Abstract
In the central nervous system, cyclin-dependent kinase 5 (Cdk5), an unusual member of the Cdk family, is implicated in the regulation of various physiological processes ranging from neuronal survival, migration and differentiation, to synaptogenesis, synaptic plasticity and neurotransmission. Dysregulation of this kinase has been demonstrated to play a critical role in the pathogenic process of neurodegenerative disorders. DNA damage is emerging as an important pathological component in various neurodegenerative conditions. In this review, we discuss the recent progress regarding the regulation and roles of Cdk5 under physiological conditions, and its dysregultion under pathological conditions, especially in neuronal death mediated by DNA damage.
Keywords: Cdk5, Central nervous system, Cell death, DNA damage, Neurodegenerative disease
1. Introduction
Cyclin-dependent kinase 5 (Cdk5), a proline-directed serine/threonine kinase predominantly expressed in the central nervous system (CNS), is a member of the cyclin-dependent kinase (Cdk) family. The amino acid sequence of Cdk5 has about 60% homology to that of human Cdk2 and cell division cycle kinase 2 (Cdc2) (Hellmich et al., 1992; Lew et al., 1992; Meyerson et al., 1992). Cdk5 is largely inactive in the cell cycle and controlled by neuronally enriched noncyclin activators, p35 and p39, or their respective truncated forms, p25 and p29 (Dhavan and Tsai, 2001).
Cdk5 plays a key role in the normal development of the CNS, regulating neuronal migration, axon growth, neurotransmission, synaptic plasticity, cognitive functions, and the maintenance of neuronal survival (Dhavan and Tsai, 2001; Gong et al., 2003; Ikiz and Przedborski, 2008; Qu et al., 2007; Tang et al., 2005). However, dysregulation of Cdk5 is neurotoxic and may contribute to the pathogenesis of neurodegenerative disorders including Alzheimer disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS) and focal cerebral ischemia (Gong et al., 2003; Shelton and Johnson, 2004; Tang et al., 2005). Interestingly, recent studies have implicated Cdk5 in neuronal DNA damage response. Neuronal DNA damage caused by various neurotoxins or DNA repair gene mutations is related to the aging of the human brain (Huang et al., 2010; Mattson and Magnus, 2006; Rass et al., 2007; Segura Aguilar and Kostrzewa, 2004). While DNA damage typically triggers cell-cycle checkpoints in proliferating cells, it reactivates cell-cycle machinery in post-mitotic neurons and induces their death (Tian et al., 2009). Cdk5 regulates neuronal apoptosis and DNA repair pathway through phosphorylation of a number of novel substrates such as the phosphatidylinositol-3-kinase-like kinase ATM (ataxia telangiectasia mutated), antioxidant enzyme peroxiredoxin-2 (Prx2), p53 and base excision repair enzyme apurinic/apyrimidinic endonuclease I (Ape1) (Huang et al., 2010; Kim et al., 2008; Lee et al., 2007; Tian et al., 2009). The nuclear level of Cdk5 has been proposed to be associated with maintenance of the post-mitotic status in neurons (Zhang et al., 2010). Thus, DNA damage response mediated by Cdk5 is emerging as a critical pathological component during neuronal stress and degenerative response.
2. Regulation of Cdk5
Unlike cell cycle-related Cdks, Cdk5 is activated by binding to its neuronally enriched activators, p35 and p39, and therefore, displays its function predominantly in post-mitotic neurons (Dhavan and Tsai, 2001). p35 appears to play a major role in the cerebral cortex while p39 is more important in cerebellum (Ko et al., 2001). Cdk5 activity is tightly regulated in vivo. While defect of Cdk5 is destructive to the CNS, its hyperactivation is also toxic to neurons (Gong et al., 2003). In general, Cdk5 activity is regulated by transcriptional control of p35 or Cdk5 genes, degradation of p35, phosphorylation of Cdk5 or p35, the interaction between Cdk5 and p35, the association of this complex with membranes, and the cleavage of p35 to p25 (Ishiguro, 2008). p25 is a C-terminal proteolytic fragment of p35 generated by calpain. Since p25 is more stable than p35, it keeps Cdk5 hyperactivated. In addition, binding to p25 may change Cdk5 subcellular localization from membrane to cytoplasm and nucleus. Cdk5 phosphorylates various substrates at the serine/threonine site in the context of (S/T) PX (K/H/R) (Dhavan and Tsai, 2001). The number of Cdk5 substrates in vitro is ever growing although not all of them have been confirmed in vivo. Cdk5 directly phosphorylates many proteins involved in modulating cell morphology and motility, in neural development, and in neuronal survival or death (Dhariwala and Rajadhyaksha, 2008; Plattner et al., 2008).
The amount of p35 or p39 protein in neurons is a key determinant of Cdk5 kinase activity. During neuronal differentiation, neuronal growth factors or extracellular matrix proteins contribute to the stimulation of the synthesis of p35, which coincides with the increase in Cdk5 activity. Brain-derived neurotrophic factor, glial-derived neurotrophic factor, nerve growth factor (NGF), retinoic acid, laminin and neuregulin have all been reported to up-regulate Cdk5 activity. On the other hand, degradation of p35 or p39 by proteasome results in downregulation of Cdk5 activity. During this process, Cdk5 phosphorylates p35 and induces its ubiquitination and subsequent degradation (Kamei et al., 2007). In addition, phosphorylation of p35 regulates the membrane association of Cdk5/p35 complex. The kinase activity of Cdk5/p35 complex can be suppressed by binding to membranes (Sato et al., 2007; Zhu et al., 2005).
