Abstract
Background
Hypothermia, defined as a core body temperature less than 36°C (96.8°F), is a relatively common occurrence in the unwarmed surgical patient. A mild degree of perioperative hypothermia can be associated with significant morbidity and mortality. A threefold increase in the frequency of surgical site infections is reported in colorectal surgery patients who experience perioperative hypothermia. As part of the Surgical Care Improvement Project, guidelines aim to decrease the incidence of this complication.
Methods
We review the physiology of temperature regulation, mechanisms of hypothermia, effects of anesthetics on thermoregulation, and consequences of hypothermia and summarize recent recommendations for maintaining perioperative normothermia.
Results
Evidence suggests that prewarming for a minimum of 30 minutes may reduce the risk of subsequent hypothermia.
Conclusions
Monitoring of body temperature and avoidance of unintended perioperative hypothermia through active and passive warming measures are the keys to preventing its complications.
Keywords: Hypothermia, perioperative, prewarming, thermoregulation
INTRODUCTION
The operating room (OR) is generally not considered an extreme environment; however, it can be for many patients. As homeothermic (warm-blooded) animals, humans require near constant internal body temperatures to maintain optimal function of multiple organs and systems. ORs are typically kept below 23°C (73.4°F); this is the temperature required to maintain normothermia for all but the shortest procedures.1 Surgical patients consistently comment on the cold temperature in the OR. Room temperature is the single most critical factor influencing actual heat loss because the temperature gradient determines this rate. Most OR personnel find the temperatures required for normothermia uncomfortably warm, however, and lower OR temperatures are the norm. Surgeons are particularly vulnerable to warm ORs because of the high level of stress during surgery and because they must wear multiple layers of clothing, including sterile gowns and lead aprons. Physicians and other staff may perspire into a surgical incision if the OR is not kept cool. Warm temperatures may also impair the performance of OR personnel by decreasing their vigilance.
Up to 20% of patients experience unintended perioperative hypothermia (UPH) defined as a core temperature below 36°C (96.8°F) perioperatively.2 Anesthesia eliminates behavioral modification, humans' most effective defense for avoiding temperature extremes; the anesthetized patient cannot move to a warmer environment. Anesthesia also alters thermoregulatory mechanisms, thereby allowing unwarmed patients to become hypothermic. More than a decade of research has convincingly illustrated the adverse effects of mild hypothermia in perioperative patients. These include a threefold increase in the incidence of morbid cardiac outcomes, increases in surgical blood loss, a 20% increase in allogeneic transfusion, and a tripling of surgical site infections (SSIs).3-10
The Surgical Care Improvement Project (SCIP) has focused institutional efforts on preventing hypothermic complications during and after surgery. Compliance with SCIP measures is increasing nationally.11 For example, during the most recent quarters in 2010, Ochsner Medical Center in New Orleans had 100% compliance with SCIP INF 10, the application of warming devices or perioperative normothermia (Ochsner internal audit presented to Performance Improvement Committee, October 2010). An understanding of the basic science of thermal regulation, the mechanisms of hypothermia in the perioperative setting, and the adverse effects of UPH is essential for personnel providing anesthesia care.
TEMPERATURE MONITORING
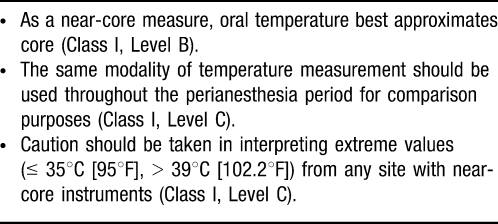
The American Society of Anesthesiologists' standards for basic anesthetic monitoring state, “Every patient receiving anesthesia shall have temperature monitored when clinically significant changes in body temperature are intended, anticipated, or suspected.”12 This guideline does not define the means of temperature monitoring, when to monitor, or the duration of monitoring, leading to inconsistency in patient care and wide variation in clinical practice. Guidelines from the American Society of Perianesthesia Nurses (ASPAN) in Table 1 provide evidence-based recommendations for temperature monitoring.
Table 1. .
Temperature Measurement Supported by “Strong” Evidence
Core temperature represents the temperature of the deep thoracic, abdominal, and central nervous system tissues. This temperature is tightly controlled, usually 2-4°C (3.6-7.2°F) warmer than skin temperature. Core temperatures are measured at the distal esophagus, bladder (with high urine flow), nasopharynx, pulmonary artery, etc and are commonly assessed in general anesthetic cases. Core temperature, although by no means completely characteristic of body heat content and distribution, is the single best indicator of thermal status in humans.13
Near-core temperatures obtained from axillary, rectal, bladder (with low urine flow), and oral measurements are more commonly used in regional anesthesia cases and in the perioperative period. These measurements are generally easier to obtain but are affected by external influences (ambient temperature) and thermoregulatory functions (regional skin blood flow) of the body. Although this concept makes sense when dealing with axillary and oral temperature measurements, the discrepancies with rectal and bladder temperatures are not as obvious.
Rectal temperatures normally correlate very closely with core temperatures.14,15 However, during cases of heat stroke and malignant hyperthermia, these measures lag behind.16,17 Additionally, during cardiopulmonary bypass, the rectal temperature lags behind true core temperature and is thus considered a near-core temperature in deliberately cooled patients. Thus, one must exercise caution in using rectal temperature measurements.
Bladder temperature is equal to pulmonary artery (core) temperature when urine flow is high but approximates rectal temperature when urine flow is low.18 Because of the dependence on urine flow, bladder temperature is also considered a near-core measure. Thus, each of these measures is limited in the ability to reflect core temperature.
The optimal source for monitoring temperature in the preoperative, intraoperative, and postoperative periods is an area of debate, typically depending on patient age. In the preoperative period, oral temperature is most commonly used for adults, oral or axillary temperature is used for pediatric patients, and axillary measurements are used for neonates.
Intraoperatively, core temperature is measured by an esophageal probe whenever possible. This method is low risk and low cost and provides the most accurate assessment of thermal status. Additionally, this measure is most accurate for large changes in temperature. Unfortunately, esophageal measurement is more difficult in regional/monitored anesthesia care cases and in the postoperative period. Core temperatures can be estimated from near-core measurements with reasonable accuracy except during extreme thermal perturbations.14,15
Another common means of thermal monitoring is the liquid-crystal temperature strip applied to the forehead. These devices are inexpensive, noninvasive, and easy to use. Although skin temperatures are considerably lower than core values and are more affected by ambient room temperature, when adjusted with an appropriate offset (∼ 0.5°C for awake adults, ∼ 1°C for anesthetized adults), these strips will give a reasonable reflection of core temperatures.19 During more extreme temperature changes such as malignant hyperthermia (MH), liquid-crystal temperature strips are unreliable in detecting increases in temperature in porcine models.20 These monitors have not been evaluated for this purpose in humans and are not recommended for detection of MH.21
With these issues in mind, either core or near-core temperatures may be used in the perioperative period after a risk versus benefits assessment has been performed and with the understanding that clinical circumstances may require an additional method.
