Abstract
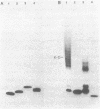
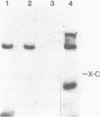
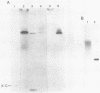
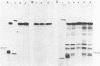
Unsubstituted oligodeoxynucleotides, or oligodeoxynucleotides linked to poly-(L)-lysine, when hybridized to a 322 base long template, did not inhibit the production of full length DNA copies by the Klenow fragment of E. coli DNA polymerase I. However, synthesis was inhibited if the cysteamine derivative of the same oligomer was cross-linked to the template via PtII. Truncated products were formed by termination of DNA synthesis a small number of bases upstream from the 5'-end of the cross-linked oligomer. AMV reverse transcriptase behaved similarly but was also slightly inhibited by the hybridized oligomer or its poly-lysine derivative.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Bridson P. K., Orgel L. E. Catalysis of accurate poly(C)-directed synthesis of 3'-5'-linked oligoguanylates by Zn2+. J Mol Biol. 1980 Dec 25;144(4):567–577. doi: 10.1016/0022-2836(80)90337-x. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Ligation of oligonucleotides to nucleic acids or proteins via disulfide bonds. Nucleic Acids Res. 1988 May 11;16(9):3671–3691. doi: 10.1093/nar/16.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G., Wu R. Terminal transferase: use of the tailing of DNA and for in vitro mutagenesis. Methods Enzymol. 1983;100:96–116. doi: 10.1016/0076-6879(83)00047-6. [DOI] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova L. S., Popova T. L., Ivanova N. A., Zibina E. A., Ivanova V. L. Epidemiologicheskii analiz nositel'stva virusa grippa A u zdorovykh detei. Vopr Virusol. 1983 May-Jun;(3):286–289. [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean J. M., Murakami A., Blake K. R., Cushman C. D., Miller P. S. Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger RNA. Biochemistry. 1988 Dec 27;27(26):9113–9121. doi: 10.1021/bi00426a008. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Murakami A., Blake K. R., Lin S. B., Miller P. S. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with single-stranded DNA. Biochemistry. 1988 May 3;27(9):3197–3203. doi: 10.1021/bi00409a011. [DOI] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Aurelian L., Blake K. R., Murakami A., Reddy M. P., Spitz S. A., Ts'o P. O. Control of ribonucleic acid function by oligonucleoside methylphosphonates. Biochimie. 1985 Jul-Aug;67(7-8):769–776. doi: 10.1016/s0300-9084(85)80166-8. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Zarytova V. F., Kutiavin I. V., Mamaev S. V., Podyminogin M. A. Complementary addressed modification and cleavage of a single stranded DNA fragment with alkylating oligonucleotide derivatives. Nucleic Acids Res. 1986 May 27;14(10):4065–4076. doi: 10.1093/nar/14.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T. R., Matteucci M. D. Hybridization triggered cross-linking of deoxyoligonucleotides. Nucleic Acids Res. 1986 Oct 10;14(19):7661–7674. doi: 10.1093/nar/14.19.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zerial A., Thuong N. T., Hélène C. Selective inhibition of the cytopathic effect of type A influenza viruses by oligodeoxynucleotides covalently linked to an intercalating agent. Nucleic Acids Res. 1987 Dec 10;15(23):9909–9919. doi: 10.1093/nar/15.23.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]