Abstract
Human epidemiological studies have shown that diets enriched in n-3 polyunsaturated fatty acids (n-3 PUFA) are associated with a lower incidence of cancers including breast cancer. Our previous studies showed that the n-3 PUFA, docosahexaenoic acid (DHA), upregulated syndecan-1 (SDC-1) expression to induce apoptosis in the human breast cancer cell line MCF-7. We now present evidence of a signaling pathway that is impacted by SDC-1 in these cells and in mouse mammary tissues to result in apoptosis. In MCF-7 cells and SK-BR-3 cells, DHA and a SDC-1 ectodomain impaired signaling of the p44/42 mitogen-activated protein kinase (MAPK) pathway by inhibiting the phosphorylation of MAPK/Erk (MEK)/extracellular signal-regulated kinase (Erk) and Bad to induce apoptosis. SDC-1 siRNA significantly enhanced phosphorylation of these signal molecules and blocked the inhibitory effects of DHA on their phosphorylation. SDC-1 siRNA diminished apoptosis of MCF-7 cells, an effect that was markedly blocked by MEK inhibitor, PD98059. In vivo studies used (i) Fat-1 mice, a genetic model able to convert n-6 to n-3 PUFA to result in higher SDC-1 levels in Fat-1 mammary tissue compared with that of wild-type (wt) mice. Phosphorylation of MEK, Erk and Bad was lower in the Fat-1 versus wt tissue and (ii) SDC-1−/− mice that demonstrated markedly higher levels of phosphorylated MEK, Erk and Bad in mammary gland tissue compared with those of SDC+/+ mice. These data elucidate a pathway whereby SDC-1, upregulated by DHA, induces apoptosis in breast cancer cells through inhibition of MEK/Erk/Bad signaling.
Introduction
Human epidemiological studies have shown that a high dietary intake of fish is associated with a lower incidence of cancers including breast cancer (1–3). Animal studies clearly support the idea that dietary supplementation with fish oil or its constituent, fatty acids not only slows the growth of both xenograft (4–6) and chemically induced tumors (7,8) but also increases sensitivity to chemotherapy (9,10) and inhibits metastases (4,5). There are two main omega-3 polyunsaturated fatty acids (n-3 PUFA) in fish oil: eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3). DHA in particular was shown to be a potent enhancer of tumor cell chemosensitivity (11,12) and ongoing studies are showing promise of DHA improving outcome of chemotherapy in patients with metastatic breast cancer (13). We previously reported that DHA and EPA were delivered to MCF-7 cells by n-3 PUFA-enriched low-density lipoproteins or by albumin to result in major changes in gene transcription (14).
In agreement with reports that the transmembrane heparan sulfate proteoglycan, syndecan-1 (SDC-1) is downregulated in malignant tumors (15–22) and tumor cells (23), our previous studies showed that levels of SDC-1 in human breast cancer cells were lower than those of non-cancer cells (14). SDC-1 has been shown to act as a tumor suppresser by inhibiting cell proliferation (24) and inducing apoptosis (25). Importantly, we showed that DHA upregulated SDC-1 expression in human breast cancer cells (26) and moreover, that DHA induced apoptosis, an effect that was blocked by SDC-1 silencing (27). That is to say, DHA induced apoptosis of human breast cancer cells through SDC-1.
The present studies were conducted to determine the intracellular signaling pathways impacted by DHA–SDC-1 in breast cancer cells to promote apoptosis. Activation of the p44/42 mitogen-activated protein kinase (MAPK) pathway plays a major role in regulating cell growth and survival in breast cancer cells (28) and is protective against apoptosis through phosphorylation of Bad (29). Previous studies have indicated that this pathway may be a target for differential effects of n-3 and n-6 PUFA. DHA was shown to inhibit, whereas the n-6 PUFA, linoleic acid (LA), increased extracellular signal-regulated kinase (Erk) phosphorylation in human lymphocytes (30). LA enhanced Erk phosphorylation in colorectal cancer cells (31). DHA, but not EPA, was shown to decrease Erk activation in mesangial cells (32). Furthermore, it was reported that minican, a truncated form of SDC-1 inhibited Erk phosphorylation of S115 mouse mammary carcinoma cells (33). Our study is the first to examine the involvement of this pathway in the growth-inhibitory effects of DHA mediated through SDC-1 in breast cancer cells. The results demonstrate that SDC-1 impairs signaling of the MAPK pathway by inhibiting phosphorylation of MEK, Erk and Bad. This results in apoptosis induction in the breast cancer cells. In addition, we have used two animal models to demonstrate the in vivo relevance of this pathway. Firstly, the Fat-1 mouse is a model engineered by Kang et al. (34) to express the Fat-1 transgene from Caenorhabditis elegansthat encodes an n-3 desaturase to convert n-6 to n-3 fatty acids. This results in tissues enriched in n-3 PUFA (34–36) and higher levels of SDC-1 in mammary glands of Fat-1 compared with those of wild-type (wt) mice (37). This model was used to demonstrate that endogenous synthesis of n-3 PUFA in the mammary gland is associated with a decrease in phosphorylation of MEK, Erk and Bad. Secondly, we have used SDC-1 null mice (38) to demonstrate that MAPK signaling is elevated when SDC-1 is absent. Together, these data indicate that SDC-1, upregulated by DHA, induces apoptosis of breast cancer cells by inhibiting the activity of the MEK/Erk signaling pathway.