Three phosphorylation sites at Thr14, Tyr15 and Ser159, have been identified in Cdk5. Phosphorylation on Tyr15 of Cdk5 elevates its kinase activity while phosphorylation at Thr14 shows an inhibitory effect. Phosphorylation of Ser159 is not necessary for Cdk5 activation. However, when this site is phosphorylated by casein kinase 1, the catalytic activity of Cdk5/p25 complex is increased (Dhavan and Tsai, 2001).
The literature on Cdk5 and cyclins remains controversial. Several studies have reported that Cdk5 may interact with normal cyclins, including cyclins D, E and even DNA polymerase component, PCNA (Guidato et al., 1998; Mao, 2008; Matsunaga, 2000; Xiong et al., 1992). Moreover, Cdk7/cyclin H are shown to phosphorylate Cdk5 at Ser159 (Rosales et al., 2003). Although far from universal acceptance, these findings hint at the possibility that cyclins may be involved in the regulation of Cdk5. Consistent with this, Cdk5 mRNA and protein expression are also detected in non-neuronal tissues and cells where p35 or p39 levels are believed to be low. These include kidney, testes, ovary, myocytes, pancreatic β-cells, glial cells, monocytic and neutrophilic leucocytes (Dhavan and Tsai, 2001; Rosales and Lee, 2006; Smith et al., 2001). Non-neuronal cell functions modulated by Cdk5 include differentiation/development, exocytosis, gene expression/transcription, adhension/migration, tissue regeneration/wound healing, senescence, hormone regulation and Endothelial cell migration and angiogenesis (Dhavan and Tsai, 2001; Liebl et al., 2010; Rosales and Lee, 2006).
Neurotoxins can cause the dysregulation of Cdk5 through generating p25 from p35 or stabilizing Cdk5/p35 complex. Selective inhibition of p25 has been explored as a strategy to specifically disrupt the hyperactivation of Cdk5 (Zheng et al., 2010; Zheng et al., 2005). Chemical inhibitors of Cdks such as roscovitine, olomoucine, and butyrolactone I are used to inhibit Cdk5/p35 and Cdk5/p25 complexes, allowing one to test the involvement of Cdk5 in particular neuronal activities. Other protein factors are also able to regulate Cdk5 kinase activity. For example, both casein kinase 2 and C42 protein inhibit Cdk5 activity through preventing the p35-associated activation of Cdk5 (Ching et al., 2002; Lim et al., 2004).
3. Physiological functions of Cdk5 in the CNS
Cdk5 activity is essential for a variety of neuronal functions in both developing and mature neurons (Dhavan and Tsai, 2001; Smith et al., 2001). To perform these diverse functions, Cdk5 displays extensive crosstalks with various regulatory pathways including mitogen-activated protein kinase-receptor tyrosine kinases (Zheng et al., 2007), c-Jun N-terminal kinase 3 (Li et al., 2002), phosphoinositol-3-kinase/protein kinase B (Liu et al., 2008) and glycogen synthase kinase 3β signaling pathways (Kanungo et al., 2009; Morfini et al., 2004).
Cdk5-deficienct (Cdk5−/−) mice provide an essential tool to understand Cdk5 functions in the development of the CNS. The prenatal mortality of Cdk5−/− mice is more than 60%, and the newborns die within 12 h of birth (Ohshima et al., 1996). This early lethality is suggested to be caused by degenerative changes in large neurons of the brain stem, such as motor neurons in the lower cranial nerve nuclei and spinal cord (Pareek and Kulkarni, 2008). Perturbations of neuronal migration are considered to be the main neuronal pathology in Cdk5−/− mice, which exhibit morphological abnormalities in the structure of cerebral cortex with neurogenic gradient inverted from the normal inside-out development. The role of Cdk5 in neuronal migration is well supported by its substrates such as nestin, focal adhesion kinase, and doublecortin as well as its regulation of actin, microtubule, and intermediate-filament cytoskeletal components (Lambert de Rouvroit and Goffinet, 2001; Xie and Tsai, 2004). Cdk5−/− mice also display abnormalities at the pre- and post-synaptic neuromuscular junction, abundant intramuscular nerve projection and anomalous branching patterns (Fu et al., 2005; Gilmore et al., 1998). Due to the redundancy of p39, p35−/− mice show a milder phenotype than Cdk5−/− mice, but p35 and p39 double knockout mice display an identical phenotype as that of Cdk5−/− mice (Chae et al., 1997; Ohshima et al., 2001).
Cdk5 activity is associated with axonal and neurite growth. Cdk5, p35, and p39 are all present in the growth cones of extending neurites (Fu et al., 2002). Cdk5−/− mice display defects in elongation of axons (Paglini and Caceres, 2001; Smith and Tsai, 2002). In cortical neurons, inhibition of Cdk5 activity attenuates NGF-mediated dendrite outgrowth by inhibiting transcription factor Egr-1, while phosphorylation of transcription factor signal transducer and activator of transcription 3 (STAT3) leads to NGF-induced neurite outgrowth (Nikolic et al., 1996). Aside from NGF, other extracellular cues such as neurotrophins, semaphorins and ephrins have been shown to control extension and arborization of dendrites. Cdk5 is known to mediate some of these signals via phosphorylation of the receptor tyrosine kinases Trk receptor B, the Wiskott–Aldrich syndrome protein-family verprolin homologous protein 1, or collapsing response mediator protein 2 (Cheung and Ip, 2007; Kim et al., 2006; Uchida et al., 2005).
