Abstract
Aberrant dopamine-mediated behaviors are a hallmark of a number of psychiatric disorders, including substance use disorders and schizophrenia. It has been demonstrated recently that rodent models of these diseases display enhanced dopamine neuron activity throughout the ventral tegmental area (VTA). It is known, however, that the VTA is not a homogeneous structure, and that the dopamine neuron population provides discrete, topographical innervation of nucleus accumbens sub-regions. In addition, these ventromedial and ventrolateral dopamine systems are known to subserve complementary but distinct aspects of goal-directed behavior. Using in vivo extracellular recordings of identified dopamine neurons in chloral hydrate anesthetized rats, we examined the level of dopamine neuron population activity across the mediolateral extent of the VTA following amphetamine sensitization or gestational methylazoxymethanol acetate (MAM) treatment, a verified rodent model of schizophrenia. Here we demonstrate that both models display an augmented medial VTA-ventromedial striatal dopamine system function that correlates with the augmented locomotor response to amphetamine observed in both models. In contrast, only MAM-treated rats exhibit an increase in VTA-ventrolateral striatal dopamine system function. This latter finding is consistent with human imaging studies in schizophrenia patients. In summary, we demonstrate that, although a number of disorders involving a hyperdopaminergic state demonstrate an increase in dopamine neuron population activity, there is divergence in the exact populations of neurons affected. This distinction likely underlies the observed differences in disease symptomatology.
Keywords: dopamine, schizophrenia, amphetamine sensitization, stress, nucleus accumbens, associative striatum
Introduction
Hyper-functioning of the mesolimbic dopamine systems is a consistent observation of a number of disorders including schizophrenia and drug abuse. We have previously demonstrated an increase in dopamine neuron activity in both a verified rodent model of schizophrenia as well as following repeated amphetamine administration (Lodge and Grace, 2007; Lodge and Grace, 2008). More specifically, the administration of a mitotoxin, methylazoxymethanol acetate (MAM) to pregnant dams on gestational day 17 recapitulated a range of deficits in adult offspring associated with the positive, negative and cognitive symptoms of the disease (for review see (Lodge and Grace, 2009)), including an increase in dopamine system function and augmented response to psychomotor stimulants (Lodge and Grace, 2007). A similar increase in dopamine system function was observed following the behavioral sensitization to amphetamine. Thus, the repeated administration of amphetamine (5 days, 1.5mg/kg/day i.p.), followed by a period of withdrawal (5 days) resulted in an increase in dopamine neuron activity and an enhanced locomotor response to an amphetamine challenge (Lodge and Grace, 2008).
Importantly, the augmented dopamine neuron activity, measured in chloral hydrate anesthetized rats, was restricted to increases in the number of spontaneously active neurons; a standard measure of the population activity of dopamine neurons, with no changes in either firing rate or pattern (Lodge and Grace, 2007; Lodge and Grace, 2008). Thus, it has been demonstrated that a significant proportion of ventral tegmental area (VTA) dopamine neurons, in vivo, are hyperpolarized and not spontaneously active (Grace and Bunney, 1985), likely due to a tonic drive from the GABAergic neurons of the ventral pallidum (VP) (Floresco et al., 2001; Floresco et al., 2003). Afferents that attenuate VP activity can therefore modulate this GABAergic tone and set the gain of the dopamine system (Fig. 1). One region that is known to regulate dopamine system function via this mechanism is the ventral hippocampus (vHipp) (Floresco et al., 2001; Floresco et al., 2003). Moreover, we have previously demonstrated that the vHipp is hyperactive in a number of rodent models of neuropsychiatric disease including schizophrenia (Lodge and Grace, 2007) and drug abuse (Lodge and Grace, 2008) and the ventral subiculum (vSub) to nucleus accumbens (NAc) pathway is augmented following acute and chronic stress exposure (Valenti and Grace, 2008). Thus, an enhanced vHipp output drives spike firing in the NAc that, in turn, inhibits tonic activity within the VP, increasing the number of dopamine neurons that are spontaneously active (Floresco et al., 2001; Floresco et al., 2003). Since a dopamine neuron must be depolarized and firing in order to be phasically activated (Lodge and Grace, 2006), this increase in dopamine neuron population activity is proposed to represent the level of amplification, or “gain,” of a phasic event (Lodge and Grace, 2006) and can be directly correlated with changes in dopamine efflux measured by microdialysis in the NAc (Floresco et al., 2003). Therefore, aberrant vHipp-NAc activity results in a significant increase in dopamine neuron population activity and an enhanced response to psychomotor stimulants such as amphetamine (Lodge and Grace, 2007; Lodge and Grace, 2008; Valenti and Grace, 2008).
