Abstract
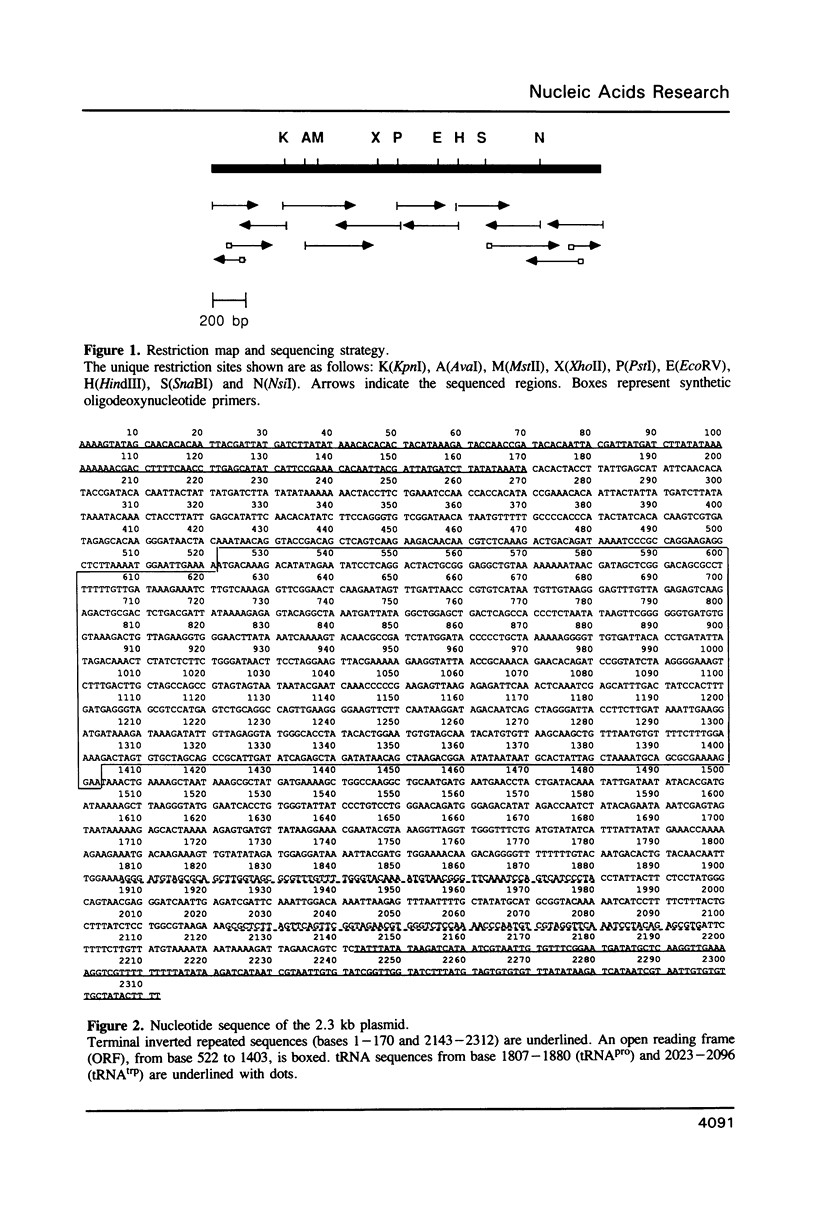
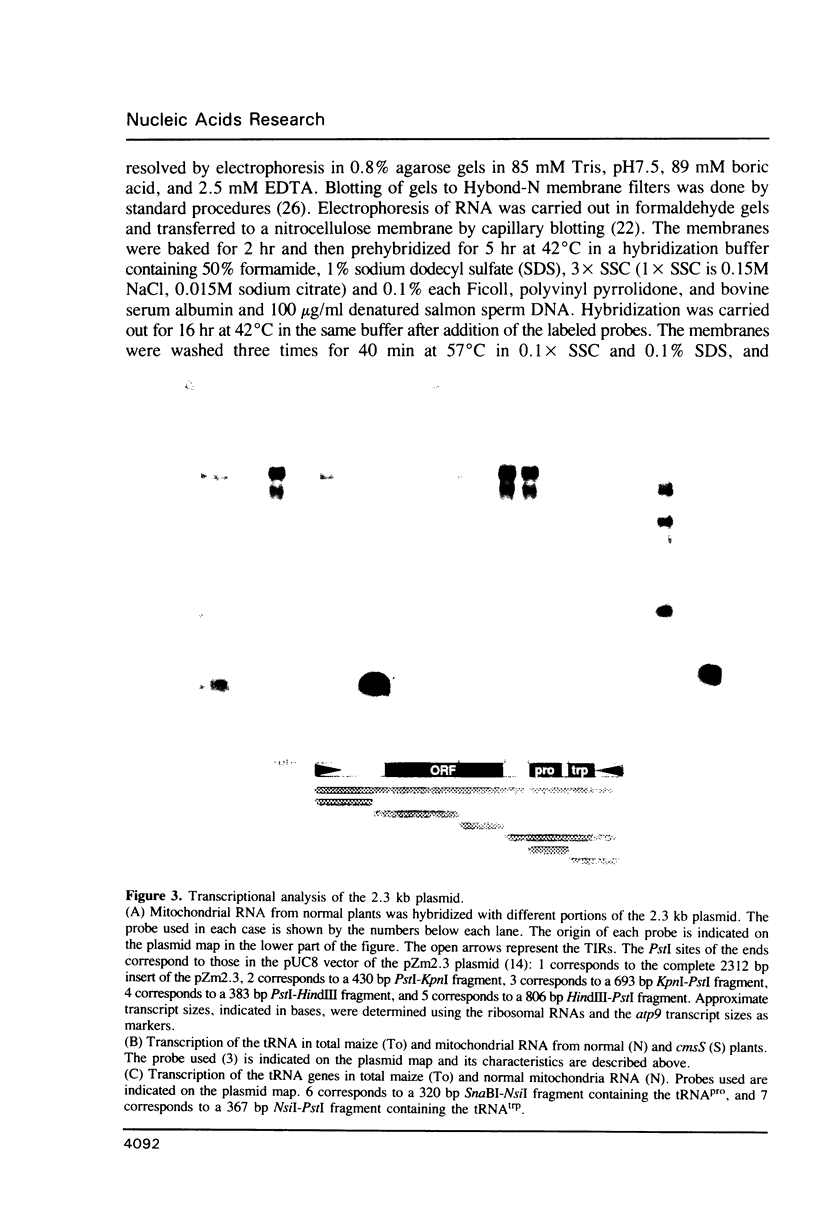
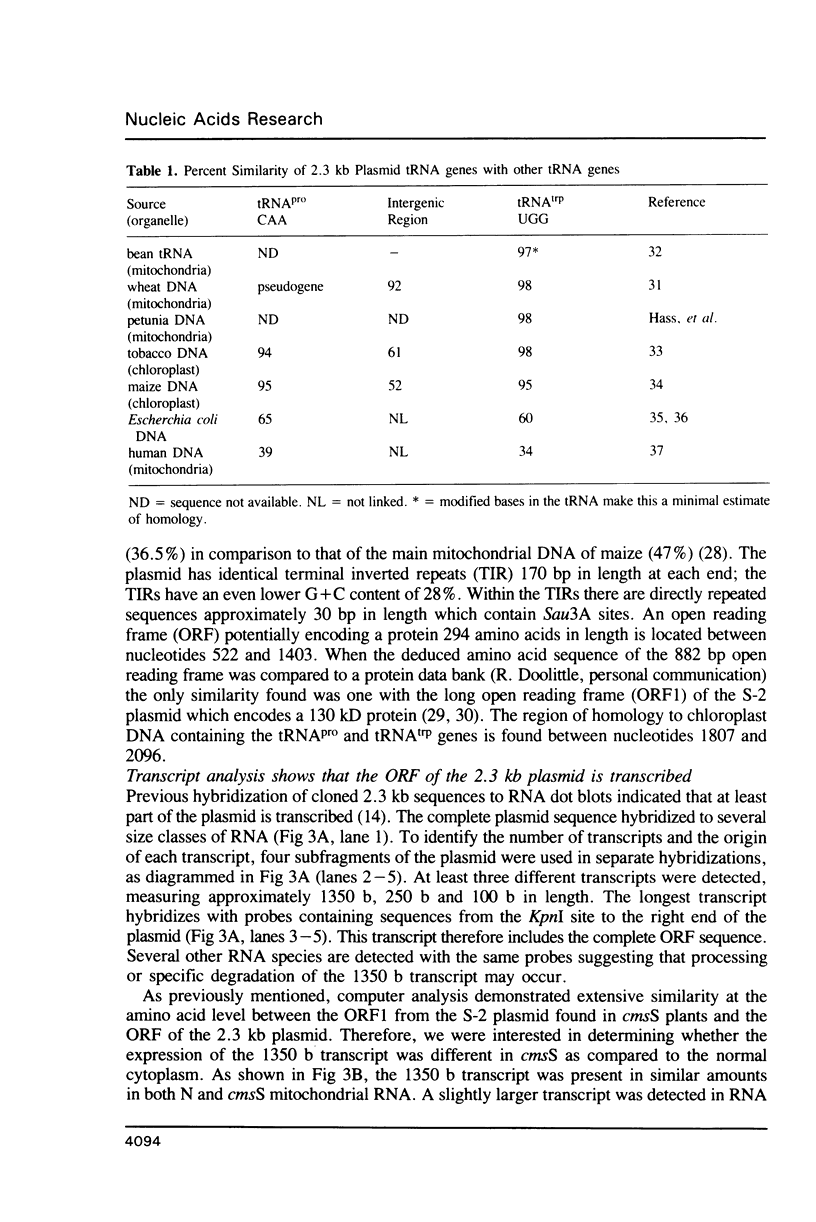
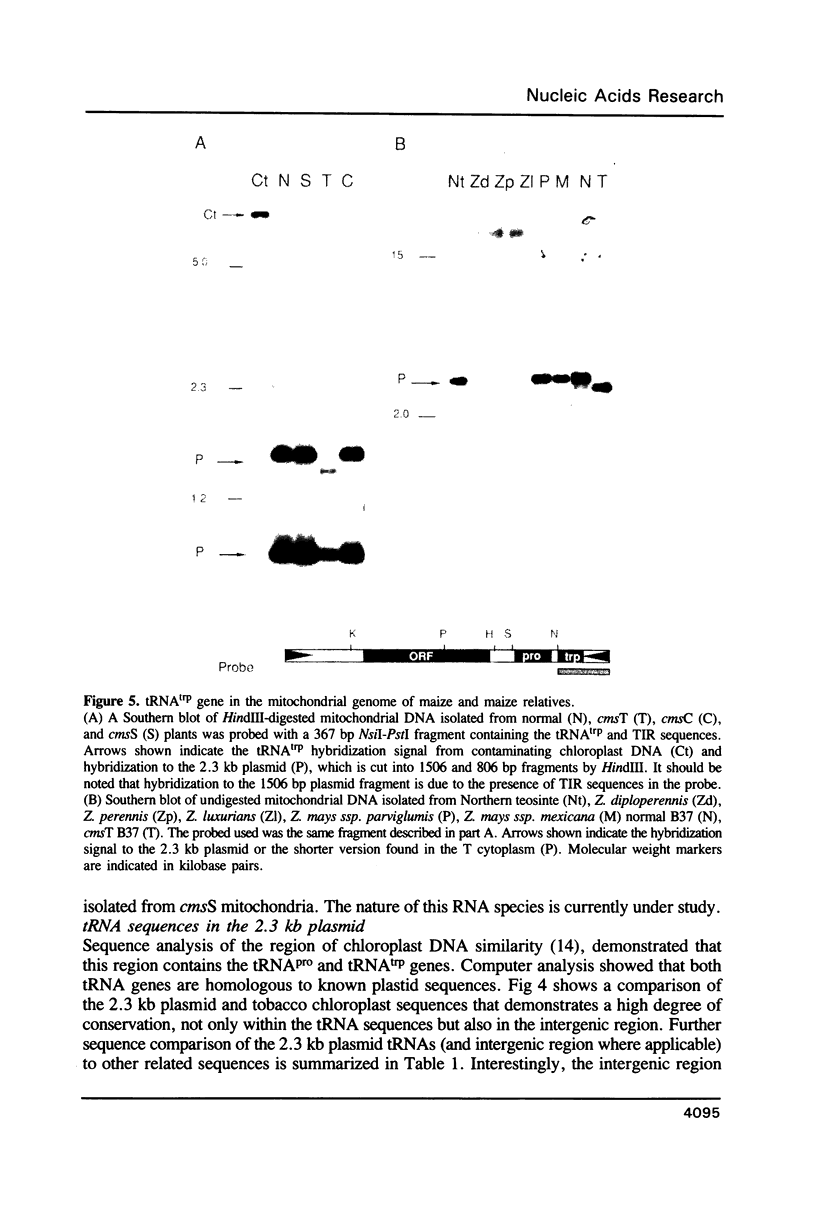
The nucleotide sequence and transcription pattern of the linear 2.3 kb plasmid of maize mitochondria was analyzed in order to elucidate its possible function in the organelle. The plasmid has 170 bp inverted repeats at its termini composed, in turn, of shorter repetitive sequences. An open reading frame within the plasmid is transcribed and can potentially specify a 33 kD product. In addition the plasmid contains two tRNA genes homologous to chloroplast sequences; the tRNApro(CAA) and the tRNAtrp(UGG). Both of the tRNA genes of the plasmid are transcribed, but apparently only the tRNAtrp is processed to the correct size. These tRNA sequences are found in the main mitochondrial genome of all higher plants tested, and in most maize relatives. An exception is the close maize relative Northern teosinte in which the tRNAtrp gene is also carried on a plasmid. These results suggest that the 2.3 kb plasmid has acquired the tRNA sequences from the main mitochondrial DNA. It is possible that the plasmid-encoded tRNAtrp gene is essential for organelle function thereby ensuring the maintenance of the plasmid in the mitochondrion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bedinger P., de Hostos E. L., Leon P., Walbot V. Cloning and characterization of a linear 2.3 kb mitochondrial plasmid of maize. Mol Gen Genet. 