Cdk5 phosphorylates many downstream substrates such as Rho and Ras family small GTPases (e.g., Pak1, Rac1, RhoA, Cdc42) or microtubule-binding proteins (e.g., Tau, MAP2, MAP1b), and modulates actin dynamics (Paglini et al., 1998) to regulate neurite growth and/or spine morphogenesis (Fu et al., 2001; Rimer, 2003).
In the mature CNS, through phosphorylation of a number of substrates associated with neurotransmitter release and synapse plasticity, Cdk5 regulates multiple steps critical for neurotransmitter synthesis, synaptic vesicle exocytosis, vesicles fusion with the presynaptic membrane, and endocytosis (Benavides and Bibb, 2004; Bibb, 2003; Lagace et al., 2008; Zheng et al., 1998). For example, Cdk5 phosphorylates tyrosine hydroxylase and may be involved in the synthesis of catecholamine neurotransmitters, such as norepinephrine, dopamine, and epinephrine. Cdk5 phosphorylates exocytosis associated proteins such as Muc18, Sept5, Synapsin 1, and Pctaire 1 as well as endocytosis associated proteins such as dynamin I, amphiphysin 1 and synaptojanin 1 at synaptic terminals (Chung, 2008; Tomizawa et al., 2003). Through controlling these downstream effectors, Cdk5 may act as either a positive or a negative regulator of exocytosis/endocytosis depending on certain physiological conditions (Nguyen and Bibb, 2003; Tan et al., 2003).
Cdk5 phosphorylates its synaptic substrates to regulate structural and functional plasticity. For example, phosphorylation of N-methyl-D-aspartate (NMDA) receptor subunit NR1 and NR2 by Cdk5 controls channel properties and localization. Cdk5 also affects calpain-mediated NR2B degradation and thus influences NMDA receptor conductance (Lai and Ip, 2009). The functions of Cdk5 and its activators in learning, memory and pain response have begun to be illustrated. Cdk5 activity is observed to be elevated during associative learning and fear conditioning (Fischer et al., 2002). In p35−/− mice, spatial learning and long-term depression induction are both impaired (Ohshima et al., 2005). On the other hand, long-term potentiation induction is enhanced in high-level p25-expressing mice, accompanied by an increase in spine density (Fischer et al., 2005). p35−/− mice also display abnormalities in pain signaling. Cdk5−/− pups show no response to noxious cutaneous pinching (Fu et al., 2005). Similarly, mice lacking Cdk5 expression predominantly in C-fiber neurons have evident hypoalgesia in response to thermal activation (Pareek et al., 2007).
4. Cdk5 and neurodegenerative disease
Neuronal death is an important contributor to the pathology of neurodegenerative diseases. Though Cdk5 has been shown to promote cell survival by direct activation of the anti-apoptotic proteins Bcl-2 and STAT3 or negative regulation of c-Jun N-terminal kinase 3 activity (Cheung et al., 2008; Courapied et al., 2010; Li et al., 2002; Zheng et al., 2007), dysregulation of Cdk5 activity is toxic to neurons and contributes to neuronal death in various neurodegenerative models/diseases such as AD, ALS, PD, and Huntington’s disease. This can happen via aberrant elevation of p25 levels. Under neurotoxic stress and neuronal injury conditions, p35 is cleaved by calpain to generate p25 in response to increased intracellular calcium. The elevated level of p25, when in complex with Cdk5, leads to subcellular misallocation as well as the hyperactivation of Cdk5 (Ko et al., 2001). Mislocalized activation of Cdk5 may result in altered substrate specificity. In support of the above notion, aberrant activation of Cdk5 in the nucleus leads to direct phosphorylation of a known neuronal survival factor transcriptional factor myocyte-specific enhancer factor 2D (MEF2D) (Gong et al., 2003; Tang et al., 2005). Phosphorylation by Cdk5 at Ser444 converts MEF2D into a good substrate for caspases, leading to a caspase-dependent degradation and subsequently neuronal death. This mechanism is relevant in vivo since neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces phosphorylation of MEF2D by Cdk5 in dopaminergic neurons in the substantial nigra region, contributing to the neuronal death in a mouse model of PD (Smith et al., 2006). In addition, Cdk5 can phosphorylate Parkin and Prx2, impair mitochondrial functions, and mediate neural cell death in models of PD (Avraham et al., 2007; Sun et al., 2008). Consistent with these reports, both increased levels of p25 and Cdk5 are observed in the brain of PD patients (Alvira et al., 2008).
Cdk5 has been hypothesized to contribute to tau pathology, a hallmark of AD (Flaherty et al., 2000; Sengupta et al., 1997). Hyperphosphorylation of tau by Cdk5 disrupts its ability to bind and stabilize microtubule, leading to the disruption of axonal transport and neuronal death. Familial mutations in tau contributes to neurodegeneration in frontotemporal dementia with parkinsonism associated with chromosome 17 (FTDP-17) (Sakaue et al., 2005). Hyperphosphorylation of FTDP-mutant tau is evident in p25 transgenic animal brains. Tau and AD-like pathologies disappear following mutation of all SP/TP sites in transgenic Drosophila overexpressing tau (Steinhilb et al., 2007). Silencing of Cdk5 by RNA interference (RNAi) can also attenuate the phosphorylation of tau in vitro and in vivo (Piedrahita et al., 2010). Together with GSK3β, Cdk5 can generate disease-associated phospho-epitopes on Tau and regulate Aβ production (Cruz et al., 2006; Imahori and Uchida, 1997; Phiel et al., 2003).