Materials and methods
Preparation of fatty acid (FA)–bovine serum albumin (BSA) complexes. FA–BSA complexes were prepared as our previous report (26). FA-free BSA (Sigma, St Louis, MO) was prepared as a 125 μM solution in Dulbecco’s modified Eagle medium (DMEM)/Ham’s F-12. FA salts (including DHA, EPA and LA) were solublized to 600 μM stocks in the BSA media as described (4:1 ratio of FA:BSA) and stored in aliquots at −20°C.
Preparation of SDC-1 ectodomain
The SDC-1 ectodomain was prepared as described in our previous report (27). The SDC-1 ectodomain expression construct was designed to encode a C-terminal polyhistidine fusion protein and was created using a two-step cloning process as follows. The SDC-1 ectodomain complementary DNA (cDNA) was amplified by polymerase chain reaction using the SDC-1 plasmid (OriGene Technologies, Rockville, MD) as the source of template, 5′-gcagaattcggcagcatgaggcgcgcggcgctct-3′ as the forward primer and 5′-gcaggatcctttcctgtccaggaggccctgtga-3′ as the reverse primer. The resulting cDNA was cut with BamHI and EcoRI restriction endonucleases and ligated into the pTcam4 expression plasmid (39). In the second round of polymerase chain reaction, 5′-gcaaagcttgaattcggcagcatgaggcgcgcg-3′ (forward) and 5′-gcactcgagttagtgatggtgatgatggtgctgcag-3′ (reverse) primers were used to amplify the SDC-1 ectodomain cDNA from the modified pTcam4 plasmid. The amplified cDNA was treated with HindIII and XhoI and subsequently ligated into the pCEP4 (Invitrogen, Corp., Carlsbad, CA) expression plasmid. The resulting plasmid was transfected into 293-EBNA cells and stable expressing cells selected by the addition of 200 μg/ml hygromycin B. The cells were then amplified and grown to confluence in T175 flasks. Upon confluence, the culture media was replaced with serum-free DMEM. Media was then collected every 48 h and fresh media added back to the cells. Under these conditions, the transfected cells remain viable for several weeks allowing for the collection of conditioned media over an extended period of time. After collecting several liters of conditioned media, recombinant SDC-1 ectodomain was purified. Conditioned media was first concentrated using a Millipore Pellicon 2 tangential flow system (Millipore Corporation, Bedford, MA). Concentrated conditioned media was then subjected to anion exchange chromatography using an Amersham Biosciences ÄKTAexplorer chromatography system equipped with a 5 ml HiTrap Q column and bound SDC-1 ectodomain eluted with a linear gradient of 0–2 M sodium chloride in a buffer consisting of 20 mM Tris, 0.2% 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate, pH 8.0.
Cell culture
MCF-7 cell line was obtained from ATCC (Rockville, MD) and maintained in DMEM/F12 supplemented with 5% fetal bovine serum (FBS), 10 mg/ml porcine insulin (Sigma), penicillin/streptomycin and l-glutamine at 37°C in 5% CO2. In experiments measuring phosphorylation of MAPK signaling molecules, the cells grown in 0.5% FBS of medium were treated with 30 μM DHA, 30 μM EPA and 30 μM LA for 48 h or with a range of concentrations of SDC-1 ectodomain for 4 h. SK-BR-3 cells were cultured in RPMI containing 10% FBS, penicillin/streptomycin and l-glutamine at 37°C in 5% CO2. To determine the effect of n-3 fatty acid and SDC-1 ectodomain on phosphorylation of Erk, SK-BR-3 cells grown in 1% FBS of medium were treated with 30 μM DHA for 48 h or with a range of concentrations of SDC-1 ectodomain for 4 h.
Fat-1 and SDC-1−/− mice
All animal care was conducted in compliance with the state and federal Animal Welfare Acts and standards and policies of the Department of Health and Human Services. The protocol was approved by the Wake Forest University Animal Care and Use Committee. Fat-1 transgenic mice were generously supplied by Dr Jing X.Kang, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts. The genetic groups (Fat-1 and wt littermates) consisted of five animals. All animals were housed in an isolated environment in barrier cages and a fed specific diet for 7 weeks after weaning. Diets were prepared by the custom animal diet laboratory of the Animal Resources Program at Wake Forest University and contained 397 kcal/100 g; 30% of energy was from fat, 50% from carbohydrates and 30% from proteins. The fatty acid content was 30% saturated (16:0 + 18:0), 26% monounsaturated (18:1) and 43% n-6 polyunsaturated (18:2). Detailed diet composition has been described previously (40). At termination, mice were fasted for 4 h and then euthanized. SDC-1−/− mice were obtained from Dr Caroline Alexander, University of Wisconsin–Madison and fed a chow diet for 7 weeks after weaning and prior to termination.