1986 Nov;205(2):206–212. doi: 10.1007/BF00430428. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Escote L. J., Gabay-Laughnan S. J., Laughnan J. R. Cytoplasmic reversion to fertility in cms-S maize need not involve loss of linear mitochondrial plasmids. Plasmid. 1985 Nov;14(3):264–267. doi: 10.1016/0147-619x(85)90011-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Brennicke A. Cytochrome oxidase subunit II gene in mitochondria of Oenothera has no intron. EMBO J. 1983;2(12):2173–2178. doi: 10.1002/j.1460-2075.1983.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. M., Klee H. J., Zagorski J., Fournier M. J. Structure of an Escherichia coli tRNA operon containing linked genes for arginine, histidine, leucine, and proline tRNAs. J Bacteriol. 1984 Jun;158(3):934–942. doi: 10.1128/jb.158.3.934-942.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Thompson R. D. S1 and S2, the linear mitochondrial DNAs present in a male sterile line of maize, possess terminally attached proteins. Nucleic Acids Res. 1982 Dec 20;10(24):8181–8190. doi: 10.1093/nar/10.24.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin E. V., Levchenko I. V., Zaitseva G. N. S2 plasmid from cms-S-maize mitochondria potentially encodes a specific RNA polymerase. Nucleic Acids Res. 1988 May 11;16(9):4177–4177. doi: 10.1093/nar/16.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Kim B. D., Pring D. R., Conde M. F., Mans R. J., Laughnan J. R., Gabay-Laughnan S. J. Cytoplasmic Reversion of cms-S in Maize: Association with a Transpositional Event. Science. 1980 Aug 29;209(4460):1021–1023. doi: 10.1126/science.209.4460.1021. [DOI] [PubMed] [Google Scholar]