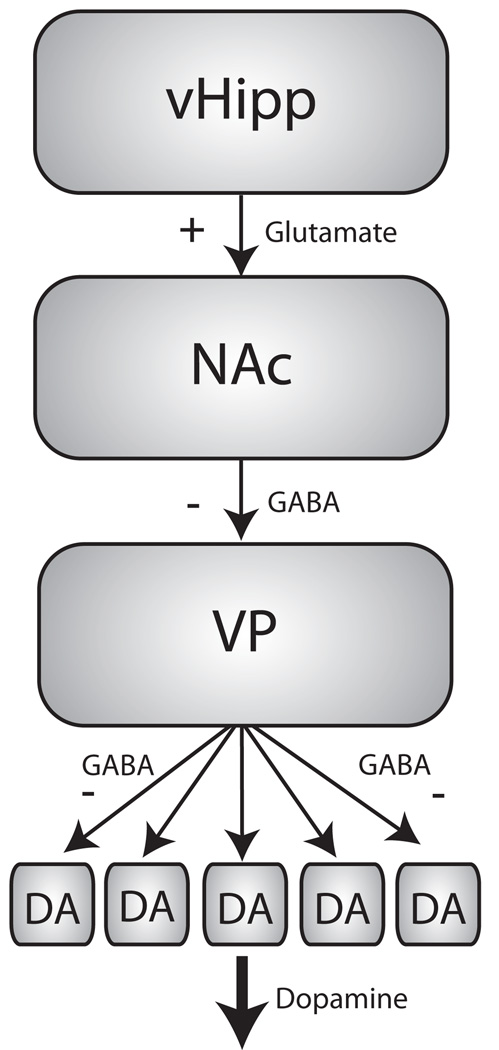
Figure 1.
The ventral hippocampus (vHipp) regulates VTA dopamine neuron activity via a polysynaptic pathway. Glutamatergic inputs from the vHipp synapse onto GABAergic medium spiny neurons (msn) in the nucleus accumbens (NAc). The msn’s, in turn, provide an inhibitory input to the ventral pallidum (VP). Under resting conditions, the VP is tonically active and results in the hyperpolarization of a subset of dopamine neurons in the VTA. Thus, increases in vHipp activity result in the disinhibition of dopamine neuron activity, expressed as an increase in the proportion of DA neurons firing spontaneously and therefore capable of being phasically activated by appropriate stimuli.
The dopamine neurons of the midbrain provide a topographic innervation of both the dorsal and ventral striatum. Thus, dopamine neurons located in the medial VTA more specifically innervate ventromedial regions of the striatum, including the medial shell of the NAc, whereas more laterally located dopamine neurons project to more dorsal regions including the core of the NAc (For review see: Ikemoto, 2007). Importantly, these afferent targets have been associated with the regulation of distinct but complementary behaviors. Thus, the medial shell of the NAc is often associated with the motivational salience of stimuli, whereas the core is thought to mediate the associative aspects of goal-directed behavior (Ikemoto, 2007). Given that these divergent dopamine systems regulate distinct behavioral outcomes, it is prudent to determine whether elevations in dopamine system function, previously reported in rodent models of schizophrenia and drug use, are restricted to distinct medial or lateral populations of dopamine neurons. Moreover, while there are similarities between some of the behavioral characteristics of MAM-exposed and amphetamine-sensitized rats, i.e. an augmented locomotor response to psychomotor stimulants, there are also considerable differences between these models that likely reflect, in part, involvement of complementary but distinct neuronal systems. Indeed, we now report that while both models display an augmented medial VTA-ventromedial striatal dopamine system function, only MAM-treated rats exhibit an increase in VTA-ventrolateral striatal dopamine system function.
