Abstract
The human prostate gland is one of the only internal organs that continue to enlarge throughout adulthood. The specific mechanisms that regulate this growth, as well as the pathological changes leading to the phenotype observed in the disease benign prostatic hyperplasia (BPH), are essentially unknown. Recent studies and their associated findings have made clear that many complex alterations occur, involving persistent and chronic inflammation, circulating hormonal level deregulation, and aberrant wound repair processes. BPH has been etiologically characterized as a progressive, albeit discontinuous, hyperplasia of both the glandular epithelial and stromal cell compartments coordinately yielding an expansion of the prostate gland and clinical symptoms. Interestingly, the inflammatory and repair responses observed in BPH are also key components of general wound repair in post-natal tissues. These responses include altered expression of chemokines, cytokines, matrix remodeling factors, chronic inflammatory processes, altered immune surveillance and recognition, as well as the formation of a prototypical ‘reactive’ stroma which is similar to that observed across various fibroplasias and malignancies of a variety of tissue sites. Stromal tissue, both embryonic mesenchyme, and adult reactive stroma myofibroblasts, has been shown to exert potent and functional regulatory control over epithelial proliferation and differentiation as well as immunoresponsive modulation. Thus, the functional biology of a reactive stroma, within the context of an adult disease typified by epithelial and stromal aberrant hyperplasia, is critical to understand within the context of prostate disease and beyond. The mechanisms that regulate reactive stroma biology in BPH represent targets of opportunity for new therapeutic approaches that may extend to other tissue contexts. Accordingly, this review seeks to address the dissection of important factors, signaling pathways, genes, and other regulatory components that mediate the interplay between epithelium and stromal responses in BPH.
MeSH Keywords: reactive stroma, BPH, tenascin-C, IL-8, CXCL12, hyperplasia
Benign Prostatic Hyperplasia and the Reactive Phenotype
The human prostate gland is composed of secretory epithelium arranged in glandular acini within a fibromuscular stroma composed primarily of smooth muscle. The stromal compartment also contains fibroblasts, vasculature, nerves and immune components. In an interactive manner, each of these epithelial and stromal components is likely involved in the genesis and evolution of benign prostatic hyperplasia (BPH). Understanding prostate gland development is helpful in understanding some of the hyperplastic changes observed in BPH, since this disorder has been viewed historically as a type of re-established embryonic inductive process [1, 2].
Developmentally, prostate tissue forms via a highly conserved process termed branching morphogenesis, whereby epithelial buds from the urogenital sinus protrude into the adjacent mesenchyme, elongate and bifurcate into an arborized network of branches with terminal tips [3]. These terminal tips eventually give rise to the epithelial duct system in a process similar to that observed within the renal or bronchiolar networks, collectively providing the final size and shape of the adult prostate [3]. In humans the adult prostate gland encapsulates the initial 3 cm of the urethral tube descending from the bladder, linking the urethra and ejaculatory ducts at a diverticulum junction called the verumontanum. This structure is a vestigial remnant of the developmental layer that gives rise to the female uterus [4].
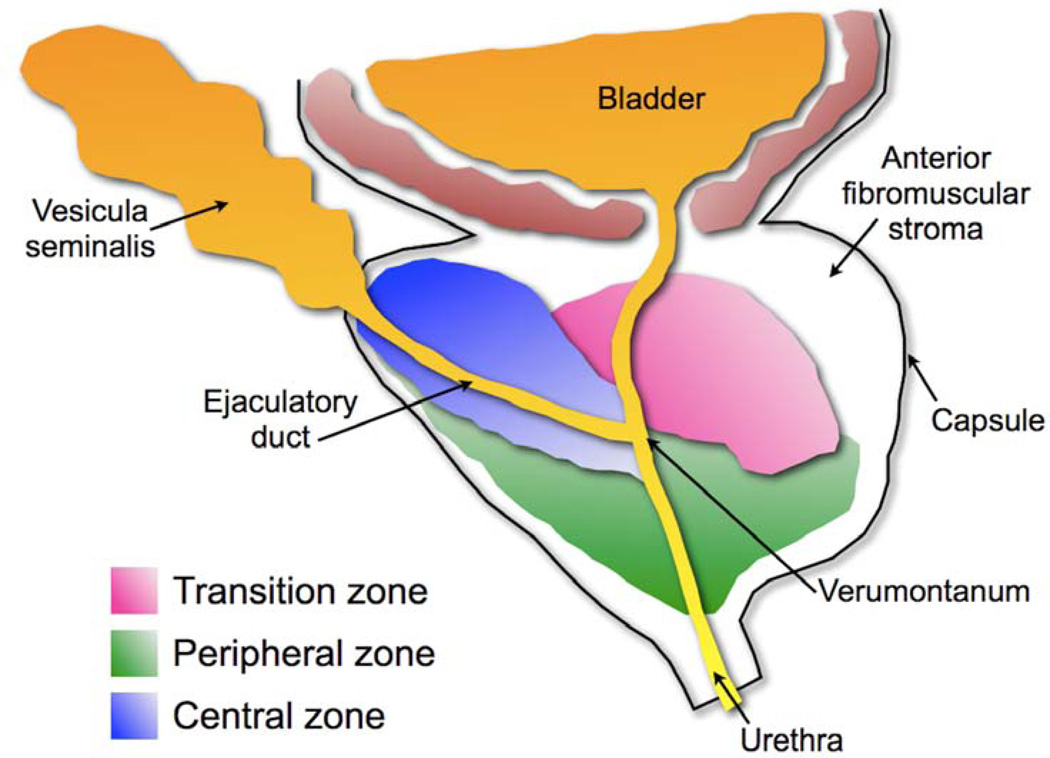
This developmental process leads to the morphogenesis of a lobar structure into 4 distinct anatomical zones: peripheral, central, transitional, and anterior (Figure 1). The central zone contains the ejaculatory duct junctions, while the fibromuscular anterior zone lacks any glandular structures. The peripheral zone represents approximately 70% of the total prostatic volume, and is where the majority of prostate adenocarcinoma forms [5]. The transitional zone represents only 5% of prostatic volume, however it is the exclusive zone where benign prostatic hyperplasia (BPH) occurs [5] (Figures 1 and 2). The prostate is enclosed within a thin, vascularized capsule external to a concentric smooth muscle layer that is continuous with the tissue layer surrounding the base of the bladder [4] (Figure 1).
Figure 1. Schematic of the zonal anatomy of the human prostate.
As indicated, each zone houses distinct sections of the prostatic urethra. The central zone, where little if any disease develops, houses the ductal tube from the vesicular seminalis. The peripheral zone, the primary site of pre-cancerous and cancerous lesions, houses the descending penile urethra. The transition zone, the only site of benign prostatic hyperplasia, houses the transitional urethra composed of descending bladder and prostatic urethral sections. The verumontanum is the junction between the ejaculatory ducts and the prostatic urethra.
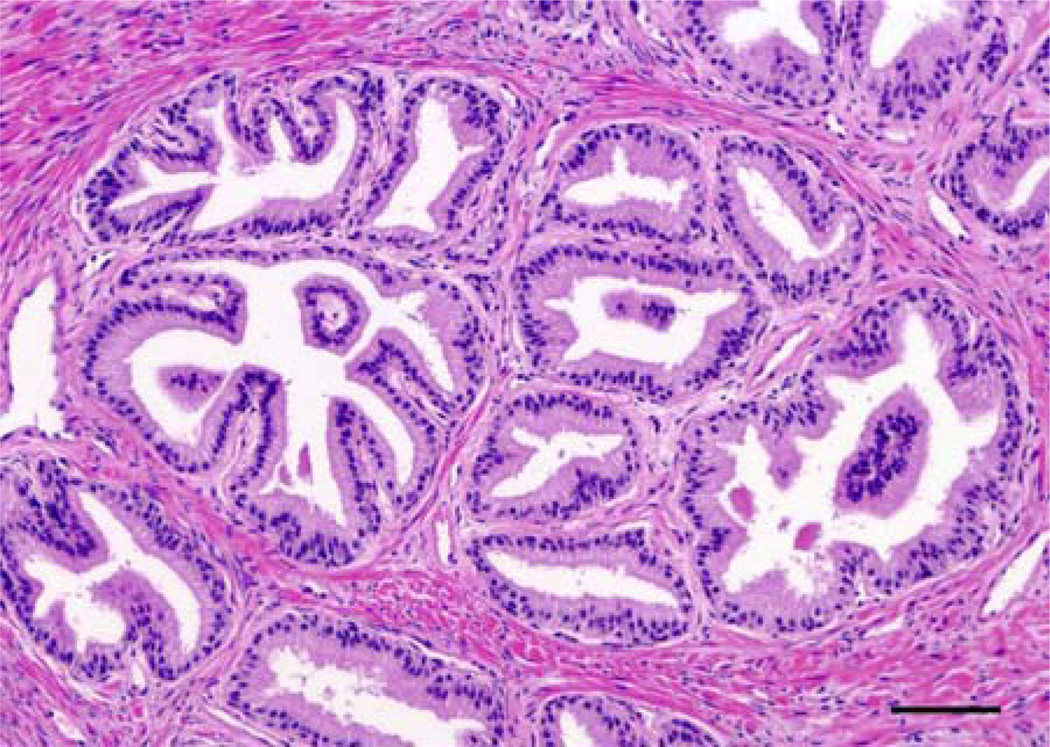
Figure 2. Histomorphology of Nodular Benign Prostatic Hyperplasia.