- Levings C. S., Sederoff R. R. Nucleotide sequence of the S-2 mitochondrial DNA from the S cytoplasm of maize. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4055–4059. doi: 10.1073/pnas.80.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Garber R. C., Yoder O. C. Nucleotide sequence of a fungal plasmid-like DNA containing the mitochondrial ATPase subunit 6 gene. Nucleic Acids Res. 1988 Oct 25;16(20):9875–9875. doi: 10.1093/nar/16.20.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens J. H., Bogorad L. Nucleotide sequence containing the maize chloroplast proline (UGG) and tryptophan (CCA) tRNA genes. Nucleic Acids Res. 1988 Jun 10;16(11):5192–5192. doi: 10.1093/nar/16.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson J. C., Liddell A. D., Leaver C. J., Murray K. A protein specific to mitochondria from S-type male-sterile cytoplasm of maize is encoded by an episomal DNA. EMBO J. 1986 Nov;5(11):2775–2780. doi: 10.1002/j.1460-2075.1986.tb04567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal L., Runeberg-Roos P., Grienenberger J. M., Colin J., Weil J. H., Lejeune B., Quetier F., Lonsdale D. M. Homology in the region containing a tRNA(Trp) gene and a (complete or partial) tRNA(Pro) gene in wheat mitochondrial and chloroplast genomes. Curr Genet. 1987;12(2):91–98. doi: 10.1007/BF00434662. