Abstract
Purpose
Despite aggressive therapies, median survival for malignant gliomas is less than 15 months. Patients with unmethylated O6-methylguanine–DNA methyltransferase (MGMT) fare worse, presumably because of temozolomide resistance. AdV-tk, an adenoviral vector containing the herpes simplex virus thymidine kinase gene, plus prodrug synergizes with surgery and chemoradiotherapy, kills tumor cells, has not shown MGMT dependency, and elicits an antitumor vaccine effect.
Patients and Methods
Patients with newly diagnosed malignant glioma received AdV-tk at 3 × 1010, 1 × 1011, or 3 × 1011 vector particles (vp) via tumor bed injection at time of surgery followed by 14 days of valacyclovir. Radiation was initiated within 9 days after AdV-tk injection to overlap with AdV-tk activity. Temozolomide was administered after completing valacyclovir treatment.
Results
Accrual began December 2005 and was completed in 13 months. Thirteen patients were enrolled and 12 completed therapy, three at dose levels 1 and 2 and six at dose level 3. There were no dose-limiting or significant added toxicities. One patient withdrew before completing prodrug because of an unrelated surgical complication. Survival at 2 years was 33% and at 3 years was 25%. Patient-reported quality of life assessed with the Functional Assessment of Cancer Therapy-Brain (FACT-Br) was stable or improved after treatment. A significant CD3+ T-cell infiltrate was found in four of four tumors analyzed after treatment. Three patients with MGMT unmethylated glioblastoma multiforme survived 6.5, 8.7, and 46.4 months.
Conclusion
AdV-tk plus valacyclovir can be safely delivered with surgery and accelerated radiation in newly diagnosed malignant gliomas. Temozolomide did not prevent immune responses. Although not powered for efficacy, the survival and MGMT independence trends are encouraging. A phase II trial is ongoing.
INTRODUCTION
From an annual 22,000 diagnoses of primary brain tumors, there are about 13,000 deaths, most of which are from malignant gliomas such as glioblastoma multiforme (GBM) and anaplastic astrocytomas (AAs). GBMs are the most common and have the most dismal prognosis. Even after gross total resection, tumors recur because of infiltration outside the main mass. Temozolomide was approved for use with radiation on the basis of an improvement in median survival from 12.1 to 14.6 months.1 Tumors with an unmethylated O6-methylguanine–DNA methyltransferase (MGMT) promoter, and consequently high expression of the MGMT DNA repair gene, are not as responsive to temozolomide.2 For recurrent GBM, median survival is less than 6 months.3 Bevacizumab was approved for recurrent GBM on the basis of objective response rates.3 Novel therapies are greatly needed for this devastating disease.
Immunotherapy such as Sipuleucel-T (Provenge; Dendreon, Seattle, WA), which was recently approved for castration-resistant prostate cancer, also has potential for malignant gliomas.4,5 The approach described here generates a systemic vaccine effect through local delivery of an adenoviral vector containing the herpes simplex virus thymidine kinase gene (AdV-tk), followed by an antiherpetic prodrug. This approach kills tumor cells via necrosis and apoptosis, elicits danger signals, and stimulates antitumor T-cell proliferation.6–9 The most well-known effect is the cytotoxic component whereby herpes simplex virus thymidine kinase (HSV-tk) phosphorylates the antiherpetic prodrugs and converts them into nucleotide analogs, which are toxic to dividing or DNA-repairing cells and neighboring cells through the bystander effect.10–13 Normal, quiescent cells are less susceptible to this effect. DNA-damaging agents, such as radiation and some chemotherapies, increase DNA repair activity and consequently increase susceptibility. Proliferating endothelial cells from growing tumor vessels are highly sensitive to local AdV-tk plus prodrug cytotoxicity.9,14 This antiangiogenic effect and a potent immune-stimulatory effect are the less well-appreciated but equally or even more important effects of AdV-tk in the clinic.
The immunotherapeutic effect was observed in animal studies when AdV-tk plus prodrug led not only to local tumor response and increased survival but also to protection against metastases and tumor rechallenge, which was potentiated by radiation.8,15,16 Increased efficacy of HSV-tk plus prodrug occurred in immune-competent compared with immune-deficient models.17–19 Specific immunogenic effects included increased heat shock protein expression,7 increased expression of the toll-like receptor ligand HMGB1,20 increased costimulatory molecule expression on antigen-presenting cells,21 infiltration of macrophages and T cells, and Th1 cytokine expression.6,16,22 In addition, the immunostimulatory effect can be enhanced by addition of other immune effectors, such as Flt3 ligand or interleukin-2.20,23,24 These systemic effects may be particularly important for malignant brain tumors, which often have tumor cells infiltrating normal brain that cannot be removed by surgery or radiation and often lead to local recurrence or progression.
