Abstract
Despite therapeutic advances, the poor prognoses for acute myeloid leukemia (AML) and intermediate and high-risk myelodysplastic syndromes (MDS) point to the need for better treatment options. AML and MDS cells express the myeloid marker CD33, making it amenable to CD33-targeted therapy. Lintuzumab (SGN-33), a humanized monoclonal anti-CD33 antibody undergoing clinical evaluation, induced meaningful responses in a Phase 1 clinical trial and demonstrated anti-leukemic activity in preclinical models. Recently, it was reported that 5-azacytidine (Vidaza™) prolonged the overall survival of a group of high risk MDS and AML patients. To determine whether the combination of lintuzumab and 5-azacytidine would be beneficial, a mouse xenograft model of disseminated AML was used to evaluate the combination. There was a significant reduction in tumor burden and an increase in overall survival in mice treated with lintuzumab and 5-azacytidine. The effects were greater than that obtained with either agent alone. As the in vivo anti-leukemic activity of lintuzumab was dependent upon the presence of mouse effector cells including macrophages and neutrophils, in vitro effector function assays were used to assess the impact of 5-azacytidine on lintuzumab activity. The results show that 5-azacytidine significantly enhanced the ability of lintuzumab to promote tumor cell killing through antibody-dependent cellular cytotoxicity (ADCC) and phagocytic (ADCP) activities. These results suggest that lintuzumab and 5-azacytidine act in concert to promote tumor cell killing. Additionally, these findings provide the rationale to evaluate this combination in the clinic.
Key words: CD33, monoclonal antibody, immunotherapy, myeloid malignancies, 5-azacytidine, epigenetic therapies, hypermethylation, effector function
Introduction
Myelodysplastic syndromes (MDS) refer to a class of hematologic malignancies that affect the bone marrow and are characterized by abnormalities in cell proliferation, maturation and survival.1,2 Approximately 10,000–20,000 new cases are diagnosed annually in the United States and Europe (NCI SEER and WHO Globocan). There is a high incidence of transformation to acute myeloid leukemia (AML) and many patients also die due to complications associated with the disease.2–4 MDS and AML are pre-dominantly a disease of patients over the age of 60, and as such, the incidence will increase as the population ages. For 2009, the American Cancer Society (Cancer Facts and Figs. 2009) projected 13,000 new cases and 9,000 deaths from AML in the US alone.
5-azacytidine (Vidaza™) and 5-aza-2′deoxycytidine (decitabine, Dacogen™) are nucleoside analogs that belong to a class of epigenetic therapeutics capable of inducing tumor cell killing through the disruption of protein synthesis and inhibition of DNA methylation.5–8 Re-expression of tumor suppressor genes and cell cycle regulators are a result of their interactions with DNA methyltransferase I.9 Decitabine was reported to be ∼10-fold more potent than 5-azacytidine.10,11 Elevated levels of DNA methyltransferases and hypermethylated DNA were found in blood and bone marrow samples from MDS and AML patients.12–15 Altering the methylation status of DNA in leukemic samples promoted the differentiation of tumor cells,16–18 resulting in reduced tumor cell growth,19 possibly by making them amenable to natural killer (NK) cell killing.18
5-azacytidine was the first drug approved by the FDA for the treatment of MDS.20 Expanded approval was granted recently based upon the outcome of a multi-center, controlled Phase III clinical trial where 5-azacytidine-treated patients demonstrated improved overall survival, increased quality of life and reduced risk of transformation to AML compared to conventional care regimens.21,22 Similar outcomes were described for elderly patients with low blast counts (reclassified as AML under WHO criteria).23 Despite these achievements, multiple treatment cycles of 5-azacytidine were needed to obtain a response and most of the patients who responded to treatment eventually relapsed.22–25 Additionally, modest benefit was observed in patients with relapsed/refractory disease26,27 or in high-risk MDS and AML patients with unfavorable cytogenetics.25 Clearly better therapy options are needed.
Lintuzumab, also known as SGN-33 or HuM195,28 is a humanized monoclonal antibody (mAb) in clinical development that targets CD33, a myeloid lineage-specific antigen normally expressed on precursor myeloid cells and most monocytic cells.29 CD33 is an important drug target expressed on AML and MDS tumor cells.30–32 In ongoing clinical trials, the antibody is under evaluation in patients with myeloid malignancies who are not considered candidates for intensive chemotherapy. The results from a multiple dose, single arm dose escalation Phase 1 study showed that the antibody is well-tolerated, with the most common adverse event being transient chills with the initial infusion.33 Clinical response was observed in 7 (four complete remissions) of 17 AML patients with blast percentages ranging from 29–63%.33
In a previous study, we reported that lintuzumab significantly prolonged the survival of mice in multiple models of AML.34 Additionally, lintuzumab interacts with effector cells to mediate tumor cell killing through antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) activities. In the current study, experiments were undertaken to assess the impact of 5-azacytidine on the ability of lintuzumab to effect anti-leukemic activity. The results show that 5-azacytidine enhanced lintuzumab-mediated effector functions in vitro and promoted significant anti-tumor effects in vivo. These findings demonstrate that greater antitumor activity may be achieved with the combination of lintuzumab and 5-azacytidine than with either agent alone, providing the rationale for this combination to undergo clinical evaluation as an alternative option for the treatment of CD33+ myeloid diseases. A Phase II clinical trial to gauge the activity of this combination in previously untreated MDS patients has been initiated.
Results
5-Azacytidine enhances the in vivo activity of lintuzumab in a disseminated model of AML.