Methods
Animals
All experiments were performed in accordance with the guidelines outlined in the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
MAM treatments were performed as described previously (Moore et al., 2006). In brief, timed pregnant female Sprague Dawley rats were obtained at GD15 and housed individually in plastic breeding tubs. MAM (diluted in saline, 20mg/kg, i.p., n=5) was administered on GD17. Control rats received injections of saline (1ml/kg i.p, n=5). Electrophysiology was performed on adult male offspring (>12 weeks of age). All experiments were performed on multiple litters of MAM and saline-treated rats. For sub-chronic amphetamine treatments, male Sprague Dawley rats (300–400g) were housed individually in plastic breeding tubs and injected daily for 5 days (between 1500–1700 hours) with either D-amphetamine sulfate (1.5mg/kg/day i.p., n=7) or saline (1ml/kg/day i.p., n=6). Neurophysiological studies were performed following a 5-day drug-free period. Other dopamine neuron data from these groups of animals have been published previously (Lodge and Grace, 2007; Lodge and Grace, 2008).
VTA Dopamine Neuron Extracellular Recordings
Rats were anaesthetized with chloral hydrate (400 mg/kg i.p.) and placed in a stereotaxic apparatus. Anesthesia was maintained by supplemental administration of chloral hydrate as required to maintain suppression of limb compression withdrawal reflex and a core body temperature of 37°C was sustained by a thermostatically controlled heating pad. Extracellular microelectrodes (impedance 6–14MΩ) were lowered into the VTA (A/P −5.3 to −5.7, M/L +0.6 to 1.0 mm from bregma and −6.5 to −9.0mm ventral of brain surface) using a hydraulic microdrive. 6–9 vertical passes, separated by 200µm, were performed in a predetermined pattern to enable the comparison between dopamine neurons located throughout the medial and lateral areas of the VTA. A typical pattern involved starting at the most rostral and medial location and subsequently recording three tracks across the medial-lateral extent of the VTA. The electrode was then moved caudally and the process repeated until the final track was examined at the most caudal and lateral location. Spontaneously active dopamine neurons were identified with open filter settings (low pass: 50Hz, high pass: 16kHz) using previously established electrophysiological criteria (Grace and Bunney, 1983) and once isolated, their activity was recorded for 2–3 mins. Three parameters of activity were measured: i) population activity (defined as the number of spontaneously active dopamine neurons recorded per electrode track), ii) basal firing rate, and (iii) the proportion of action potentials occurring in bursts (defined as the occurrence of two spikes with an interspike interval of <80ms, and the termination of the burst defined as the occurrence of an interspike interval of >160ms (Grace and Bunney, 1984)). At the cessation of the electrophysiology experiments, the recording site was marked via electrophoretic ejection of Pontamine sky blue from the tip of the recording electrode (−25µA constant current: 20–30 mins). All rats were decapitated and their brains removed, fixed for at least 48 hours (8% w/v paraformaldehyde in phosphate buffered saline: PBS), and cryoprotected (25% w/v sucrose in PBS) until saturated. Brains were sectioned (60µm coronal sections), mounted onto gelatin-chrom alum coated slides and stained with Cresyl violet for histochemical verification of electrode and/or cannula sites. All histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1986).
Materials
Methyl azoxymethanol acetate (MAM) was purchased from Midwest Research Institute (Kansas City, MO). Chloral hydrate and D-amphetamine sulfate were all purchased from Sigma (St Louis, MO). All other chemicals and reagents were of either analytical or laboratory grade and purchased from various suppliers.
Analysis
Electrophysiological analysis of DA neuron activity was performed using custom-designed computer software (Neuroscope). All data are represented as the mean ± standard error of the mean (SEM). All statistics were calculated using the Prism software program (GraphPad Software Inc). Significant differences are calculated with 2-way ANOVAs followed by a Bonferroni posthoc test when appropriate.