The AdV-tk vector has been evaluated in brain, prostate, pancreatic, and ovarian cancer, retinoblastomas, and other cancers with more than 400 patient doses delivered to more than 250 patients.25–32 Overall, the approach has demonstrated a good safety profile and encouraging efficacy results. The first clinical trial was a dose escalation study in recurrent malignant gliomas.25 No significant toxicity was observed on the first three dose levels (2 × 109 to 2 × 1011 vector particles [vp]), whereas two patients treated at the highest dose, 2 × 1012 vp, experienced CNS toxicity. Three of 13 patients survived more than 25 months, one with stable disease and another with transient reduction in tumor dimensions; two had a post-treatment interval of reduced steroid requirement. Inflammation and necrosis were seen within tumors whereas extratumoral inflammation was not observed.25 Of particular interest, at autopsy of one patient, significant lymphocytic infiltrate was observed in a contralateral untreated tumor, suggesting induction of distant antitumor immunity. Other groups have reported comparable results with similar vectors.33,34
To capitalize on the synergy with radiation and the expectation that immunotherapy would be more effective in the up-front adjuvant setting to prevent or delay recurrence, a phase IB dose escalation study was planned. Before initiating this phase IB study, a preclinical study requested by the US Food and Drug Administration that evaluated the combination of AdV-tk with radiation in the limited space of the cranium demonstrated no increased toxicity and reduced neurologic symptoms with the combination.35 Radiation did not alter the immunohistologic detection of AdV-tk expression in the tumors. Those data supported the use of an accelerated timing for radiation to maximize the potential for AdV-tk synergy such that radiation was targeted to start within 1 week of AdV-tk injection in this phase IB trial. The primary question for the clinical trial was whether AdV-tk plus valacyclovir would be safe in up-front disease in combination with surgery and radiation.
PATIENTS AND METHODS
Study Design
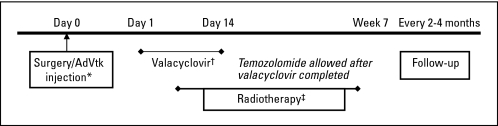
Three dose levels were planned using a standard three- to six-patient-per-dose design. AdV-tk was delivered to residual tumor cells in the tumor bed during surgery followed by valacyclovir for 14 days starting on day 1 to 3 and radiation starting 3 to 7 days after AdV-tk injection (Fig 1). Institutional review boards of the participating institutions approved the protocol and informed consent documents. Specific written informed consent was obtained from each patient before enrollment.
Fig 1.
Study design. An adenoviral vector containing the herpes simplex virus thymidine kinase gene (AdVtk) was injected at the time of surgery in a total volume of 1 mL divided into 10 injection sites in residual tumor in the surgical wall. Valacyclovir was administered at 2 gm three times per day for 14 days starting on days 1 to 3. Radiation was started 4 to 9 days after AdVtk injection to overlap with AdVtk activity and valacyclovir administration. Temozolomide was administered after completing valacyclovir. Differences in the study design compared with other recent studies46 with adenoviral delivery of herpes simplex virus thymidine kinase to newly diagnosed malignant gliomas include vector delivery: 1 mL in 10 sites versus 10 mL in 30 to 70 injection sites; prodrug: oral valacyclovir versus intravenous ganciclovir over 1 hour twice per day; radiation timing: accelerated radiation targeting within 7 days after surgery versus radiation delayed to 3 to 6 weeks after surgery. Differences highlighted in the figure as follows: (*) vector delivery; (†) prodrug; (‡) radiation timing.
Patients
Patients were required to be 18 years of age or older and to be diagnosed with presumed malignant glioma on the basis of clinical and radiologic evaluation. Pathologic confirmation at the time of surgery, if not previously determined, was required for continued participation. The tumor site had to be amenable to injection and not be located in the brainstem, midbrain, within the ventricular system, or in the infratentorial region. Patients were required to have a Karnofsky performance score ≥ 70, AST within 3× upper limit of normal, platelets more than 100,000/μL, WBC more than 3,000/μL, and serum creatinine less than 2 mg/dL.
AdV-tk Vector Description and Administration
AdV-tk has been previously described.36 GliAtak represents its current trade name for glioma (Advantagene, Auburndale, MA). Three dose levels were evaluated: 3 × 1010, 1 × 1011, and 3 × 1011 vp.
On day 0, craniotomy and tumor resection, total or subtotal, were performed. Stereotactic methods, intraoperative navigational guidance, magnetic resonance imaging (MRI), and/or other radiologic guidance were used at the neurosurgeons' discretion. AdV-tk was administered by using tuberculin syringes into each of 10 sites at least 1 cm apart. For each site, the needle was inserted into the tumor bed to a depth of 1 to 2 cm and then 100 μL was slowly injected over approximately 60 seconds. The neurosurgeon selected sites that avoided adjacent motor or speech cortex, the cerebral ventricle, or spillage into the subarachnoid space. Routine wound closure was performed with sutures, not staples, to avoid interference with simulation for radiation therapy. Standard perioperative antibiotics, corticosteroids, anticonvulsants, and analgesics were used as clinically indicated.
Valacyclovir Prodrug
Valacyclovir (Valtrex, provided by GlaxoSmithKline, Philadelphia, PA) was administered at 2,000 mg orally three times per day for 14 days starting 1 to 3 days after AdV-tk injection.
Chemoradiotherapy
Radiation was initiated within 9 days after surgery at 2 Gy per day for approximately 6 weeks to a total of 55 to 60 Gy. Standard temozolomide was started after completion of valacyclovir.