Emerging evidence suggests that several strategies aimed at reducing Cdk5 activity protect neurons from toxicity in models of neuronal stresses. For example, both calpain inhibitor calpastatin, which blocks cleavage of p35 to p25, and dominant-negative Cdk5 protect neurons against MPTP toxicity (Smith et al., 2003; Smith et al., 2006). The Cdk5/p25-specific inhibitory peptide, or its derivative p5, has been tested for the treatment of ischemic stroke in models of the disease (Slevin and Krupinski, 2009; Zheng et al., 2010; Zheng et al., 2005).
5. Cdk5 and DNA damage
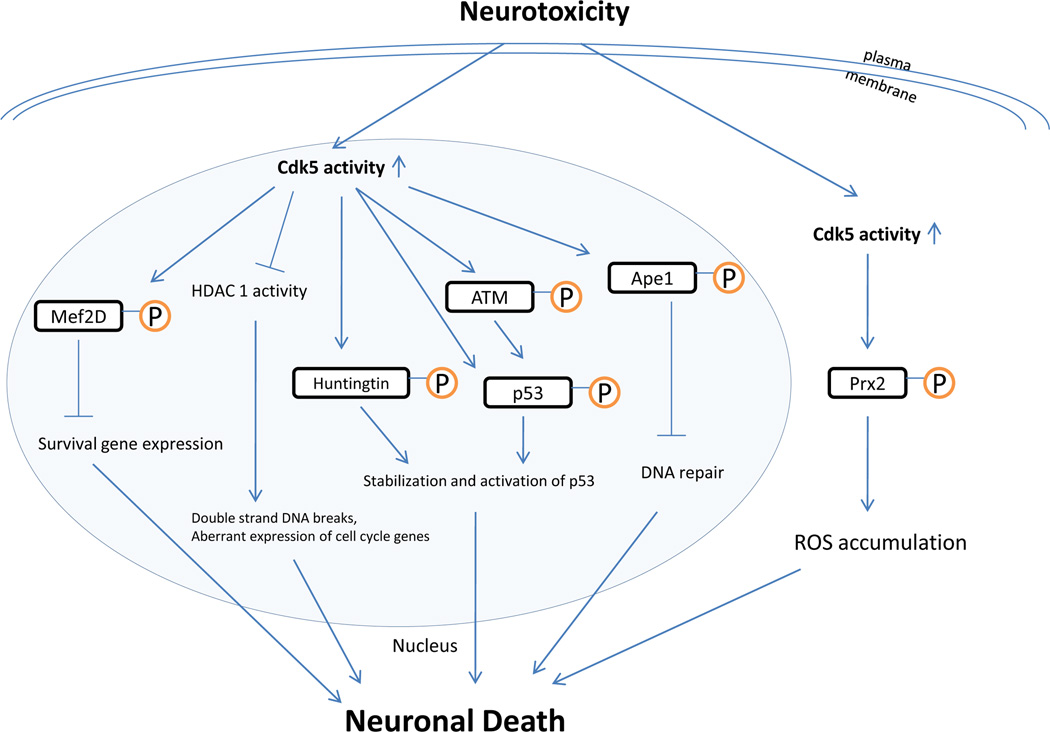
DNA damage is known to stimulate the protease calpain, which mediates the conversion of p35 to the more potent Cdk5 activator p25. Cdk5 activity is found to be increased during the early phases of DNA damage (Lu et al., 2004). Aberrant Cdk5 activity is associated with dysregulation of cell cycle events in neurons. Aside from this general correlation, several recent studies indicate that Cdk5 plays a more direct and important role in modulating DNA damage response in neurons (Figure 1). The phosphatidylinositol-3-kinase-like kinase ATM is identified as a novel substrate of Cdk5. ATM is a serine/threonine kinase and plays an essential role in the maintenance of genomic integrity. ATM regulates cell cycle checkpoint control, DNA repair, and apoptosis in response to DNA damage by phosphorylating a number of critical proteins including p53, H2AX, breast-cancer-associated 1, p53-binding protein 1 and the checkpoint kinase CHK2 (McKinnon, 2004). Oxidation of ATM can directly induce ATM activation (Guo et al., 2010). How ATM activity is regulated by DNA damage signal was not entirely clear. It has been shown now that camptothecin damages DNA and induces calpain-dependent cleavage of p35 to p25 to activate Cdk5. Cdk5, in turn, directly phosphorylates ATM at Ser794 in neurons. Ser794 phosphorylation seems to be an upstream initiation event because it precedes and is required for ATM autophosphorylation at Ser1981, leading to activation of ATM kinase activity. In post-mitotic neurons, Cdk5-ATM signal regulates phosphorylation of p53 and H2AX, resulting in expression of neuronal death genes such as puma and bax as well as γH2AX foci formation (Tian et al., 2009). This Cdk5-ATM pathway is utilized by neurons in response to a number of other DNA damage regents in addition to camptothecin such as etoposide and bleomycin. Down-regulation of Cdk5 by inhibitor roscovitine or Cdk5 RNAi blocks ATM phosphorylation at both Ser794 and Ser1981 and inhibits DNA damage-induced cell cycle reentry, consistent with previous observation that repression of such ectopic cell-cycle activity attenuates neuronal apoptosis (Liu and Greene, 2001). Cdk5 can also directly interact with p53 following mitomycin C-induced DNA damage (Tian et al., 2009). Phosphorylation of p53 by Cdk5 at Ser15, Ser33 and Ser46 enhances its stabilization and induces transactivation of p53 target genes (Lee et al., 2007).
