Abstract
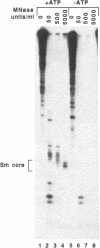
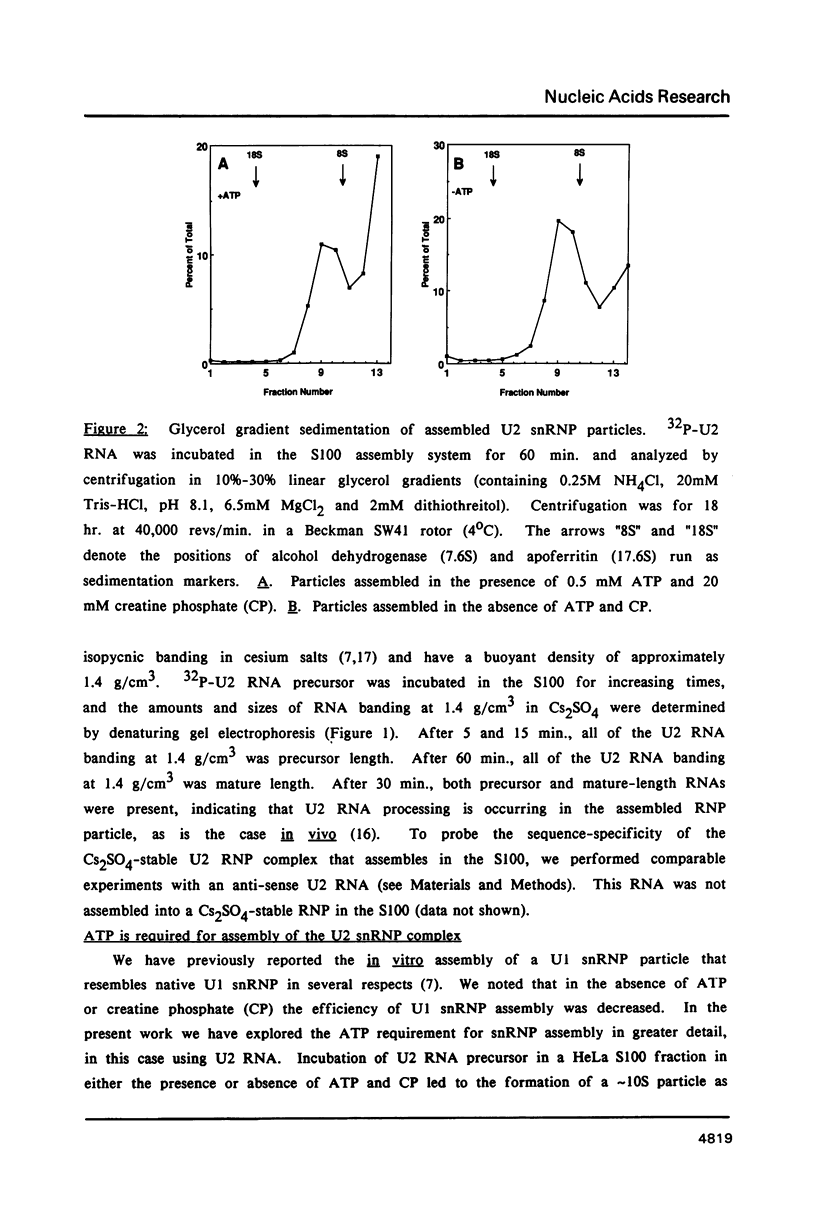
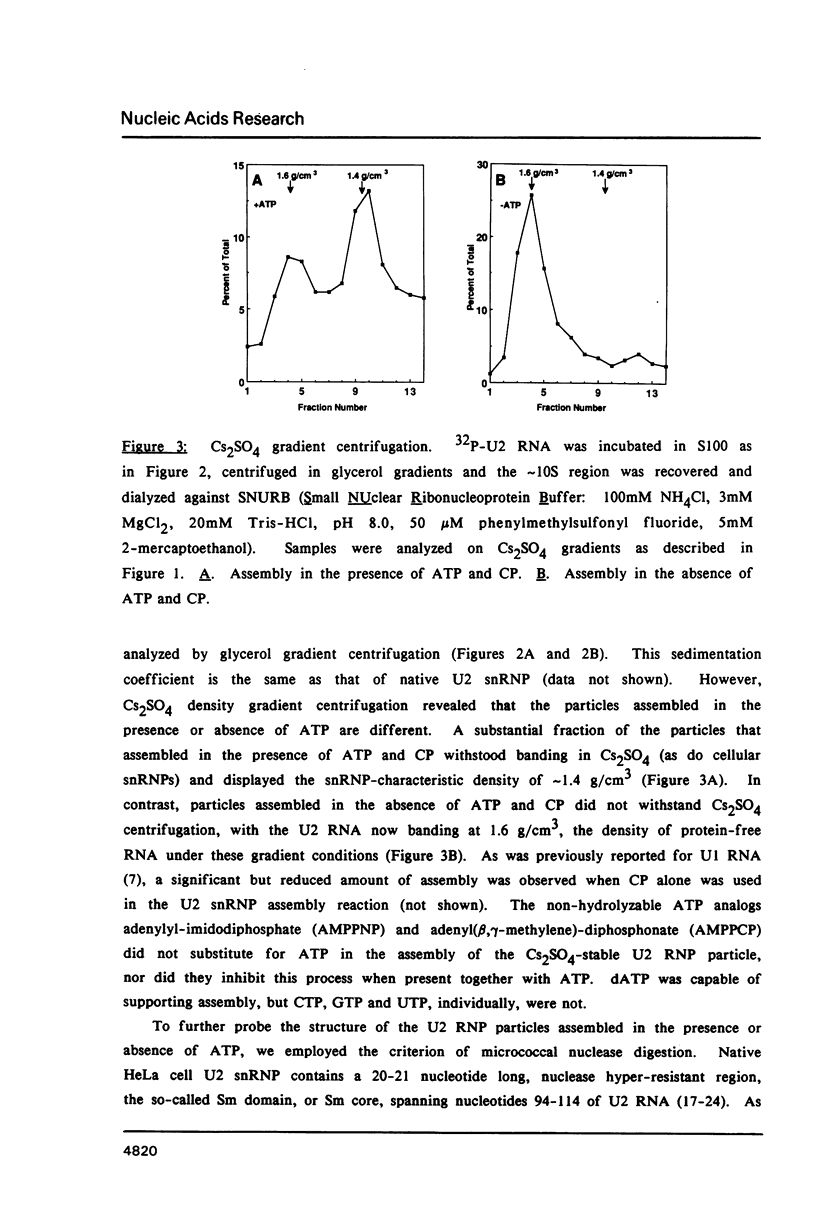
Incubation of a SP6-transcribed human U2 RNA precursor molecule in a HeLa cell S100 fraction resulted in the formation of ribonucleoprotein complexes. In the presence of ATP, the particles that assembled had several properties of native U2 snRNP, including resistance to dissociation in Cs2SO4 gradients, their buoyant density, and pattern of digestion by micrococcal nuclease. These particles also reacted with Sm monoclonal antibody and a human autoantibody with specificity for the U2 snRNP-specific proteins A' and B", but not with antibodies for U1 snRNP-specific proteins. In contrast, the particles that formed in the absence of ATP did not have these properties. ATP analogs with non-hydrolyzable beta-gamma bonds did not substitute for ATP in U2 snRNP assembly. Additional experiments with a mutant U2 RNA confirmed that nucleotides 154-167 of U2 RNA are required for binding of the U2 snRNP-specific proteins but not of the "Sm" core proteins. Pseudouridine formation, a major post-transcriptional modification of U2 RNA, was enhanced under assembly permissive conditions.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arena F., Ciliberto G., Ciampi S., Cortese R. Purification of pseudouridylate synthetase I from Salmonella typhimurium. Nucleic Acids Res. 1978 Dec;5(12):4523–4536. doi: 10.1093/nar/5.12.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Kammen H. O., Penhoet E. E. Purification and properties of a mammalian tRNA pseudouridine synthase. J Biol Chem. 1982 Mar 25;257(6):3045–3052. [PubMed] [Google Scholar]
- Habets W., Hoet M., Bringmann P., Lührmann R., van Venrooij W. Autoantibodies to ribonucleoprotein particles containing U2 small nuclear RNA. EMBO J. 1985 Jun;4(6):1545–1550. doi: 10.1002/j.1460-2075.1985.tb03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., Kazmaier M., Mattaj I. W. In vitro assembly of U1 snRNPs. EMBO J. 1987 Nov;6(11):3479–3485. doi: 10.1002/j.1460-2075.1987.