Söderhäll K. Crustacean hemocytes and haematopoiesis. Aquaculture. 2000;191:45–52. [Google Scholar]
- Kaslin J, Ganz J, Geffarth M, Grandel H, Hans S, Brand M. Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J.Neurosci. 2009;29:6142–6153. doi: 10.1523/JNEUROSCI.0072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. Adult Neurogenesis. New York: Oxford University Press; 2006. [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kim YF. Honors thesis. Wellesley, MA: Wellesley College; 2009. Differentiation and survival of adult-born neurons in the brain of Cherax destructor. [Google Scholar]
- Kim YF, Beltz BS. Adult neurogenesis: quantitative analysis of proliferation, survival and rate of differentiation of newborn neurons in the brain of the crayfish, Cherax destructor. Soc. Neurosci. Abstr. 2008;34 423.26. [Google Scholar]
- Kohyama J, Abe H, Shimazaki T, Koizumi A, Nakashima K, Gojo S, Taga T, Okano H, Hata J, Umezawa A. Brain from bone: efficient "meta-differentiation" of marrow stroma derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation. 2001;68:235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Ann. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz H, Tsutsumi V, Arechiga H. Morphological and biochemical characerization of Procambarus clarkii blood cells. Dev. Comp. Immunol. 1993;17:389–397. doi: 10.1016/0145-305x(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Lemieux G, Baverel G, Vinay P, Wadoux P. Glutamine synthetase and glutamyltransferase in the kidney of man, dog and rat. Am. J. Physiol. 1976;231:1068–1073. doi: 10.1152/ajplegacy.1976.231.4.1068. [DOI] [PubMed] [Google Scholar]
- Lennington JB, Yang Z, Conover JC. Neural stem cells and the regulation of adult neurogenesis. Reprod. Biol. Endocrin. 2003;1:99. doi: 10.1186/1477-7827-1-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Söderhäll K, Söderhäll I. Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, importance in hemocyte homeostasis. BMC Immunol. 2008;9:58. doi: 10.1186/1471-2172-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Novotny M, Söderhäll K, Söderhäll I. Ancient cytokines -the role of astakines as hematopoietic growth factors. J. Biol. Chem. 2010;285:28577–28586. doi: 10.1074/jbc.M110.138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog. Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Linser PJ, Trapido-Rosenthal HG, Orona E. Glutamine synthetase is a glial-specific marker in the olfactory regions of the lobster (Panulirus argus) nervous system. Glia. 1997;20:275–283. [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Ma D-K, Ming G-L, Gage FH, Song H. Neurogenic niches in the adult mammalian brain. Chapter 11 in Adult Neurogenesis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. [PubMed] [Google Scholar]
- Martini FJ, Valdeolmillos M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J. Neurosci. 2010;30:8660–8670. doi: 10.1523/JNEUROSCI.1962-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Role of nitric oxide in subventricular zone neurogenesis. Brain Res. Rev. 2005;49:355–366. doi: 10.1016/j.brainresrev.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J. Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Benveniste P, Iscove NN, van der Kooy D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med. 2002;8:268–273. doi: 10.1038/nm0302-268. [DOI] [PubMed] [Google Scholar]
- Morris NR, Efimov VP, Xiang X. Nuclear migration, nucleokinesis and lissencephaly. Trends Cell Biol. 1998;8:467–470. doi: 10.1016/s0962-8924(98)01389-0. [DOI] [PubMed] [Google Scholar]
- Nam SC, Kim Y, Dryanovski D, Walker A, Goings G, Woolfrey K, Kang SS, Chu C, Chenn A, Erdelyi F, Szabo G, Hockberger P, Szele FG. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. J. Comp. Neurol. 2007;505:190–208. doi: 10.1002/cne.21473. [DOI] [PubMed] [Google Scholar]