SDC-1 siRNA transfection
The SDC-1 siRNA, #142557 was purchased from Ambion, Austin, TX. For transfection, 50 nM siRNA was added to 2.0 × 105 cells in six-well plates using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and DMEM/F12 medium lacking serum and antibiotics. Control cells were transfected with a negative control siRNA with no known messenger RNA target designed by Ambion (AM4624). At 6 h after transfection, each well was supplemented with 2 ml of complete growth medium. Twenty-four hours after transfection, the medium was replaced with 2 ml of DMEM/F12 medium containing 5% FBS for another 24 h and the cells were harvested to determine the effect of SDC-1 siRNA on phosphorylation of MEK, Erk and Bad and on apoptosis. Alternatively, 24 h after transfection, the medium was replaced with 2 ml of DMEM/F12 medium containing 0.5% FBS and cells were treated with DHA (30 μM) for an additional 48 h before cell harvest.
Western blot analysis
Cells were washed twice with ice-cold phosphate-buffered saline and lysed for 10 min on ice; debris was then removed by centrifugation and equivalent amounts of protein were separated by 10 or 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane. The membranes were blocked with 10 mM Tris–base, 100 mM NaCl, 0.1% Tween-20, pH 7.5 (TBST) containing 5% nonfat dry milk for 2 h at room temperature and then were washed three times with TBST for 5 min and exposed to anti-MEK, Erk, Bad and poly (ADP ribose) polymerase (PARP) (Cell Signaling Technology, Beverly, MA) antibodies in TBST containing 3% BSA at 4°C overnight, followed by three washes with TBST. They were then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, washed with TBST and developed using the Super Signal West Pico Kit (Super Signal West Pico; Pierce, Rockford, IL). The band densities on photographic films were analyzed using Image J software. For phosphorylated MEK, Erk and Bad measurement, band densities were normalized to total MEK, Erk and Bad and presented as the fractional change from control values.
Apoptosis assays
MCF-7 cells were seeded in 96-well plates at a density of 3 × 103 cells per well in DMEM/F12 with 5% FBS and pretreated with 50 μM MEK inhibitor PD98059 (EMD Chemicals, Gibbstown, NJ) for 24 h and then transfected with SDC-1 siRNA for 48 h. Apoptotic activity in adherent cells was measured with the Caspase-Glo 3/7®. Assay in which 30 μl of Caspase-Glo 3/7 reagent was added to each well and incubated for 1 h at room temperature. Luminescence was measured by a Reporter Microplate Luminometer (Turner Biosystems). To measure the levels of cleaved PARP, MCF-7 cells were planted in six-well plates at a density of 1.5 × 105 cells per well in DMEM/F12 with 5% FBS and pretreated with 50 μM MEK inhibitor PD98059 for 24 h and then transfected with SDC-1 siRNA for 48 h. Apoptotic activity was determined by cleaved PARP using western blots.
Data analysis
Data were analyzed by analysis of variance and Student’s t-test. The assays were carried out in triplicate and the averages are shown, together with standard error.
Results
DHA inhibits phosphorylation of MEK, Erk and Bad in MCF-7 and SK-BR-3 cells
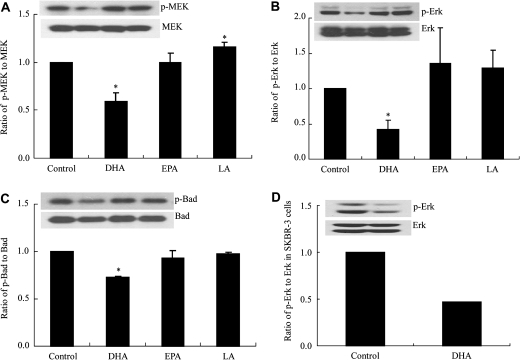
Previously, we had reported that 30 μM DHA–BSA, a concentration close to that obtained physiologically through dietary supplementation (41), can induce apoptosis through SDC-1 in MCF-7 cells. EPA was ineffective at concentrations up to 100 μM (27) and the n-6 PUFA, LA likewise had no apoptotic activity (14). To investigate the mechanism by which DHA and SDC-1 induce apoptosis in these cells, we examined the effect of DHA on the MEK/Erk/Bad signaling pathway. MCF-7 cells were treated with 30 μM DHA–BSA, EPA-BSA and LA-BSA for 48 h. Consistent with the effect of DHA on apoptosis (27), the results showed that DHA, but not EPA and LA, significantly diminished phosphorylation of MEK, Erk and Bad (Figure 1A, B and C). Compared with control, the relative phosphorylated MEK, Erk and Bad levels were, respectively, reduced 25–40%, 49–67% and 26–28% by DHA in three separate experiments. The effect was not specific to MCF-7 cells since SK-BR-3 cells also demonstrated a marked reduction of phosphorylation of p-Erk (Figure 1D), p-MEK and p-Bad (data not shown) when similarly treated with DHA.
Fig. 1.
DHA inhibits phosphorylation of MEK, Erk and Bad in MCF-7 and SK-BR-3 cells. MCF-7 cells were preincubated in media containing 0.5% FBS for 18 h and then media was supplemented with 30 μM DHA, EPA or LA for 48 h. SK-BR-3 cells were preincubated in media containing 1% FBS for 18 h and then media was supplemented with 30 μM DHA for 48 h. Cell protein extracts were used for western blot analysis of phosphorylated (p)-MEK, MEK, p-Erk, Erk, p-Bad (ser 112) and Bad. Bargraphs (A–C) show means ± standard error of triplicate experiments performed in triplicate and represent ratios of p to total MEK, Erk and Bad, respectively, in MCF-7 cells. D represents a ratio of p-Erk to Erk in SK-BR-3 cells. Inserts show a representative blot for each protein. Values with asterisk are significantly different from control (P < 0.05).