BPH is histologically defined as oblong hyperplastic tissue nodules, most often composed of epithelia and stroma. Pure stromal nodules, lacking any epithelial component, are rare but observed. Nodular growth proximal to the transitional zone urethral tube, constricting urine flow (see Figure 1), accounts for the majority of BPH patient symptoms. H&E, magnification X200, Scale bar = 50µm
Functionally, the adult prostate is an exocrine accessory reproductive gland that propels a complex proteolytic solution composed of acid phosphatase, citric acid, fibrinolysin, prostate specific antigen, and other enzymes and nutrients into the urethra during ejaculation [4]. The expelled prostatic secretions liquefy the ejected seminiferous solution in order to improve spermatozoan motility, as well as alkalinize the vaginal canal to promote increased viability [4].
It is of interest that the human prostate gland is one of the only internal organs that continue to enlarge past development, past the androgenic surge at puberty, and throughout adulthood (Figure 3). The specific mechanisms that regulate this enlargement, as well as the pathological changes leading to the BPH phenotype, are essentially unknown. However, it is becoming clearer that many complex alterations occur that involve chronic inflammatory and wound repair processes. BPH is characterized by a progressive, but discontinuous, hyperplasia of both glandular epithelial and stromal cells leading to expansion of the prostate gland and clinical symptoms [1, 6]. BPH occurs as a definitive function of age specifically in the transition zone, while cancer foci occur primarily along the proximal peripheral zone. The biological distinction dictating this zonal specificity in prostate disease is as yet uncharacterized. Gene expression profiling of each zone within the prostate has revealed specific differences between the peripheral (cancer) and transitional zones (BPH) in gene products that modulate cell-cell stromal-epithelial interaction, which strongly suggests that prostate disease susceptibility is zone-dependent [7].
Figure 3. The human prostate continues to enlarge throughout life.
The human prostate passes through several phases of rapid growth and relative quiescence, but is the only male organ internal organ that continues to grow through all of adulthood. The various disease states reflect a resumption of rapid growth beyond normal control, including BPH. (Reproduced with kind permission from Springer Science+Business Media: Cell Tissue Research, Early prostate development and its association with late-life prostate disease, Vol 322, 2005, Figure 1 p174, Risbridger G.P. et al., ©Springer-Verlag).
Beginning at age 40 the prevalence of BPH escalates in a growth incidence pattern that nearly mirrors age. Fifty percent of males can exhibit BPH symptoms by age 51–60 [8]. Seventy percent of males present with BPH by the age of 70, and incidence increases to eighty percent by the age of 85 [9]. The volume of the transition zone can nearly double by age 55–60 [2, 6]. Cell proliferation is greatly elevated in BPH compared to normal equivalent tissue regions: epithelial cell proliferation was 9-fold higher, while stromal cell proliferation was 37-fold higher, in a retrospective study [10]. Prostate epithelial proliferation results in enlarged glandular nodules (Figure 2), whereas stromal proliferation produces a more diffuse hyperplasia with elevated matrix production, such as collagen type I [11], typical of a pro-fibrotic lesion. Nodular BPH growth in the transitional zone can go undetected for decades, unless nodules initiate near, and eventually impinge upon, the prostatic section of the urethral tube.
With over 300,000 BPH surgeries performed annually in the US alone, and nearly $4 billion spent annually [12], there is a clear need to direct research efforts towards an understanding of specific mechanisms that could be targeted with novel therapeutic approaches. We now understand that BPH is associated with a general reactive phenotype in both the epithelial and the stromal compartment. Inflammation induces host responses typical of a stromal wound repair, and our studies and those of others suggest that BPH hyperplasia is similar to an altered wound repair response typically observed in inflammation-induced fibroses, such as those observed in the lung, liver and kidney. McNeal suggested that BPH stroma could be defined as "reminiscent of embryonic mesenchyme" and a "partial maturation of fibroblast-like cells into smooth muscle" [1]. We now know that reactive stroma in BPH contains reactive myofibroblasts [13], common in fibrotic diseases and in the reactive stroma associated with cancer and other proliferative disorders [14, 15]. Recent studies have concluded that BPH myofibroblast stromal cells exhibit markers consistent with marrow-derived mesenchymal / myeloid stem cells [16], and recruitment of myeloid cells is a hallmark of local inflammatory responses. Although the activation of genes that regulate developmental processes most certainly are important in the stromal responses in BPH, considerable evidence also points to a repair-centric biology of priority (Figure 4) that involves recruitment of myofibroblasts that are common to most stromal repair sites, including BPH.
Figure 4. The Biology of Priority.
Mammalian embryonic development occurs within the confines of a sterile environment, following a specified pattern of symmetrical and asymmetrical cell division and organ / tissue specification. Adult differentiated biology homeostasis can be disrupted by injury or microbial insult, transitioning to a state of repair-focused biology. Once the insult or injury is sufficiently resolved and tissue is repaired, the biological priority returns back to differentiated functional biology. However, it is likely that senescence, chronic inflammation, and other disease processes associated with aging, affect the efficiency of transition from repair-focused biology back to differentiated homeostasis.
The specific mechanisms are unknown and are likely to be considerably complex. Whereas embryonic development occurs in a sterile, closely-regulated, physiologically protected environment, wound repair of adult tissue is post-natal and occurs in a damaged, microbe-rich external environment and hence necessarily involves inflammatory, surveillance, and rapid stromal response mechanisms that are fundamental to survival of the species. Thus, it is likely that prototypical and chronic wound repair responses occur during the generation and evolution of BPH as a disease. Understanding the fundamental reasons for these repair responses is therefore vital to understanding the mechanisms leading to the BPH reactive phenotype. An examination of the relationship between the biology of altered epithelial barrier function and the concurrent reactive stromal response biology provide possible clues as to how these interactions may affect the BPH phenotype.
Epithelial Barrier Function and the Reactive Stroma Response
Epithelia, though specialized for unique functions throughout the body, share several common morphological features. In general, epithelia line a given surface, directly apposed one to another, yielding a layer. Epithelial tight junctions and other junctional complexes allow for a tight and fairly robust structural integrity of this epithelial lining layer, thus protecting against frictional expansion and contraction within dynamic exocrine organs, such as the prostate. Moreover, the apical side of the epithelium is always in direct contact with the outside environment, whether this epithelium is a surface epithelium (e.g. lung), epithelium lining ducts, or a secretory / absorptive epithelium (e.g. intestinal). Accordingly, a key concept in understanding stromal responses is that all epithelial layers function as a first line barrier to external environmental pathogens, in addition to having tissue-specific secretory and/or absorptive functions.
Another universal feature is that epithelium is positioned atop a basal lamina, a layer of filaments and extracellular matrix. The basal lamina appears to be a key feature of the epithelial barrier function and reactive stromal response. Invasive agents or inflammatory states that produce a weakened epithelial integrity and/or a breach of the epithelial basal lamina result in a predictable stromal response. This property of epithelia was demonstrated in a mouse model of prostate epithelial over-expression of hepsin, a serine protease that disrupts basal lamina, wherein excessive laminar disruption was not only associated with prostatic inflammation, but also promoted deregulated epithelial growth and hyperplasia [17].
Perturbation of the epithelia and the basal lamina has been shown to result in the release of inflammatory mediators. This was demonstrated in a model of human pulmonary inflammation, wherein matrix metalloproteinase 12 (MMP-12)-mediated ECM destruction directly induced lung epithelial cells to over-produce interleukin-8 (IL-8) in a concentration-dependent manner via extracellular signal-regulated kinases 1/2 (Erk1/2) activation [18]. Similarly, epithelia also recognize and respond to alterations in osmotic pressure, as hyperosmolar stress induced IL-8 expression in primary human limbic epithelial cells [19]. Furthermore, overall disease response can depend on epithelial secretion of inflammatory chemokines as a mechanism of barrier function. In inflamed gastrointestinal mucosal phagocytosis, recruitment of mononuclear phagocyte progenitors is dependent on epithelial expression of IL-8 [20]. Another example of this includes insult-dependent progression of cystic fibrosis, the development of which relies on sustained IL-8 production by airway epithelium [21]. Similarly, response to some ocular inflammatory diseases may rely on glial cell stimulation via IL-8 released from retinal epithelial cells [22]. Finally, in corneal wound healing disruption of the Bowman layer resulted in an epithelial-induced keratopathy characterized by a fibroblast-mediated scarring [23]. Therefore, it is clear that the epithelium is poised as a sentinel, detecting inconsistencies evoked by damage to the epithelial barrier function, including basal lamina damage, and initiating an acute inflammation-mediated chemokine up regulation [24] that in general leads to inflammation and stromal repair responses. However, not fully understood is the role of acute or prolonged inflammation in the biology of benign disease initiated in prostate epithelia, and the specific molecular mechanisms of how this impacts the biology of stromal compartment.