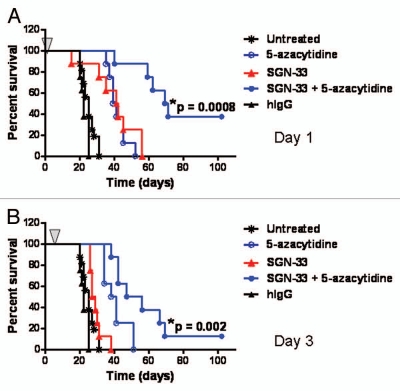
We recently reported that lintuzumab (SGN-33) prolonged the survival of mice in multiple preclinical models of AML.34 In the current study, an aggressive HL60cy model of AML was used to assess the effect of 5-azacytidine on the in vivo activity of lintuzumab. In the HL60cy model, the mice displayed signs of disease (scruffy coat, significant weight loss, hind limb paralysis, buffalo head and presence of palpable tumors) almost three times faster (within 20–30 d) than the HL60 model we reported (40–80 d for disease symptoms to develop).34 The results of dosing tumor-bearing mice with lintuzumab and 5-azacytidine 1 d (referred to as Day 1) or 3 d (referred to as Day 3) after intravenous injection of the tumor cells are shown in Figure 1. The mice were given a single dose of lintuzumab (0.3 mg/kg) and the maximum tolerated dose of 5-azacytidine (5 mg/kg, every 3 d × 5 doses). The dose of lintuzumab tested was sufficient to offer reasonable activity on Day 1 and adequate for testing in combination with 5-azacytidine. These treatments did not show any negative side effects on the health of the mice (data not shown). A single dose of lintuzumab given on Day 1 significantly improved the survival of the mice compared to untreated or hIgG1 treated mice (median survival time of 41.5 d for lintuzumab compared to 27 d for untreated and 22 d for hIgG1-treated mice, p = 0.012, log rank test, Fig. 1A). 5-Azacytidine-treated mice also showed greater survival compared to untreated mice (p < 0.0001, Fig. 1A) but survival was not significantly different compared to lintuzumab alone (Fig. 1A). Interestingly, the combination of lintuzumab with 5-azacytidine provided greater survival benefit compared to treatment with either agent alone (median survival time of 70 d for the combination compared to 40 d for 5-azacytidine, p = 0.0005 or 41.5 d for lintuzumab, p = 0.0008, Fig. 1A). In the Day 3 study, 5-azacytidine alone significantly increased the survival of mice (p < 0.0001, Fig. 1B) whereas a single dose of lintuzumab exhibited a modest survival advantage that was not significantly different from untreated (Fig. 1B). Similar to treatment initiation on Day 1, the combination of lintuzumab with 5-azacytidine significantly increased survival relative to either lintuzumab or 5-azacytidine alone when mice were dosed 3 d after the administration of the tumor cells (median survival time of 51.5 d for the combination compared to 39.5 d for 5-azacytidine alone, p = 0.019 or 28 d for lintuzumab, p = 0.002, Fig. 1B). Comparable results were observed with lintuzumab dosed at 1 mg/kg (data not shown). In experiments where treatment was initiated 7 d after injection of tumor cells, the survival of mice was significantly prolonged by the combination of lintuzumab and 5-azacytidine compared to lintuzumab alone (data not shown).
Figure 1.
5-Azacytidine significantly enhances the in vivo activity of lintuzumab (SGN-33) in the HL60cy disseminated disease model of AML. Mice (n = 8–10/group) were injected with 5 million cells and dosed 1 day (A) or 3 days later (B) with a single dose of 0.3 mg/kg lintuzumab or hIgG1 (arrow) and 5-azacytidine (5 mg/kg, q3d × 5). The combination of lintuzumab + 5-azacytidine significantly enhanced survival of mice dosed on Day 1 (p = 0.0008 to untreated mice or mice treated with hIgG, SGN-33 alone, or 5-azacytidine alone by log-rank test) or Day 3 (p = 0.002 to untreated mice or mice treated with hIgG, SGN-33 alone, and p = 0.02 to treatment with 5-azacytidine alone by log-rank test). The results shown are representative of data obtained from two separate experiments.
Lintuzumab does not increase the direct cytotoxic activity of 5-azacytidine against AML cell lines in vitro.
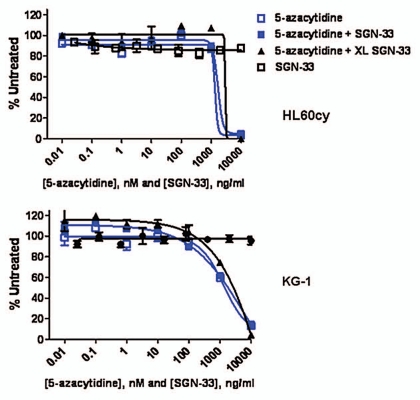
Since 5-azacytidine showed single-agent activity in the HL60cy model (Fig. 1), we examined the effects of 5-azacytidine and lintuzumab against AML cell lines in vitro. In both HL60cy and KG-1 cell lines, 5-azacytidine significantly decreased cell viability at concentrations higher than 100 nM (Fig. 2). On the other hand, lintuzumab at concentrations up to 10 µg/mL had no direct effect on cell viability. The addition of lintuzumab alone, or cross-linked by a secondary antibody, also did not enhance the cytotoxic effect of 5-azacytidine (Fig. 2). These data suggest that increased direct cytotoxicity against AML cells might not be the dominant mechanism underlying the combined effect of lintuzumab and 5-azacytidine in vivo.
Figure 2.
Lintuzumab (SGN-33) does not enhance the direct cytotoxic effect of 5-azacytidine against AML cell lines in vitro. HL60cy and KG-1 AML cell lines were incubated with increasing concentrations of 5-azacytidine, lintuzumab or 5-azacytidine in combination with 10 µg/ml lintuzumab or cross-linked lintuzumab (XL-SGN-33) as described in Materials and Methods. Similar results were obtained with HEL9217 cells (data not shown). Data shown are mean ± SEM of duplicate values graphed as % untreated (untreated = 100%).
Anti-leukemic activity of lintuzumab in vivo requires effector cells.