SDC-1 diminishes phosphorylation of MEK, Erk and Bad in MCF-7 and SK-BR-3 cells
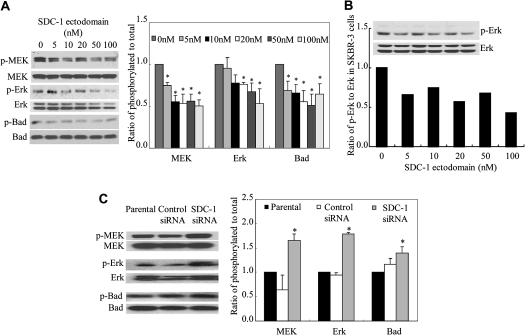
Our previous studies had shown that like DHA, SDC-1 ectodomain can induce apoptosis in MCF-7 cells (27). To determine whether SDC-1 can block phosphorylation of MEK, Erk and Bad, cells were cultured for 4 h in the presence of human recombinant SDC-1 ectodomain, which resulted, as shown in Figure 2A, in a dose-dependent decrease in phosphorylation of MEK, Erk and Bad. Similarly, SDC-1 ectodomain inhibited the phosphorylation of Erk (Figure 2B), MEK and Bad (data not shown) in SK-BR-3 cells, showing that SDC-1 suppression of this pathway is also effective in breast cancer cells that over express Her-2/neu. To further confirm an effecter role for SDC-1 in suppression of this signaling pathway, SDC-1 siRNA was transfected into the MCF-7 cells to silence SDC-1 expression. Consistent with previous data (27), this reagent was shown to effectively inhibit SDC-1 messenger RNA by 80 ± 2.8, 86 ± 9.2, 70 ± 1.3% at 24, 48 and 72 h, respectively, after transfection. As shown in Figure 2C, compared with untransfected cells or cells transfected with a control siRNA (no known target), phosphorylation of MEK, Erk and Bad was markedly enhanced in cells transfected with the SDC-1 siRNA. Thus, SDC-1 silencing resulted in enhancement of progrowth/survival signals in the cells.
Fig. 2.
SDC-1 decreases phosphorylation of MEK, Erk and Bad in MCF-7 cells. (A) MCF-7 cells and (B) SK-BR-3 cells were incubated in media containing indicated concentrations of SDC-1 ectodomain for 4 h. (C) MCF-7 cells were transfected with control siRNA or SDC-1 siRNA for 48 h. Cell protein extracts were used for western blot analysis of phosphorylated (p)-MEK, MEK, p-Erk, Erk, p-Bad (ser 112) and Bad. Data are presented relative to control (=1.0) and are means ± standard error of three independent experiments performed in triplicate. Values with asterisk are significantly different from control (P < 0.05).
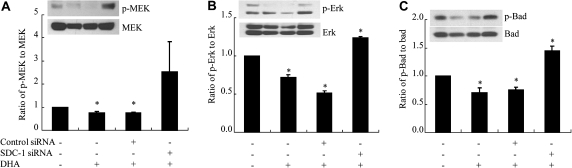
DHA inhibits phosphorylation of MEK, Erk and Bad through SDC-1 in MCF-7 cells
We previously demonstrated that DHA induced apoptosis in MCF-7 cells through upregulation of SDC-1 (27). To test the hypothesis that inhibition of phosphorylation of MEK, Erk and Bad by DHA in these cells is mediated by SDC-1, SDC-1 siRNA was transfected into the cells to silence SDC-1 expression and after 24 h, the cells were incubated in media with or without DHA (30 μM) for 48 h. As shown in Figure 3, the reduced levels of phosphorylated MEK (Figure 3A), Erk (Figure 3B) and Bad (Figure 3C) achieved by DHA treatment of cells transfected with control siRNA were lost in cells transfected with SDC-1 siRNA, thus indicating that the inhibitory effect of DHA on phosphorylation of MEK, Erk and Bad is controlled by SDC-1. This result is in agreement with and furthers our understanding of the mechanism by which DHA induces apoptosis of MCF-7 cells (27).
Fig. 3.
DHA inhibits phosphorylation of MEK, Erk and Bad through SDC-1 in MCF-7 cells. MCF-7 cells were transfected with control siRNA or SDC-1 siRNA for 24 h and then treated with DHA (30 μM) for 48 h. Cell protein extracts were used for western blot analysis of phosphorylated (p)-MEK, MEK, p-Erk, Erk, p-Bad (ser 112) and Bad. Data are presented relative to control (=1.0) and are means ± standard error of three independent experiments performed in triplicate. Values with asterisk are significantly different from control (P < 0.05).