BACKGROUND BASIC SCIENCE
Heat loss from a patient to the environment may occur by 4 mechanisms. Of these, radiation and convection are the largest contributors.22
Radiation (Thermal)
All surfaces that exist at a temperature above absolute zero radiate heat (infrared radiation). All surrounding surfaces absorb this radiated heat. Therefore, the patient radiates heat into the surrounding environment. Radiation likely represents the major type of heat loss during surgery.
Convection
Normally, a thin layer of still air adjacent to the skin acts as an insulator and limits conductive heat loss to surrounding air molecules. When air currents disrupt this layer, the insulating properties are markedly diminished and heat loss increases. This is referred to as convection and is the basis for the concept of the wind chill factor. In non-OR hospital settings, room air is typically exchanged 4 times per hour, while in typical ORs the air exchange occurs 15 times per hour. This minimally perceptive movement of air throughout the OR makes these rooms feel subjectively colder. Surgical drapes act as thermal insulators to minimize convective heat loss. Despite draping, convective heat loss is considered the second most significant source of heat loss in the OR.
Conduction
Conduction is the transmission of heat through a conducting medium without perceptible motion of the medium. The rate of heat transfer depends on the temperature difference between the 2 media and the heat conductivity of the material. Conduction plays a minor role in heat loss during surgery because the patient is in direct contact with the foam insulating mattress on the OR table.
Evaporation
Evaporation is the change of a liquid into a vapor while below the boiling point. This takes place at the surface of a liquid where molecules with the highest kinetic energy are able to escape, lowering the kinetic energy and decreasing temperature. This type of heat loss typically occurs when sterile preparation solutions are applied. Evaporative losses from surgical wounds may contribute as well.22
PHYSIOLOGY OF TEMPERATURE REGULATION
Under normal conditions, human thermoregulatory systems maintain a constant body temperature within a few tenths of a degree centigrade; normal body temperature is approximately 37°C (98.6°F). In the OR, however, a combination of altered thermoregulatory mechanisms and cooler room temperatures typically causes a decrease in core temperature. Hypothermia, defined as a core temperature less than 36°C (96.8°F), is a relatively common occurrence among surgical patients, with an incidence of up to 20%.2 It is not uncommon for a relatively healthy patient to experience a decrease in core temperature of 0.5-1.5°C (0.9-2.7°F)23 in the first hour of a procedure.
A system of afferent thermal sensing, central regulation, and efferent responses constitutes physiologic thermoregulation. In hypothermia, afferent thermal sensing arises from cold-sensitive cells located in the brain, spinal cord, deep abdominal tissue, thoracic tissue, and skin surface.24 The hypothalamus and to a lesser degree the spinal cord centrally regulate body temperature. The efferent response to hypothermia is manifested primarily through behavioral modification but also through vasoconstriction and shivering in adults. Neonates also demonstrate nonshivering thermogenesis, which is discussed in more detail later.
ANESTHETIC EFFECTS ON THERMOREGULATION
Under normal circumstances, body temperature is well maintained by regulating blood flow through arteriovenous shunts located in the skin's surface. This blood flow may account for up to 10% of the cardiac output, and vasoconstriction may increase mean arterial blood pressure by approximately 15 mmHg.25 Anesthetics have a profound effect on thermoregulatory mechanisms. General anesthetics inhibit cold-induced vasoconstriction, which promotes the development of patient hypothermia in the presence of a cold environment.
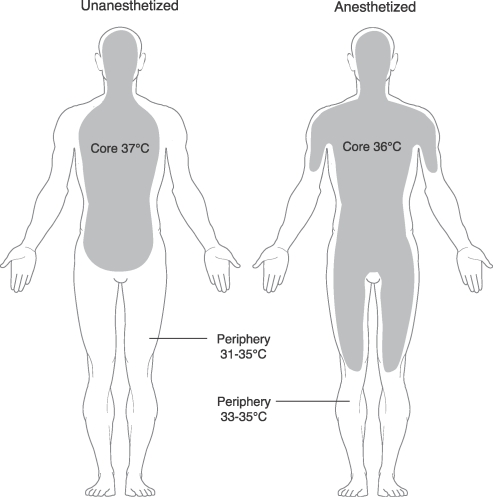
The body's heat is not uniformly distributed. Rather, heat is normally concentrated in the core region, mainly the head and truncal areas, while the periphery remains cooler. Interestingly, as the body is stressed by cooler OR temperatures and the vasodilatory effects of a general anesthetic occur because of a loss of sympathetic tone, the heat in the core area flows outward towards the periphery23 (Figure 1). This shift results in a rapid core temperature decrease of 0.5-1.5°C (0.9-2.7°F) measured within the first hour of surgery. This redistribution hypothermia is not actual heat loss but rather a shift in thermal energy from the core to the periphery, occurring as a result of the vasodilation properties of general anesthetics. Subsequently, the warmer periphery caused by general anesthetics also results in a greater risk of the patient losing heat into the OR environment.
Figure 1. .
Redistribution of core heat during general anesthesia.
Reprinted with permission from Miller's Anesthesia.22
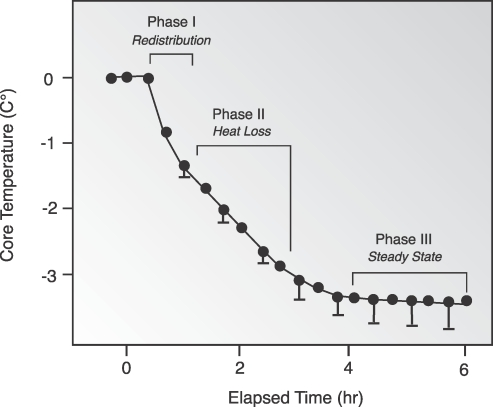
Hypothermia during general anesthesia has a characteristic pattern of an initial rapid decrease in core temperature (Phase I), followed by a linear reduction in core temperature (Phase II). Finally, a plateau phase (Phase III) occurs in which core temperature stabilizes (Figure 2). Phase I rapid heat loss in the first hour may be attributed to thermal redistribution. In Phase II (redistribution from the warmer periphery to the environment), the slow linear reduction that follows over the next 2-4 hours, heat loss exceeds metabolic heat production.26 Phase III (thermal steady state) occurs after 3 to 4 hours, when peripheral vasoconstriction is triggered by core temperatures of 33-35°C (91.4-95°F).27
Figure 2. .