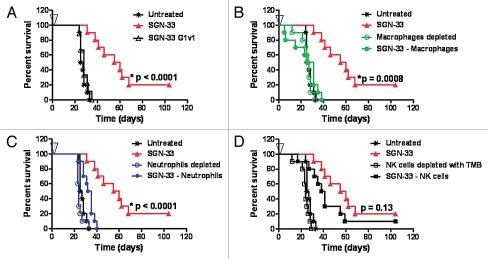
Lintuzumab (SGN-33) interacts with effector cells in vitro to mediate tumor cell killing.34 Two approaches were applied to determine the role of antibody effector functions and host innate immune system in the anti-leukemic activity of lintuzumab in the HL60cy xenograft model. First, a variant of lintuzumab (SGN-33 G1v1) with amino acid substitutions in the heavy chain constant domains to reduce Fcγ receptor binding was engineered.35 Similar to a G1v1 variant of an anti-CD70 antibody,36 SGN-33 G1v1 does not bind to the human Fcγ receptor I (CD64), Fcγ receptor IIIA (CD16), or to the murine Fcγ receptor IV (the functional counterpart of CD16) (data not shown). The G1v1 engineering of SGN-33 completely abolished the anti-leukemic activity of the antibody (Fig. 3A, p < 0.0001 to SGN-33), suggesting that a functional interaction between the Fc domain of lintuzumab and host Fcγ receptor-expressing immune effector cells was required for anti-tumor activity in this model. In the second approach, immune effector cells in tumor-bearing mice were selectively removed. Depletion of macrophages with clodronate-encapsulated liposomes (CEL, Fig. 3B) or neutrophils with an anti-GR-1 antibody (Fig. 3C) almost eliminated the activity of the antibody (p = 0.0008 and p < 0.0001, respectively to SGN-33 alone). In contrast, abrogating NK activity with anti-Tm-β1 antibody marginally affected activity (p = 0.13 to SGN-33 alone, Fig. 3D) and results were similar to depletion of NK cells with an anti-asialo GM-1 antibody (data not shown).
Figure 3.
The in vivo activity of lintuzumab (SGN-33) in a disseminated disease model of AML is dependent up the presence of murine effector cells. In the HL60cy model, mice (n = 10/group) were treated one day prior or the same day with agents to deplete murine effector cells as described in Materials and Methods. Mice were injected with 5 million cells and dosed 1 d later with a single dose of 10 mg/kg lintuzumab or the mutant, SGN-33G1v1 (arrow, A). The anti-leukemic activity of lintuzumab is dependent upon the presence of murine macrophages (B, p = 0.0008, log rank test) and neutrophils (C, p < 0.0001) but less on NK cells (D, p = 0.13). Results shown for depletion of NK cells with anti-TM-β1 (TMB) was similar to that obtained using anti-asialo GM-1 (ASGM). The results shown are representative of data obtained from two separate experiments.
5-azacytidine enhances lintuzumab-mediated ADCP activity in vitro.
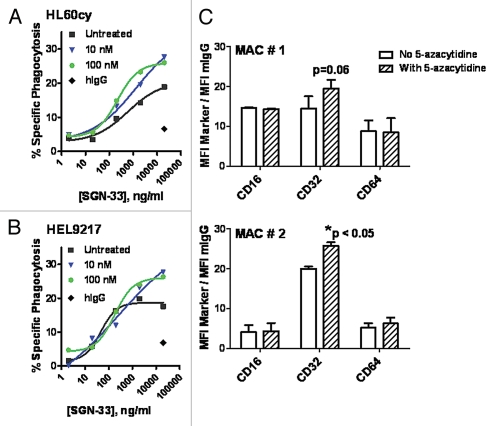
Having demonstrated the combination effect of lintuzumab and 5-azacytidine in vivo as well as the importance of Fc-Fcγ receptor interaction in mediating lintuzumab activity, we proceeded to examine the effect of 5-azacytidine on lintuzumab-mediated effector functions. In the presence of primary human macrophages, lintuzumab mediated the phagocytosis of HL60cy and HEL9217 cells in a dose-dependent manner whereas the hIgG1κ control antibody demonstrated minimal activity (Fig. 4A and B), in agreement with previously reported findings.34 Pretreatment of monocyte-derived macrophages with 5-azacytidine significantly increased lintuzumab-mediated phagocytosis of HL60cy cells compared to untreated cells (p = 0.018, 2-way ANOVA, Fig. 4A). The concentrations of 5-azacytidine tested were significantly lower than that required to directly kill tumor cells (IC50 in a 96 h cytotoxicity assay for HL60cy and HEL9217 = 1.5 µM, Fig. 2 and data not shown) and comparable to plasma values reportedly found in patients.37,38 There was a detectable increase in overall cell lysis (20% in untreated to a maximum of 34% in treated) as well as a significant shift in EC50 values. For 5-azacytidine-treated macrophages, the EC50 (∼200 ng/ml) was 3-fold lower than that found for untreated HL60cy cells (∼600 ng/ml) (Fig. 4A). Prior treatment of macrophages with 5-azacytidine also increased the specific phagocytosis from 18–28% when HEL9217 cells were used as target cells (Fig. 4B) but did not significantly alter the EC50 values. Increasing the concentration of 5-azacytidine from 100 nM to 10 µM also did not change the maximum activity (data not shown). Pretreatment of HL60cy or HEL9217 target cells with 5-azacytidine for 18 h did not alter CD33 levels, suggesting that enhanced phagocytosis was not a result of 5-azacytidine-induced CD33 overexpression on the target cells (data not shown). 5-Azacytidine pretreatment also did not render HL60cy or HEL9217 cells more susceptible to phagocytosis by macrophages. Collectively, these data suggest that the observed increase in phagocytosis was an effect of 5-azacytidine on macrophages rather than on target cells.
Figure 4.
5-Azacytidine significantly enhances lintuzumab (SGN-33)-mediated ADCP activity and increased the expression of CD32 on human macrophages. Macrophages isolated from human PBMCs cultured long-term in GM-CSF were pre-treated with 10 nM or 100 nM 5-azacytidine prior to addition of lintuzumab-treated AML cell lines, HL60cy (A) or HEL9217 (B). 5-azacytidine significantly enhanced lintuzumab-mediated ADCP activity (p < 0.018, 2-way ANOVA). Data shown are mean ± SEM of triplicate values from two to three separate experiments using macrophages from different donors. (C) Flow cytometry analyses of the Fcγ receptors CD32, CD16 and CD64 on human macrophages incubated with 100 nM 5-azacytidine or vehicle for 18 h. Data (gating on live cells) are expressed as ratio of MFI for each marker to MFI for the isotype control. Data from two separate experiments using cells from two different donors (MAC # 1 and MAC # 2) are shown and expressed as mean ± SD of duplicate values. *p < 0.05 to No 5-azacytidine.