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme M., Kamogashira T., Shinozoki K., Sugiura M. Locations and sequences of tobacco chloroplast genes for tRNAPro(UGG), tRNATrp, tRNAfMet and tRNAGly(GCC): the tRNAGly contains only two base-pairs in the D stem. Nucleic Acids Res. 1984 Sep 11;12(17):6741–6749. doi: 10.1093/nar/12.17.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Physical and gene mapping of chloroplast DNA from Atriplex triangularis and Cucumis sativa. Nucleic Acids Res. 1982 Mar 11;10(5):1593–1605. doi: 10.1093/nar/10.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B., Marcu K., Dudock B. The isolation and sequence analysis of transfer RNA: the use of plaskon chromatography (RPC-5). Biochim Biophys Acta. 1973 Aug 10;319(1):25–36. doi: 10.1016/0005-2787(73)90037-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederoff R. R., Ronald P., Bedinger P., Rivin C., Walbot V., Bland M., Levings C. S., 3rd Maize mitochondrial plasmid S-1 sequences share homology with chloroplast gene psbA. Genetics. 1986 Jun;113(2):469–482. doi: 10.1093/genetics/113.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Thompson R. D., Kemble R. J., Flavell R. B. Variations in mitochondrial DNA organisation between normal and male-sterile cytoplasms of maize. Nucleic Acids Res. 1980 May 10;8(9):1999–2008. doi: 10.1093/nar/8.9.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Young R. A. Transcription termination in the Escherichia coli ribosomal RNA operon rrnC. J Biol Chem. 1979 Dec 25;254(24):12725–12731. [PubMed] [Google Scholar]