Results
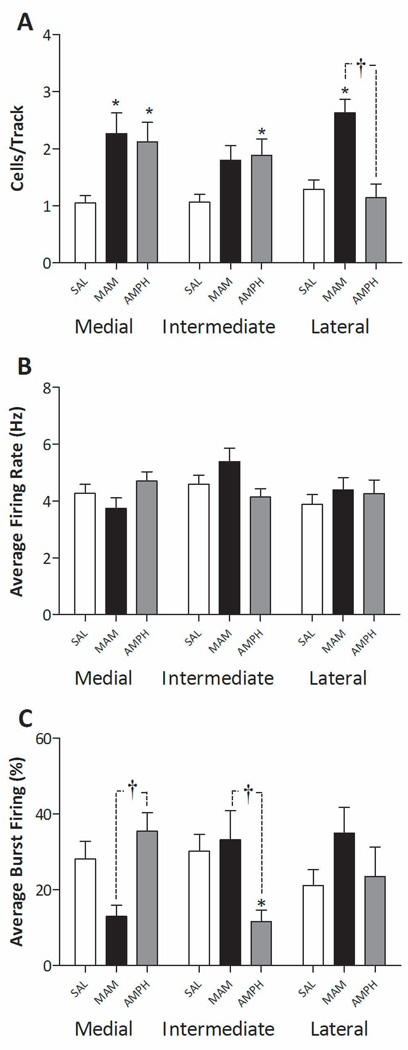
The effects of MAM and amphetamine on dopamine neuron population activity have been reported previously (Lodge and Grace, 2007; Lodge and Grace, 2008); however, the regional specificity of these treatments has not been reported. Here we expand our previous analysis of these rats to evaluate the effect of MAM or sub-chronic amphetamine administration on dopamine neuron activity states based on their relative location throughout the mediolateral divisions of the VTA. Rats that received saline injections on GD17 (n = 5 rats) or adulthood (n = 6 rats) were not significantly different (2-way ANOVA: F=0.29, p=0.60) and their data were combined. Saline treated rats did not display any significant differences in the distribution of spontaneously active dopamine neurons (Saline- medial tracks: 1.05 ± 0.13, intermediate tracks: 1.06 ± 0.14, lateral tracks: 1.29 ± 0.16 cells/track; 1-way ANOVA, F=0.88, p=0.42, n= 11 rats/group), average firing rate (Saline- medial tracks: 4.27 ± 0.32, intermediate tracks: 4.59 ± 0.32, lateral tracks: 3.88 ± 0.35 Hz; 1-way ANOVA, F=1.15, p=0.32, n= 30–32 cells/group), or average percent burst firing (Saline- medial tracks: 28.2 ± 4.6, intermediate tracks: 30.2 ± 4.4, lateral tracks: 21.1 ± 4.1 %; 1-way ANOVA, F=1.16, p=0.32, n= 30–32 cells/group).
Prenatal MAM administration (n = 5 rats – Figure 2) resulted in a significant increase in dopamine neuron population activity compared to saline administration. Moreover, this increase was observed uniformly throughout the VTA with significant differences observed in both medial and lateral recording locations (MAM- medial tracks: 2.27 ± 0.36 p<0.05, intermediate tracks: 1.80 ± 0.25 n.s., lateral tracks: 2.63 ± 0.24 cells/track p<0.05; 2-way ANOVA, F=25.56, p<0.0001). There were no significant changes in either average firing rate (MAM- medial tracks: 3.74 ± 0.37, intermediate tracks: 5.39 ± 0.47, lateral tracks: 4.39 ± 0.44 Hz; 2-way ANOVA, F=2.26, p=0.14) or average percent burst firing (MAM- medial tracks: 13.0 ± 2.9, intermediate tracks: 33.2 ± 7.7, lateral tracks: 34.9 ± 6.8 %; 2-way ANOVA, F=0.01, p=0.92) at any location in MAM-treated rats.
Figure 2.
Divergent activation of ventromedial and ventrolateral dopamine systems following gestational MAM or repeated amphetamine administration. Three parameters of activity were recorded: population activity (number of spontaneously firing dopamine neurons per electrode track (A)), average firing rate (B), and average percent spikes fired in bursts (C). MAM-treated rats (GD17 - 20mg/kg i.p.) display a significantly greater population activity throughout both medial and lateral regions of the VTA, with no significant differences in burst firing or average firing rate. In contrast, the repeated administration of amphetamine (1.5mg/kg i.p. BID – 5 days) resulted in a significantly greater population activity in medial VTA regions, with no significant differences observed throughout the lateral VTA. * represents statistically significant difference from control (saline administration) wheras † represents statistically significant difference between MAM and amphetamine treated rats (p<0.05 2 way ANOVA: Bonferroni post hoc: n = 5–11 rats/group; error bars represent SEM).