Interaction between the Fc domain of the antibody and the Fcγ receptors expressed on macrophages is a key step toward phagocytosis. We examined the effect of 5-azacytidine on CD16 (Fcγ receptor IIIA), CD32 (Fcγ receptor II), and CD64 (Fcγ receptor I) expression on macrophages by flow cytometry (Fig. 4C). In two independent experiments (MAC #1 and MAC#2, Fig. 4C), we did not detect any marked changes in CD16 or CD64 expression; however, in the presence of 5-azacytidine, a detectable trend in the upregulation in CD32 expression was observed (Fig. 4C).
5-Azacytidine enhances lintuzumab-mediated ADCC activity in vitro.
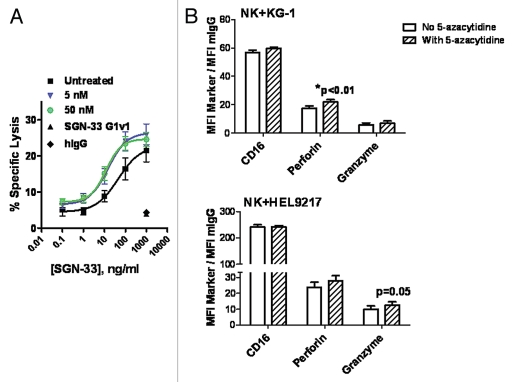
Lintuzumab mediated the tumor cell killing of KG-1 cells in the presence of primary human NK cells in a dose-dependent manner (Fig. 5A), in agreement with previous findings.34 In contrast, the hIgG1κ control antibody and SGN-33 G1v1 construct demonstrated minimal activity even at a saturating concentration of 1 µg/mL (Fig. 5A). Pretreatment of NK cells with 5-azacytidine at 5 nM or 50 nM for 2 h significantly increased lintuzumab-mediated tumor cell killing compared to untreated NK cells (Fig. 5A, p < 0.0002, 2-way ANOVA). As was observed with the ADCP assay, the concentrations of 5-azacytidine required to affect ADCC activity were significantly lower than those required to directly kill the tumor cells (IC50 in a 96 h cytotoxicity assay for KG-1 = 1.5 µM, Fig. 2) and comparable to plasma values reportedly found in patients.37,38 While the effect on maximal cell lysis was modest, there was an approximately 3-fold shift in EC50 values. For cells treated with 5 nM or 50 nM 5-azacytidine, the EC50 was 12–14 ng/ml compared to 45 ng/ml for untreated cells (Fig. 5A). Similar increases in ADCC activity were obtained when NK cells were pretreated with a more potent DNA demethylating agent, 5-aza-2′deoxycytidine11 (data not shown). Pretreatment of KG-1 target cells for 2 h or 18 h with 5-azacytidine did not alter CD33 levels and did not render them more susceptible to lysis by NK cells in the ADCC assay (data not shown).
Figure 5.
5-Azacytidine significantly enhanced the lintuzumab (SGN-33)-mediated ADCC tumor cell killing activity and increased the expression of human NK lytic enzymes, perforin and granzyme B. (A) NK cells isolated from FcγRIIIA 158V/V donors were pre-incubated for 2 h with 5 nM or 50 nM 5-azacytidine. ADCC activity against KG-1 tumor cells was assessed in three separate experiments as described in Materials & Methods using cells from two different donors. Pretreatment of NK cells with 5-azacytidine significantly increased SGN-33 mediated ADCC activity (p < 0.0002, 2-way ANOVA). (B) Flow cytometry analyses of CD16, perforin and granzyme B on human NK cells incubated with 50 to 100 nM 5-azacytidine or vehicle in the presence of KG-1 or HEL9217 AML cell lines for 4–6 h. Data (gating on live NK cells) are expressed as ratio of MFI for each marker to MFI for the isotype control. Data using NK cells from two different donors are shown, expressed as mean ± SD of duplicate values. *p < 0.01 to No 5-Azacytidine.
Flow cytometry was used to assess the effects of 5-azacytidine on the levels of phenotypic markers associated with functionally mature NK cells including CD16, perforin and granzyme B (Fig. 5B). Co-incubation of NK cells with AML cells (KG-1 or HEL9217) in the presence of 5-azacytidine did not affect CD16 expression; however, a trend for an increase in the levels of perforin and granzyme B in NK cells was detected (Fig. 5B). These changes in the expression of perforin and granzyme B did not appear to result from an increase in RNA synthesis since significant changes in mRNA levels were not seen by RT-PCR assays (data not shown).
Discussion
In this study, the effects of an epigenetic therapeutic agent, 5-azacytidine, on the anti-leukemic activity of the humanized anti-CD33 antibody, lintuzumab, were investigated and characterized. The results suggest that targeting AML and MDS with a combination of lintuzumab with 5-azacytidine may prove to be clinically relevant. Indeed, a clinical trial has recently been initiated to evaluate this combination in previously untreated MDS patients.