Figure 1.
Schematic representation demonstrates Cdk5-mediated neuronal death in response to neurotoxic signals.
Cdk5 plays a role in neurotoxin-induced DNA damage response in models of PD. Experimental models of PD can be developed following administration of neurotoxic compounds such as MPTP, metoclopramide, butyrophenones, rotenone and reserpine (Langston et al., 1983; Mocko et al., 2010; Ogata et al., 1997). In animals, MPTP is converted to its toxic metabolite, 1-methyl-4-phenyl-pyridinium (MPP+), by glia. After specific uptake by dopaminergic neurons, MPP+ perturbs calcium homeostasis and mitochondrial dysfunction, leading to oxidative stress (Wang and Yuen, 1994). The levels of oxidative stress, which can induce DNA strand breaks, are high in the brain of PD patients and believed to be a critical mediator of damage in PD (Caldecott, 2004; Rhee et al., 2005). Reactive oxygen species is reduced by enzymatic antioxidants, including glutathione, catalase, thioredoxins, and peroxiredoxins (Prxs). Peroxidase Prx2 is an antioxidative enzyme (Low et al., 2008) localized in the neuronal cytoplasm. It plays a protective role against the oxidative damage by utilizing cysteine residues to decompose hydrogen peroxide (Han et al., 2005). MPTP activates Cdk5, which in turn phosphorylates Prx2 at Thr89 and decreases its peroxidase activity. This is believed to contribute to increased oxidative stress. Inhibition of Cdk5, p35, or calpain decreases MPTP-induced loss of dopaminergic cell bodies and behavioral deficits (Qu et al., 2007; Smith et al., 2003).
Another nuclear Cdk5 substrate implicated in DNA damage repair is Ape1. The base excision repair (BER) pathway is responsible for cleaning up damaged nucleotides and apurinic/apyrimidinic (AP) sites and restoring the chemical integrity of DNA. Ape1 functions as a key enzyme in the BER pathway. As a reduction-oxidation factor, Ape1 not only enhances the DNA binding activity of a number of transcription factors such as AP-1, NFκB, HIF-1α, and p53 but also maintains them in a reduced state liable for transcription (Evans et al., 2000). Ape1 is also associated with the control of the apoptosis process (Vanlerberghe et al., 2002). MPP+ treatment leads to Cdk5-dependent phosphorylation of Ape1 at Thr232 in neurons. This reduces its AP endonuclease activity and interferes with its repair function. Phospho-Ape1 has been observed in the nuclei of dopaminergic neurons from patients with PD or AD (Huang et al., 2010), suggesting that the defects of Ape1 function may contribute to the pathogenic process in some neurodegenerative disorders.
Huntington's disease is a dominant genetic neurodegenerative disorder caused by the mutation in the gene encoding the protein huntingtin. Huntingtin is an anti-apoptotic protein. Phosphorylation of huntingtin by Cdk5 at Ser1181 and Ser1201 protects neurons against polyglutamine expansion as well as DNA damage mediated toxicity (Anne et al., 2007).
In addition to phosphorylation of proteins, Cdk5 may also affect DNA integrity through histone deacetylase 1 (HDAC1) independent of its kinase activity. Histone acetylation elevates transcription via chromatin modification and is critical for neuronal proliferation and differentiation. HDACs regulate chromatin structure and gene expression by removing acetyl groups from histones to attenuate the accessibility of transcription factors to DNA (Cerna et al., 2006). Different classes of HDACs are implicated in a number of neuronal functions. HDAC1 is involved in the repression of cell cycle genes required for cell proliferation, such as p21/WAF, E2F1, cyclins A and E, Hus1, Rad1and Rad9 (Brehm et al., 1998; Cai et al., 2000; Lagger et al., 2002; Rayman et al., 2002; Stiegler et al., 1998). In addition, HDAC1 plays a role in gene silencing through the cooperation with the methyl-binding proteins and DNA methyltransferases (Dobosy and Selker, 2001). Cdk5/p25 interacts with and inhibits HDAC1 (Kim et al., 2008). Cdk5-induced inhibition of HDAC1 function in neurons correlates with increased DNA damage, dysregulation of cell cycle activity, and neuronal death.
6. Summary
Recently, several pathways connecting Cdk5 and neuronal death in response to DNA damage have been delineated. It is now clear that Cdk5 plays a much more prominent role in regulating neuronal response to DNA damage than previously realized. Although certain aspects of this newly discovered role for Cdk5 remain to be further clarified, it now appears that the neurotoxic effects of Cdk5 dysregulation involve, at least in part, defective DNA damage response. Cdk5 constitutes a molecular link between processes involving DNA damage, irregular cell cycle progression, and neuronal stress response. Identifying key Cdk5 substrates/effectors associated with these processes will provide potential therapeutic targets for neurodegenerative disorders.
Research Highlights.
DNA damage is emerging as an important pathological component in various neurodegenerative conditions. Based on most recent relevant findings we discuss the progress regarding the roles of Cdk5 in mediating DNA damage signal in neurons.