Clinical Assessment
Toxicity was assessed by using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Dose-limiting toxicities (DLTs) were defined as any grade 3 or 4 toxicity requiring interruption in radiation therapy for more than 7 days. Adverse events up to 1 week after completion of valacyclovir (weeks 1-3) were considered acute and were considered late if they occurred during the remainder of radiation therapy (weeks 4-8). Investigators evaluated the association as definitely, probably, possibly, unlikely, or not related to the investigational agent.
Total resection was defined as removal of more than 95% of the enhancing tumor. Classification of prognostic groups was based on the original Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis definition,37 except that baseline mental status was determined by using the Mini-Mental S Examination (MMSE) with abnormal defined as a score of ≤ 26 of 30. Progression was defined according to the Macdonald criteria as ≥ 25% increase in tumor area, appearance of a new lesion or neurologic deterioration, and corticosteroid dose stable or increasing.38
Quality-of-Life Assessment
The Functional Assessment of Cancer Therapy-Brain (FACT-Br) version 4 was used to assess patient-reported outcomes at baseline and at follow-up visits. The FACT-Br instrument is a 50-item questionnaire with physical, social, emotional, functional, and brain-specific domains validated for use in patients with brain tumors.39
MGMT Analysis
Tumor DNA harvested from frozen samples was analyzed by using the methylation-specific polymerase chain reaction.2
Immunohistochemistry
Paraffin-embedded tumor sections were stained with antibodies specific for T cells (CD3, CD4, and CD8), B cells (CD20), and macrophages (CD68).
Statistical Methods
The primary method of data analysis for this phase I study was descriptive. Progression-free survival (PFS) and overall survival (OS) rates were calculated from the time of AdV-tk injection by using the Kaplan-Meier method.
RESULTS
Patients and Treatment
Thirteen patients were enrolled and one patient withdrew during prodrug administration because of an intraoperative vascular complication that was unrelated to the trial. Patient characteristics for the 12 evaluable patients are listed in Table 1. Valacyclovir was started 1 to 3 days (mean, 2 days) after AdV-tk injection. One patient stopped after 10 days because of nausea and vomiting. Radiation was started by day 7 after surgery except for one patient who started on day 8 for logistical reasons and one who started on day 9 because staples rather than sutures had been used for surgical wound closure. Temozolomide was administered after completion of valacyclovir.
Table 1.
Patient Demographics and Outcomes
| Patient ID | AdV-tk Dose (vp) | Age(years) | Baseline MMSE | Baseline KPS | Tumor Pathology | RPA | Resection | MGMT Status | OS(months) | PFS(months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1B2 | 3 × 1010 | 59 | 30 | 100 | AA | 2 | Subtotal | N/A | > 56.1 | 49.9 |
| 1B3 | 3 × 1010 | 41 | 25 | 90 | GBM | 3 | Total | U | 46.4 | 27.8 |
| 1B4 | 3 × 1010 | 47 | 23 | 80 | GBM | 4 | Total | N/A | 11.1 | 6.5 |
| 2B1 | 1 × 1011 | 72 | 10 | 70 | GBM | 5 | Subtotal | U | 6.5 | 2.9 |
| 2B2 | 1 × 1011 | 69 | 27 | 90 | GBM | 4 | Subtotal | N/A | 13.7 | 8 |
| 2B3 | 1 × 1011 | 59 | 21 | 90 | GBM | 5 | Total | U | 8.7 | 4.7 |
| 3B1 | 3 × 1011 | 64 | 30 | 90 | GBM | 4 | Total | N/A | 15.8 | 10.2 |
| 3B2 | 3 × 1011 | 62 | 17 | 80 | GBM | 5 | Total | M | 7.7 | 5.4 |
| 3B3 | 3 × 1011 | 52 | 27 | 100 | GBM | 4 | Subtotal | N/A | 33.9 | 22.3 |
| 3B4 | 3 × 1011 | 60 | 24 | 90 | GBM | 5 | Subtotal | N/A | 2.0 | 2.0 |
| 3B5 | 3 × 1011 | 60 | 30 | 90 | GBM | 4 | Subtotal | M | 10.6 | 10.2 |
| 3B6 | 3 × 1011 | 43 | 27 | 100 | AA | 1 | Subtotal | N/A | > 47 | > 47 |
Abbreviations: AA, anaplastic astrocytoma; AdV-tk, an adenoviral vector containing the herpes simplex virus thymidine kinase gene; GBM, glioblastoma multiforme; ID, identification number; KPS, Karnofsky performance score; M, methylated; MGMT, O6-methylguanine–DNA methyltransferase; MMSE, mini-mental state examination; N/A, not available; OS, overall survival; PFS, progression-free survival; RPA, recursive partitioning analysis; U, unmethylated; vp, vector particles.
Safety
DLT was not observed, and dose escalation proceeded with three patients each at dose levels 1 and 2 and six patients at dose level 3. Laboratory and symptomatic abnormalities reported in patients while on study are summarized in Table 2 with grading by the clinical investigators according to CTCAE version 3.0. None of the events were definitely or probably related; possibly related events are indicated with an asterisk in Table 2. Most events were deemed expected and related to the underlying disease or standard of care (SOC) treatments. Laboratory abnormalities, which were considered possibly related and included increased ALT, AST, or creatinine and hyponatremia, were transient.