Epigenetic compounds including 5-azacytidine can modify the biology of transformed cells in multiple ways to achieve activity. Aberrant methylation leading to silencing of tumor suppressor genes has been reported in MDS and AML.12–15 Exposure of tumor cells to 5-azacytidine and 5-aza-2′deoxycytidine promotes the differentiation of the tumor cells, resulting in changes in morphology, phenotype, and various cellular properties.17,18 In the presence of 5-azacytidine, increased differentiation of AML cells was associated with augmented expression of the NKG2 ligand, UL16 binding protein, resulting in enhanced NK cell activity and tumor cell lysis.18 On the other hand, high concentrations of 5-azacytidine and 5-aza-2′deoxycytidine directly killed tumor cells.7,11,39 The IC50 for 5-azacytidine in a 96 h in vitro cytotoxicity assay against KG-1, HL60cy or HEL9217 cell lines was approximately 2 µM (Fig. 2). Based on previously published pharmacokinetic parameters for 5-azacytidine observed in mice,8,40 it is likely that the 5 mg/kg dose used in the current study could result in plasma 5-azacytidine levels that were cytotoxic to the implanted AML cells. The significant benefit and improved overall survival obtained when mice were treated with the combination of lintuzumab and 5-azacytidine might therefore result from an enhanced cytotoxic activity of 5-azacytidine in the presence of lintuzumab; however, this hypothesis is not supported by the observation that in vitro, the combination of 5-azacytidine with lintuzumab did not yield enhanced killing of AML cells (Fig. 2).
One potential mechanism by which lintuzumab exerts its anti-tumor activity is through interactions with immune cells to mediate effector functions, including ADCP and ADCC activities.28,34 The importance of effector cells in mediating the antileukemic effect of lintuzumab in preclinical xenograft models was demonstrated using antibodies and other reagents that ablate effector cells. Depletion of Fcγ receptor-expressing cells, in particular macrophages and neutrophils, eliminated the protective effect of lintuzumab in an aggressive HL60cy mouse model of AML.
The impact of 5-azacytidine on lintuzumab-mediated tumor cell killing was assessed in vitro using effector function assays. 5-Azacytidine increased the phagocytosis of lintuzumab-coated AML cells that was associated with a decrease in EC50 for lintuzumab. Incubation with 5-azacytidine did not seem to affect CD33 expression on AML cells or macrophages. 5-Azacytidine can promote the differentiation of many cell types.41–44 Indeed, 5-azacytidine enhanced the expression of the macrophage mannose receptor (CD206) on a mouse macrophage cell line in culture, suggesting that it may modulate cell surface receptors to regulate their phagocytic function;41 however, with the exception of a slight upregulation in CD32 expression, 5-azacytidine did not induce any pronounced changes in CD16 or CD64 on human macrophages in the current study. Additional experiments are needed to better define how 5-azacytidine improves the functions of differentiated immune effector cells and whether these changes in phenotypic marker expression are sufficient to contribute to significant activity in vivo.
Treatment of NK cells with 5-azacytidine improved the lintuzumab-mediated lysis of tumor cells. Increased ADCC activity was associated with elevated specific lysis and a 2- to 4-fold decrease in EC50. As was observed in the ADCP study, the effect of this demethylating agent on ADCC activity was achieved with concentrations much lower than those needed for direct tumor cell killing. These findings are in line with previous reports that the compounds produced hypomethylation at levels lower than that required to affect cell viability.10,39 Demethylating agents have also been reported to modify antibody-independent NK lytic activity,45,46 possibly through changes in chromatin structure.19,47,48 Treatment of primary human NK cells or NK cell lines with micromolar quantities of 5-azacytidine suppressed cytolytic activity, resulting in decreased lysis of NK-sensitive cell lines.45,46 Loss of antibody-independent NK killing activity was associated with overexpression of inhibitory KIRs (killer immunoglobulin-like regulatory receptors) and impaired release of perforin and granzymes.45 Interestingly, in the current study, we found that sub-micromolar concentrations of 5-azacytidine actually improved lintuzumab-mediated ADCC against AML target cells. The underlying mechanism responsible for increased ADCC activity needs to be further defined, although there was a trend for 5-azacytidine treatment to increase the in vitro expression of perforin and granzyme B in NK cells without altering their mRNA levels. It has been reported that treatment of human CD4+ and CD8+ T cells with 5-azacytidine enhanced perforin expression.49 Hence, demethylating agents may trigger common signaling pathways responsible for the release of lytic enzymes upon ligand binding to Fcγ receptor III and the T-cell receptor.50
Additional studies are needed to further refine our understanding of how epigenetic changes in immune effector cells enhanced the anti-leukemic activity of lintuzumab in preclinical models. The in vitro data demonstrating lintuzumab-mediated antibody effector functions34 and the requirement for effector cells to mediate in vivo activity clearly support a significant role for the interaction between lintuzumab and host innate immune functions. Perhaps the NK cells that contributed minimal benefit when lintuzumab was tested as a single agent in vivo became a larger factor in the presence of 5-azacytidine. It is also possible that the effects of 5-azacytidine in vitro on phenotypic marker expression, i.e., CD32 for macrophages and perforin and granzyme B for NK cells, and on ADCP and ADCC activities translated to sufficiently greater anti-tumor activities in vivo.
While 5-azacytidine improved overall survival and quality of life for MDS and AML patients in a recent clinical trial,21–23 several shortcomings exist, including the association of early relapse with cessation of treatment.21,22 Therefore, there is a need for improved therapeutic options. The findings from the current study demonstrate that lintuzumab and 5-azacytidine work in concert to effectively mediate anti-tumor activity in vitro and in vivo. Greater anti-tumor activity was achieved with the combination of lintuzumab and 5-azacytidine compared with either agent alone. These data provide the rationale to investigate the combination of lintuzumab and 5-azacytidine in the clinic.
Materials and Methods
Cell lines.
The human AML cell lines HL60 and HEL9217 were obtained from ATCC (Manassas, VA, USA) while KG-1 cells were purchased from DSMZ (Braunschweig, Germany). The cells were grown in RPMI-1640 media (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine sera (Invitrogen). HL60cy cells were obtained upon long-term culture of the HL60 cells. HL60cy cells exhibited a more aggressive growth pattern in vitro and in vivo but expressed the same phenotypic markers as the parent cell line except for higher levels of CD33 (16,800 copies compared to 12,000 copies).
Cytotoxicity assay.