Characteristic pattern of temperature change seen with general anesthesia.
Reprinted with permission from Miller's Anesthesia.22
Neuraxial anesthesia also disrupts physiologic thermoregulation, but by a different mechanism than general anesthetics. Epidural28,29 and spinal30,31 anesthesia each decrease the shivering and vasoconstriction thresholds by about 0.6°C (1.08°F). Much of the regulation of core temperature actually depends on afferent thermal input from skin sensors in the legs. In a typical OR, continuous cold signals are generated from the periphery.31 In regional anesthesia, however, thermal input is halted throughout blocked regions. The resultant absence of cold signaling is interpreted centrally as relative leg warming that ultimately reduces the shivering and vasoconstriction thresholds. Thus, a regionally anesthetized patient perceives himself to be warm when he is actually being cooled. Neuraxial anesthesia is often supplemented with sedatives and analgesics that impair thermoregulatory control even further.32-34
RISK FACTORS
Ideally, practitioners should identify risk factors for UPH prior to any surgical procedure to optimize intraoperative thermal management. ASPAN utilized an evidence-based practice approach to identify risk factors.35 This system ranked the strength and quality of evidence according to the evidence rating scale by Stetler and colleagues.36 Guidelines were derived and ranked according to a modified version of the American College of Cardiology/American Heart Association (ACC/AHA) classifications that address the risk/benefit ratio and amount and quality of evidence supporting the recommendations. These classes are defined as follows:37
Class I: The benefit far outweighs the risk, and the recommendation should be performed or administered.
Class IIa: The benefit outweighs the risk, and it is reasonable to perform or administer the recommendation.
Class IIb: The benefit is equal to the risk, and it is not unreasonable to perform or administer the recommendation.
Class III: The risk outweighs the benefit, and the recommendation should not be performed or administered.
These recommendations are supported by 3 levels of evidence:
Level A: Evidence from multiple randomized trials or meta-analyses evaluating multiple (3-5) populations, with general consistency of direction and magnitude of effect.
Level B: Evidence from single randomized trials or nonrandomized studies evaluating limited (2-3) populations.
Level C: Evidence from case studies, standards of care, or expert opinion involving very limited (1-2) populations.
Table 2 presents the ASPAN list of risk factors for UPH. Unfortunately, none of the identified risk factors is supported by strong evidence, suggesting that further research is needed in this area. These factors imply correlation but not necessarily causation; thus, a patient may have risk factors and not develop hypothermia. Hopefully through identification of susceptible patients during the preoperative assessment, strategies may be developed to help ensure normothermic conditions during the perioperative period.
Table 2. .
Risk Factors for Perioperative Hypothermia35
WARMING METHODS
In the OR, the initial decrease in core temperature results from the redistribution of heat to the periphery. Prewarming in the preoperative area, when applied for at least 30 minutes, may prevent redistribution of body heat and resultant hypothermia.38 Forced air warmers are one of the most effective means of warming a patient and are best used preoperatively and intraoperatively to prevent UPH. These warmers work better intraoperatively when the patient's periphery is vasodilated.6 Without prewarming, however, intraoperative warming techniques, including those employing forced air warming technology throughout the case, still fail to eliminate the initial fall in temperature.
Other warming modalities aim to prevent radiant heat transfer; they include water blankets and warmed cotton blankets. Conventional water mattresses or blankets by themselves have been consistently reported to be nearly ineffective.39 This ineffectiveness may be a result of the small surface area (when compared to forced air blankets) and the minimal amount of heat lost into the foam insulation present on most OR tables. Additionally, the risk for tissue damage is increased by the decreased local perfusion on the backs of patients in the supine position. This scenario may result in tissue damage in cases even when the water temperature does not exceed 40°C (104°F).40 More recent has been the development of circulating-water garments that increase the warmed surface area and materials that facilitate conduction.41,42 One layer of cotton blankets can decrease heat loss by approximately 30%. Additional layers do not provide very much additional insulation.
Heat loss from administration of cold intravenous fluids also contributes to hypothermia. Fluid warmers are commonly used in the OR and are recommended whenever blood or large amounts of intravenous fluid are administered. They are not recommended for patient rewarming because the fluid temperature cannot substantially exceed the patient's body temperature without causing hemolysis, thereby materially limiting warming effectiveness.
Initially, warming of inspired gases was considered to be an important component in maintaining normothermia. The dry gases are cold and thus represent a potential source of heat loss for patients receiving general anesthetics. However, on further review researchers noted that little heat is actually lost via respiration and that active airway heating and humidification minimally influence core temperature.26 This finding has led to the elimination of heated anesthesia circuits in contemporary practice.
MORBIDITY AND MORTALITY OF HYPOTHERMIA
Although therapeutic indications for perioperative hypothermia exist, they are limited to very specific circumstances where benefits have been demonstrated in clinical trials. One area of intense research deals with brain protection. Initially, the benefits were thought to result from the decrease in tissue metabolic rate associated with hypothermia; however, similar decreases in tissue metabolic rate with other agents such as high-dose isoflurane or barbiturates did not achieve similar protection as hypothermia.43 Other mechanisms such as decreased release of excitatory amino acids have been proposed as a mechanism for the protective actions of hypothermia.44 Examples of therapeutic use of hypothermia include
After intracranial aneurysm surgery57
Although therapeutic hypothermia has recently demonstrated benefits, for the vast majority of surgical patients, UPH has long been associated with increased morbidity, mortality, and expense.
Surgical Site Infections
One of the most important causes of morbidity associated with hypothermia in patients is SSIs. Several mechanisms for hypothermia-related susceptibility to SSIs have been identified. Cooler temperatures may directly impair neutrophil function. Hypothermia may also trigger thermoregulatory vasoconstriction; the consequent reduction in cutaneous blood flow leads to subcutaneous tissue hypoxia and failure of humoral immune defense systems to reach target areas to fight infection.58,59 Hypothermia is associated with a threefold increase in SSIs in colon resections3 and with a significant increase of infection in patients undergoing cholecystectomy.60 Considering all of the complications that may be affected by anesthetics, wound infections are likely the most significant cause of morbidity, greater than all other anesthetic complications combined.61 It is estimated that SSIs increase postoperative hospitalization by an average of 4 days and result in an increased attributable cost of $8,00062 to $25,000 (Ochsner estimates from the Infection Control Department, calendar year 2010) for each patient.