Table 2.
Adverse Events After AdV-tk Injection During Valacyclovir and the First 2 Weeks of Radiation (weeks 1-3) and After Valacyclovir Through Completion of Radiation (weeks 4-8)
| Adverse Events | Weeks 1-3 |
Weeks 4-8 |
||||||
|---|---|---|---|---|---|---|---|---|
| CTC Grade |
CTC Grade |
|||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Cardiac | ||||||||
| Ischemia | 1 | |||||||
| Constitutional | ||||||||
| Fatigue | 1 | 2 | 1 | |||||
| Fever | 1* | |||||||
| Insomnia | 1 | |||||||
| Dermatologic | ||||||||
| Rash | 1 | |||||||
| Wound complication | 1* | |||||||
| GI | ||||||||
| Anorexia/weight loss | 1 | |||||||
| Constipation | 3 | 2 | 1 | |||||
| Nausea/vomiting | 1 | 1* | 2 | |||||
| Hematologic | ||||||||
| Hemoglobin | 3 | 2 | 1 | 1 | ||||
| Leukopenia | 1 | |||||||
| Platelets | 1 | 2 | ||||||
| Hemorrhage | ||||||||
| Epistaxis | 1 | |||||||
| Hematoma, while on heparin | 1 | |||||||
| Infection | ||||||||
| Sinus | 1 | |||||||
| Laboratory | ||||||||
| ALT | 7* | 2* | 1* | 1* | ||||
| AST | 4* | 1* | 1* | |||||
| Bilirubin | 1 | |||||||
| Creatinine | 1 | 1* | 2 | |||||
| Hyponatremia | 5 | 1* | ||||||
| Lymphatics | ||||||||
| Edema, lower extremities | 1 | 1 | ||||||
| Neurologic | ||||||||
| Ataxia | 1 | |||||||
| Confusion | 1* | |||||||
| CSF leak | 1 | |||||||
| Dizziness | 1 | |||||||
| Hydrocephalus | 1* | |||||||
| Mood alteration, anxiety | 1 | |||||||
| Mood alteration, depression | 1* | 1 | 2 | |||||
| Neuropathy, cranial | 2 | 1 | ||||||
| Neuropathy, motor | 1* | |||||||
| Seizure | 1 | |||||||
| Speech impairment | 1* | 1 | ||||||
| Pain | ||||||||
| Headache | 1* | 1* | ||||||
| Chest, not cardiac | 3 | |||||||
| Postoperative | 1 | |||||||
| Pulmonary | ||||||||
| Dyspnea (COPD flare-up) | 1 | |||||||
| Renal/genitourinary | ||||||||
| Urinary retention with UTI | 1 | |||||||
| Vascular | ||||||||
| Deep venous thrombosis | 1 | 1 | ||||||
NOTE. Most events were deemed unrelated to the adenoviral vector containing the herpes simplex virus thymidine kinase gene (AdV-tk) + prodrug except those marked with an asterisk which were deemed possibly related. These events were transient.
Abbreviations: COPD, chronic obstructive pulmonary disease; CSF, cerebrospinal fluid; CTC, Common Terminology Criteria for Adverse Events version 3.0; UTI, urinary tract infection.
Two serious adverse events were deemed possibly related to the investigational agent. The first was in the fifth patient on dose level 3, who was hospitalized for fever, hyponatremia, and confusion 1 week after surgery and AdV-tk injection. Grade 2 AST and ALT and creatinine increases were also noted. Extensive evaluations for the cause of fever did not result in a diagnosis, and intermittent fevers continued for several weeks. The laboratory abnormalities resolved, and mental status returned to baseline. Valacyclovir was discontinued after 10 days because of nausea and vomiting. The patient completed radiation therapy per SOC. The second event was in the fourth patient on dose level 3 who was hospitalized 7 weeks after AdV-tk injection for placement of a subdural peritoneal shunt for progressive extra-axial fluid collection underlying the surgical wound closure. Radiation therapy and concomitant temozolomide were interrupted 3 days before completion of therapy. The patient remained hospitalized for complications following shunt placement, including a seizure, fever, confusion, and agitation. Cultures of the subdural fluid were negative. The patient improved and was due to resume radiation therapy but developed pneumonia and possible sepsis which progressed to acute respiratory distress syndrome and death in week 8. The clinical investigator deemed the fluid collection possibly related but the postshunt operative issues not related.
Starting radiation therapy within 7 days of surgery was well tolerated without wound complications such as dehiscence or infections.
Clinical Outcome
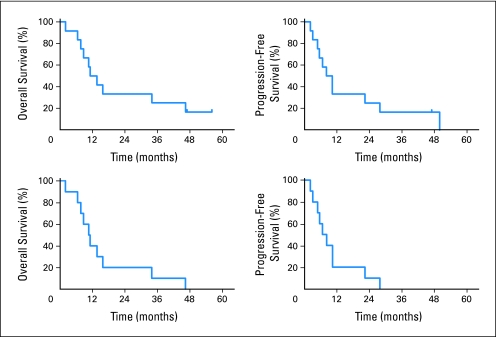
Accrual was completed in 2007 and follow-up continues with two patients with AA still alive. Patient demographics and outcomes are provided in Table 1, and survival curves are shown in Figure 2. Median OS was 12.4 months, median PFS was 9.1 months, 2-year survival was 33%, and 3-year survival was 25%.