Acknowledgements
This work was supported in part by NIH grants to Z.M. (AG023695, NS048254, ES015317, ES016731-0002) and Michael J. Fox Foundation (Z.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alvira D, Ferrer I, Gutierrez-Cuesta J, Garcia-Castro B, Pallas M, Camins A. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson's disease. Parkinsonism Relat Disord. 2008;14:309–313. doi: 10.1016/j.parkreldis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Anne SL, Saudou F, Humbert S. Phosphorylation of huntingtin by cyclin-dependent kinase 5 is induced by DNA damage and regulates wild-type and mutant huntingtin toxicity in neurons. J Neurosci. 2007;27:7318–7328. doi: 10.1523/JNEUROSCI.1831-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham E, Rott R, Liani E, Szargel R, Engelender S. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem. 2007;282:12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- Bibb JA. Role of Cdk5 in neuronal signaling, plasticity, and drug abuse. Neurosignals. 2003;12:191–199. doi: 10.1159/000074620. [DOI] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- Cai RL, Yan-Neale Y, Cueto MA, Xu H, Cohen D. HDAC1, a histone deacetylase, forms a complex with Hus1 and Rad9, two G2/M checkpoint Rad proteins. J Biol Chem. 2000;275:27909–27916. doi: 10.1074/jbc.M000168200. [DOI] [PubMed] [Google Scholar]
- Caldecott KW. DNA single-strand breaks and neurodegeneration. DNA Repair (Amst) 2004;3:875–882. doi: 10.1016/j.dnarep.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Cerna D, Camphausen K, Tofilon PJ. Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol. 2006;73:173–204. doi: 10.1016/S0070-2153(05)73006-4. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Gong K, Ip NY. Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J Neurosci. 2008;28:4872–4877. doi: 10.1523/JNEUROSCI.0689-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. The roles of cyclin-dependent kinase 5 in dendrite and synapse development. Biotechnol J. 2007;2:949–957. doi: 10.1002/biot.200700056. [DOI] [PubMed] [Google Scholar]
- Ching YP, Pang AS, Lam WH, Qi RZ, Wang JH. Identification of a neuronal Cdk5 activator-binding protein as Cdk5 inhibitor. J Biol Chem. 2002;277:15237–15240. doi: 10.1074/jbc.C200032200. [DOI] [PubMed] [Google Scholar]
- Chung S-H. Cyclin-Dependent Kinase 5: A Critical Regulator of Neurotransmitter Release. In: Tsai NYIaL-H., editor. Cyclin Dependent Kinase. Vol. 5. Springer; 2008. [Google Scholar]
- Courapied S, Sellier H, de Carne Trecesson S, Vigneron A, Bernard AC, Gamelin E, Barre B, Coqueret O. The cdk5 kinase regulates the STAT3 transcription factor to prevent DNA damage upon topoisomerase I inhibition. J Biol Chem. 2010;285:26765–26778. doi: 10.1074/jbc.M109.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH. p25/cyclindependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Dobosy JR, Selker EU. Emerging connections between DNA methylation and histone acetylation. Cell Mol Life Sci. 2001;58:721–727. doi: 10.1007/PL00000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000;62:463–472. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Fu AK, Fu WY, Cheung J, Tsim KW, Ip FC, Wang JH, Ip NY. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat Neurosci. 2001;4:374–381. doi: 10.1038/86019. [DOI] [PubMed] [Google Scholar]
- Fu AK, Ip FC, Fu WY, Cheung J, Wang JH, Yung WH, Ip NY. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc Natl Acad Sci U S A. 2005;102:15224–15229. doi: 10.1073/pnas.0507678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WY, Wang JH, Ip NY. Expression of Cdk5 and its activators in NT2 cells during neuronal differentiation. J Neurochem. 2002;81:646–654. doi: 10.1046/j.1471-4159.2002.00856.x. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Tang X, Wiedmann M, Wang X, Peng J, Zheng D, Blair LA, Marshall J, Mao Z. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Guidato S, McLoughlin DM, Grierson AJ, Miller CC. Cyclin D2 interacts with cdk-5 and modulates cellular cdk-5/p35 activity. J Neurochem. 1998;70:335–340. doi: 10.1046/j.1471-4159.1998.70010335.x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Han YH, Kim HS, Kim JM, Kim SK, Yu DY, Moon EY. Inhibitory role of peroxiredoxin II (Prx II) on cellular senescence. FEBS Lett. 2005;579:4897–4902. doi: 10.1016/j.febslet.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Qu D, Zhang Y, Venderova K, Haque ME, Rousseaux MW, Slack RS, Woulfe JM, Park DS. The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat Cell Biol. 2010;12:563–571. doi: 10.1038/ncb2058. [DOI] [PubMed] [Google Scholar]
- Ikiz B, Przedborski S. A sequel to the tale of p25/Cdk5 in neurodegeneration. Neuron. 2008;60:731–732. doi: 10.1016/j.neuron.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Imahori K, Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J Biochem. 1997;121:179–188. [PubMed] [Google Scholar]
- Ishiguro S-iHaK. The Kinase Activity of Cdk5 and Its Regulation. In: Tsai NYIaL-H., editor. Cyclin Dependent Kinase. Vol. 5. Springer; 2008. pp. 171–190. [Google Scholar]
- Kamei H, Saito T, Ozawa M, Fujita Y, Asada A, Bibb JA, Saido TC, Sorimachi H, Hisanaga S. Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J Biol Chem. 2007;282:1687–1694. doi: 10.1074/jbc.M610541200. [DOI] [PubMed] [Google Scholar]