It is becoming very clear that the stromal compartment response to acute wound repair in adult tissue is governed by a biology of priority, beginning when the epithelial layer is breached. Host response mechanisms within repairing tissue follow a repair-centric biology of priority, as compared with a developmental biology of priority, as factors, mechanisms and the cell/tissue biology is different in many ways. Following a Darwinian model, damaged tissue, whether acute or chronic, must repair quickly in the face of injury or insult in order to assure survival of the organism and therefore of the species (Figure 4). In general, damaged tissues switch their biology of priority from differentiated function, to a biology directed toward an expedient and effective repair process that involves an immediate / acute response, and when unresolved, chronic responses. These responses include inflammation, immune surveillance, matrix remodeling, angiogenesis and the formation of reactive stroma. The state of repair-centric biology is generally modulated back to differentiated function once the tissue is capable of sustained normal paracrine interaction, allowing for a return back to the homeostatic state of adult differentiated biology.
The transition of adult functional organ systems to repair biology mode is overly emphasized in response to aging, in the context of cellular and tissue senescence. The biology of priority emphasizes relatively quick repair that likely sacrifices functional differentiated biology in order to retain microbial-resistant epithelial barrier and lining function. Thus, inappropriate epithelial hyperplasia within the tissue context of BPH could be considered the result of prolonged inflammatory and senescence-mediated stasis within the repair phase of this so-called biology of priority (Figure 4). These principles have been highlighted in several recent reviews by Judith Campisi focusing on the role of senescent inflammatory activation of tissue ECM fibroblasts. Campisi suggests that fibroblast senescence within the context of tumorigenesis or aberrant hyperplasia mobilizes a detrimental version of both the systemic and local repair-mediated activation program [25]. Furthermore, tumor-activated senescent fibroblasts display the acquisition of a senescent-associated secretory phenotype, characterized by a prominent increase in the secretion of pro-inflammatory cytokines that stimulate tumor progression [26, 27]. Taken within the context of a benign disease, this scenario would be consistent with the chronic inflammation, epithelial hyperplasia, and the reactive stromal repair-type responses commonly observed in BPH.
Reactive Stroma Repair and Myofibroblasts
Although the mechanisms of genesis and/or recruitment of myofibroblasts to diseased tissue regions is as yet unresolved [28], myofibroblasts play a central role in fibrotic diseases of the skin, liver, pancreas, kidney and urogenital tissues, among others. Myofibroblasts exhibit properties of both smooth muscle cells and fibroblasts, secreting a host of growth factors and chemokines [29], and are defined here histologically as co-expressing vimentin, smooth muscle α actin (α-SMA), tenascin-C, and pro-collagen type 1, while negative or low for calponin (Figure 5) [14, 15, 30–33]. Myofibroblast involvement in skin fibrosis is displayed within the context of TGF-β1-driven matrix expression by myofibroblasts as a major contributor to systemic cutaneous sclerosis [34]. Similarly, the role that myofibroblasts play in facilitating liver fibrogenesis was illustrated via specific antibody-targeted myofibroblast apoptosis, significantly reducing liver fibrosis [35]. This is also observed within the inflamed pancreas, where pancreatic stellate cells phenotypically stress-convert to myofibroblasts in response to inflammatory chemokines. Activated pancreatic myofibroblasts then initiate ECM filament synthesis and deposition, playing a pivotal role in pancreatic fibrogenesis [36].
Figure 5. Phenotypic markers of stromal cell differentiation.
Myofibroblasts and fibroblasts are both proliferative and synthetic, while smooth muscle is primarily quiescent. In the prostate gland, stromal cell types can be distinguished histologically, and by overlapping expression patterns of vimentin, α-SMA, intracellular synthesis of pro-collagen type 1, deposition of the glycoprotein tenascin-C, and calponin. (With adaptation from The Journal of Urology, Vol 166, Tuxhorn J. et al., Reactive Stroma in Prostate Cancer Progression, Figure 2 on p2474, ©2001 American Urological Association, Inc., with permission from Elsevier).
Additionally, in kidney fibrosis, unregulated proliferation and excessive synthesis of matrix components by myofibroblasts was critical to disease pathogenesis [37]. This was further illustrated by observation of reduced synthetic contribution in progression towards tubulointerstitial renal fibrosis via Erk1/2-mediated myofibroblast inhibition [38]. This is also observed in the male reproductive tract disorder, Peyronie’s disease, wherein proliferative myofibroblasts partially mediate fibrotic plaque formation following induction by transformaing growth factor β1 (TGF-β1) [39]. In females, this process is observed during the pathogenesis of endometriotic lesions, as stromal compartment response to IL-8 is associated with inflammatory regulation [40]. Furthermore, a mechanism of the stromal cell response to IL-8 is mediated through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), among other signals, via mitogenic transduction through p38-mitogen activated protein kinases (p38-MAPK) [41, 42]. Collectively, the role of IL-8 in promoting disease progression of prostate cancer, within the peripheral zone specifically, is well documented [43].
Our own studies have identified the myofibroblast as a critical component of the ‘reactive’ nature of the stroma associated with pre-neoplastic disease in the prostate peripheral zone [15, 32]. Moreover, we have shown that myofibroblasts are associated spatially with BPH acini that overexpress IL-8 [13] and the regulation of myofibroblast differentiation and tenascin-C expression by IL-8 [44], implicating myofibroblasts and IL-8 action in the genesis of BPH. Therefore, IL-8 is involved in the stromal myofibroblast response observed across multiple fibrotic pathologies, but its potentially inductive role in the stromal response to benign hyperplasia within the human prostate transition zone is not well understood.
Benign Prostatic Hyperplasia and Chronic Inflammation
At present, there is no consensus on the etiology of BPH. There have been many suggestions, some mechanical in nature, such as altered urodynamic function due to increased prostatic urethral angulation [45]. Some have identified potential molecular events gone awry, such as elevated oxidative stress [46, 47], ischemic damage due to vascular impairment [48], loss of negative regulators of cell cycle control [49], or age-related changes in hormone levels [50, 51]. However, the majority of etiological postulations point toward prostatic inflammation as an initiator of BPH [52–57]. Although there is still no agreement as to whether inflammation is simply a parallel occurrence or a direct cause, several within the field have found a significant correlation between inflammation and BPH [53, 55, 58].
The etiology of BPH is likely complex and multi-factorial, although it is clear that this disorder is progressive and associated with chronic inflammation [52, 53, 59–63]. Risk for BPH symptoms has been shown to be significantly higher in men with chronic intraprostatic inflammation [64]. The histopathology is suggestive of other fibrotic disorders where elevated cytokines and chemokines are associated with a chronic inflammation or a chronic wound repair environment. BPH could be considered a reactive phenotype in response to a fundamental alteration in tissue homeostasis associated with aging, altered hormonal levels, and chronic inflammation. Several studies, including our own, have shown that altered cytokine/chemokine expression is associated with BPH foci and local stromal responses in human BPH [13, 65, 66]. Our studies implicate IL-8 as a key upstream factor, and Penna has concluded that among the 11 cytokines/chemokines examined, "IL-8 appears to be the most reliable and predictive surrogate marker" for the diagnosis of chronic prostatitis and BPH [66]. Studies by Macoska have shown that expression of both chemokine [C-X-C motif] ligand (CXCL) 5 and CXCL12 are elevated in aged prostate stromal cells, and that CXCL5 is significantly higher in men with BPH and prostatitis [65, 67]. It is becoming clearer that inflammation, elevated chemokine expression, and induction of a tissue repair type of fibrotic / hyperplastic responses are interrelated in the development of BPH.
Conditions leading to inflammation and the up regulation of chemokines and cytokines in the aged human prostate gland are not understood and are likely to be quite complex. In addition to the conditions already discussed, these conditions could include altered ratios of sex steroids (testosterone/estrogen ratios) [68–71], increased oxidative stress [72], metabolic syndromes [73, 74], chronic inflammation [52, 53, 63], and altered activity of autonomic nerves [75, 76]. Each of these individually, or differentially in combination, could lead to altered prostate gland homeostasis and a compensatory response.
It is likely that a combination of conditions result in the manifestation of BPH clinical symptoms. This makes the modeling of BPH with mice a difficult endeavor. However, a major advancement in therapeutic concepts would be made with the identification of a drugable target pathway or series of pathways that would at least regulate specific aspects of the BPH hyperplastic phenotype. With the keratinocyte-derived chemokine (KC; murine homolog of human IL-8) transgenic mouse reported by our laboratory [44], it is at least possible to address the effects, biology, and key downstream mediators induced by over-expression of a specific chemokine candidate implicated in the BPH phenotype. It is also possible to test whether uncoupling this biology via drugs or induced gene expression would attenuate the hyperplastic phenotype. Our project is focused on this approach and we believe that IL-8 action and pathways are targets of opportunity for new therapeutic advancements.