Fig 2.
Kaplan-Meier analysis of overall survival and progression-free survival. The top two curves include all evaluable patients at dose levels 1, 2, and 3, and the bottom two curves include the 10 patients with glioblastoma multiforme.
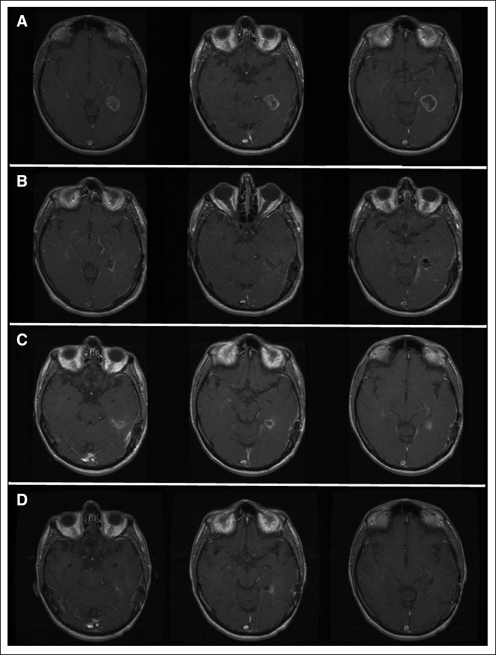
Three patients had post-treatment MRI findings suggestive of progression that gradually resolved without change in SOC treatment. One dose-level 1 patient with GBM developed ring enhancement 4 weeks postradiation that continued to resolve through 8 months (Fig 3). This patient was clinically well without progression through 28 months, despite stopping temozolomide after four cycles because of noncompliance. A patient with GBM who was on dose level 3 had a new focus of enhancement on MRI that remained stable for more than 1 year and finally progressed at 22 months. A third patient had a 5.6-cm residual AA tumor 1 month after radiation that decreased to 3.1 cm 4 months later on adjuvant temozolomide, increased gradually over the next 5 months followed by some gradual decrease, and has remained stable for more than 47 months since AdV-tk injection without additional therapy.
Fig 3.
Slow resolution of magnetic resonance imaging enhancement; magnetic resonance imaging of patient 1B3 at dose level 1. Before surgery and injection (panel A) imaging shows a glioblastoma multiforme in the left temporal lobe. Postoperative images (panel B) reveal a total resection. Residual ring enhancement is apparent 4 weeks after completion of radiation (panel C), which gradually resolved over the next 6 months. Decreased enhancement 4 months after radiation completion is shown in panel D. Adjuvant temozolomide was stopped after four cycles because of noncompliance. However, the patient did not develop progression until 27 months and survived for 46 months.
Quality of Life
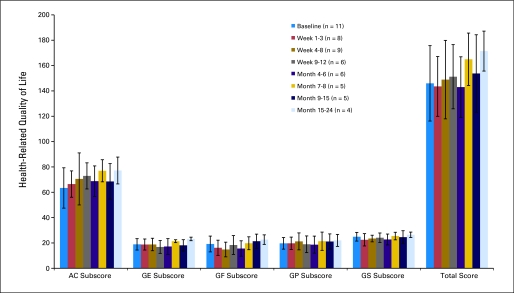
Health-related quality of life, evaluated by using the FACT-Br questionnaire as a patient-reported outcome measure, was stable or improved after treatment (Appendix Fig A1, online only).
Molecular and Histologic Analyses
MGMT promoter methylation was available for five of 12 evaluable patients (Table 1). No specific correlation between survival and MGMT methylation was observed from this small sample. The one long-term survivor with GBM (shown in Figure 3) possessed an unmethylated MGMT promoter.
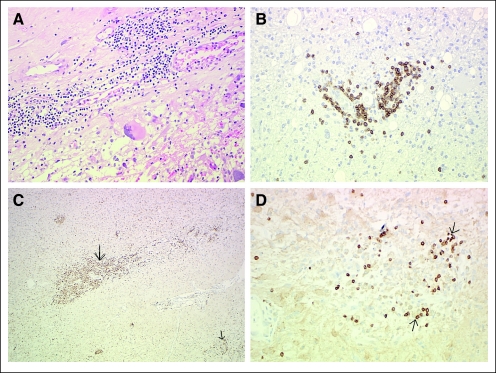
Evidence suggestive of immune stimulation was observed with a significant CD3+ T-cell infiltrate in four of four patients at 7 to 22 months after AdV-tk injection (Fig 4). Significant CD68+ macrophages were also detected. Further analysis in one patient (3B3) revealed the T-cell subset to be predominantly CD8+ (data not shown).
Fig 4.