Myocardial Ischemia
Research also demonstrates that surgical patients with cardiovascular disease who are hypothermic are three times more likely to have adverse myocardial outcomes than their normothermic counterparts.4 Perioperative hypothermia elevates blood pressure, heart rate, and plasma catecholamine concentrations (principally norepinephrine levels, which may be increased fivefold over baseline,63 contributing to patient discomfort64 in addition to cardiovascular risk in susceptible populations. Hypothermia also shifts the oxyhemoglobin dissociation curve to the left, which increases oxygen binding to hemoglobin, thus reducing available oxygen for tissues. This combination of increased myocardial oxygen demand (hypertension and tachycardia) and decreased myocardial oxygen supply (shorter diastolic filling time and increased hemoglobin affinity for oxygen) may shift the myocardial oxygen balance into a net deficit, resulting in ischemia.
Prolongation of Drug Effects
By decreasing drug metabolism, even mild hypothermia can lead to delayed awakening and prolonged postoperative anesthesia care unit (PACU) stay.65,66 Hypothermia alters the effects of many classes of drugs, including muscle relaxants, volatile agents, and intravenous anesthetic agents.67-69 A general prolongation of pharmacologic effect results from multiple causes. Both hepatic and renal blood flow are diminished with mild hypothermia, which in turn decreases metabolism and drug excretion, respectively, with resultant decreases in plasma clearance and increases in drug effects. The prolonged duration of action seen in nondepolarizing muscle relaxants is a result of changes in volume of distribution, altered local diffusion receptor affinity, changes in pH at the neuromuscular junction, and the net effect of cooling on the various components of neuromuscular transmission.70 With volatile anesthetics, the minimal alveolar concentration of an agent (a measure of potency) is diminished, and thus a lower amount of agent is required for a given effect.71
Bleeding Diatheses
Increased surgical bleeding has been attributed to hypothermia. In a study of hip arthroplasty patients, a decrease of 1.6°C (2.9°F) in body temperature increased blood loss by 500 mL and increased the need for allogeneic blood transfusions.6 This reaction is a result of 3 different mechanisms in the coagulation system: platelet function, the coagulation cascade, and fibrinolysis. Coagulation is impaired by cold-induced defects in platelet function72 related to local temperature. These defects result from reduced levels of thromboxane B2 at the site of tissue injury.73 Hypothermia also directly impairs temperature-dependent enzyme function in the coagulation cascade to a lesser degree.74 Interestingly, these perturbations in coagulation will not be apparent during routine coagulation screening because these tests are performed at 37°C (98.6°F). When the tests are performed at hypothermic temperatures, however, the defect becomes apparent.75 Fibrinolysis is also enhanced with hypothermia, which destabilizes clots and predisposes to increased bleeding.76
Shivering
Shivering occurs during neuraxial anesthetics and after general anesthetics. This means of heat production can increase metabolic heat production along with oxygen consumption by 50-400%.77 Initial concerns that the increased metabolic rate caused by shivering would result in myocardial ischemia have had poor clinical correlation, possibly explained by the fact that oxygen consumption/heat production rarely doubles and then only during extreme circumstances.78,79 Shivering is generally impaired in patients with a high risk for cardiac complications.80 Hypoxia actually inhibits shivering and thus should decrease the incidence.81 Lastly, several studies indicate that perioperative myocardial ischemia is unrelated to shivering.4,82
Skin Integrity and Length of Hospital Stay
Hypothermia has also been linked to pressure ulcer development83 and increased length of hospital stay for surgical patients.3,4
Patient Satisfaction
Maintaining normothermia is also important to track patient comfort, and patients who feel cold may report lower satisfaction with their care.84 Studies show that patients report greater satisfaction and less anxiety with prewarming.85
Economics
Though some authors argue that normothermia may be difficult to attain and requires significant personnel, equipment, and time costs,3,86,87 the forced air warmer disposable blanket is a one-time cost, usually less than $10.88 The use of such devices requires minimal training and time for personnel to implement appropriately. Standardization of warming procedures can decrease the incidence of UPH89 and thus should mitigate the unexpected delays in discharge from the PACU resulting from hypothermia. These delays may result in the OR suites being placed on hold until a PACU bed becomes available. OR costs are approximately $1,600/hour (personal communication with Associate Vice President of Perioperative Services, Ochsner Clinic Foundation, New Orleans, LA, January 2010), and therefore any such delays have substantive financial impact.
HYPOTHERMIA CLINICAL PERFORMANCE INDICATORS
The question of exactly which measure of hypothermia is the most important to track has yet to be answered. Is it the nadir, the mean, the time temperature integral (a measure of cumulative hypothermic time),90 or the initial PACU temperature? This question has been brought to light by Lehtinen et al91 using a nested, matched, case-control study to determine if hypothermia was an independent risk factor for SSI in gastrointestinal (GI) surgeries. Use of SCIP measures showed no independent association between perioperative hypothermia and SSIs. The researchers concluded, “Pay for performance measures focusing on perioperative normothermia may be of limited value in preventing SSI after GI surgery” and recommended further studies. In the future, use of larger national electronic databases, such as those of the Multicenter Perioperative Outcomes Group and the Anesthesia Quality Institute, may be helpful in determining which measures are most pertinent.
Current SCIP measures require hospitals to report the percentage of patients in which active warming was used or who had at least one body temperature reading greater than or equal to 36°C (96.8°F) within 30 minutes before or 15 minutes after anesthesia end time. Reporting included all patients undergoing general or neuraxial anesthesia for 60 minutes or longer.92
PEDIATRIC HYPOTHERMIA
Although the majority of literature on hypothermia concerns the adult population, pediatric patients undergoing surgery are even more susceptible to UPH. This susceptibility results from a combination of decreased heat production and increased heat loss during surgery, both of which occur to a greater degree in children than in adults.
Pediatric patients undergoing general anesthesia experience a 20% decrease in metabolic rate,93 which is similar to the decrease seen in the adult population.94 Additionally, approximately half of the oxygen consumption and concomitant heat generation in neonates can be attributed to the work of respiration.95 Most pediatric patients undergo general anesthesia where mechanical ventilation is partially or completely provided by the anesthesia team, so that very little heat is generated from the work of breathing.
