Abstract
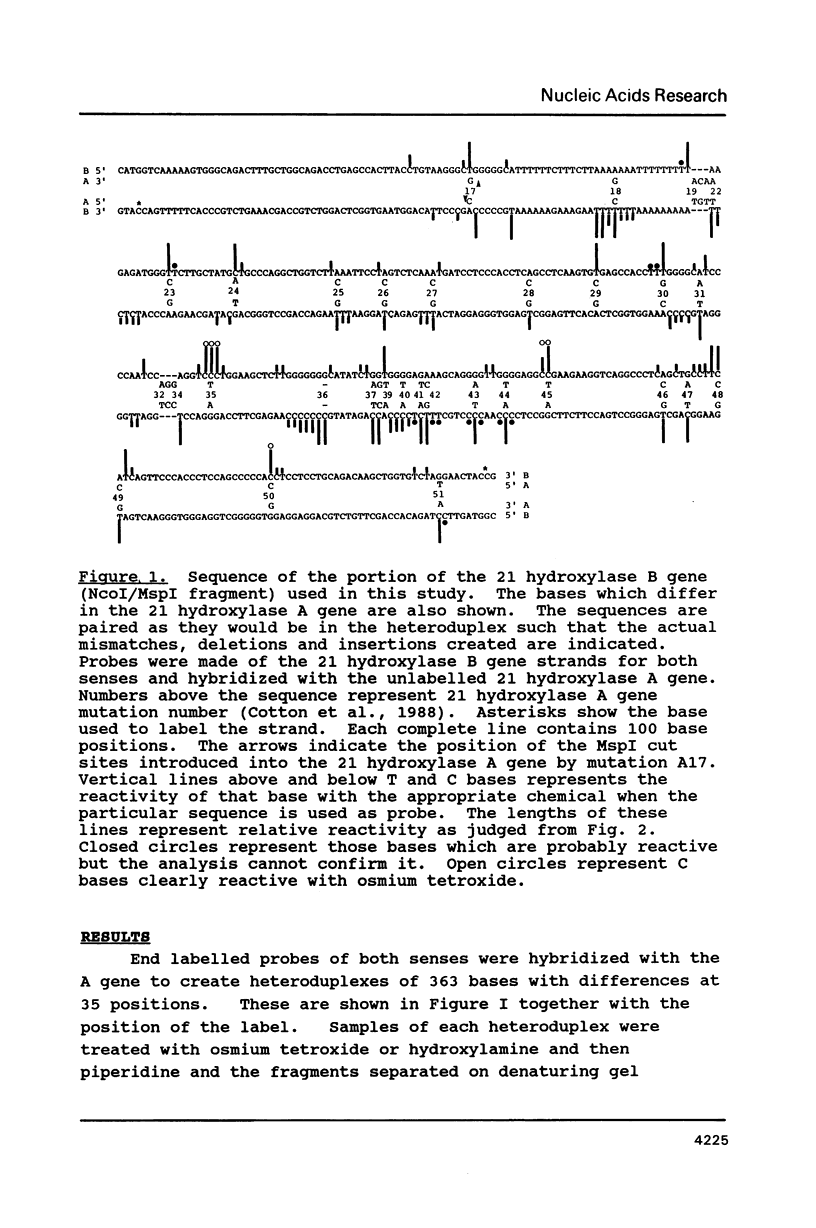
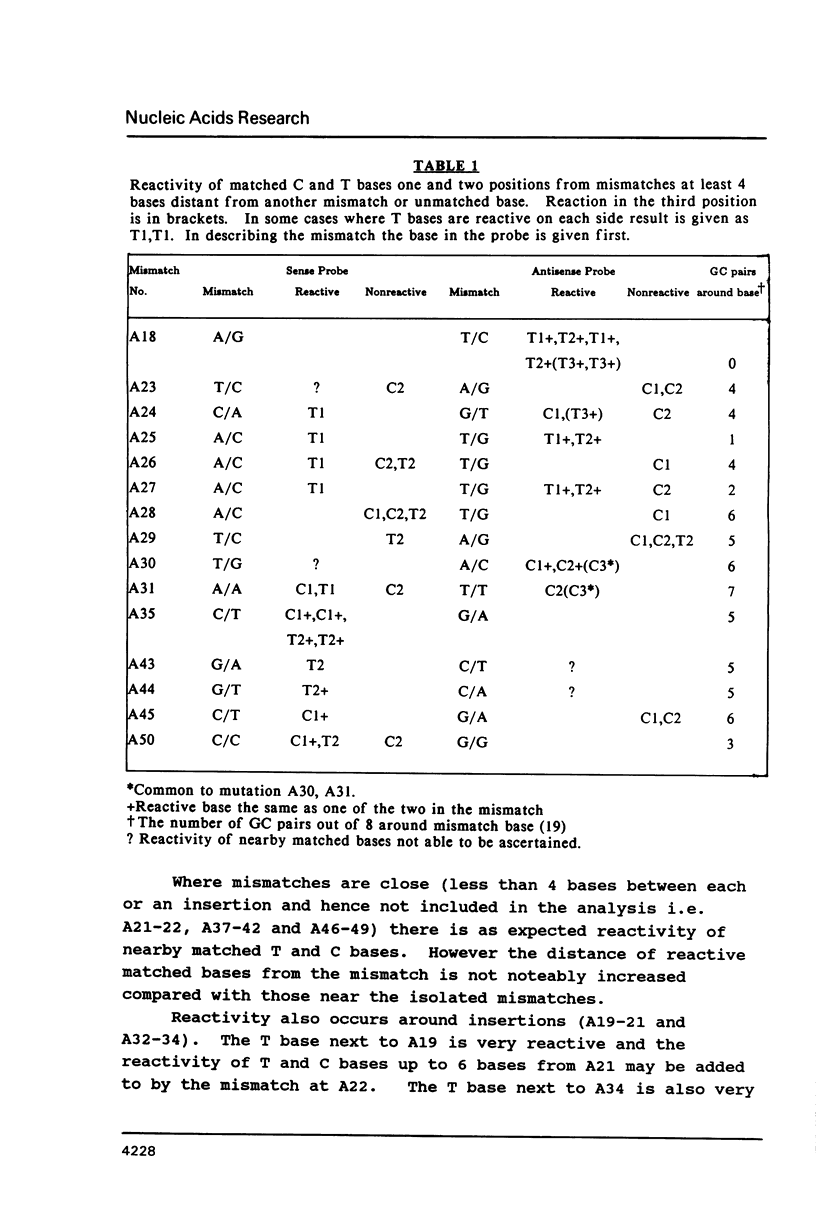
The chemical reactivity of matched T and C bases to osmium tetroxide and hydroxylamine near mismatched and unmatched bases in a heteroduplex between two strands of DNA with multiple differences was examined. Data was available for matched bases one or two positions away from 24 mismatches. Reactive bases were found near 16 of the mismatches and were usually one or two bases away. This reactivity is consistent with structural studies indicating perturbation of the duplex around mismatches and will allow another mode of study of the effect of mismatches. The reactivity of these bases was found not to be strongly correlated with mismatch type or GC basepair content of the basepairs around the mismatches. Extra reactivity may have been promoted by the presence of either T or C in the mismatch allowing increased reactivity of nearby T or C. The utility of the phenomenon for the detection of mutations is discussed. Unmatched bases in the heteroduplex also gives rise to reactive matched bases nearby.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer F. Three-dimensional structure of tRNA. Prog Nucleic Acid Res Mol Biol. 1971;11:391–421. doi: 10.1016/s0079-6603(08)60333-5. [DOI] [PubMed] [Google Scholar]
- Duckett D. R., Murchie A. I., Diekmann S., von Kitzing E., Kemper B., Lilley D. M. The structure of the Holliday junction, and its resolution. Cell. 1988 Oct 7;55(1):79–89. doi: 10.1016/0092-8674(88)90011-6. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., Quignard E., Woisard A., Guschlbauer W., van der Marel G. A., van Boom J. H., Jones M., Radman M. Structures of mismatched base pairs in DNA and their recognition by the Escherichia coli mismatch repair system. EMBO J. 1986 Dec 20;5(13):3697–3703. doi: 10.1002/j.1460-2075.1986.tb04702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. L., Patel D. J. NMR studies of A.C mismatches in DNA dodecanucleotides at acidic pH. Wobble A(anti).C(anti) pair formation. J Biol Chem. 1987 Dec 15;262(35):16973–16984. [PubMed] [Google Scholar]