Histologic analysis of re-resected tumors. Re-resection of glioblastoma multiforme at 30 weeks in patient 1B4 with demonstration of intratumoral lymphocytic infiltrate (A) via staining with hematoxylin and eosin, which was found to be CD3+ T cells by immunohistochemistry. (B) Anti-CD3 antibody. Re-resection of glioblastoma multiforme at 36 weeks in patient 2B2 with demonstration of macrophage (C) and cell (D) infiltration. (C) Anti-CD68 antibody. (D) Anti-CD3 antibody. Anti-CD20 staining for B cells was negative (not shown). Arrows point to positive cells.
DISCUSSION
This study is unique in capitalizing on AdV-tk synergy with radiation by starting radiation within 1 week of injection to overlap the therapeutic effects. The safety of AdV-tk and other adenoviral-based approaches has been demonstrated in recurrent malignant gliomas.25,33,34,40–43 This phase IB study demonstrated that AdV-tk/prodrug can be safely delivered intracranially up front in combination with surgery, intensive timing radiation, and chemotherapy. In addition, the use of valacyclovir, an oral antiherpetic prodrug, significantly decreased inconvenience, toxicity, and cost compared with intravenous ganciclovir used in previous studies. Although DLT was not reached, further dose escalation was not pursued. Early AdV-tk data in prostate cancer suggested that efficacy was not dose dependent.26,27 Toxicity, however, was seen at 2 × 1012 vp in our phase I study in recurrent malignant glioma.25 Thus, escalation beyond 5 × 1011 vp was not considered necessary.
The vector, as well as the transgene, is a key component of the approach. A phase III study with a retroviral vector failed, in large part because of poor transduction.44–46 Adenoviral vectors significantly enhance in vivo gene transfer to malignant gliomas.47 In a small controlled study in malignant gliomas, an adenoviral-tk vector led to a doubling of mean survival compared with a retroviral-tk vector or an adenoviral-marker vector.40 The infiltrative nature of malignant gliomas and natural inhibitory factors, such as inflammatory and immune response, suggest that achieving vector delivery to all tumor cells is unlikely. The potential immune stimulation by AdV-tk/valacyclovir could provide an innovative tumor vaccine mechanism to inhibit residual tumor cells after debulking therapy.
A European group has evaluated a similar adenoviral vector, AdvHSV-tk, in malignant gliomas. A phase II study48 that included primary and recurrent patients showed a significant survival advantage. A subsequent phase III study randomly assigned 251 patients to AdvHSV-tk versus SOC alone. The study suffered from patient and SOC variability and was not sufficiently powered for definitive evaluation. Nonetheless, it showed a trend toward improved outcomes for a composed primary end point of time to reintervention and OS.
AdV-tk has shown synergy with all components of malignant glioma SOC, including surgery,49 radiation,8 and temozolomide.50 For radiation and surgery, synergistic systemic effects suggest a mechanism involving potentiation of TK-mediated immunostimulation.8,49 Synergy with temozolomide has been shown to be due to inhibition of DNA cross-link repair by phosphorylated prodrug.50 Temozolomide inhibition of regulatory T cells may augment tumor vaccine effects.51,52 The study design, based in part on these proposed mechanisms, included accelerated radiation timing, which was well tolerated, and temozolomide per SOC.
AdV-tk immunostimulation, recently reviewed,53 is the result of a complex interaction from the delivery vehicle being a virus, the mode of cell death induced, the immunogenic properties of the transgene itself, and the synergy of AdV-tk with a local insult such as radiation.8,17–19,27,54–56 In vivo, the systemic antitumor effect requires prodrug, indicating that tumor cytotoxicity is required to release antigens to be used by antigen-presenting cells. In addition, CD8+ T-depletion studies showed that these cells are critical for the response.54 Preclinical data support the proposed mechanism of local cytotoxicity in the context of an immune stimulatory milieu leading to a polyvalent tumor vaccine effect. If accurate, this mechanism is not limited to a specific antigen, and it reduces the potential of immunologic escape from single-antigen vaccines.57,58
The observed pseudoprogressions, with subsequent gradual resolution, may reflect an immune reaction. In a mesothelioma study that used a similar AdV-tk delivered to the pleural space, two patients showed no initial imaging response but had long-term durable responses (> 6.5 years) after gradual resolution of the initial tumor many months after treatment.59 Immunotherapies may require re-evaluation of traditional response measures. In addition to imaging abnormalities, median survival may not reflect a real benefit. Immunotherapy may not have any impact on patients with aggressive tumors that progress early. If 50% of the treatment population had aggressive tumors, the median would not change. Patients with minimal residual disease, in which immunotherapy is most likely to be effective, may be represented in the tail of the survival curve. This may be addressed by selecting against rapid progressors, as in the EGFRvIII [epidermal growth factor receptor variant III] vaccine trial for GBM58 that selected patients who had not progressed 3 months after surgery. However, this approach delays immunotherapy while the residual tumor mass increases. The observation that immunotherapy may not meet traditional end points but may still prolong survival was demonstrated in two randomized phase III trials of Sipuleucel-T.60
MGMT promoter methylation would not be expected to affect immunotherapy. In an EGFRvIII vaccine trial, the PFS and OS were actually longer in patients with unmethylated MGMT.58 The evaluable tumors from this study were insufficient to make conclusions, but there were no evident patterns, and one patient with GBM with an unmethylated tumor survived more than 46 months.