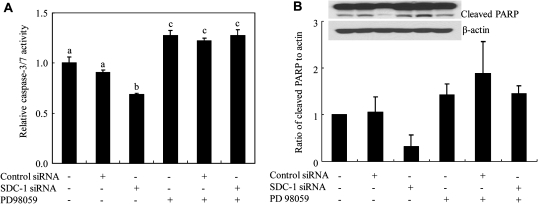
MEK inhibitor blocks the inhibitory effect of SDC-1 siRNA on apoptosis of MCF-7 cells
To further confirm that SDC-1 induces apoptosis by diminishing phosphorylation of MEK and Erk, MCF-7 cells were pretreated with MEK inhibitor PD98059 for 24 h and then a control or SDC-1 siRNA was transfected into the cells. The results showed that SDC-1 silencing resulted in a decrease in apoptosis as measured by caspase activity. This effect was blocked when MEK was inhibited by PD98059. As expected, MEK inhibition resulted in increased caspase activity (Figure 4A). A similar effect was observed using a second indicator of apoptosis: measurement of PARP cleavage product (Figure 4B). Compared with control, the cleaved PARP level in cells treated with PD98059 was increased 42%, and in the cells transfected with SDC-1 siRNA, the MEK inhibitor enhanced the cleaved PARP by 3.7-fold. Thus, the inhibitory effect of silencing SDC-1 on PARP cleavage was lost when the cells were treated with the MEK inhibitor. These observations further support the hypothesis that SDC-1 promotes apoptosis of human breast cancer cells through inhibition of MEK and Erk phosphorylation.
Fig. 4.
MEK inhibitor diminishes the inhibitory effect of SDC-1 siRNA on apoptosis of MCF-7 cells. Cells were pretreated with PD98059 (50 μM) for 24 h and then transfected with SDC-1 siRNA for 48 h. (A) Caspases-3/7 activity was determined with Caspase-Glo® 3/7 assay following the manufacture’s protocol. Data are means ± standard errors. of three experiments performed in triplicate. Values with different letters are significantly different (P < 0.05). (B) Apoptotic activity was determined by cleaved PARP using western blots. Data are presented relative to control (=1.0) and are means ± standard errors of two independent experiments.
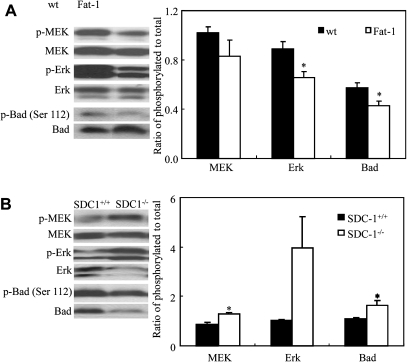
SDC-1 and n-3 PUFA decrease phosphorylation of MEK, Erk and Bad in vivo
We have demonstrated elevated level of SDC-1 in the n-3 PUFA-enriched mammary glands of Fat-1 mice compared with their wt littermates (37). To further confirm that SDC-1 and n-3 PUFA induce apoptosis through inhibition of MEK, Erk and Bad phosphorylation, the mammary glands of female Fat-1 and wt mice were examined for levels of these three phosphorylated signaling molecules. The ratios of phosphorylated to unphosphorylated Erk and Bad were lower in Fat-1 than in wt mice but those of MEK did not reach statistical significance in this small group of animals (Figure 5A). We also examined levels of phosphorylated MEK, Erk and Bad in mammary glands of female SDC-1−/− mice. Higher levels of these phosphorylated signaling proteins were measured in the SDC-1−/− compared with SDC-1+/+ littermates (Figure 5B). This supports the idea that SDC-1 may act as an inhibitor of the MEK/Erk/Bad signaling pathway and thus reduce pro-proliferative signals in favor of proapoptotic signals in the cells.
Fig. 5.
Levels of phosphorylated MEK, Erk and Bad in mammary glands of Fat-1 and SDC-1−/− mice. Levels of phosphorylated (p) to total MEK, Erk and Bad in mammary glands of (A) female Fat-1 and wt mice; (B) female SDC-1−/− mice and SDC+/+mice measured by western analysis of tissue extracts. A representative blot for each protein is shown. Data are means ± standard errors of five Fat-1 or three SDC-1−/− mice. Values with asterisk are significantly different from control (P < 0.05).
Discussion
Although our previous studies have shown that DHA induces apoptosis of human breast cancer cells through SDC-1 (27), the intracellular signals impacted by SDC-1 to trigger apoptosis remained unknown. The present studies have identified SDC-1 as an important effecter of the MEK/Erk/Bad cascade in human breast cancer cells. The most striking findings are that SDC-1 is a key controller in the ability of DHA to inhibit phosphorylation of MEK, Erk and Bad in these cells and further, that there is evidence for this regulation in breast tissue in vivo. This represents a novel mechanism for the chemopreventive properties of DHA in breast cancer cells.
Consistent with previous studies showing that DHA but not EPA is effective in apoptosis induction in human breast cancer cells (27), we found that DHA, but not EPA and LA, inhibits phosphorylation of MEK, Erk and Bad proteins in these cells. This finding is supported by other reports although their data were from human lymphocytes (30) and mesangial cells (32). We have shown previously that in both breast (27) and prostate (42) cancer cells, the sdc1 gene is a target for PPARγ and that DHA upregulates SDC-1 through activation of PPARγ. This transcriptional activity was not shared by either EPA or LA although both are known to bind PPARγ (43). The differential results between binding and activation may be indirect and resulting from metabolism of the different PUFA to more active compounds (43). It is also of interest to note that PPARγ is phosphorylated by Erk-1/2 and this results in reducing its ligand binding and gene-regulating activity (44). Thus, a reduction in Erk activity by DHA may have a positive effect on PPARγ activity.