- Nyfelar Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U, Taylor V. Jagged 1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005;24:3504–3515. doi: 10.1038/sj.emboj.7600816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58:865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc. Natl. Acad. Sci. USA. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. T. Roy. Soc. B. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Tsai LH. Nucleokinesis illuminated. Nat. Neurosci. 2004;7:1169–1170. doi: 10.1038/nn1104-1169. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR. Neural Cells Derived from Adult Bone Marrow and Umbilical Cord Blood. J. Neurosci. Res. 2002;69:880–893. doi: 10.1002/jnr.10337. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Cardozo-Pelaez F, Song S. Differentiation of neuron-like cells from bone marrow stromal cells. Mov. Disord. 1998;13 Suppl:122. [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, Stedeford T, Chopp M, Sanberg PR. Expression of neural markers in human umbilical cord blood. Exp. Neurol. 2001;171:109–115. doi: 10.1006/exnr.2001.7748. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Bazin F, Beltz BS. Adult neurogenesis: evolutionary parallels in crustaceans and mammals. Arthropod Struct. Dev. 2011 doi: 10.1016/j.asd.2011.03.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman DC, Benton JL, Beltz BS. An identified serotonergic neuron regulates neurogenesis in the crayfish brain. Dev. Neurobiol. 2009;69:530–545. doi: 10.1002/dneu.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman D, Beltz B, Sandeman R. Crayfish brain interneurons that converge with serotonin giant cells in accessory lobe glomeruli. J. Comp. Neurol. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol. Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. "Impoverished" and "enriched" living conditions influence the proliferation and survival of neurons in crayfish brain. J. Neurobiol. 2000;45:215–226. [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the Nervous System. Oxford, U.K: Elsevier Academic Press; 2006. [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Res. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. J. Neurobiol. 2001;48:181–203. doi: 10.1002/neu.1050. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Identification of putative neuroblasts at the base of adult neurogenesis in the olfactory midbrain of the spiny lobster, Panulirus argus. J. Comp. Neurol. 2007;503:64–84. doi: 10.1002/cne.21366. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Harzsch S. Ontogeny of the ventral nerve cord in malacostracan crustaceans: a common plan for neuronal development in Crustacea, Hexapoda and other Arthropoda? Arthropod Struct. Dev. 1999;32:17–37. doi: 10.1016/S1467-8039(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Scotto Lomassese S, Strambi C, Strambi A, Charpin P, Augier R, Aouane A, Cayre M. Influence of environmental stimulation on neurogenesis in the adult insect brain. J. Neurobiol. 2000;45:162–171. [PubMed] [Google Scholar]
- Shapiro EM, Gonzalez-Perez O, Garcia-Verdugo M, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in theadult rodent brain. Neuroimage. 2006;32:1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang S-M, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:3289–3300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderhäll I, Kim Y-A, Jiravanichpaisal P, Lee S-Y, Söderhäll K. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J. Immunol. 2005;174:6153–6160. doi: 10.4049/jimmunol.174.10.6153. [DOI] [PubMed] [Google Scholar]
- Söderhäll I, Bangyeekhun E, Mayo S, Söderhäll L. Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev. Comp. Immunol. 2003;27:661–672. doi: 10.1016/s0145-305x(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Söderhäll K, Smith VJ. Separation of the haemocyte populations of Carcinus maenas and other decapods, and prophenoloxidase distribution. Dev. Comp. Immunol. 1983;7:229–239. doi: 10.1016/0145-305x(83)90004-6. [DOI] [PubMed] [Google Scholar]
- Song CK, Johnstone LM, Schmidt M, Derby CD, Edwards DH. Social domination increases neuronal survival in the brain of juvenile crayfish Procambarus clarkii. J. Exp. Biol. 2007;210:1311–1324. doi: 10.1242/jeb.02758. [DOI] [PubMed] [Google Scholar]