This study has demonstrated the potential for neoadjuvant use of AdV-tk plus valacyclovir and accelerated radiation in treatment of malignant gliomas. In addition, although not powered to evaluate clinical efficacy, the 2- and 3-year survival rates of 33% and 25%, respectively, are encouraging, especially since two patients surviving more than 2 years had unfavorable GBMs, one with unmethylated MGMT and the other with a subtotal resection. A phase II study to further evaluate safety, MGMT status, and potential efficacy is ongoing.
Appendix
Fig A1.
Patient-reported outcomes from the Functional Assessment of Cancer Therapy-Brain (FACT-Br) questionnaire before and after treatment. Colored bars show average and black vertical bars show standard deviation for each subscore and the total score for each time period. n, number of patients evaluated in each time period. AC, Additional Concerns section (brain subscale); GE, General Emotional Well-Being; GF, General Functional Well-Being; GP, General Physical Well-Being; GS, General Social/Family Well-Being.
Footnotes
Supported in part by Grant No. 5R44CA107745 from the National Cancer Institute.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008, and at the Annual Meeting of the Society for Neuro-Oncology, New Orleans, LA, October 22-24, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00751270.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Laura K. Aguilar, Advantagene (C); Carissa M. Monterroso, Advantagene (C); Andrea G. Manzanera, Advantagene (C); Estuardo Aguilar-Cordova, Advantagene (C) Consultant or Advisory Role: None Stock Ownership: Laura K. Aguilar, Advantagene; Estuardo Aguilar-Cordova, Advantagene Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: E. Antonio Chiocca, Laura K. Aguilar, Estuardo Aguilar-Cordova, Pamela Z. New
Financial support: Estuardo Aguilar-Cordova
Administrative support: E. Antonio Chiocca, Laura K. Aguilar,Susan D. Bell, Carissa Monterroso, Andrea G. Manzanera,Estuardo Aguilar-Cordova
Provision of study materials or patients: E. Antonio Chiocca, Susan D. Bell, Balveen Kaur, Robert Cavaliere, John McGregor, Simon Lo, Abhik Ray-Chaudhuri, Arnab Chakravarti, Herbert Newton, Robert G. Grossman, Todd W. Trask, David S. Baskin,Pamela Z. New
Collection and assembly of data: E. Antonio Chiocca, Laura K. Aguilar, Susan D. Bell, Balveen Kaur, Jayson Hardcastle, Robert Cavaliere, John McGregor, Simon Lo, Abhik Ray-Chaudhuri, Arnab Chakravarti, Herbert Newton, Kimbra S. Harris, Robert G. Grossman, Todd W. Trask, David S. Baskin, Carissa Monterroso, Andrea G. Manzanera, Estuardo Aguilar-Cordova, Pamela Z. New
Data analysis and interpretation: E. Antonio Chiocca, Laura K. Aguilar, Balveen Kaur, Jayson Hardcastle, Robert Cavaliere, John McGregor, Simon Lo, Abhik Ray-Chaudhuri, Arnab Chakravarti, John Grecula, Herbert Newton, Robert G. Grossman, Todd W. Trask, David S. Baskin, Estuardo Aguilar-Cordova, Pamela Z. New
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 3.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 4.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 5.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 6.Vile RG, Castleden S, Marshall J, et al. Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intratumoural cytokine expression. Int J Cancer. 1997;71:267–274. doi: 10.1002/(sici)1097-0215(19970410)71:2<267::aid-ijc23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Melcher A, Todryk S, Hardwick N, et al. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 8.Chhikara M, Huang H, Vlachaki MT, et al. Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3:536–542. doi: 10.1006/mthe.2001.0298. [DOI] [PubMed] [Google Scholar]
- 9.Ayala G, Satoh T, Li R, et al. Biological response determinants in HSV-tk + ganciclovir gene therapy for prostate cancer. Mol Ther. 2006;13:716–728. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Eastham JA, Chen SH, Sehgal I, et al. Prostate cancer gene therapy: Herpes simplex virus thymidine kinase gene transduction followed by ganciclovir in mouse and human prostate cancer models. Hum Gene Ther. 1996;7:515–523. doi: 10.1089/hum.1996.7.4-515. [DOI] [PubMed] [Google Scholar]
- 11.Fyfe JA, Keller PM, Furman PA, et al. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl) guanine. J Biol Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 12.Freeman SM, Abboud CN, Whartenby KA, et al. The “bystander effect”: Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 13.Fick J, Barker FG, 2nd, Dazin P, et al. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc Natl Acad Sci U S A. 1995;92:11071–11075. doi: 10.1073/pnas.92.24.11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ram Z, Walbridge S, Shawker T, et al. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg. 1994;81:256–260. doi: 10.3171/jns.1994.81.2.0256. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Cruet MJ, Trask TW, Chen SH, et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39:506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- 16.Hall SJ, Mutchnik SE, Chen SH, et al. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer. 1997;70:183–187. doi: 10.1002/(sici)1097-0215(19970117)70:2<183::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Vile RG, Nelson JA, Castleden S, et al. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 18.Kuriyama S, Kikukawa M, Masui K, et al. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer. 1999;83:374–380. doi: 10.1002/(sici)1097-0215(19991029)83:3<374::aid-ijc13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Gagandeep S, Brew R, Green B, et al. Prodrug-activated gene therapy: Involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]