In a recent review on BPH pathophysiology and therapy Sampson and Berger 2008 identify altered cytokine and chemo-attractant production by remodeled BPH stroma as promoting local inflammation, elevated inflammatory cytokines and reactive oxygen/nitrogen species, and local hypoxia as a result of increased oxygen demands of proliferating cells as key events in developing a reactive stroma. Further, the authors identify prospective BPH therapeutic targets that include selective hormone antagonists/agonists, antioxidant or redox reactants like hypoxia-inducible factor 1α (HIF-1α), analogues of vitamin D, and targets inducing reactive stroma [77]. Interestingly, elocalcitol (or the pharmaceutical BXL-628), a vitamin D receptor agonist previously shown to arrest BPH patient prostate growth [78], significantly inhibited IL-8-mediated proliferation and NF-κB-activation of BPH stromal cells [79]. Specifically, elocalcitol attenuated IL-8-induced myosin phosphatase target 1 (MYPT-1) phosphorylation, membrane translocation of Ras-homolog gene family member A (RhoA), cyclooxygenase-2 (COX-2) expression, prostaglandin-E 2 production, and arrested nuclear translocation of NF-κB p65, collectively exerting an anti-proliferative, anti-inflammatory effect on BPH activated stromal cells [79]. The altered expression and biology induced by several chemokines and cytokines in BPH lends further evidence that inflammation related events mediate much of the BPH phenotype.
Chemokines and Cytokines in BPH reactive stroma
Many chemokines and cytokines have been shown to be associated with BPH. The CXC chemokines shown to be specifically associated with BPH include IL-1α [80], IL-2 [81], IL-8 [66, 82], IL-15 and IL-17 [83, 84]. Among these, IL-8 was shown to be expressed specifically in BPH epithelial cells, and to stimulate expression of fibroblast growth factor 2 (FGF2) in vitro [82, 85]. Additionally, IL-8 was shown to promote the proliferation of prostate epithelial cells in culture [82] and was identified as the most reliable predictive biomarker of BPH [66]. Furthermore, IL-8 was shown to augment the intensity of localized inflammation and tissue damage in periapical pathologies [86] and to regulate cell proliferation, migration and angiogenesis in tumorigenic or fibrotic pathologies within multiple organs, including the prostate, breast, ovaries, pancreas, and pulmonary system [87–91]. Thus, the protective effect of a critically fast and forceful response by α-chemokines for defense against infection has the potential to be subverted in a feed-forward scenario of extensive and chronic tissue damage [92]. IL-8 is therefore a critical mediator of inflammation, and does directly impact epithelial phenotype, a particularly apparent component of BPH nodular growth. Our group has shown that epithelial expression of IL-8 expression is elevated within some BPH hyperplastic acini and this is spatially associated with a prototypical myofibroblast reactive stroma with altered expression of tenascin-C, a marker of reactive stroma repair tissue [13]. Furthermore, we showed that expression of KC targeted to prostate gland epithelium in a transgenic mouse elicited phenotypic changes in both epithelium and adjacent stromal cells, consistent with a BPH phenotype [44]. Hence, emerging literature implicates IL-8 as a key factor in the induction of prototypical reactive stroma responses in several disorders that include both BPH and prostatic carcinoma.
Stromal-derived factor 1α (SDF-1α), also termed CXCL12, is also emerging as a key factor in regulating reactive stroma responses. SDF-1α activates its cognate chemokine receptor [C-X-C motif] chemokine receptor 4 (CXCR4) to facilitate proliferation, migration, and metastatic potential in a variety of cancers, including prostate [93], breast [94], lung [95], multiple myeloma [96], leukemia [97], and colon and pancreatic [98]. A handful of recent articles have discussed the potential for reactive stroma expression of SDF-1α contributing to cancer progression [99–102]. However, the role of SDF-1α in facilitating a reactive stroma in BPH is not completely understood. Co-culture or conditioned medium from prostate cancer-associated fibroblasts expressing high levels of CD90, a marker indicating mesenchymal stem cell lineage, increased CXCR4 expression in BPH-1 non-malignant prostate epithelial cells, and in vitro communication was partially dependent on TGF-β, leading to a reduced apoptotic index for BPH-1 cells [103]. These data indicate that acquisition of elevated CXCR4 expression and activation in BPH epithelium may in part be driven by enhanced secretion and activation by SDF-1α in BPH reactive stromal cells.
Macoska and colleagues have shown that prostate stromal fibroblasts isolated from elderly men aged 63–81 years of age express and secrete SDF-1α/CXCL12 and CXCL5 (or ENA-78) at higher levels than those isolated from younger men, activating a CXCR4-mediated signaling induction of proliferation [104]. Furthermore, Macoska and colleagues showed that in prostate disease patients presenting with low serum PSA, total SDF-1α and CXCL5 blood levels may be useful biomarkers to distinguish between prostate cancer (SDF-1α high) and BPH (CXCL5 high) [65]. Interestingly, Macoska and colleagues have also shown by transcriptome profiling that aging prostate reactive stroma displays an up-regulation of several secreted inflammatory chemokines including CXCL1, CXCL2, CXCL5, CXCL6, CXCL12, as well as IL-11, IL-33 and cytokine-like 1 (CYTL1) [105]. Further, it was confirmed at the protein level that CXCL1, CXCL5, and CXCL6 secretion was elevated in primary BPH stromal fibroblasts, and that CXCL1, CXCL5, CXCL6, and CXCL12 all activated a sustained proliferative response in prostate stromal fibroblasts and epithelial cells [105]. The authors postulate that “inflammatory mediators are secreted by prostatic stroma consequent to aging” which facilitate “low-level increases in the proliferative rate” potentially explaining the “low-level, but cumulative, proliferation of both epithelial and fibroblastic/myofibroblastic cell types that characterizes the aging-associated development of benign prostatic hypertrophy” [105]. Therefore, it is clear that CXCL5, and perhaps CXCL12, signaling axes play a dominant role in facilitating the reactive stroma response to BPH.
A recent study identified chemokine [C-C] ligand (CCL) 5 secreted by aged prostate activated-fibroblasts as critical to increased proliferation, elevated migration, and significant induction of angiogenesis-related genes in non-tumorigenic BPH-1 prostate epithelial cells [106]. In an effort to detect age-altered gene expression in mouse prostate glandular-adjacent stromal cells, Bianchi-Frias and colleagues utilized young and old micro dissected mouse prostate fibroblasts for protein array analysis [107]. This study showed a fibroblast-specific increase in transcripts that regulate inflammation, such as CCL8 and CCL12, directly associated with increased age. Aging-correlated expression was also observed for fibroblast-secreted factors altering the cellular response to genotoxic or oxidative stress, like apolipoprotein D and Serpinb5, as well as paracrine-acting ECM regulators like cysteine-rich protein 61 [107]. Furthermore, in aged fibroblasts, there was a noted decrease in expression of several collagen genes, including Col1a1 and Col3a1 [107]. Thus, considerable work points to secreted chemokines (IL-8, CXCL5, CXCL12) and cytokines (CCL5, CCL8) as driving-force factors in promoting a BPH reactive stroma, strongly implicating these factors as potentially viable therapeutic targets.
Interleukin-8 and Benign Prostatic Hyperplasia
Interleukins, in general, are mediators of inflammatory processes and are central to induction of wound repair responses and have been implicated in fibrosis. In the prostate gland, IL-8, IL-1α and IL-6 have each gained attention. Over expression of IL-8 is universally observed in many proliferative disorders and sites of inflammation. IL-8 is a multifunctional chemokine interleukin that regulates many immune host responses and wound repair mechanisms. The elevated expression of several interleukins and other cytokines known to be associated with tissue repair, have also been reported in BPH [57, 109, 110]. Over expression of these factors over time would be expected to induce a reactive stroma wound repair response as a compensatory event. Moreover, epithelial proliferation may be induced indirectly, again as a compensatory repair mechanism.
There is considerable evidence that implicates IL-8 in hyperplastic disorders. IL-8 is an interleukin most often associated with induced wound repair, having coordinating effects on leukocyte infiltration, stromal proliferation, angiogenesis, re-epithelialization and expression of putative downstream factors such as FGF-2. In vitro studies have shown that IL-8 is over-expressed in human BPH epithelial cells and induced expression of FGF-2 in human prostate stromal cells [13, 82, 85]. Both IL-8 and FGF-2 are potent inducers of reactive stroma and angiogenesis [13, 111, 112]. IL-8 is also over expressed in asymptomatic prostatitis [113] and chronic prostatitis/chronic pelvic pain syndrome [114]. Remarkably, in a screen of 11 cytokines/chemokines, IL-8 was shown to be the most reliable surrogate marker in seminal plasma to diagnose chronic prostatitis and BPH [66]. This study showed a correlation between inflammatory infiltrate and IL-8 producing cells in prostate specimens. IL-8 is a potent chemotactic factor for neutrophil recruitment and infiltration [115] and has been shown to be mitogenic and chemotactic to endothelial cells when studied in prostate cancer models [87, 116]. In agreement with these studies, more recent studies show that IL-8 can directly stimulate angiogenesis [117] via the CXCR2 receptor and Phosphatidylinositol 3-kinase (PI3K) and Erk1/2 pathways [118].