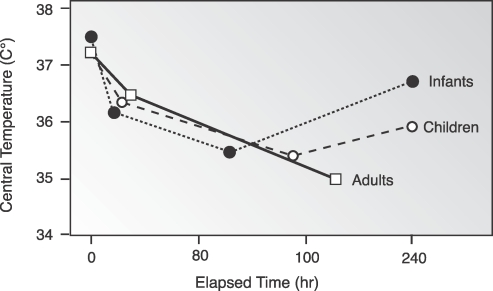
Other contributors to increased risk of UPH are central thermoregulatory inhibition and redistribution of heat within the body, which are similar to those that occur in adults; however, the slopes of each phase are steeper in the younger age groups.96 One adaptation specific to neonates that is helpful in maintaining normothermia is nonshivering thermogenesis,97 a metabolic process that occurs in brown fat. This is reflected in the phase III rewarming slope that is unique to neonates (Figure 3).
Figure 3. .
Characteristic temperature change at various ages.
Reprinted with permission from Miller's Anesthesia.22
Factors that result in greater transmittance of heat to the environment in pediatric patients also predispose them to UPH. Infants have a greater surface-area-to-volume ratio98 compared to adults. Also, infants have less insulating subcutaneous adipose tissue. Both of these factors result in increased thermal conductance and proportionally more metabolic heat loss through the skin in the youngest patients.
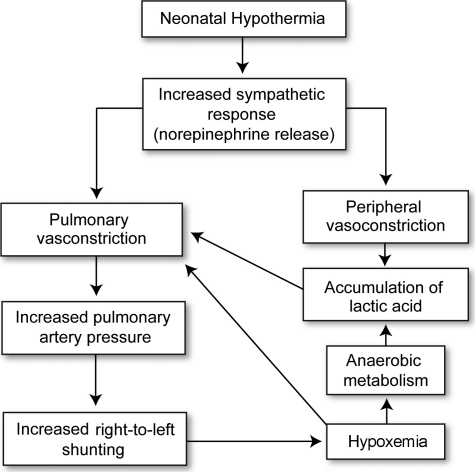
Neonates and infants are most vulnerable to the adverse consequences of UPH. Cold stress in neonates causes increased sympathetic activity (Figure 4) and norepinephrine release, resulting in increased oxygen and substrate utilization.99 Consequences may include increased pulmonary artery pressure, arrhythmias, decreased peripheral perfusion, hypoglycemia, hypoxia, tachypnea, metabolic acidosis, and potentially death. In low-birth-weight and preterm neonates, these effects may be more pronounced. As a result, maintenance of normothermia in pediatric patients is a major priority during the perioperative period. Because cutaneous heat loss is increased in neonates, OR temperature is kept at or above 26°C (78.9°F). Circulating hot water blankets, infrared radiant heaters, and convection heaters are commonly employed during surgery. Head caps may also be used because a larger fraction of metabolic heat is lost through the head of infants and children compared to adults.100-102
Figure 4. .
Physiologic changes associated with hypothermia in the neonate.
Adapted from Avery's Neonatology.99
PREVENTING PERIOPERATIVE HYPOTHERMIA
Tables 3-5 are a brief summary of the ASPAN Evidence-Based Clinical Practice Guideline for the Promotion of Perioperative Normothermia.35
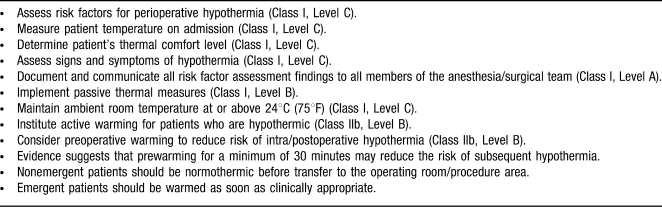
Table 3. .
Preadmission/Preoperative Recommendations
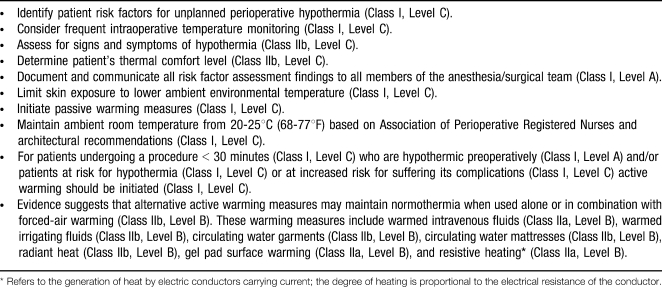
Table 4. .
Intraoperative Recommendations
Table 5. .
Phase I/II Postoperative Anesthesia Care Unit Patient Management Recommendations
CONCLUSIONS
UPH is a common occurrence in the unwarmed surgical patient and is associated with significant morbidity and mortality. Monitoring of body temperature and avoidance of UPH through active and passive warming measures are the keys to preventing its complications that may include surgical site infection, delayed wound healing, adverse myocardial outcome, and increased bleeding. Uniform implementation of evidence-based perioperative warming guidelines can be expected to reduce adverse outcomes and improve patient satisfaction, although prospective validation of SCIP measure optimization has not yet convincingly proven causality.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Morris RH. Operating room temperature and the anesthetized, paralyzed patient. Arch Surg. 1971;102(2):95–97. doi: 10.1001/archsurg.1971.01350020005002. [DOI] [PubMed] [Google Scholar]
- 2.Kurz A. Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol. 2008;22(4):627–644. doi: 10.1016/j.bpa.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 4.Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–1134. [PubMed] [Google Scholar]
- 5.Luna GK, Maier RV, Pavlin EG, Anardi D, Copass MK, Oreskovich MR. Incidence and effect of hypothermia in seriously injured patients. J Trauma. 1987;27(9):1014–1018. doi: 10.1097/00005373-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347(8997):289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 7.Lenhardt R, Negishi C, Sessler DI. Perioperative fever. Acta Anaesthesiol Scand Suppl. 1997;111:325–328. [PubMed] [Google Scholar]
- 8.Carli F, Emery PW, Freemantle CA. Effect of preoperative normothermia on postoperative protein metabolism in elderly patients undergoing hip arthroplasty. Br J Anaesth. 1989;63(3):276–282. doi: 10.1093/bja/63.3.276. [DOI] [PubMed] [Google Scholar]
- 9.Kurz A, Plattner O, Sessler DI, Huemer G, Redl G, Lackner F. The threshold for thermoregulatory vasoconstriction during nitrous oxide/isoflurane anesthesia is lower in elderly than in young patients. Anesthesiology. 1993;79(3):465–469. doi: 10.1097/00000542-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Peng RY, Bongard FS. Hypothermia in trauma patients. J Am Coll Surg. 1999;188(6):685–696. doi: 10.1016/s1072-7515(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 11.Chassin MR, Loeb JM, Schmaltz SP, Wachter RM. Accountability measures—using measurement to promote quality improvement. N Engl J Med. 2010;363(7):683–688. doi: 10.1056/NEJMsb1002320. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Anesthesiologists. 2010. Standards for basic anesthetic monitoring. http://www.asahq.org/For-Healthcare-Professionals/∼/media/For%20Members/documents/Standards%20Guidelines%20Stmts/Basic%20Anesthetic%20Monitoring%202011.ashx. Accessed January 13, 2011. [Google Scholar]
- 13.Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109(2):318–338. doi: 10.1097/ALN.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissonnette B, Sessler DI, LaFlamme P. Intraoperative temperature monitoring sites in infants and children and the effect of inspired gas warming on esophageal temperature. Anesth Analg. 1989;69(2):192–196. [PubMed] [Google Scholar]