If DHA inhibits phosphorylation of MEK, Erk and Bad in MCF-7 cells through SDC-1, SDC-1 should have a similar function and our data confirm this property. We have shown previously that an SDC-1 ectodomain can induce apoptosis in MCF-7 cells (27). In the present studies, SDC-1 ectodomain, like DHA, blocked phosphorylation of MEK, Erk and Bad in a dose-dependent manner, thus converting a mitogenic cascade to one that promotes apoptosis. Minican, a truncated SDC-1, was shown previously to inhibit growth of murine mammary cancer cells through inhibition of Erk phosphorylation, however, measures of apoptosis were not reported (33). To confirm that SDC-1 is a key mediator in DHA-inhibited phosphorylation of these signaling molecules in MCF-7 cells, an SDC-1 siRNA was transfected into the cells to silence SDC-1 expression. In the absence of DHA, compared with untransfected or cells transfected with a control siRNA, the SDC-1 siRNA greatly enhanced phosphorylation of MEK, Erk and Bad. When SDC-1 was silenced by its siRNA and the transfected cells were then grown in the presence of DHA the inhibitory effect of DHA on phosphorylation of MEK, Erk and Bad was blocked thus confirming a key role for SDC-1 in the ability of DHA to regulate this signaling pathway. Furthermore, the MEK inhibitor PD98059 was shown to block the inhibitory effect of SDC-1 siRNA on apoptosis, thus indicating the involvement of MEK activity in SDC-1-induced apoptosis. The increase in apoptosis resulting from MEK inhibition is consistent with in vivo data showing a marked reduction in colon cancer in mice treated with a MEK inhibitor (45). Bad phosphorylation on serine 112 is MEK dependent and inhibits Bad association with the Bcl-xL survival protein; thus inhibition of MEK promotes Bad-Bcl-xL interaction and leads to apoptosis (46).
Several studies have implicated elevated activity of the MAPK pathway in human breast cancer (47–49). Sivaraman et al. (47) first reported increased expression and activity of MAPK in breast carcinoma versus benign tissue as well as in metastatic cells within positive lymph nodes. In a subsequent study, MAPK activity in primary breast tumors was demonstrated in only a subset of tumors and trended to be negatively associated with relapse-free survival (49). This kinase cascade presents an obvious target for the development of new anticancer drugs. To date, the focus has been on small molecule inhibitors of the various kinases (50). To our knowledge, there is no previous evidence to show that n-3 PUFA inhibits phosphorylation of MEK and Erk in mammary tissue or the involvement of SDC-1 in this process. Our in vivo studies have shown (i) an elevation of SDC-1 in the n-3 PUFA-enriched mammary glands of Fat-1 mice (37) that we now show is accompanied by reduced phosphorylation of MEK, Erk and Bad and (ii) that levels of phosphorylated MEK, Erk and Bad are higher in mammary glands of SDC-1−/− mice compared with SDC-1+/+ mice. These interesting findings, together with the in vitro data, imply that the inhibitory effect of DHA on phosphorylation of MEK, Erk and Bad is mediated by SDC-1.
Several details of the mechanistic link between DHA, SDC-1 and MEK/Erk inhibition are still unclear. Our studies have shown that DHA upregulates SDC-1 in breast cancer cells through activation of PPARγ and results in apoptosis that can be blocked by SDC-1 siRNA (27). Apoptosis can also be induced in these cells by exogenous SDC-1 ectodomain. These observations suggest that shedding of the ectodomain may be required for its proapoptotic activity. The SDC-1 ectodomain is known to undergo constitutive and inducible shedding by proteolytic cleavage of its core protein at a specific membrane-proximal site, G245-L246 in human (51) and A243-S244 in mouse (52). Although the identity of the enzyme active in breast cancer cells is not known, matrix metalloproteinase (MMP)-7 (matrilysin), MMP-9 (gelatinase B) and MMP-14 (MMT-1-MMP) have been identified as SDC-1 sheddases (51,53–55). In addition, heparanase indirectly promotes SDC-1 shedding through enhanced expression and activity of MMP-9 (56). Shedding results in a pool of soluble SDC-1 that competes with the same ligands as the membrane-bound proteoglycan and may result in different physiological effects on the cell (57). It is unknown whether shed SDC-1 ectodomain interacts with the cell by binding to a specific cell surface receptor or whether it enters the cell and whether any of these processes are impacted by DHA.
In summary, our data highlight a novel pathway, SDC-1/MEK/Erk/Bad, through which DHA promotes apoptosis of human breast cancer cells. Furthermore, this report is the first to demonstrate that SDC-1 inhibits phosphorylation of MEK/Erk in vivo. We have no evidence to date that SDC-1 is directly interacting with MEK/Erk/Bad or that SDC-1 is directly inhibiting their phosphorylation. Additional studies are clearly needed to identify both upstream and downstream mediators to further define how SDC-1 inhibits this important signaling pathway in human breast cancer cells and how this may be impacted by increasing dietary intake of DHA.