In contrast to that observed in the MAM model, rats treated repeated with amphetamine and tested 5 days after withdrawal (n = 7 rats – Figure 2) exhibited a significant increase in dopamine neuron population activity only in the medial areas of the VTA (amphetamine-medial tracks: 2.12 ± 0.34 p<0.05, intermediate tracks: 1.88 ± 0.29 p<0.05, lateral tracks: 1.14 ± 0.24 cells/track n.s; 2-way ANOVA, F=8.77, p=0.01). There were no significant changes in average firing rate (amphetamine - medial tracks: 4.70 ± 0.33, intermediate tracks: 4.14 ± 0.29, lateral tracks: 4.27 ± 0.47 Hz; 2-way ANOVA, F=0.09, p=0.77) although average percent burst firing was decreased in the intermediate tracks (amphetamine - medial tracks: 35.5 ± 4.8 n.s., intermediate tracks: 11.6 ± 3.0 p<0.05, lateral tracks: 23.5 ± 7.8 % n.s.; 2-way ANOVA – no effect of treatment F=0.24, p=0.62).
Discussion
We have previously reported that disorders involving a hyperdopaminergic state, such as amphetamine sensitization (Lodge and Grace, 2008), stress exposure (Valenti and Grace, 2008), and the MAM developmental disruption model of schizophrenia (Lodge and Grace, 2007), are associated with increases in dopamine neuron population activity. These previous studies assessed the VTA as a homogeneous group of neurons with only cursory histological analyses to confirm that the effects were not associated with recordings that lay outside of the predefined region of the VTA. However, it is clear that there are significant behavioral differences between these models that likely reflect, in part, involvement of complementary but distinct neuronal systems. Thus, while amphetamine sensitized rats display robust increases in psychostimulant-induced locomotion, other behaviors typically associated with the symptoms of schizophrenia, such as pre-pulse inhibition of startle and working memory deficits, are not consistently affected (Featherstone et al., 2007). Given an increasing literature demonstrating divergent functions of dopamine in limbic versus associative regions of the ventral striatum, the aim of the current study was to examine whether the augmented dopamine system function observed in these models, , is consistent across the mediolateral extent of the VTA. We now report that, while MAM-treated rats display an augmented dopamine system function throughout the entire mediolateral extent of the VTA, the increase in dopamine neuron firing observed in amphetamine-sensitized rats is restricted to the medial VTA (Figure 3). Similarly, we have recently demonstrated that footshock-stress-induced increases in dopamine system function are localized to the medial regions of the VTA (Valenti and Grace, 2008). These data are therefore consistent with the premise that reward- or affect-related stimuli affect ventromedial dopamine transmission, whereas conditions affecting associative information processing are likely to recruit lateral VTA projections to associative areas of the NAc (Ikemoto, 2007).
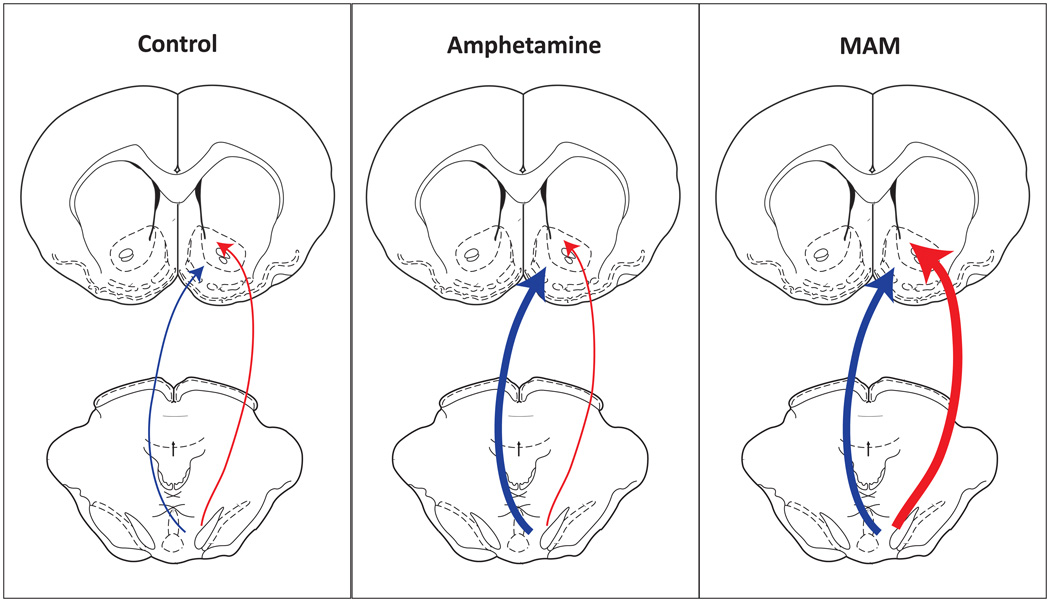
Figure 3.