- 15.Cork RC, Vaughan RW, Humphrey LS. Precision and accuracy of intraoperative temperature monitoring. Anesth Analg. 1983;62(2):211–214. [PubMed] [Google Scholar]
- 16.Iaizzo PA, Kehler CH, Zink RS, Belani KG, Sessler DI. Thermal response in acute porcine malignant hyperthermia. Anesth Analg. 1996;82(4):782–789. doi: 10.1097/00000539-199604000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Ash CJ, Cook JR, McMurry TA, Auner CR. The use of rectal temperature to monitor heat stroke. Mo Med. 1992;89(5):283–288. [PubMed] [Google Scholar]
- 18.Horrow JC, Rosenberg H. Does urinary catheter temperature reflect core temperature during cardiac surgery. Anesthesiology. 1988;69(6):986–989. doi: 10.1097/00000542-198812000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda T, Sessler DI, Marder D, Xiong J. Influence of thermoregulatory vasomotion and ambient temperature variation on the accuracy of core-temperature estimates by cutaneous liquid-crystal thermometers. Anesthesiology. 1997;86(3):603–612. doi: 10.1097/00000542-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Iaizzo PA, Kehler CH, Carr RJ, Sessler DI, Belani KG. Prior hypothermia attenuates malignant hyperthermia in susceptible swine. Anesth Analg. 1996;82(4):803–809. doi: 10.1097/00000539-199604000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Larach MG, Gronert GA, Allen GC, Brandom BW, Lehman EB. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010;110(2):498–507. doi: 10.1213/ANE.0b013e3181c6b9b2. [DOI] [PubMed] [Google Scholar]
- 22.Sessler DI. Temperature regulation and monitoring. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, editors. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone/Elsevier; 2010. pp. 1533–1536. [Google Scholar]
- 23.Matsukawa T, Sessler DI, Sessler AM, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82(3):662–673. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Jessen C, Feistkorn G. Some characteristics of core temperature signals in the conscious goat. Am J Physiol. 1984;247(3 Pt 2):R456–R464. doi: 10.1152/ajpregu.1984.247.3.R456. [DOI] [PubMed] [Google Scholar]
- 25.Greif R, Laciny S, Rajek A, Doufas AG, Sessler DI. Blood pressure response to thermoregulatory vasoconstriction during isoflurane and desflurane anesthesia. Acta Anaesthesiol Scand. 2003;47(7):847–852. doi: 10.1034/j.1399-6576.2003.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynson JM, Sessler DI. Intraoperative warming therapies: a comparison of three devices. J Clin Anesth. 1992;4(3):194–199. doi: 10.1016/0952-8180(92)90064-8. [DOI] [PubMed] [Google Scholar]
- 27.Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69(3):357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Sessler DI, Ponte J. Shivering during epidural anesthesia. Anesthesiology. 1990;72(5):816–821. doi: 10.1097/00000542-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki M, Kurz A, Sessler DI, et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology. 1994;81(2):282–288. doi: 10.1097/00000542-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Kurz A, Sessler DI, Schroeder M, Kurz M. Thermoregulatory response thresholds during spinal anesthesia. Anesth Analg. 1993;77(4):721–726. doi: 10.1213/00000539-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Rajek A, Greif R, Sessler DI. Effects of epidural anesthesia on thermal sensation. Reg Anesth Pain Med. 2001;26(6):527–531. doi: 10.1053/rapm.2001.25924. [DOI] [PubMed] [Google Scholar]
- 32.Matsukawa T, Kurz A, Sessler DI, Bjorksten AR, Merrifield B, Cheng C. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82(5):1169–1180. doi: 10.1097/00000542-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Kurz A, Go JC, Sessler DI, Kaer K, Larson MD, Bjorksten AR. Alfentanil slightly increases the sweating threshold and markedly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83(2):293–299. doi: 10.1097/00000542-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Kurz A, Ikeda T, Sessler DI, et al. Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold. Anesthesiology. 1997;86(5):1046–1054. doi: 10.1097/00000542-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Hooper VD, Chard R, Clifford T, et al. ASPAN's evidence-based clinical practice guideline for the promotion of perioperative normothermia. J Perianesth Nurs. 2009;24(5):271–287. doi: 10.1016/j.jopan.2009.09.001. Erratum in: J Perianesth Nurs. 2010;25(2):111. [DOI] [PubMed] [Google Scholar]
- 36.Stetler CB, Brunell M, Giuliano KK, Morsi D, Prince L, Newell-Stokes V. Evidence-based practice and the role of nursing leadership. J Nurs Adm. 1998;28(7-8):45–53. doi: 10.1097/00005110-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Eagle KA, Brundage BH, Chaitman BR, et al. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) J Am Coll Cardiol. 1996;27(4):910–948. doi: 10.1016/0735-1097(95)99999-x. [DOI] [PubMed] [Google Scholar]
- 38.National Institute for Health and Clinical Evidence. Clinical practice guideline: The management of inadvertent perioperative hypothermia in adults. http://www.nice.org.uk/nicemedia/pdf/CG65Guidance.pdf. Accessed June 7, 2011. [Google Scholar]
- 39.Morris RH, Kumar A. The effect of warming blankets on maintenance of body temperature of the anesthetized, paralyzed adult patient. Anesthesiology. 1972;36(4):408–411. doi: 10.1097/00000542-197204000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Crino MH, Nagel EL. Thermal burns caused by warming blankets in the operating room. Anesthesiology. 1968;29(1):149–150. doi: 10.1097/00000542-196801000-00038. [DOI] [PubMed] [Google Scholar]