- Ikuta S., Takagi K., Wallace R. B., Itakura K. Dissociation kinetics of 19 base paired oligonucleotide-DNA duplexes containing different single mismatched base pairs. Nucleic Acids Res. 1987 Jan 26;15(2):797–811. doi: 10.1093/nar/15.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen C., Nielsen P. E. Detection of intercalation-induced changes in DNA structure by reaction with diethyl pyrocarbonate or potassium permanganate. Evidence against the induction of Hoogsteen base pairing by echinomycin. FEBS Lett. 1988 Apr 11;231(1):172–176. doi: 10.1016/0014-5793(88)80725-7. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Johnston B. H. The S1-sensitive form of d(C-T)n.d(A-G)n: chemical evidence for a three-stranded structure in plasmids. Science. 1988 Sep 30;241(4874):1800–1804. doi: 10.1126/science.2845572. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClellan J. A., Palecek E., Lilley D. M. (A-T)n tracts embedded in random sequence DNA--formation of a structure which is chemically reactive and torsionally deformable. Nucleic Acids Res. 1986 Dec 9;14(23):9291–9309. doi: 10.1093/nar/14.23.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Hare D. Nuclear magnetic resonance and distance geometry studies of DNA structures in solution. Annu Rev Biophys Biophys Chem. 1987;16:423–454. doi: 10.1146/annurev.bb.16.060187.002231. [DOI] [PubMed] [Google Scholar]
- Portugal J., Fox K. R., McLean M. J., Richenberg J. L., Waring M. J. Diethyl pyrocarbonate can detect a modified DNA structure induced by the binding of quinoxaline antibiotics. Nucleic Acids Res. 1988 May 11;16(9):3655–3670. doi: 10.1093/nar/16.9.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rairkar A., Rubino H. M., Lockard R. E. Chemical probing of adenine residues within the secondary structure of rabbit 18S ribosomal RNA. Biochemistry. 1988 Jan 26;27(2):582–592. doi: 10.1021/bi00402a013. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Dunham I., Yu C. Y., Carroll M. C., Porter R. R., Campbell R. D. Molecular characterization of the HLA-linked steroid 21-hydroxylase B gene from an individual with congenital adrenal hyperplasia. EMBO J. 1987 Jun;6(6):1653–1661. doi: 10.1002/j.1460-2075.1987.tb02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Werntges H., Steger G., Riesner D., Fritz H. J. Mismatches in DNA double strands: thermodynamic parameters and their correlation to repair efficiencies. Nucleic Acids Res. 1986 May 12;14(9):3773–3790. doi: 10.1093/nar/14.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]