HL60cy and KG-1 AML tumor cell lines were incubated with increasing concentrations of 5-azacytidine (Sigma, St. Louis, MO, USA), lintuzumab, or 5-azacytidine in combination with 10 µg/ml lintuzumab or cross-linked lintuzumab for 96 h. Lintuzumab was cross-linked by incubating the antibody at room temperature for 20 minutes with a 4-fold amount of goat anti-human IgG polyclonal antibody (Jackson Immunoresearch, West Grove, PA, USA). Cell viability was measured with Celltiter-Glo (Promega, Madison, WI, USA). Results were reported as IC50, the concentration of compound needed to yield a 50% reduction in viability compared to untreated cells (control = 100%).
Antibody-dependent cellular phagocytosis (ADCP).
ADCP was assessed using a previously described method.51 Macrophages, generated from normal human PBMCs cultured with 500 U/ml human granulocyte-macrophage colony-stimulating factor (GM-CSF, PeproTech, Rocky Hill, NJ, USA), were treated for 18 h with vehicle or 5-azacytidine. Target AML cells were incubated with the fluorescent dye PKH67 (Sigma) prior to addition of lintuzumab or hIgG1 and primary human macrophages. The macrophages were labeled with a PE/Cy5-conjugated CD11b antibody (BD Pharmingen, San Diego, CA, USA). To mitigate the possibility of lintuzumab binding to the macrophages and interfering with the assay, target AML cell lines were first coated with lintuzumab and washed several times before they were incubated with the macrophages. Uptake of the target cells by the macrophages (phagocytosis) was assessed by flow cytometry and visualized by immunofluorescence using a Carl Zeiss Axiovert 200M microscope.
Macrophages that had been treated with vehicle or 5-azacytidine were also assessed by flow cytometry to evaluate the expression of relevant phenotypic markers including CD16, CD32 and CD64 using commercially available antibodies from BD Pharmingen.
Antibody-dependent cellular cytotoxicity (ADCC).
ADCC activity was measured using the standard 51Cr-release assay as previously described.51 Briefly, the KG-1 target tumor cells were labeled with 100 µCi Na51CrO4, washed and pre-incubated with lintuzumab prior to addition of effector (natural killer, NK) cells. NK cells were prepared from non-adherent peripheral blood mononuclear cells (PBMCs) obtained from normal FcγRIIIA 158V/V donors (Lifeblood, Memphis, TN) with immunomagnetic beads (EasySep, StemCell Technologies, Vancouver, BC, CA). Viable NK cells (CD16+ CD56+) were pre-incubated with 5-azacytidine for 2 h prior to addition to target cells at an effector to target cell ratio of 10:1. A human IgG1κ (Ancell, Bayport, MN, USA) and SGN-33G1v1 were used as negative controls in this assay.
NK cells that had been treated with vehicle or 5-azacytidine and incubated with or without KG-1 or HEL9217 AML cell lines for 4 to 6 h were also assessed by flow cytometry to evaluate the expression of relevant phenotypic markers including CD16, perforin, and granzyme B (BD Pharmingen). In one study, 1 µg/ml brefeldin (BD GolgiPlug, BD Biosciences, San Jose, CA, USA) was added to the cells, but the results were not significantly different from that obtained without the protein transport inhibitor.
Anti-leukemic activity of lintuzumab and its in vivo mechanism of action.
Animal experiments were conducted in an AALAC (Association for Assessment of Laboratory Animal Care) accredited facility and under IACUC (Institutional Animal Care and Use Committee) guidelines and approval. An aggressive model of disseminated AML was established in female C.B-17 SCID mice (Harlan, Indianapolis, IN) with the HL60cy tumor cells (5 × 106 cells/mouse) administered intravenously. The animals were routinely monitored for signs of disease, including a significant weight loss of 15–20%, development of scruffy coats, buffalo head, hind limb paralysis, and presence of palpable tumors. In contrast to a previously described HL60 model,34 the onset of disease with the HL60cy cell line occurred between 20–30 days after injection of the cells. Mice were euthanized upon onset of symptoms. Flow cytometric analyses of blood, bone marrow, lymph node and tumor masses confirmed the presence of human CD33+ tumor cells in these tissues.
To determine the in vivo mechanism of action of lintuzumab, SCID mice were treated on the same day or one day prior to tumor cell injection, with depletion reagents to selectively remove effector cells as described previously.52,53 Briefly, mice were given a total of six doses once every 5 days of anti-asialo GM-1 (ASGM, 1.25 mg/kg, Wako Pure Chemical industries, Richmond, VA, USA) or anti-GR-1 (4 mg/kg, BD Biosciences) antibodies to deplete NK cells and neutrophils, respectively. An additional group of animals was given 100 mg anti-TM-β1 (IL-2Rβ) antibody (TMB, BioLegend, San Diego, CA, USA) once every 21 days for a total of two doses. The TMB antibody blocks binding of IL-2 to its receptor and abrogates NK activity.54 To deplete macrophages, the mice were dosed a total of seven times once every 3 days with clodronate-encapsulated liposomes (CEL, 80 mg/kg).53 One day after tumor cell injection, the mice were treated with a single dose of 10 mg/kg lintuzumab or SGN-33G1v1, a variant of lintuzumab which had been engineered to reduce Fcγ receptor binding.35 Depletion of effector cells was confirmed by flow cytometry.
For activity experiments, SCID mice were injected intravenously with tumor cells. The mice were then pooled, randomly distributed into treatment groups, and dosed intraperitoneally one day or 3 days later with a single dose of a nonbinding control antibody or lintuzumab (0.3 mg/kg) and the maximum tolerated dose of 5-azacytidine (5 mg/kg, every 3 days × 5 doses).
Data were plotted and analyzed using the Kaplan Meier Survival Curves and logarithmic rank test in Prism (GraphPad Software, San Diego, CA, USA).
Acknowledgements
We are grateful to Muriel Siadek for her help with reviewing the manuscript.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- AML
acute myeloid leukemia
- MDS
myelodysplastic syndromes
- PBMC
peripheral blood monocytic cells
- NK
natural killer cells
- MFI
mean fluorescence intensity
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/12203
Disclosure
All authors except Nico van Rooijen are employees of Seattle Genetics, Inc.