- 41.Stanley TO, Grocott HP, Phillips-Bute B, Mathew JP, Landolfo KP, Newman MF Neurologic Outcome Research Group C.A.R.E; Investigators of the Duke Heart Center. Preliminary evaluation of the Arctic Sun temperature-controlling system during off-pump coronary artery bypass surgery. Ann Thorac Surg. 2003;75(4):1140–1144. doi: 10.1016/s0003-4975(02)04545-9. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi A, Ratnaraj J, Kabon B, et al. Effects of a circulating-water garment and forced-air warming on body heat content and core temperature. Anesthesiology. 2004;100(5):1058–1064. doi: 10.1097/00000542-200405000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todd MM, Warner DS. A comfortable hypothesis reevaluated. Cerebral metabolic depression and brain protection during ischemia. Anesthesiology. 1992;76(2):161–164. [PubMed] [Google Scholar]
- 44.Conroy BP, Lin CY, Jenkins LW, et al. Hypothermic modulation of cerebral ischemic injury during cardiopulmonary bypass in pigs. Anesthesiology. 1998;88(2):390–402. doi: 10.1097/00000542-199802000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 46.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. Erratum in: N Engl J Med. 2002;346(22):1756. [DOI] [PubMed] [Google Scholar]
- 47.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 48.Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 49.Frank SM, Parker SD, Rock P, et al. Moderate hypothermia, with partial bypass and segmental sequential repair for thoracoabdominal aortic aneurysm. J Vasc Surg. 1994;19(4):687–697. doi: 10.1016/s0741-5214(94)70043-5. [DOI] [PubMed] [Google Scholar]
- 50.Kouchoukos NT, Rokkas CK. Hypothermic cardiopulmonary bypass for spinal cord protection: rationale and clinical results. Ann Thorac Surg. 1999;67(6):1940–1942; discussion 1953-1958. doi: 10.1016/s0003-4975(99)00442-7. [DOI] [PubMed] [Google Scholar]
- 51.Drenger B, Parker SD, McPherson RW, et al. Spinal cord stimulation evoked potentials during thoracoabdominal aortic aneurysm surgery. Anesthesiology. 1992;76(5):689–695. doi: 10.1097/00000542-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Cambria RP, Davison JK, Zannetti S, et al. Clinical experience with epidural cooling for spinal cord protection during thoracic and thoracoabdominal aneurysm repair. J Vasc Surg. 1997;25(2):234–241; discussion 241-243. doi: 10.1016/s0741-5214(97)70365-3. [DOI] [PubMed] [Google Scholar]
- 53.Hilgenberg AD. Spinal cord protection for thoracic aortic surgery. Cardiol Clin. 1999;17(4):807–813, x. Review. doi: 10.1016/s0733-8651(05)70116-8. [DOI] [PubMed] [Google Scholar]
- 54.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 55.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 56.Shiozaki T, Hayakata T, Taneda M, et al. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg. 2001;94(1):50–54. doi: 10.3171/jns.2001.94.1.0050. [DOI] [PubMed] [Google Scholar]
- 57.Todd MM, Hindman BJ, Clarke WR, Torner JC Intraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352(2):135–145. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- 58.van Oss CJ, Absolom DR, Moore LL, Park BH, Humbert JR. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc. 1980;27(6):561–565. [PubMed] [Google Scholar]
- 59.Sheffield CW, Sessler DI, Hopf HW, et al. Centrally and locally mediated thermoregulatory responses alter subcutaneous oxygen tension. Wound Repair Regen. 1996;4(3):339–345. doi: 10.1046/j.1524-475X.1996.40310.x. [DOI] [PubMed] [Google Scholar]
- 60.Flores-Maldonado A, Medina-Escobedo CE, Ríos-Rodríguez HM, Fernández-Domínguez R. Mild perioperative hypothermia and the risk of wound infection. Arch Med Res. 2001;32(3):227–231. doi: 10.1016/s0188-4409(01)00272-7. [DOI] [PubMed] [Google Scholar]
- 61.Bremmelgaard A, Raahave D, Beier-Holgersen R, Pedersen JV, Andersen S, Sørensen AI. Computer-aided surveillance of surgical infections and identification of risk factors. J Hosp Infect. 1989;13(1):1–18. doi: 10.1016/0195-6701(89)90090-x. [DOI] [PubMed] [Google Scholar]
- 62.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA., Jr Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199(4):531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 63.Roundtable summary: perioperative temperature management. Anesthesiology News. http://www.anesthesiologynews.com. October 2005. Accessed December 21, 2007. [Google Scholar]
- 64.Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology. 1995;82(1):83–93. doi: 10.1097/00000542-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 65.Bissonnette B, Sessler DI. Mild hypothermia does not impair postanesthetic recovery in infants and children. Anesth Analg. 1993;76(1):168–172. doi: 10.1213/00000539-199301000-00028. [DOI] [PubMed] [Google Scholar]
- 66.Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87(6):1318–1323. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80(5):1007–1014. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 68.Fritz HG, Bauer R, Walter B, Moertiz KU, Reinhart K. Effects of hypothermia (32°C) on plasma concentration of fentanyl in piglets (abstract) Anesthesiology. 1999;91(3A):A444. [Google Scholar]
- 69.Heier T, Caldwell JE, Sessler DI, Miller RD. Mild intraoperative hypothermia increases duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide-isoflurane anesthesia in humans. Anesthesiology. 1991;74(5):815–819. doi: 10.1097/00000542-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Miller RD, Agoston S, van der Pol F, Booij LH, Crul JF, Ham J. Hypothermia and the pharmacokinetics and pharmacodynamics of pancuronium in the cat. J Pharmacol Exp Ther. 1978;207(2):532–538. [PubMed] [Google Scholar]
- 71.Eger EI, 2nd, Johnson BH. MAC of I-653 in rats, including a test of the effect of body temperature and anesthetic duration. Anesth Analg. 1987;66(10):974–976. [PubMed] [Google Scholar]
- 72.Staab DB, Sorensen VJ, Fath JJ, Raman SB, Horst HM, Obeid FN. Coagulation defects resulting from ambient temperature-induced hypothermia. J Trauma. 1994;36(5):634–638. doi: 10.1097/00005373-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205(2):175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20(10):1402–1405. doi: 10.1097/00003246-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108(1):71–77. doi: 10.1097/01.anes.0000296719.73450.52. [DOI] [PubMed] [Google Scholar]