IL-8 also affects stromal cell migration and biology. In airway disorders, IL-8 was shown to affect human airway smooth muscle cell migration and contraction, consistent with tissue repair biology [119]. Mechanistically, IL-8 stimulates phosphorylation of Cbl and Akt [120]. Inhibitors of PI3K blocked IL-8 induced chemotaxis of neutrophils [120]. Accordingly, the PI3K and Akt pathways are key IL-8 signaling mechanisms in chemotaxis of IL-8 target cells, however, this has not been examined carefully in stromal cells.
IL-8 over expression has also been associated with aging, which is clearly correlated with the genesis of BPH. Michael Ittmann and colleagues showed that IL-8 levels are elevated when prostate epithelial cells undergo senescence [82]. This study went on to show that IL-8 promotes proliferation of human primary and immortalized prostatic epithelial cells in vitro [82]. This was the first report that proposed IL-8 as a key factor in the genesis of BPH. Others, using prostate cancer models have shown similar results. Prostate cancer cells over expressing IL-8 showed elevated tumorigenesis and neovascularization [87, 116]. In addition, IL-8 was also shown to stimulate androgen-independent proliferation and migration of LNCaP cells [54]. Another study showed that over expression of IL-8 in prostate cancer cells resulted in elevated motility, invasion, and microvessel density [121]. IL-8 also stimulated migration [122] and invasion [123] of breast cancer cells. Expression of IL-8 in various epithelial cells has been shown to be induced by oxidative stress [124], Toll-like receptor-4 [125], insulin-like growth factor 1 [126], IL-1α [58], acetylcholine [127], and various infectious agents [128], consistent with a role in mediating inflammation and repair. Much about the potential role of IL-8 in regulating tissue homeostasis is also predicted based on the known biology of the murine KC chemokine.
Murine KC (keratinocyte-derived chemokine) is the functional homolog of human IL-8 in mice [129]. KC and IL-8 are both CXC α-chemokines, and belong to the ELR motif family. The ELR family of α-chemokines is chemotactic to neutrophils and plays a key role in inflammation, angiogenesis and wound healing/fibrosis [130, 131]. KC, like IL-8, is a primary regulator of neutrophil recruitment, epithelial migration and wound closure [129]. CXCR1 and CXCR2 are cognate receptors for IL-8 and exhibit differential expression, regulation and biological activity. For example, stimulation of human endothelial cell proliferation and chemotaxis, important in angiogenesis, is regulated through CXCR2 [118, 132]. Activation of ERK1/2 pathways has been implicated [118, 133]. In mice KC is rapidly expressed by epithelial cells at sites of wounding or infection [129], similar to IL-8 and binds to human IL-8 CXCR2 (type b) receptor (corresponds to murine CXCR2 receptor) at a site competitive with IL-8 and Gro-alpha/MGSA [134]. Murine CXCR2 is considered to be a cognate receptor for KC (Kd = 5nM) [134]. Knockout of CXCR2 in mice led to defective wound repair, decreased angiogenesis, decreased re-epithelialization, and low neutrophil recruitment, decreased macrophage recruitment and lower vascular cell adhesion molecule 1 (VCAM-1) expression, although mice are viable [115, 135]. The expression pattern of KC in wounding (in mice) is nearly identical to IL-8 in humans [136]. As discussed previously, the overexpression of KC in a transgenic mouse prostate gland produced a moderate glandular and reactive stromal phenotypes that included elevated expression of tenascin-C. Each of these changes is consistent with some of the changes observed in human BPH.
These studies taken together suggest that IL-8 is a key factor in regulating a reactive microenvironment in BPH, typified by both epithelial and stromal cell hyperplasia. IL-8 is over expressed at nearly every site of altered tissue homeostasis, wound repair, inflammation, fibroses, and in BPH. Our data have shown that elevated IL-8 expression is significantly higher in BPH as compared to normal prostate, and that IL-8 over expression mediated altered deposition patterns of tenascin-C in human prostate stromal cells. Several chemokines and cytokines regulate the biology of matrix proteins and glycoproteins, including tenascin-C, which exhibits several important biological functions during wound repair. It seems likely therefore, that the biology of myofibroblasts and the extracellular matrix are critical to mediating the stromal-directed effects of altered chemokines and cytokines in the development of epithelial hyperplasia in BPH.
Extracellular Matrix in Reactive Stroma and BPH
Tenascin-C is an extracellular matrix glycoprotein that exhibits altered expression and deposition patterns in human BPH. We have shown that this is statistically correlated with elevated epithelial expression of IL-8 [13]. Moreover, tenascin-C is deposited in activated fibroblasts/myofibroblasts immediately adjacent to BPH epithelial acini. These cells have a phenotype very similar to what we have reported for reactive stroma in prostate cancer. Reactive stroma consisting of activated fibroblasts and myofibroblasts is a common phenotype associated with both transition zone BPH and peripheral zone cancer and these cells are not observed in the normal human prostate gland [14, 15]. Our studies have shown that IL-8 alters focal expression / deposition of tenascin in normal human prostate stromal cells in vitro and that these IL-8 treated fibroblasts also exhibited a reactive phenotype.
There is considerable evidence that tenascin-C is a regulator of a reactive fibroblasts/myofibroblasts in wound repair and stromal hyperplasia. Functional roles have been addressed by generating tenascin-C null mice. Tenascin-C is up regulated in chronic liver disease. Knockout of tenascin-C attenuated liver fibrosis induced by chronic hepatitis [137]. Recruitment of myofibroblasts to sites of liver fibrosis was inhibited. Tenascin-C knockout mice show normal development, however have defective wound repair during an induced glomerulonephritis and dermatitis [138, 139], defective neointimization after vascular surgery, and interestingly, defective myofibroblast recruitment in repair to myocardial injury [140]. These studies went on to show that tenascin-C stimulated myofibroblast differentiation in activated stromal cells. Tenascin-C also stimulated smooth muscle proliferation/hyperplasia during neointimization of arterial bypass grafts leading to graft failure [141]. Inhibition of tenascin-C expression resulted in a decreased smooth muscle hyperplasia in this model.
Several factors known to be overexpressed at sites of wound repair and hyperplasia function to regulate tenascin-C levels. Stretch-induced strain on the cell and growth factors, including TGF-β, has been shown to stimulate tenascin expression in fibroblasts [142]. TGF-β regulation of tenascin in fibroblasts is dependent on functional Smad3, Ets1, Sp1 and CBP/p300 [143]. Other modulators of wound repair stroma also stimulate tenascin expression in fibroblasts. For example, platelet-derived growth factor (PDGF), a potent regulator of fibroblast proliferation during wounding upregulates tenascin in fibroblasts through a PI3K/Akt pathway [143]. McKean showed that fibroblast migration depended on interaction with tenascin and activation of focal adhesion kinase (FAK) [144]. These studies showed that FAK stimulated tenascin expression via FAK induction of the transcription factor paired-related homeobox 1 (Prx1).
Along with these many functions promoting cell proliferation and migration, apoptosis is also affected by tenascin-C. Tenascin-C stimulated stromal cell adhesion and survival by activating Akt in human chondrosarcoma cells [145]. Tenascin-C also promoted epithelial cell proliferation and migration. Branching morphogenesis in lung development is dependent on tenascin-C, which is regulated by oxygen tension [146]. Here, tenascin-C is expressed at the growing tips and it was proposed that it promotes the penetration of epithelial branching into the surrounding mesenchyme, as well as branching of the expanding airways [147]. Similar biological processes are regulated by tenascin-C in diseases. Invasion by colon cancer cells is mediated by myofibroblast-expressed tenascin-C, which in turn, stimulated invasion via RhoA/Rac mechanisms [148]. Other studies have shown synergistic effects of TGF-β and tenascin in breast cancer epithelial cell invasion in vitro through stimulating MMP-9 expression [149].
Tenascin-C also interacts with other matrix proteins known to function in cell adhesion. Tenascin-C interacts specifically with fibronectin and integrin receptors to regulate cell proliferation and migration [150]. Tenascin-C was shown to interact directly with heparin sulfate proteoglycans [151]. This interaction stimulated fibroblast adhesion to tenascin-C. Integrin-β3 has been implicated specifically in fibroblasts. Integrin-β3 ligand binding and activation of Src/MAPK/MMP modulated the deposition of tenascin-C [152].
Together, each of these studies, as well as other literature not cited here, show that tenascin-C is a potent modulator of tissue biology during development, remodeling, wound repair, fibrosis, and stromal hyperplasia. Since others and we have shown that tenascin expression is altered in BPH stroma, and we now know that tenascin is regulated by IL-8, tenascin-C emerges as a likely candidate regulator of the BPH hyperplastic phenotype. The up regulation of tenascin by TGF-β and PDGF is consistent with its putative role in BPH, however the specific molecular signaling and migratory activation roles of tenascin-C in BPH stromal activation have yet to be characterized.