References
- 1.Atallah E. Evolving treatment strategies for patients with myelodysplastic syndrome. Am J Oncol Rev. 2006;5:4–9. [Google Scholar]
- 2.Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 3.Shi J, Shao ZH, Liu H, Bai J, Cao YR, He GS, et al. Transformation of myelodysplastic syndromes into acute myeloid leukemias. Chin Med J (Engl) 2004;117:963–967. [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman S. The inhibition of DNA(cytosine-5)methylases by 5-azacytidine. The effect of azacytosine-containing DNA. Mol Pharmacol. 1981;19:314–320. [PubMed] [Google Scholar]
- 6.Jones PA, Taylor SM, Wilson VL. Inhibition of DNA methylation by 5-azacytidine. Recent Results Cancer Res. 1983;84:202–211. doi: 10.1007/978-3-642-81947-6_15. [DOI] [PubMed] [Google Scholar]
- 7.Li LH, Olin EJ, Buskirk HH, Reineke LM. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970;30:2760–2769. [PubMed] [Google Scholar]
- 8.Presant CA, Valeriote F, Vietti TJ. Biological characterization of a prolonged antileukemic effect of 5-azacytidine. Cancer Res. 1977;37:376–381. [PubMed] [Google Scholar]
- 9.Issa JP. DNA methylation as a therapeutic target in cancer. Clin Cancer Res. 2007;13:1634–1637. doi: 10.1158/1078-0432.CCR-06-2076. [DOI] [PubMed] [Google Scholar]
- 10.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 11.Momparler RL, Momparler LF, Samson J. Comparison of the antileukemic activity of 5-AZA-2′-deoxycytidine, 1-beta-D-arabinofuranosylcytosine and 5-azacytidine against L1210 leukemia. Leuk Res. 1984;8:1043–1049. doi: 10.1016/0145-2126(84)90059-6. [DOI] [PubMed] [Google Scholar]
- 12.Desmond JC, Raynaud S, Tung E, Hofmann WK, Haferlach T, Koeffler HP. Discovery of epigenetically silenced genes in acute myeloid leukemias. Leukemia. 2007;21:1026–1034. doi: 10.1038/sj.leu.2404611. [DOI] [PubMed] [Google Scholar]
- 13.Gebhard C, Schwarzfischer L, Pham TH, Schilling E, Klug M, Andreesen R, et al. Genome-wide profiling of CpG methylation identifies novel targets of aberrant hypermethylation in myeloid leukemia. Cancer Res. 2006;66:6118–6128. doi: 10.1158/0008-5472.CAN-06-0376. [DOI] [PubMed] [Google Scholar]
- 14.Herman JG, Civin CI, Issa JP, Collector MI, Sharkis SJ, Baylin SB. Distinct patterns of inactivation of p15INK4B and p16INK4A characterize the major types of hematological malignancies. Cancer Res. 1997;57:837–841. [PubMed] [Google Scholar]
- 15.Melki JR, Vincent PC, Clark SJ. Concurrent DNA hypermethylation of multiple genes in acute myeloid leukemia. Cancer Res. 1999;59:3730–3740. [PubMed] [Google Scholar]
- 16.Attadia V. Effects of 5-aza-2′-deoxycytidine on differentiation and oncogene expression in the human monoblastic leukemia cell line U-937. Leukemia. 1993;7:9–16. [PubMed] [Google Scholar]
- 17.Laurenzana A, Petruccelli LA, Pettersson F, Figueroa ME, Melnick A, Baldwin AS, et al. Inhibition of DNA methyltransferase activates tumor necrosis factor alpha-induced monocytic differentiation in acute myeloid leukemia cells. Cancer Res. 2009;69:55–64. doi: 10.1158/0008-5472.CAN-08-0245. [DOI] [PubMed] [Google Scholar]
- 18.Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar-Filipowicz A. Differentiation-promoting drugs upregulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res. 2007;31:1393–1402. doi: 10.1016/j.leukres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, March TH, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- 20.Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10:176–182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 21.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain N, Rossi A, Garcia-Manero G. Epigenetic therapy of leukemia: An update. Int J Biochem Cell Biol. 2009;41:72–80. doi: 10.1016/j.biocel.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 24.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921 and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 25.Ravandi F, Issa JP, Garcia-Manero G, O'Brien S, Pierce S, Shan J, et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer. 2009;115:5746–5751. doi: 10.1002/cncr.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borthakur G, Huang X, Kantarjian H, Faderl S, Ravandi F, Ferrajoli A, et al. Report of a phase 1/2 study of a combination of azacitidine and cytarabine in acute myelogenous leukemia and high-risk myelodysplastic syndromes. Leuk Lymphoma. 51:73–78. doi: 10.3109/10428190903318329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czibere A, Bruns I, Kroger N, Platzbecker U, Lind J, Zohren F, et al. 5-Azacytidine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome who relapse after allo-SCT: a retrospective analysis. Bone Marrow Transplant. 2010;45:872–876. doi: 10.1038/bmt.2009.266. [DOI] [PubMed] [Google Scholar]
- 28.Caron PC, Co MS, Bull MK, Avdalovic NM, Queen C, Scheinberg DA. Biological and immunological features of humanized M195 (anti-CD33) monoclonal antibodies. Cancer Res. 1992;52:6761–6767. [PubMed] [Google Scholar]
- 29.Dinndorf PA, Andrews RG, Benjamin D, Ridgway D, Wolff L, Bernstein ID. Expression of normal myeloid-associated antigens by acute leukemia cells. Blood. 1986;67:1048–1053. [PubMed] [Google Scholar]
- 30.D'Emilio A, Montaldi A, Stella M, Celli P, Guercini N, Tremul L, et al. Hematologic and cytogenetic analyses of 99 cases of de novo myelodysplastic syndromes. Haematologica. 1993;78:61–65. [PubMed] [Google Scholar]