Disorders involving a hyperdopaminergic state demonstrate a divergence in the localization of dopamine neurons affected. Amphetamine sensitization induces a selective increase in ventromedial dopamine transmission (center, bold arrow). In contrast, prenatal MAM administration, a rodent model of schizophrenia, results in a global increase in dopamine neuron population activity that includes activation of ventrolateral dopamine neurons known to innervate associative areas of the ventral striatum (right, bold arrows).
An understanding of the divergent roles for ventral striatal subregions has come largely from research examining the neurological basis for substance-use disorders. More specifically, there are significant data demonstrating a role for ventromedial dopamine transmission in the regulation of positive affect and heightened behavioral activation. Rats will learn to self-administer dopamine agonists, including cocaine, directly into the shell, but not core, of the NAc (Ikemoto et al., 1997; Rodd-Henricks et al., 2002). Thus, it appears that the positive affect associated with drugs of abuse are more likely associated with increases in ventromedial dopamine transmission. Indeed, we now report that amphetamine sensitization significantly augments dopamine neuron activity only in the medial regions of the VTA.
An additional role of the ventromedial pathway is the activation of locomotor behavior to enable the procurement of a salient incentive or the avoidance of a negative stimulus. Thus, administration of a number of diverse pharmacological compounds into the posterior-medial VTA results in an increase in spontaneous locomotion (Ikemoto, 2004; Joyce et al., 1981; Zangen et al., 2006). Similarly, dopamine agonists administered directly to the medial shell, but not core, of the NAc increase spontaneous locomotion (Ikemoto, 2002; Swanson et al., 1997). These data suggest that the ventromedial dopamine system underlies, at least in part, the locomotor-enhancing effects of stimulant drugs (Ikemoto, 2007). Indeed, an enhanced locomotor response to amphetamine was observed in all conditions shown to activate dopamine neurons in the medial VTA; i.e. amphetamine sensitization, stress, and prenatal MAM administration (Lodge and Grace, 2007; Lodge and Grace, 2008; Valenti and Grace, 2008).
In contrast to the restricted activation observed following amphetamine or stress, MAM-treated rats display a more widespread increase in dopamine neuron activity, including a prominent activation of lateral VTA regions. This difference parallels distinct behavioral differences between amphetamine sensitization, stress, and rodent models of schizophrenia. Thus, while all three models of dopamine hyperfunction demonstrate an enhanced locomotor response to amphetamine administration, MAM-treated rats also display a number of behavioral deficits known to involve associative information processing and correlate with clinical symptoms of schizophrenia (For review see: (Lodge and Grace, 2009)). Schizophrenia is a chronic mental illness with the positive symptoms of the disease thought to involve increases in dopamine neurotransmission. This dopamine hypothesis was initially based on the observation that all available antipsychotic drugs are dopamine D2 receptor antagonists (Kapur et al., 2000; Meltzer et al., 1989a; Meltzer et al., 1989b). In addition, schizophrenia patients are more sensitive to indirect dopamine agonists, such as amphetamine (Janowsky et al., 1973; Laruelle et al., 1996), whereas high doses of these drugs can precipitate psychosis in the general population (Angrist and Gershon, 1970; Disclafani Ii et al., 1981). Since this time, evidence for an augmented dopamine transmission has been more directly examined using brain imaging techniques. Thus, a number of studies have demonstrated an increase in striatal dopamine release in human schizophrenia patients (Abi-Dargham, 2004; Abi-Dargham et al., 2000; Laruelle and Abi-Dargham, 1999; Laruelle et al., 1996). Similarly, we have recently demonstrated an increase in dopamine neuron population activity in the MAM GD17 model of schizophrenia (Lodge and Grace, 2007). The data presented demonstrate that, compared to amphetamine, the most profound difference in MAM-treated rats was in lateral regions of the VTA. These data are in agreement with recent imaging studies examining the baseline occupancy of dopamine D2 receptors and displacement by amphetamine throughout the functional subdivisions of the striatum in schizophrenia patients. Specifically, the greatest α-MPT-induced increases in dopamine receptor availability, an index of baseline dopamine levels, were found to occur in the associative regions of the striatum; i.e. regions which receive a prominent innervation from the more lateral regions of the ventral mesencephalon (Kegeles et al., 2010). Moreover, this associative region of the striatum was also found to exhibit the largest amphetamine-induced raclopride displacement in schizophrenia patients, with this increased release proportionate to the amount of α-MPT-induced increase in dopamine receptor availability (Abi-Dargham et al., 2009) Again, this is consistent with the current study showing that, compared to amphetamine, the most profound difference in MAM-treated rats was the high level of dopamine neuron firing in the VTA region that preferentially innervated more associative areas of the striatum.