In order to further define the role of tenascin-C in mouse prostate development and differentiation, Ishii and colleagues found that tenascin-C knockout mice displayed an age-dependent protrusion of epithelial cell clusters into the ductal lumen of the mouse anterior and dorso-lateral prostate lobes, as well as an increase in binucleated epithelial cells concurrent with a decrease in androgen receptor-positive epithelia [153]. The authors verified this effect on cytodifferentiation, morphogenesis, and androgen receptor expression using sub-renal implanted tissue recombinants of dorso-lateral prostate epithelium and tenascin-C knockout urogenital mesenchyme [153]. These data strongly suggest that tenascin-C plays a role in epithelial cell maintenance and proliferation, and thus elevated tenascin-C expression by BPH reactive stroma cells likely fosters a similar promotion of epithelial hyperplastic behavior, although this has not been studied directly.
It is likely that several other matricellular proteins and factors are involved in the genesis of BPH, although not much is understood about these factors. Versican has been studied in BPH. Stromal-expressed versican is another proteoglycan member of the complex group of ECM molecules elevated in BPH [154]. Versican immunoreactivity was increased specifically in the reactive stroma of BPH nodules, as was higher mRNA transcript levels of versican variants V0, V1, and V3, relative to non-nodular transition zone or peripheral zone parenchyma controls [154].
These studies and several others suggest that elevated expression of versican, tenascin-C and several other matrix-associated factors may play a role in reactive stroma formation and promotion of BPH nodular growth. Much additional work is needed to identify and understand the complex biology regulated by matrix-associated factors in BPH.
Summary and Conclusions
The etiology and specific mechanisms that lead to phenotypic changes that manifest as benign prostate disease remains poorly understood. Recent data suggest that pathophysiological signaling mechanisms are complex, likely involving age-related and chronic defects in tissue homeostasis that lead to compensatory and reactive changes in both the stroma and the epithelial tissue compartments. The historical perspective of BPH biology suggests that the compensatory biology likely involves reactivation of embryonic developmental patterns. However, it is clear that a repair-compensatory biology in adult tissues involves far more that developmental programming alone. Involved also, are inflammatory and repair responses that are usually only observed in post-natal tissues. These responses include altered expression of chemokines, cytokines, matrix remodeling, chronic inflammation, altered immune surveillance, and the formation of a prototypical reactive stroma similar to that observed at other tissue sites of wound repair. Since stromal tissue, both embryonic mesenchyme, and adult reactive stroma myofibroblasts, has been shown to have potent regulatory functions on epithelial proliferation and differentiation, as well as immune modulatory functions, the biology of this reactive stroma in an adult disease typified by epithelial and stromal hyperplasia becomes an important biology to understand. The mechanisms that regulate reactive stroma biology in BPH are targets of opportunity for new therapeutic approaches. Accordingly, the dissection of important factors, signaling pathways, genes, and other regulatory components that mediate the interplay between epithelium and stromal responses in BPH is a key priority.
Acknowledgments
Funding Sources: Supported by NIH grants R01 DK083293 and R01 CA58093
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17(3):477–486. [PubMed] [Google Scholar]
- 2.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15(4):340–345. [PubMed] [Google Scholar]
- 3.Risbridger GP, Almahbobi GA, Taylor RA. Early prostate development and its association with late-life prostate disease. Cell Tissue Res. 2005;322(1):173–181. doi: 10.1007/s00441-005-1121-9. [DOI] [PubMed] [Google Scholar]
- 4.Hunter D, Davies J. The Glandular Organ Development Database. Medical Research Council; 1997. An Overview of Prostate Gland Development. [Google Scholar]
- 5.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2(1):35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 6.Price H, McNeal JE, Stamey TA. Evolving patterns of tissue composition in benign prostatic hyperplasia as a function of specimen size. Hum Pathol. 1990;21(6):578–585. doi: 10.1016/s0046-8177(96)90002-7. [DOI] [PubMed] [Google Scholar]
- 7.van der Heul-Nieuwenhuijsen L, et al. Gene expression profiling of the human prostate zones. BJU Int. 2006;98(4):886–897. doi: 10.1111/j.1464-410X.2006.06427.x. [DOI] [PubMed] [Google Scholar]
- 8.Berry SJ, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs JT. Etiology of benign prostatic hyperplasia. Eur Urol. 1994;25 Suppl 1:6–9. doi: 10.1159/000475324. [DOI] [PubMed] [Google Scholar]
- 10.Claus S, et al. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol Res. 1993;21(5):305–308. doi: 10.1007/BF00296825. [DOI] [PubMed] [Google Scholar]
- 11.Morrison C, Thornhill J, Gaffney E. The connective tissue framework in the normal prostate, BPH and prostate cancer: analysis by scanning electron microscopy after cellular digestion. Urol Res. 2000;28(5):304–307. doi: 10.1007/s002400000123. [DOI] [PubMed] [Google Scholar]
- 12.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;173(4):1309–1313. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 13.Schauer IG, et al. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72(1):205–213. doi: 10.1016/j.urology.2007.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166(6):2472–2483. [PubMed] [Google Scholar]
- 15.Tuxhorn JA, et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- 16.Lin VK, et al. Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property. Prostate. 2007;67(12):1265–1276. doi: 10.1002/pros.20599. [DOI] [PubMed] [Google Scholar]
- 17.Klezovitch O, et al. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6(2):185–195. doi: 10.1016/j.ccr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Le Quement C, et al. Mmp-12 Induces Il-8/Cxcl8 Secretion through Egfr and Erk1/2 Activation in Epithelial Cells. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.00489.2007. [DOI] [PubMed] [Google Scholar]
- 19.Li DQ, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smythies LE, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80(3):492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 21.Joseph T, Look D, Ferkol T. NF-kappaB activation and sustained IL-8 gene expression in primary cultures of cystic fibrosis airway epithelial cells stimulated with Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L471–L479. doi: 10.1152/ajplung.00066.2004. [DOI] [PubMed] [Google Scholar]
- 22.Malgorzata Goczalik I, et al. The activation of IL-8 receptors in cultured guinea pig Muller glial cells is modified by signals from retinal pigment epithelium. J Neuroimmunol. 2005;161(1–2):49–60. doi: 10.1016/j.jneuroim.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Obata H, Tsuru T. Corneal wound healing from the perspective of keratoplasty specimens with special reference to the function of the Bowman layer and Descemet membrane. Cornea. 2007;26(9 Suppl 1):S82–S89. doi: 10.1097/ICO.0b013e31812f6f1b. [DOI] [PubMed] [Google Scholar]
- 24.Ratner AJ, et al. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem. 2006;281(18):12994–12998. doi: 10.1074/jbc.M511431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campisi J. Cellular senescence: putting the paradoxes in perspective. Current opinion in genetics & development. 2011;21(1):107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davalos AR, et al. Senescent cells as a source of inflammatory factors for tumor progression. Cancer metastasis reviews. 2010;29(2):273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppe JP, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual review of pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5(3):334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell DW, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 30.Ayala G, et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–4801. [PubMed] [Google Scholar]
- 31.McAlhany SJ, et al. Promotion of angiogenesis by ps20 in the differential reactive stroma prostate cancer xenograft model. Cancer Res. 2003;63(18):5859–5865. [PubMed] [Google Scholar]
- 32.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17(4):411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 33.Yang F, et al. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65(19):8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- 34.Farina G, et al. Cartilage-oligomeric-matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor-{beta} Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.086850. [DOI] [PubMed] [Google Scholar]
- 35.Douglass A, et al. Antibody-targeted myofibroblast apoptosis reduces fibrosis during sustained liver injury. J Hepatol. 2008 doi: 10.1016/j.jhep.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu K. Pancreatic stellate cells: molecular mechanism of pancreatic fibrosis. J Gastroenterol Hepatol. 2008;23 Suppl 1:S119–S121. doi: 10.1111/j.1440-1746.2007.05296.x. [DOI] [PubMed] [Google Scholar]
- 37.Gao X, et al. Connective tissue growth factor stimulates renal cortical myofibroblast-like cell proliferation and matrix protein production. Wound Repair Regen. 2008;16(3):408–415. doi: 10.1111/j.1524-475X.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Pena AB, et al. Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int. 2008 doi: 10.1038/ki.2008.160. [DOI] [PubMed] [Google Scholar]
- 39.Cantini LP, et al. Profibrotic Role of Myostatin in Peyronie's Disease. J Sex Med. 2008 doi: 10.1111/j.1743-6109.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirota Y, et al. Activation of protease-activated receptor 2 stimulates proliferation and interleukin (IL)-6 and IL-8 secretion of endometriotic stromal cells. Hum Reprod. 2005;20(12):3547–3553. doi: 10.1093/humrep/dei255. [DOI] [PubMed] [Google Scholar]