- Song CK, Johnstone LM, Edwards DH, Derby CD, Schmidt M. Cellular basis of neurogenesis in the brain of crayfish, Procambarus clarkii: neurogenic complex in the olfactory midbrain from hatchlings to adults. Arth. Struct. Dev. 2009;38:339–360. doi: 10.1016/j.asd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Spitzer N, Edwards DH, Baro DJ. A crustacean serotonin receptor: cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J. Comp. Neurol. 2004;473:526–537. doi: 10.1002/cne.20092. [DOI] [PubMed] [Google Scholar]
- Spitzer N, Edwards DH, Baro DJ. Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii. J. Exp. Biol. 2008;211:92–105. doi: 10.1242/jeb.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cate HS, Derby CD. A spatiotemporal wave of turnover and functional maturation of olfactory receptor neurons in the spiny lobster Panulirus argus. J. Neurosci. 2000;20:3282–3294. doi: 10.1523/JNEUROSCI.20-09-03282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Neural pathways connecting the deutocerebrum and lateral protocerebrum in the brains of decapod crustaceans. J. Comp. Neurol. 2001;441:9–22. doi: 10.1002/cne.1394. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J. Neurobiol. 2005a;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils of the crayfish brain. J. Comp. Neurol. 2005b;48:118–126. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Adult neurogenesis in the central olfactory pathway in the absence of receptor neuron turnover in Libinia emarginata. Eur. J. Neurosci. 2005c;22:2397–2402. doi: 10.1111/j.1460-9568.2005.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Beltz BS. Serotonin depletion in vivo inhibits the branching of olfactory projection neurons in the lobster deutocerebrum. J. Neurosci. 2000;20:7716–7721. doi: 10.1523/JNEUROSCI.20-20-07716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: a common strategy across diverse species. J. Comp. Neurol. 2007a;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Beltz BS. Characterization of a putative stem/progenitor cell niche in the brain of an adult invertebrate, the crayfish Procambarus clarkii. Soc. Neurosci. Abstr. 2005;31 366.4. [Google Scholar]
- Sullivan JM, Sandeman DC, Benton JL, Beltz BS. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. J. Mol. Histol. 2007b;38:527–542. doi: 10.1007/s10735-007-9112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreys R, Velde GV, Krylychkina O, Vellema M, Verhoye M, Timmermans JP, Baekelandt V, Van der Linder A. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: Validation of various MPIO labeling strategies. Neuroimage. 2010;49 doi: 10.1016/j.neuroimage.2009.10.034. 2094-2013. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wells WA. Is transdifferentiation in trouble? J. Cell Biol. 2002;157:15–18. doi: 10.1083/jcb.200203037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen CM, Cheng YH, Huang YF, Wang CS. Isolation and characterization of a neural progenitor cell line from tilapia brain. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;149:167–180. doi: 10.1016/j.cbpa.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Wen CM, Huang JY, Ciou JH, Kao YL, Cheng YH. Immunochemical and molecular characterization of GBC4 as a tanycyte-like cell line derived from grouper brain. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;153:191–201. doi: 10.1016/j.cbpa.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Wu C, Söderhäll I, Kim Y-A, Liu H, Söderhäll K. Hemocyte-lineage marker proteins in a crustacean, the freshwater crayfish, Pacifastacus leniusculus. Proteomics. 2008;8:4226–4235. doi: 10.1002/pmic.200800177. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beltz BS. Glutamine synthetase, a functionally active enzyme in the crayfish brain. Soc. Neurosci. Abstr. 2010;36 737.14. [Google Scholar]
- Zhang Y, Allodi S, Sandeman DC, Beltz BS. Adult neurogenesis in the crayfish brain: proliferation, migration and possible origin of precursor cells. Dev. Neurobiol. 2009;69:415–436. doi: 10.1002/dneu.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Benton JL, Beltz BS. 5-HT receptors mediate lineage-dependent effects of serotonin on adult neurogenesis. Neural Dev. 2011;6:2. doi: 10.1186/1749-8104-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. Neurogenesis and neuronal regeneration in the adult fish brain. J. Comp. Physiol. A. 2006;192:649–670. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]