Funding
The American Institute for Cancer Research (05A071) to I.J.E. and the National Institutes of Health (R01CA115958 to IJE); (P01CA106742) to Y.Q.C. and I.J.E.
Acknowledgments
We gratefully acknowledge the provision of Fat-1 mice by Dr Jing X.Kang, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts; and the provision of SDC-1−/− mice by Dr Caroline M. Alexander, Department of Oncology, University of Wisconsin, Madison, WI. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BSA
bovine serum albumin
- cDNA
complementary DNA
- DHA
docosahexaenoic acid
- DMEM
Dulbecco’s modified Eagle medium
- EPA
eicosapentaenoic acid
- Erk
extracellular signal-regulated kinase
- FA, fatty acid; FBS
fetal bovine serum
- LA
linoleic acid
- MAPK
mitogen-activated protein kinase
- PARP, poly (ADP ribose) polymerase; PUFA
polyunsaturated fatty acid
- SDC-1
syndecan-1
References
- 1.Sasaki S, et al. An ecological study of the relationship between dietary fat intake and breast cancer mortality. Prev. Med. 1993;22:187–202. doi: 10.1006/pmed.1993.1016. [DOI] [PubMed] [Google Scholar]
- 2.Kaizer L, et al. Fish consumption and breast cancer risk: an ecological study. Nutr. Cancer. 1989;12:61–68. doi: 10.1080/01635588909514002. [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, et al. Types of dietary fat and the incidence of cancer at five sites. Prev. Med. 1990;19:242–253. doi: 10.1016/0091-7435(90)90025-f. [DOI] [PubMed] [Google Scholar]
- 4.Rose DP, et al. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J. Natl Cancer Inst. 1993;85:1743–1747. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 5.Rose DP, et al. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl Cancer Inst. 1995;87:587–592. doi: 10.1093/jnci/87.8.587. [DOI] [PubMed] [Google Scholar]
- 6.Kang KS, et al. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurkowski JJ, et al. Dietary effects of menhaden oil on the growth and membrane lipid composition of rat mammary tumors. J. Natl Cancer Inst. 1985;74:1145–1150. [PubMed] [Google Scholar]
- 8.Braden LM, et al. Dietary polyunsaturated fat in relation to mammary carcinogenesis in rats. Lipids. 1986;21:285–288. doi: 10.1007/BF02536414. [DOI] [PubMed] [Google Scholar]
- 9.Hardman WE, et al. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin. Cancer Res. 2001;7:2041–2049. [PubMed] [Google Scholar]
- 10.Shao Y, et al. Dietary menhaden oil enhances mitomycin C antitumor activity toward human mammary carcinoma MX-1. Lipids. 1995;30:1035–1045. doi: 10.1007/BF02536289. [DOI] [PubMed] [Google Scholar]
- 11.Burns CP, et al. Adriamycin transport and sensitivity in fatty acid-modified leukemia cells. Biochim. Biophys. Acta. 1986;888:10–17. doi: 10.1016/0167-4889(86)90064-9. [DOI] [PubMed] [Google Scholar]
- 12.Germain E, et al. Enhancement of doxorubicin cytotoxicity by polyunsaturated fatty acids in the human breast tumor cell line MDA-MB-231: relationship to lipid peroxidation. Int. J. Cancer. 1998;75:578–583. doi: 10.1002/(sici)1097-0215(19980209)75:4<578::aid-ijc14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Bougnoux P, et al. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br. J. Cancer. 2009;101:1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards IJ, et al. Differential effects of delivery of omega-3 fatty acids to human cancer cells by low-density lipoproteins versus albumin. Clin. Cancer Res. 2004;10:8275–8283. doi: 10.1158/1078-0432.CCR-04-1357. [DOI] [PubMed] [Google Scholar]
- 15.Inki P, et al. Immunohistochemical localization of syndecan-1 in normal and pathological human uterine cervix. J. Pathol. 1994;172:349–355. doi: 10.1002/path.1711720410. [DOI] [PubMed] [Google Scholar]
- 16.Mukunyadzi P, et al. The level of syndecan-1 expression is a distinguishing feature in behavior between keratoacanthoma and invasive cutaneous squamous cell carcinoma. Mod. Pathol. 2002;15:45–49. doi: 10.1038/modpathol.3880488. [DOI] [PubMed] [Google Scholar]
- 17.Kumar-Singh S, et al. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. J. Pathol. 1998;186:300–305. doi: 10.1002/(SICI)1096-9896(1998110)186:3<300::AID-PATH180>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Mikami S, et al. Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Jpn. J. Cancer Res. 2001;92:1062–1073. doi: 10.1111/j.1349-7006.2001.tb01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anttonen A, et al. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br. J. Cancer. 1999;79:558–564. doi: 10.1038/sj.bjc.6690088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulkkinen JO, et al. Syndecan-1: a new prognostic marker in laryngeal cancer. Acta Otolaryngol. 1997;117:312–315. doi: 10.3109/00016489709117794. [DOI] [PubMed] [Google Scholar]
- 21.Numa F, et al. Syndecan-1 expression in cancer of the uterine cervix: association with lymph node metastasis. Int. J. Oncol. 2002;20:39–43. [PubMed] [Google Scholar]
- 22.Matsumoto A, et al. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int. J. Cancer. 1997;74:482–491. doi: 10.1002/(sici)1097-0215(19971021)74:5<482::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Nackaerts K, et al. Heparan sulfate proteoglycan expression in human lung-cancer cells. Int. J. Cancer. 1997;74:335–345. doi: 10.1002/(sici)1097-0215(19970620)74:3<335::aid-ijc18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Mali M, et al. Suppression of tumor cell growth by syndecan-1 ectodomain. J. Biol. Chem. 1994;269:27795–27798. [PubMed] [Google Scholar]