- 41.Jung YD, et al. Role of P38 MAPK, AP-1, and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 2002;18(4):206–213. doi: 10.1006/cyto.2002.1034. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 43.Waugh DJ, et al. Multi-faceted roles for CXC-chemokines in prostate cancer progression. Front Biosci. 2008;13:4595–4604. doi: 10.2741/3025. [DOI] [PubMed] [Google Scholar]
- 44.Schauer IG, Ressler SJ, Rowley DR. Keratinocyte-derived chemokine induces prostate epithelial hyperplasia and reactive stroma in a novel transgenic mouse model. Prostate. 2009;69(4):373–384. doi: 10.1002/pros.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho KS, et al. The overlooked cause of benign prostatic hyperplasia: prostatic urethral angulation. Med Hypotheses. 2008;70(3):532–535. doi: 10.1016/j.mehy.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Gradini R, et al. Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology. J Pathol. 1999;189(2):224–229. doi: 10.1002/(SICI)1096-9896(199910)189:2<224::AID-PATH422>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 47.Aryal M, et al. Oxidative stress in patients with Benign Prostate Hyperplasia. JNMA J Nepal Med Assoc. 2007;46(167):103–106. [PubMed] [Google Scholar]
- 48.Berger AP, et al. Vascular damage as a risk factor for benign prostatic hyperplasia and erectile dysfunction. BJU Int. 2005;96(7):1073–1078. doi: 10.1111/j.1464-410X.2005.05777.x. [DOI] [PubMed] [Google Scholar]
- 49.Cordon-Cardo C, et al. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90(17):1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- 50.Hulka BS, et al. Serum hormone levels among patients with prostatic carcinoma or benign prostatic hyperplasia and clinic controls. Prostate. 1987;11(2):171–182. doi: 10.1002/pros.2990110208. [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee B. The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer. Mol Cell Biochem. 2003;253(1–2):89–101. doi: 10.1023/a:1026057402945. [DOI] [PubMed] [Google Scholar]
- 52.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006;16(1):25–29. [PubMed] [Google Scholar]
- 53.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Lee KL, Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol. 2004;172(5 Pt 1):1784–1791. doi: 10.1097/01.ju.0000133655.71782.14. [DOI] [PubMed] [Google Scholar]
- 55.Nickel JC. Inflammation and benign prostatic hyperplasia. Urol Clin North Am. 2008;35(1):109–115. doi: 10.1016/j.ucl.2007.09.012. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sciarra A, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108(3–5):254–260. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005;40(3):121–128. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, et al. Chronic inflammation in benign prostatic hyperplasia: Implications for therapy. Med Hypotheses. 2008;70(5):1021–1023. doi: 10.1016/j.mehy.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Delongchamps NB, et al. Evaluation of prostatitis in autopsied prostates--is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179(5):1736–1740. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Silverio F, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43(2):164–175. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 61.Kohnen PW, Drach GW. Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J Urol. 1979;121(6):755–760. doi: 10.1016/s0022-5347(17)56980-3. [DOI] [PubMed] [Google Scholar]
- 62.Nickel JC, et al. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84(9):976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 63.Yi FX, et al. Risk factors for prostatic inflammation extent and infection in benign prostatic hyperplasia. Asian J Androl. 2006;8(5):621–627. doi: 10.1111/j.1745-7262.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 64.Mishra VC, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100(2):327–331. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- 65.Macoska JA, et al. Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate. 2008;68(4):442–452. doi: 10.1002/pros.20717. [DOI] [PubMed] [Google Scholar]
- 66.Penna G, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. discussion 533. [DOI] [PubMed] [Google Scholar]
- 67.Begley LA, et al. CXCL12 activates a robust transcriptional response in human prostate epithelial cells. J Biol Chem. 2007;282(37):26767–26774. doi: 10.1074/jbc.M700440200. [DOI] [PubMed] [Google Scholar]
- 68.Nakhla AM, et al. Estradiol causes the rapid accumulation of cAMP in human prostate. Proc Natl Acad Sci U S A. 1994;91(12):5402–5405. doi: 10.1073/pnas.91.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts RO, et al. Polymorphisms in genes involved in sex hormone metabolism may increase risk of benign prostatic hyperplasia. Prostate. 2006;66(4):392–404. doi: 10.1002/pros.20362. [DOI] [PubMed] [Google Scholar]
- 70.Sciarra F, Toscano V. Role of estrogens in human benign prostatic hyperplasia. Arch Androl. 2000;44(3):213–220. doi: 10.1080/014850100262191. [DOI] [PubMed] [Google Scholar]
- 71.Wilson JD, Griffin JE. The use and misuse of androgens. Metabolism. 1980;29(12):1278–1295. doi: 10.1016/0026-0495(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 72.Aydin A, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39(2):176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 73.Kasturi S, Russell S, McVary KT. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep. 2006;7(4):288–292. doi: 10.1007/s11934-996-0008-y. [DOI] [PubMed] [Google Scholar]
- 74.Ozden C, et al. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007;51(1):199–203. doi: 10.1016/j.eururo.2006.05.040. discussion 204-6. [DOI] [PubMed] [Google Scholar]
- 75.McVary KT, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4 Pt 1):1327–1433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 76.Yun AJ, Doux JD. Opening the floodgates: benign prostatic hyperplasia may represent another disease in the compendium of ailments caused by the global sympathetic bias that emerges with aging. Med Hypotheses. 2006;67(2):392–394. doi: 10.1016/j.mehy.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Sampson N, Madersbacher S, Berger P. Pathophysiology and therapy of benign prostatic hyperplasia. Wien Klin Wochenschr. 2008;120(13–14):390–401. doi: 10.1007/s00508-008-0986-5. [DOI] [PubMed] [Google Scholar]
- 78.Adorini L, et al. Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol) J Steroid Biochem Mol Biol. 2007;103(3–5):689–693. doi: 10.1016/j.jsbmb.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 79.Penna G, et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kappaB pathways. Prostate. 2009;69(5):480–493. doi: 10.1002/pros.20896. [DOI] [PubMed] [Google Scholar]
- 80.Giri D, Ittmann M. Interleukin-1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2000;157(1):249–255. doi: 10.1016/s0002-9440(10)64535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Royuela M, et al. IL-2, its receptors, and bcl-2 and bax genes in normal, hyperplastic and carcinomatous human prostates: immunohistochemical comparative analysis. Growth Factors. 2000;18(2):135–146. doi: 10.3109/08977190009003239. [DOI] [PubMed] [Google Scholar]
- 82.Castro P, et al. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate. 2004;60(2):153–159. doi: 10.1002/pros.20051. [DOI] [PubMed] [Google Scholar]
- 83.Handisurya A, et al. Differential expression of interleukin-15, a pro-inflammatory cytokine and T-cell growth factor, and its receptor in human prostate. Prostate. 2001;49(4):251–262. doi: 10.1002/pros.10020. [DOI] [PubMed] [Google Scholar]
- 84.Steiner GE, et al. The picture of the prostatic lymphokine network is becoming increasingly complex. Rev Urol. 2002;4(4):171–177. [PMC free article] [PubMed] [Google Scholar]
- 85.Giri D, Ittmann M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2001;159(1):139–147. doi: 10.1016/S0002-9440(10)61681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marton IJ, et al. Differential in situ distribution of interleukin-8, monocyte chemoattractant protein-1 and Rantes in human chronic periapical granuloma. Oral Microbiol Immunol. 2000;15(1):63–65. doi: 10.1034/j.1399-302x.2000.150111.x. [DOI] [PubMed] [Google Scholar]
- 87.Inoue K, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6(5):2104–2119. [PubMed] [Google Scholar]
- 88.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284(4):L566–L577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 89.Uehara H, et al. Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. Prostate. 2005;64(1):40–49. doi: 10.1002/pros.20223. [DOI] [PubMed] [Google Scholar]
- 90.Vazquez-Martin A, Colomer R, Menendez JA. Her-2/neu-induced "cytokine signature" in breast cancer. Adv Exp Med Biol. 2008;617:311–319. doi: 10.1007/978-0-387-69080-3_29. [DOI] [PubMed] [Google Scholar]
- 91.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 92.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2(2):108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 93.Gladson CL, Welch DR. New insights into the role of CXCR4 in prostate cancer metastasis. Cancer Biol Ther. 2008;7(11):1849–1851. doi: 10.4161/cbt.7.11.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27(2):97–105. doi: 10.1007/s10585-008-9210-2. [DOI] [PubMed] [Google Scholar]
- 95.Gangadhar T, Nandi S, Salgia R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biol Ther. 2010;9(6):409–416. doi: 10.4161/cbt.9.6.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ooi LL, Dunstan CR. CXCL12/CXCR4 axis in tissue targeting and bone destruction in cancer and multiple myeloma. J Bone Miner Res. 2009;24(7):1147–1149. doi: 10.1359/jbmr.090503. [DOI] [PubMed] [Google Scholar]