- 20.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramesh R, Munshi A, Abboud CN, et al. Expression of costimulatory molecules: B7 and ICAM up-regulation after treatment with a suicide gene. Cancer Gene Ther. 1996;3:373–384. [PubMed] [Google Scholar]
- 22.Barba D, Hardin J, Sadelain M, et al. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci U S A. 1994;91:4348–4352. doi: 10.1073/pnas.91.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: Humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo F, Barzon L, Franchin E, et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: Biological and clinical results. Cancer Gene Ther. 2005;12:835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 25.Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 26.Herman JR, Adler HL, Aguilar-Cordova E, et al. In situ gene therapy for adenocarcinoma of the prostate: A phase I clinical trial. Hum Gene Ther. 1999;10:1239–1249. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 27.Miles BJ, Shalev M, Aguilar-Cordova E, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum Gene Ther. 2001;12:1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 28.Shalev M, Kadmon D, Teh BS, et al. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J Urol. 2000;163:1747–1750. [PubMed] [Google Scholar]
- 29.Aguilar LK, Teh B, Mai W, et al. Five year follow up of a phase II study of cytotoxic immunotherapy combined with radiation in newly diagnosed prostate cancer. J Clin Oncol. 2006;24(suppl: abstr 4635):250s. [Google Scholar]
- 30.van der Linden RR, Haagmans BL, Mongiat-Artus P, et al. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol. 2005;48:153–161. doi: 10.1016/j.eururo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Hasenburg A, Tong XW, Fischer DC, et al. Adenovirus-mediated thymidine kinase gene therapy in combination with topotecan for patients with recurrent ovarian cancer: 2.5-year follow-up. Gynecol Oncol. 2001;83:549–554. doi: 10.1006/gyno.2001.6442. [DOI] [PubMed] [Google Scholar]
- 32.Chévez-Barrios P, Chintagumpala M, Mieler W, et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- 33.Smitt PS, Driesse M, Wolbers J, et al. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7:851–858. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 34.Germano IM, Fable J, Gultekin SH, et al. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: Preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 35.Nestler U, Wakimoto H, Siller-Lopez F, et al. The combination of adenoviral HSV TK gene therapy and radiation is effective in athymic mouse glioblastoma xenografts without increasing toxic side effects. J Neurooncol. 2004;67:177–188. doi: 10.1023/b:neon.0000021897.53969.ca. [DOI] [PubMed] [Google Scholar]
- 36.Chen SH, Shine HD, Goodman JC, et al. Gene therapy for brain tumors: Regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994;91:3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 39.Weitzner MA, Meyers CA, Gelke CK, et al. The Functional Assessment of Cancer Therapy (FACT) scale: Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 40.Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 41.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 42.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Chiocca EA, Smith KM, McKinney B, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16:618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 44.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 45.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 46.Harsh GR, Deisboeck TS, Louis DN, et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: A gene-marking and neuropathological study. J Neurosurg. 2000;92:804–811. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- 47.Puumalainen AM, Vapalahti M, Agrawal RS, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 48.Immonen A, Vapalahti M, Tyynelä K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: A randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Sukin SW, Chhikara M, Zhu X, et al. In vivo surgical resection plus adjuvant gene therapy in the treatment of mammary and prostate cancer. Mol Ther. 2001;3:500–506. doi: 10.1006/mthe.2001.0285. [DOI] [PubMed] [Google Scholar]
- 50.Rainov NG, Fels C, Droege JW, et al. Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 2001;8:662–668. doi: 10.1038/sj.cgt.7700355. [DOI] [PubMed] [Google Scholar]
- 51.Jordan JT, Sun W, Hussain SF, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: Case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: Mechanisms and clinical development. J Cell Biochem. 2011;112:1969–1977. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 54.Agard C, Ligeza C, Dupas B, et al. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001;8:128–136. doi: 10.1038/sj.cgt.7700281. [DOI] [PubMed] [Google Scholar]
- 55.Fujita T, Teh BS, Timme TL, et al. Sustained long-term immune responses after in situ gene therapy combined with radiotherapy and hormonal therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2006;65:84–90. doi: 10.1016/j.ijrobp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Vlachaki MT, Chhikara M, Aguilar L, et al. Enhanced therapeutic effect of multiple injections of HSV-TK + GCV gene therapy in combination with ionizing radiation in a mouse mammary tumor model. Int J Radiat Oncol Biol Phys. 2001;51:1008–1017. doi: 10.1016/s0360-3016(01)01698-4. [DOI] [PubMed] [Google Scholar]
- 57.Heimberger AB, Archer GE, Mitchell DA, et al. Epidermal growth factor receptor variant III (EGFRvIII) vaccine (CDX-110) in GBM. J Clin Oncol. 2009;27(suppl; abstr 2021):92s. [Google Scholar]
- 58.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sterman DH, Recio A, Vachani A, et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res. 2005;11:7444–7453. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- 60.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]