Taken together, the data presented here demonstrate that, although a number of disorders involving a hyperdopaminergic state demonstrate an increase in dopamine neuron population activity, there is divergence in the exact populations of neurons affected. Thus, increases in ventromedial dopamine transmission were observed following amphetamine sensitization, footshock-stress and MAM administration and this was correlated with increases in the locomotor response to amphetamine administration (Lodge and Grace, 2007; Lodge and Grace, 2008; Valenti and Grace, 2008). In contrast, the MAM model of schizophrenia also exhibited increases in ventrolateral dopamine transmission that may underlie the deficits in associative information processing. Such an understanding into the regulation of these divergent dopamine circuits is critical to gaining a better understanding of the pathophysiology underlying drug abuse and schizophrenia.
Acknowledgements
The authors would like to thank Niki MacMurdo for her technical assistance, and Brian Lowry for the production, development and support of the custom designed electrophysiology software (Neuroscope). This work was supported by the USPHS DA15408 and MH57440 (AAG) and a Young Investigator Award from NARSAD - The Mental Health Research Association (DJL).
Footnotes
Statement of Interest
Dr. Grace reports having lecture fees, industry research funding or consulting fees with Johnson & Johnson, Lundbeck, Merck, AstraZeneca, Lilly, Galaxo Smith Klein, and Puretech Ventures. Dr. Lodge has no interests to declare.
References
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Science. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, et al. Baseline and Amphetamine-Stimulated Dopamine Activity Are Related in Drug-Naïve Schizophrenic Subjects. Biol Psychiatry. 2009;65(12):1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Angrist BM, Gershon S. The phenomenology of experimentally induced amphetamine psychosis--preliminary observations. Biol Psychiatry. 1970;2(2):95–107. [PubMed] [Google Scholar]
- Disclafani Ii A, Hall RCW, Gardner ER. Drug induced psychosis: Emergency diagnosis and management. Psychosomatics. 1981;22(10):845–855. doi: 10.1016/S0033-3182(81)73092-5. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(8):1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, et al. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333(2):271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D<sub>1</sub>/D<sub>2</sub> agonists. Neuroscience. 2002;113(4):939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Unconditional hyperactivity and transient reinforcing effects of NMDA administration into the ventral tegmental area in rats. Psychopharmacology (Berl) 2004;172(2):202–210. doi: 10.1007/s00213-003-1651-3. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D<sub>1</sub> and D<sub>2</sub> receptors in the nucleus accumbens in mediating reward. Journal of Neuroscience. 1997;17(21):8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousel MK, Davis JM, Sekerke HJ. Provocation of schizophrenic symptoms by intravenous administration of methylphenidate. Arch Gen Psychiatry. 1973;28(2):185–191. doi: 10.1001/archpsyc.1973.01750320023004. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Koob GF, Strecker R. The behavioural effects of enkephalin analogues injected into the ventral tegmental area and globus pallidus. Brain Res Brain Res Rev. 1981;221(2):359–370. doi: 10.1016/0006-8993(81)90784-8. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, et al. Relationship between dopamine D<sub>2</sub> occupancy, clinical response, and side effects: A double-blind PET study of first-episode schizophrenia. American Journal of Psychiatry. 2000;157(4):514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Van Dyck CH, Gil R, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Science. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behav Brain Res. 2009;204(2):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin<sub>2</sub> pK(i) values. Journal of Pharmacology and Experimental Therapeutics. 1989a;251(1):238–246. [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacology Bulletin. 1989b;25(3):390–392. [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, et al. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press Australia; 1986. [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, et al. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. Journal of Pharmacology and Experimental Therapeutics. 2002;303(3):1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacology Biochemistry and Behavior. 1997;58(4):933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Valenti O, Grace AA. Acute and repeated stress induce a pronounced and sustained activation of VTA DA neuron population activity. Neuroscience Meeting Planner. 2008;479.11 [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, et al. Two brain sites for cannabinoid reward. Journal of Neuroscience. 2006;26(18):4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]