- 97.Burger JA. CXCR4 in acute myelogenous leukemia (AML): when too much attraction is bad for you. Leuk Res. 2009;33(6):747–748. doi: 10.1016/j.leukres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Yadav VR, et al. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med. 2010;88(12):1243–1253. doi: 10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eck SM, et al. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33(3–4):214–221. doi: 10.1016/j.jaut.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jotzu C, et al. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Anal Cell Pathol (Amst) 2010;33(2):61–79. doi: 10.3233/ACP-CLO-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kojima Y, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol. 2011;89(1):31–39. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 103.Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69(9):991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Begley L, et al. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell. 2005;4(6):291–298. doi: 10.1111/j.1474-9726.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 105.Begley LA, et al. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43(2):194–199. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eyman D, et al. CCL5 secreted by senescent aged fibroblasts induces proliferation of prostate epithelial cells and expression of genes that modulate angiogenesis. J Cell Physiol. 2009;220(2):376–381. doi: 10.1002/jcp.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bianchi-Frias D, et al. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moon Y, Yang H, Lee SH. Modulation of early growth response gene 1 and interleukin-8 expression by ribotoxin deoxynivalenol (vomitoxin) via ERK1/2 in human epithelial intestine 407 cells. Biochem Biophys Res Commun. 2007;362(2):256–262. doi: 10.1016/j.bbrc.2007.07.168. [DOI] [PubMed] [Google Scholar]
- 109.Eaton AD, Xu D, Garside P. Administration of exogenous interleukin-18 and interleukin-12 prevents the induction of oral tolerance. Immunology. 2003;108(2):196–203. doi: 10.1046/j.1365-2567.2003.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steiner GE, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83(8):1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 111.Abdel-Malak NA, et al. Angiopoietin-1 promotes endothelial cell proliferation and migration through AP-1-dependent autocrine production of interleukin-8. Blood. 2008;111(8):4145–4154. doi: 10.1182/blood-2007-08-110338. [DOI] [PubMed] [Google Scholar]
- 112.Li PJ, et al. Effect of hypertension on cell proliferation and apoptosis in benign prostatic hyperplasia. Zhonghua Nan Ke Xue. 2005;11(2):94–97. [PubMed] [Google Scholar]
- 113.Hochreiter WW, Weidner W. Prostatitis--a frequently unrecognized disease. Ther Umsch. 2006;63(2):117–121. doi: 10.1024/0040-5930.63.2.117. [DOI] [PubMed] [Google Scholar]
- 114.Khadra A, et al. Interleukin-8 levels in seminal plasma in chronic prostatitis/chronic pelvic pain syndrome and nonspecific urethritis. BJU Int. 2006;97(5):1043–1046. doi: 10.1111/j.1464-410X.2006.06133.x. [DOI] [PubMed] [Google Scholar]
- 115.Devalaraja RM, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115(2):234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim SJ, et al. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3(1):33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li A, et al. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8(1):63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 118.Heidemann J, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 119.Govindaraju V, et al. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am. J Physiol Cell Physiol. 2006;291(5):C957–C965. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 120.Lane HC, Anand AR, Ganju RK. Cbl and Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated chemotaxis. Int Immunol. 2006;18(8):1315–1325. doi: 10.1093/intimm/dxl064. [DOI] [PubMed] [Google Scholar]
- 121.Araki S, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67(14):6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 122.Kim SW, et al. Tid1 negatively regulates the migratory potential of cancer cells by inhibiting the production of interleukin-8. Cancer Res. 2005;65(19):8784–8791. doi: 10.1158/0008-5472.CAN-04-4422. [DOI] [PubMed] [Google Scholar]
- 123.Yao C, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121(9):1949–1957. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- 124.Boncoeur E, et al. Oxidative stress induces extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase in cystic fibrosis lung epithelial cells: Potential mechanism for excessive IL-8 expression. Int J Biochem Cell Biol. 2008;40(3):432–446. doi: 10.1016/j.biocel.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 125.Bhattacharyya S, et al. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J Biol Chem. 2008;283(16):10550–10558. doi: 10.1074/jbc.M708833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kooijman R, et al. Regulation of interleukin-8 expression in human prostate cancer cells by insulin-like growth factor-I and inflammatory cytokines. Growth Horm IGF Res. 2007;17(5):383–391. doi: 10.1016/j.ghir.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 127.Profita M, et al. Acetylcholine mediates the release of IL-8 in human bronchial epithelial cells by a NFkB/ERK-dependent mechanism. Eur J Pharmacol. 2008;582(1–3):145–153. doi: 10.1016/j.ejphar.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 128.Buchholz KR, Stephens RS. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect Immun. 2007;75(12):5924–5929. doi: 10.1128/IAI.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Q, et al. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 130.Belperio JA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68(1):1–8. [PubMed] [Google Scholar]
- 131.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72(1):9–18. [PMC free article] [PubMed] [Google Scholar]
- 132.Addison CL, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165(9):5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 133.Zhao M, et al. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J Biol Chem. 2004;279(47):49259–49267. doi: 10.1074/jbc.M405118200. [DOI] [PubMed] [Google Scholar]
- 134.Bozic CR, et al. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269(47):29355–29358. [PubMed] [Google Scholar]
- 135.Hallgren J, et al. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci U S A. 2007;104(51):20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tobler A, et al. Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood. 1992;79(1):45–51. [PubMed] [Google Scholar]
- 137.El-Karef A, et al. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211(1):86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 138.Koyama Y, et al. Effect of tenascin-C deficiency on chemically induced dermatitis in the mouse. J Invest Dermatol. 1998;111(6):930–935. doi: 10.1046/j.1523-1747.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 139.Nakao N, et al. Tenascin-C promotes healing of Habu-snake venom-induced glomerulonephritis: studies in knockout congenic mice and in culture. Am J Pathol. 1998;152(5):1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 140.Tamaoki M, et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167(1):71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fujinaga K, et al. Locally applied cilostazol suppresses neointimal hyperplasia by inhibiting tenascin-C synthesis and smooth muscle cell proliferation in free artery grafts. J Thorac Cardiovasc Surg. 2004;128(3):357–363. doi: 10.1016/j.jtcvs.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 142.Chiquet M, Sarasa-Renedo A, Tunc-Civelek V. Induction of tenascin-C by cyclic tensile strain versus growth factors: distinct contributions by Rho/ROCK and MAPK signaling pathways. Biochim Biophys Acta. 2004;1693(3):193–204. doi: 10.1016/j.bbamcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Jinnin M, et al. Tenascin-C upregulation by transforming growth factor-beta in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene. 2004;23(9):1656–1667. doi: 10.1038/sj.onc.1207064. [DOI] [PubMed] [Google Scholar]
- 144.McKean DM, et al. FAK induces expression of Prx1 to promote tenascin-C-dependent fibroblast migration. J Cell Biol. 2003;161(2):393–402. doi: 10.1083/jcb.jcb.200302126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jang JH, Chung CP. Tenascin-C promotes cell survival by activation of Akt in human chondrosarcoma cell. Cancer Lett. 2005;229(1):101–105. doi: 10.1016/j.canlet.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 146.Gebb SA, et al. Fetal oxygen tension promotes tenascin-C-dependent lung branching morphogenesis. Dev Dyn. 2005;234(1):1–10. doi: 10.1002/dvdy.20500. [DOI] [PubMed] [Google Scholar]
- 147.Roth-Kleiner M, Hirsch E, Schittny JC. Fetal lungs of tenascin-C-deficient mice grow well, but branch poorly in organ culture. Am J Respir Cell Mol Biol. 2004;30(3):360–366. doi: 10.1165/rcmb.2002-0266OC. [DOI] [PubMed] [Google Scholar]
- 148.De Wever O, et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. Faseb J. 2004;18(9):1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 149.Ilunga K, et al. Co-stimulation of human breast cancer cells with transforming growth factor-beta and tenascin-C enhances matrix metalloproteinase-9 expression and cancer cell invasion. Int J Exp Pathol. 2004;85(6):373–379. doi: 10.1111/j.0959-9673.2004.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ingham KC, Brew SA, Erickson HP. Localization of a cryptic binding site for tenascin on fibronectin. J Biol Chem. 2004;279(27):28132–28135. doi: 10.1074/jbc.M312785200. [DOI] [PubMed] [Google Scholar]
- 151.Jang JH, et al. Identification and kinetics analysis of a novel heparin-binding site (KEDK) in human tenascin-C. J Biol Chem. 2004;279(24):25562–25566. doi: 10.1074/jbc.M403170200. [DOI] [PubMed] [Google Scholar]
- 152.Yang Y, et al. Tenascin-C deposition requires beta3 integrin and Src. Biochem Biophys Res Commun. 2004;322(3):935–942. doi: 10.1016/j.bbrc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 153.Ishii K, et al. Role of stromal tenascin-C in mouse prostatic development and epithelial cell differentiation. Dev Biol. 2008;324(2):310–319. doi: 10.1016/j.ydbio.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 154.True LD, et al. The accumulation of versican in the nodules of benign prostatic hyperplasia. Prostate. 2009;69(2):149–158. doi: 10.1002/pros.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]