- Kanungo J, Zheng YL, Amin ND, Pant HC. Targeting Cdk5 activity in neuronal degeneration and regeneration. Cell Mol Neurobiol. 2009;29:1073–1080. doi: 10.1007/s10571-009-9410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, Guan JS, Lee BH, Moy LY, Giusti P, Broodie N, Mazitschek R, Delalle I, Haggarty SJ, Neve RL, Lu Y, Tsai LH. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. Embo J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Recent advances in understanding the roles of Cdk5 in synaptic plasticity. Biochim Biophys Acta. 2009;1792:741–745. doi: 10.1016/j.bbadis.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Lambert de Rouvroit C, Goffinet AM. Neuronal migration. Mech Dev. 2001;105:47–56. doi: 10.1016/s0925-4773(01)00396-3. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HS, Lee SJ, Kim KT. Stabilization and activation of p53 induced by Cdk5 contributes to neuronal cell death. J Cell Sci. 2007;120:2259–2271. doi: 10.1242/jcs.03468. [DOI] [PubMed] [Google Scholar]
- Lew J, Winkfein RJ, Paudel HK, Wang JH. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J Biol Chem. 1992;267:25922–25926. [PubMed] [Google Scholar]
- Li BS, Zhang L, Takahashi S, Ma W, Jaffe H, Kulkarni AB, Pant HC. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. Embo J. 2002;21:324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl J, Weitensteiner SB, Vereb G, Takacs L, Fuerst R, Vollmar AM, Zahler S. Cyclin dependent kinase 5 (Cdk5) regulates endothelial cell migration and angiogenesis. J Biol Chem. 2010 doi: 10.1074/jbc.M110.126177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AC, Hou Z, Goh CP, Qi RZ. Protein kinase CK2 is an inhibitor of the neuronal Cdk5 kinase. J Biol Chem. 2004;279:46668–46673. doi: 10.1074/jbc.M404760200. [DOI] [PubMed] [Google Scholar]
- Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–228. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- Liu R, Tian B, Gearing M, Hunter S, Ye K, Mao Z. Cdk5-mediated regulation of the PIKE-A-Akt pathway and glioblastoma cell invasion. Proc Natl Acad Sci U S A. 2008;105:7570–7575. doi: 10.1073/pnas.0712306105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Mao QYaZ. Regulation and Function of Cdk5 in the Nucleus. In: Tsai NYIaL-H., editor. Cyclin Dependent Kinase. Vol. 5. Springer; 2008. [Google Scholar]
- Matsunaga Y. Expression of cyclin E in postmitotic cells in the central nervous system. Kokubyo Gakkai Zasshi. 2000;67:169–181. doi: 10.5357/koubyou.67.169. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5:772–776. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. Embo J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocko JB, Kern A, Moosmann B, Behl C, Hajieva P. Phenothiazines interfere with dopaminergic neurodegeneration in Caenorhabditis elegans models of Parkinson's disease. Neurobiol Dis. 2010;40:120–129. doi: 10.1016/j.nbd.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. Embo J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Bibb JA. Cdk5 and the mystery of synaptic vesicle endocytosis. J Cell Biol. 2003;163:697–699. doi: 10.1083/jcb.200310038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Ogata A, Tashiro K, Nukuzuma S, Nagashima K, Hall WW. A rat model of Parkinson's disease induced by Japanese encephalitis virus. J Neurovirol. 1997;3:141–147. doi: 10.3109/13550289709015803. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ogawa M, Veeranna, Hirasawa M, Longenecker G, Ishiguro K, Pant HC, Brady RO, Kulkarni AB, Mikoshiba K. Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc Natl Acad Sci U S A. 2001;98:2764–2769. doi: 10.1073/pnas.051628498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Ogura H, Tomizawa K, Hayashi K, Suzuki H, Saito T, Kamei H, Nishi A, Bibb JA, Hisanaga S, Matsui H, Mikoshiba K. Impairment of hippocampal long-term depression and defective spatial learning and memory in p35 mice. J Neurochem. 2005;94:917–925. doi: 10.1111/j.1471-4159.2005.03233.x. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglini G, Caceres A. The role of the Cdk5--p35 kinase in neuronal development. Eur J Biochem. 2001;268:1528–1533. [PubMed] [Google Scholar]
- Paglini G, Pigino G, Kunda P, Morfini G, Maccioni R, Quiroga S, Ferreira A, Caceres A. Evidence for the participation of the neuron-specific CDK5 activator P35 during laminin-enhanced axonal growth. J Neurosci. 1998;18:9858–9869. doi: 10.1523/JNEUROSCI.18-23-09858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Keller J, Kesavapany S, Agarwal N, Kuner R, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc Natl Acad Sci U S A. 2007;104:660–665. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Kulkarni AB. Cdk5, a Journey from Brain to Pain: Lessons from Gene Targeting. In: Tsai NYIaL-H., editor. Cyclin Dependent Kinase. Vol. 5. Springer; 2008. [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Piedrahita D, Hernandez I, Lopez-Tobon A, Fedorov D, Obara B, Manjunath BS, Boudreau RL, Davidson B, Laferla F, Gallego-Gomez JC, Kosik KS, Cardona-Gomez GP. Silencing of CDK5 reduces neurofibrillary tangles in transgenic alzheimer's mice. J Neurosci. 2010;30:13966–13976. doi: 10.1523/JNEUROSCI.3637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Giese KP, Angelo M. Involvement of Cdk5 in Synaptic Plasticity, and Learning and Memory. In: Tsai NYIaL-H., editor. Cyclin Dependent Kinase. Vol. 5 2008. [Google Scholar]
- Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, Haque E, Zhang Y, Callaghan S, Daigle M, Rousseaux MW, Slack RS, Albert PR, Vincent I, Woulfe JM, Park DS. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Rass U, Ahel I, West SC. Defective DNA repair and neurodegenerative disease. Cell. 2007;130:991–1004. doi: 10.1016/j.cell.2007.08.043. [DOI] [PubMed] [Google Scholar]