- 25.Dhodapkar MV, et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91:2679–2688. [PubMed] [Google Scholar]
- 26.Sun H, et al. Omega-3 polyunsaturated fatty acids regulate syndecan-1 expression in human breast cancer cells. Cancer Res. 2005;65:4442–4447. doi: 10.1158/0008-5472.CAN-04-4200. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, et al. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santen RJ, et al. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 29.McCubrey JA, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorjao R, et al. Regulation of interleukin-2 signaling by fatty acids in human lymphocytes. J. Lipid Res. 2007;48:2009–2019. doi: 10.1194/jlr.M700175-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Seti H, et al. Effects of omega-3 and omega-6 fatty acids on IGF-I receptor signalling in colorectal cancer cells. Arch. Physiol. Biochem. 2009;115:127–136. doi: 10.1080/13813450902905899. [DOI] [PubMed] [Google Scholar]
- 32.Yusufi AN, et al. Differential effects of low-dose docosahexaenoic acid and eicosapentaenoic acid on the regulation of mitogenic signaling pathways in mesangial cells. J. Lab. Clin. Med. 2003;141:318–329. doi: 10.1016/S0022-2143(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 33.Viklund L, et al. Expression and characterization of minican, a recombinant syndecan-1 with extensively truncated core protein. Biochem. Biophys. Res. Commun. 2002;290:146–152. doi: 10.1006/bbrc.2001.6187. [DOI] [PubMed] [Google Scholar]
- 34.Kang JX, et al. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 35.Ma DW, et al. N-3 polyunsaturated fatty acids endogenously synthesized in fat-1 mice are enriched in the mammary gland. Lipids. 2006;41:35–39. doi: 10.1007/s11745-006-5067-9. [DOI] [PubMed] [Google Scholar]
- 36.Orr SK, et al. The fat-1 mouse has brain docosahexaenoic acid levels achievable through fish oil feeding. Neurochem. Res. 2010;35:811–819. doi: 10.1007/s11064-010-0139-x. [DOI] [PubMed] [Google Scholar]
- 37.Sun H, et al. Endogenous synthesis of n-3 polyunsaturated fatty acids in fat-1 mice is associated with increased mammary gland and liver syndecan-1. PLoS One. 2011;6:e20502. doi: 10.1371/journal.pone.0020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander CM, et al. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 39.McQuillan DJ, et al. Recombinant expression of proteoglycans in mammalian cells. Utility and advantages of the vaccinia virus/T7 bacteriophage hybrid expression system. Methods Mol. Biol. 2001;171:201–219. doi: 10.1385/1-59259-209-0:201. [DOI] [PubMed] [Google Scholar]
- 40.Berquin IM, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conquer JA, et al. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J. Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 42.Edwards IJ, et al. In vivo and in vitro regulation of syndecan 1 in prostate cells by N-3 polyunsaturated fatty acids. J. Biol. Chem. 2008;283:18441–18449. doi: 10.1074/jbc.M802107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forman BM, et al. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl Acad. Sci. USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao D, et al. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 45.Sebolt-Leopold JS, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 46.Scheid MP, et al. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 47.Sivaraman VS, et al. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J. Clin. Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salh B, et al. Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res. 1999;19:731–740. [PubMed] [Google Scholar]
- 49.Mueller H, et al. Potential prognostic value of mitogen-activated protein kinase activity for disease-free survival of primary breast cancer patients. Int. J. Cancer. 2000;89:384–388. doi: 10.1002/1097-0215(20000720)89:4<384::aid-ijc11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 50.Sebolt-Leopold JS. Development of anticancer drugs targeting the MAP kinase pathway. Oncogene. 2000;19:6594–6599. doi: 10.1038/sj.onc.1204083. [DOI] [PubMed] [Google Scholar]
- 51.Endo K, et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, et al. Constitutive and accelerated shedding of murine syndecan-1 is mediated by cleavage of its core protein at a specific juxtamembrane site. Biochemistry. 2005;44:12355–12361. doi: 10.1021/bi050620i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, et al. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 54.Brule S, et al. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16:488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- 55.Ding K, et al. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J. Cell Biol. 2005;171:729–738. doi: 10.1083/jcb.200508010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purushothaman A, et al. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J. Biol. Chem. 2008;283:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikolova V, et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis. 2009;30:397–407. doi: 10.1093/carcin/bgp001. [DOI] [PubMed] [Google Scholar]