- 31.Jilani I, Estey E, Huh Y, Joe Y, Manshouri T, Yared M, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol. 2002;118:560–566. doi: 10.1309/1WMW-CMXX-4WN4-T55U. [DOI] [PubMed] [Google Scholar]
- 32.Masuya M, Kita K, Shimizu N, Ohishi K, Katayama N, Sekine T, et al. Biologic characteristics of acute leukemia after myelodysplastic syndrome. Blood. 1993;81:3388–3394. [PubMed] [Google Scholar]
- 33.Raza A, Jurcic JG, Roboz GJ, Maris M, Stephenson JJ, Wood BL, et al. Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial. Leuk Lymphoma. 2009;50:1336–1344. doi: 10.1080/10428190903050013. [DOI] [PubMed] [Google Scholar]
- 34.Kung Sutherland M, Yu C, Lewis TS, Miyamoto JB, Morris-Tilden CA, Jonas M, et al. Anti-leukemic Activity of Lintuzumab (SGN-33) in Preclinical Models of Acute Myeloid Leukemia MAbs. 2009;1:481–490. doi: 10.4161/mabs.1.5.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchins JT, Kull FC, Jr, Bynum J, Knick VC, Thurmond LM, Ray P. Improved biodistribution, tumor targeting, and reduced immunogenicity in mice with a gamma 4 variant of Campath-1H. Proc Natl Acad Sci USA. 1995;92:11980–11984. doi: 10.1073/pnas.92.26.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonagh CF, Kim KM, Turcott E, Brown LL, Westendorf L, Feist T, et al. Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index. Mol Cancer Ther. 2008;7:2913–2923. doi: 10.1158/1535-7163.MCT-08-0295. [DOI] [PubMed] [Google Scholar]
- 37.Marcucci G, Silverman L, Eller M, Lintz L, Beach CL. Bioavailability of azacitidine subcutaneous versus intravenous in patients with the myelodysplastic syndromes. J Clin Pharmacol. 2005;45:597–602. doi: 10.1177/0091270004271947. [DOI] [PubMed] [Google Scholar]
- 38.Rudek MA, Zhao M, He P, Hartke C, Gilbert J, Gore SD, et al. Pharmacokinetics of 5-azacitidine administered with phenylbutyrate in patients with refractory solid tumors or hematologic malignancies. J Clin Oncol. 2005;23:3906–3911. doi: 10.1200/JCO.2005.07.450. [DOI] [PubMed] [Google Scholar]
- 39.Voytek P, Beisler JA, Abbasi MM, Wolpert-DeFilippes MK. Comparative studies of the cytostatic action and metabolism of 5-azacytidine and 5,6-dihydro-5-azacytidine. Cancer Res. 1977;37:1956–1961. [PubMed] [Google Scholar]
- 40.Pittillo RF, Woolley C. 5-azacytidine: microbiological assay in mouse blood. Appl Microbiol. 1969;18:284–286. doi: 10.1128/am.18.2.284-286.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diment S, Leech MS, Stahl PD. Generation of macrophage variants with 5-azacytidine: selection for mannose receptor expression. J Leukoc Biol. 1987;42:485–490. doi: 10.1002/jlb.42.5.485. [DOI] [PubMed] [Google Scholar]
- 42.Boyd AW, Schrader JW. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature. 1982;297:691–693. doi: 10.1038/297691a0. [DOI] [PubMed] [Google Scholar]
- 43.Harrington MA, Falkenburg JH, Daub R, Broxmeyer HE. Effect of myogenic and adipogenic differentiation on expression of colony-stimulating factor genes. Mol Cell Biol. 1990;10:4948–4952. doi: 10.1128/mcb.10.9.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katoh S, Tominaga A, Migita M, Kudo A, Takatsu K. Conversion of normal Ly-1-positive B-lineage cells into Ly-1-positive macrophages in long-term bone marrow cultures. Dev Immunol. 1990;1:113–125. doi: 10.1155/1990/28760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao XN, Lin J, Wang LL, Yu L. Demethylating treatment suppresses natural killer cell cytolytic activity. Mol Immunol. 2009;46:2064–2070. doi: 10.1016/j.molimm.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 46.Schmiedel B, Arelin V, Krusch M, Kanz L, Salih HR. 5-Azacytosine (Azacytidine) and 2′-deoxy-5-azacytidine (decitabine) mediate opposite effects on NK cell anti-tumor reactivity. Blood. 2008;112:2626. [Google Scholar]
- 47.Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, et al. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza- 2′-deoxycytidine. Mol Cancer Ther. 2005;4:1515–1520. doi: 10.1158/1535-7163.MCT-05-0172. [DOI] [PubMed] [Google Scholar]
- 48.Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, Kelly TK, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther. 2009;8:1579–1588. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Q, Wu A, Ray D, Deng C, Attwood J, Hanash S, et al. DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol. 2003;170:5124–5132. doi: 10.4049/jimmunol.170.10.5124. [DOI] [PubMed] [Google Scholar]
- 50.Djeu JY, Jiang K, Wei S. A view to a kill: signals triggering cytotoxicity. Clin Cancer Res. 2002;8:636–640. [PubMed] [Google Scholar]
- 51.McEarchern JA, Oflazoglu E, Francisco L, McDonagh CF, Gordon KA, Stone I, et al. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood. 2007;109:1185–1192. doi: 10.1182/blood-2006-07-034017. [DOI] [PubMed] [Google Scholar]
- 52.McEarchern JA, Smith LM, McDonagh CF, Klussman K, Gordon KA, Morris-Tilden CA, et al. Preclinical characterization of SGN-70, a humanized antibody directed against CD70. Clin Cancer Res. 2008;14:7763–7772. doi: 10.1158/1078-0432.CCR-08-0493. [DOI] [PubMed] [Google Scholar]
- 53.Oflazoglu E, Stone IJ, Gordon KA, Grewal IS, van Rooijen N, Law CL, et al. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood. 2007;110:4370–4372. doi: 10.1182/blood-2007-06-097014. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. Selective long-term elimination of natural killer cells in vivo by an anti-interleukin 2 receptor beta chain monoclonal antibody in mice. J Exp Med. 1993;178:1103–1107. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]