- Rayman JB, Takahashi Y, Indjeian VB, Dannenberg JH, Catchpole S, Watson RJ, te Riele H, Dynlacht BD. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 2002;16:933–947. doi: 10.1101/gad.969202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Rimer M. Neuregulins: primary or secondary signals for the control of synapse-specific gene expression. J Neurocytol. 2003;32:665–675. doi: 10.1023/B:NEUR.0000020615.79831.51. [DOI] [PubMed] [Google Scholar]
- Rosales J, Han B, Lee KY. Cdk7 functions as a cdk5 activating kinase in brain. Cell Physiol Biochem. 2003;13:285–296. doi: 10.1159/000074543. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- Sakaue F, Saito T, Sato Y, Asada A, Ishiguro K, Hasegawa M, Hisanaga S. Phosphorylation of FTDP-17 mutant tau by cyclin-dependent kinase 5 complexed with p35, p25, or p39. J Biol Chem. 2005;280:31522–31529. doi: 10.1074/jbc.M504792200. [DOI] [PubMed] [Google Scholar]
- Sato K, Zhu YS, Saito T, Yotsumoto K, Asada A, Hasegawa M, Hisanaga S. Regulation of membrane association and kinase activity of Cdk5-p35 by phosphorylation of p35. J Neurosci Res. 2007;85:3071–3078. doi: 10.1002/jnr.21438. [DOI] [PubMed] [Google Scholar]
- Segura Aguilar J, Kostrzewa RM. Neurotoxins and neurotoxic species implicated in neurodegeneration. Neurotox Res. 2004;6:615–630. doi: 10.1007/BF03033456. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Wu Q, Grundke-Iqbal I, Iqbal K, Singh TJ. Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol Cell Biochem. 1997;167:99–105. doi: 10.1023/a:1006883924775. [DOI] [PubMed] [Google Scholar]
- Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem. 2004;88:1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J. Cyclin-dependent kinase-5 targeting for ischaemic stroke. Curr Opin Pharmacol. 2009;9:119–124. doi: 10.1016/j.coph.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Smith DS, Greer PL, Tsai LH. Cdk5 on the brain. Cell Growth Differ. 2001;12:277–283. [PubMed] [Google Scholar]
- Smith DS, Tsai LH. Cdk5 behind the wheel: a role in trafficking and transport? Trends Cell Biol. 2002;12:28–36. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O'Hare MJ, Callaghan S, Slack RS, Przedborski S, Anisman H, Park DS. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, Vincent I, Wang X, Mao Z, Park DS. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilb ML, Dias-Santagata D, Mulkearns EE, Shulman JM, Biernat J, Mandelkow EM, Feany MB. S/P and T/P phosphorylation is critical for tau neurotoxicity in Drosophila. J Neurosci Res. 2007;85:1271–1278. doi: 10.1002/jnr.21232. [DOI] [PubMed] [Google Scholar]
- Stiegler P, De Luca A, Bagella L, Giordano A. The COOH-terminal region of pRb2/p130 binds to histone deacetylase 1 (HDAC1), enhancing transcriptional repression of the E2F-dependent cyclin A promoter. Cancer Res. 1998;58:5049–5052. [PubMed] [Google Scholar]
- Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008;107:265–278. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Tang X, Wang X, Gong X, Tong M, Park D, Xia Z, Mao Z. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2005;25:4823–4834. doi: 10.1523/JNEUROSCI.1331-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Yang Q, Mao Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat Cell Biol. 2009;11:211–218. doi: 10.1038/ncb1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Sunada S, Lu YF, Oda Y, Kinuta M, Ohshima T, Saito T, Wei FY, Matsushita M, Li ST, Tsutsui K, Hisanaga S, Mikoshiba K, Takei K, Matsui H. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol. 2003;163:813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, Kolattukudy P, Honnorat J, Goshima Y. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Robson CA, Yip JY. Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol. 2002;129:1829–1842. doi: 10.1104/pp.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Yuen PW. Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol Sci. 1994;15:412–419. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Xie Z, Tsai LH. Cdk5 phosphorylation of FAK regulates centrosome-associated miocrotubules and neuronal migration. Cell Cycle. 2004;3:108–110. [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Yabut O, Fitzpatrick H, D'Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 2010;30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Zheng YL, Amin ND, Hu YF, Rudrabhatla P, Shukla V, Kanungo J, Keshavpany S, Grant P, Albers W, Pant HC. A 24 residue peptide (P5), derived from P35, the CDK5 neuronal activator, specifically inhibits CDK5/P25 hyperactivity and tau hyperphosphorylation. J Biol Chem. 2010 doi: 10.1074/jbc.M110.134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Amin N, Albers W, Grant P, Pant HC. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. Embo J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Kanungo J, Kesavapany S, Amin N, Grant P, Pant HC. Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell. 2007;18:404–413. doi: 10.1091/mbc.E06-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YS, Saito T, Asada A, Maekawa S, Hisanaga S. Activation of latent cyclin-dependent kinase 5 (Cdk5)-p35 complexes by membrane dissociation. J Neurochem. 2005;94:1535–1545. doi: 10.1111/j.1471-4159.2005.03301.x. [DOI] [PubMed] [Google Scholar]