- 76.Yoshihara H, Yamamoto T, Mihara H. Changes in coagulation and fibrinolysis occurring in dogs during hypothermia. Thromb Res. 1985;37(4):503–512. doi: 10.1016/0049-3848(85)90096-9. [DOI] [PubMed] [Google Scholar]
- 77.Bay J, Nunn JF, Prys-Roberts C. Factors influencing arterial PO2 during recovery from anaesthesia. Br J Anaesth. 1968;40(6):398–407. doi: 10.1093/bja/40.6.398. [DOI] [PubMed] [Google Scholar]
- 78.Guffin A, Girard D, Kaplan JA. Shivering following cardiac surgery: hemodynamic changes and reversal. J Cardiothorac Anesth. 1987;1(1):24–28. doi: 10.1016/s0888-6296(87)92593-2. Erratum in: J Cardiothorac Anesth. 1987;1(5):following 501. [DOI] [PubMed] [Google Scholar]
- 79.Just B, Delva E, Camus Y, Lienhart A. Oxygen uptake during recovery following naloxone. Relationship with intraoperative heat loss. Anesthesiology. 1992;76(1):60–64. doi: 10.1097/00000542-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 80.Frank SM, Fleisher LA, Olson KF, et al. Multivariate determinants of early postoperative oxygen consumption in elderly patients. Effects of shivering, body temperature, and gender. Anesthesiology. 1995;83(2):241–249. doi: 10.1097/00000542-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Gautier H, Bonora M, Remmers JE. Effects of hypoxia on metabolic rate of conscious adult cats during cold exposure. J Appl Physiol. 1989;67(1):32–38. doi: 10.1152/jappl.1989.67.1.32. [DOI] [PubMed] [Google Scholar]
- 82.Frank SM, Beattie C, Christopherson R, et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;78(3):468–476. doi: 10.1097/00000542-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Scott EM, Buckland R. A systematic review of intraoperative warming to prevent postoperative complications. AORN J. 2006;83(5):1090–1104, 1107-1113. doi: 10.1016/s0001-2092(06)60120-8. [DOI] [PubMed] [Google Scholar]
- 84.Kurz A, Sessler DI, Narzt E, Lenhardt R, Lackner F. Morphometric influences on intraoperative core temperature changes. Anesth Analg. 1995;80(3):562–567. doi: 10.1097/00000539-199503000-00023. [DOI] [PubMed] [Google Scholar]
- 85.Wagner D, Byrne M, Kolcaba K. Effects of comfort warming on preoperative patients. AORN J. 2006;84(3):427–448. doi: 10.1016/s0001-2092(06)63920-3. [DOI] [PubMed] [Google Scholar]
- 86.Weirich TL. Hypothermia/warming protocols: why are they not widely used in the OR. AORN J. 2008;87(2):333–344. doi: 10.1016/j.aorn.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 87.Hedrick TL, Heckman JA, Smith RL, Sawyer RG, Friel CM, Foley EF. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg. 2007;205(3):432–438. doi: 10.1016/j.jamcollsurg.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 88.Sessler DI. New surgical thermal management guidelines. Lancet. 2009;374(9695):1049–1050. doi: 10.1016/S0140-6736(09)61686-X. [DOI] [PubMed] [Google Scholar]
- 89.Radauceanu DS, Dragnea D, Craig J. NICE guidelines for inadvertent peri-operative hypothermia. Anaesthesia. 2009;64(12):1381–1382. doi: 10.1111/j.1365-2044.2009.06141_18.x. [DOI] [PubMed] [Google Scholar]
- 90.Guest JD, Vanni S, Silbert L. Mild hypothermia, blood loss and complications in elective spinal surgery. Spine J. 2004;4(2):130–137. doi: 10.1016/j.spinee.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 91.Lehtinen SJ, Onicescu G, Kuhn KM, Cole DJ, Esnaola NF. Normothermia to prevent surgical site infections after gastrointestinal surgery: holy grail or false idol. Ann Surg. 2010;252(4):696–704. doi: 10.1097/SLA.0b013e3181f6c2a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Surgical Care Improvement Project. Get warmed up for SCIP-INF-10. http://www.info-haiwatch.com/20090918/kc20090918_landing_scip.html. Accessed January 13, 2011. [Google Scholar]
- 93.Dicker A, Ohlson KB, Johnson L, Cannon B, Lindahl SG, Nedergaard J. Halothane selectively inhibits nonshivering thermogenesis. Possible implications for thermoregulation during anesthesia of infants. Anesthesiology. 1995;82(2):491–501. doi: 10.1097/00000542-199502000-00019. [DOI] [PubMed] [Google Scholar]
- 94.Stevens WC, Cromwell TH, Halsey MJ, Eger EI, 2nd, Shakespeare TF, Bahlman SH. The cardiovascular effects of a new inhalation anesthetic, Forane, in human volunteers at constant arterial carbon dioxide tension. Anesthesiology. 1971;35(1):8–16. doi: 10.1097/00000542-197107000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Miller RD. Miller's Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. p. 2369. [Google Scholar]
- 96.Bissonnette B. Thermoregulation and paediatric anaesthesia. Curr Opin Anaesthesiol. 1993;6(3):537–542. [Google Scholar]
- 97.Bissonnette B, Ryan JF. Temperature regulation: normal and abnormal (malignant hyperthermia) In: Cotes CJ, Todres D, Ryan JF, Goudsouzian NG, editors. A Practice of Anesthesia for Infants and Children. Philadelphia: W.B. Saunders; 2001. p. 613. [Google Scholar]
- 98.Schiff D, Stern L, Leduc J. Chemical thermogenesis in newborn infants: catecholamine excretion and the plasma non-esterified fatty acid response to cold exposure. Pediatrics. 1966;37(4):577–582. [PubMed] [Google Scholar]
- 99.Friedman M, Baumgart S. Thermal regulation. In: McDonald MG, Seshia MMK, Mullett MD, editors. Avery's Neonatology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 100.Simbruner G, Weninger M, Popow C, Herholdt WJ. Regional heat loss in newborn infants. Part I. Heat loss in healthy newborns at various environmental temperatures. S Afr Med J. 1985;68(13):940–944. [PubMed] [Google Scholar]
- 101.Rowe MI, Weinberg G, Andrews W. Reduction of neonatal heat loss by an insulated head cover. J Pediatr Surg. 1983;18(6):909–913. doi: 10.1016/s0022-3468(83)80045-1. [DOI] [PubMed] [Google Scholar]
- 102.Stothers JK. Head insulation and heat loss in the newborn. Arch Dis Child. 1981;56(7):530–534. doi: 10.1136/adc.56.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]