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., van Santen V. L., Spritz R. A., Mattaj I. W. Loop I of U1 small nuclear RNA is the only essential RNA sequence for binding of specific U1 small nuclear ribonucleoprotein particle proteins. Mol Cell Biol. 1988 Nov;8(11):4787–4791. doi: 10.1128/mcb.8.11.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A. M., Pederson T. Accurate and efficient 3' processing of U2 small nuclear RNA precursor in a fractionated cytoplasmic extract. Mol Cell Biol. 1987 Sep;7(9):3131–3137. doi: 10.1128/mcb.7.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelay-Taha M. N., Reveillaud I., Sri-Widada J., Brunel C., Jeanteur P. RNA-protein organization of U1, U5 and U4-U6 small nuclear ribonucleoproteins in HeLa cells. J Mol Biol. 1986 Jun 5;189(3):519–532. doi: 10.1016/0022-2836(86)90321-9. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Kunkel G. R., Pederson T. Precursors of U4 small nuclear RNA. J Cell Biol. 1984 Sep;99(3):1140–1144. doi: 10.1083/jcb.99.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA-protein complexes. J Biol Chem. 1984 Feb 10;259(3):1929–1933. [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Intracellular site of U1 small nuclear RNA processing and ribonucleoprotein assembly. J Cell Biol. 1984 Jan;98(1):188–192. doi: 10.1083/jcb.98.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., De Robertis E. M. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell. 1985 Jan;40(1):111–118. doi: 10.1016/0092-8674(85)90314-9. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Habets W. J., van Venrooij W. J. Monospecific antibodies reveal details of U2 snRNP structure and interaction between U1 and U2 snRNPs. EMBO J. 1986 May;5(5):997–1002. doi: 10.1002/j.1460-2075.1986.tb04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Patton J. R., Patterson R. J., Pederson T. Reconstitution of the U1 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1987 Nov;7(11):4030–4037. doi: 10.1128/mcb.7.11.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. R., Pederson T. The Mr 70,000 protein of the U1 small nuclear ribonucleoprotein particle binds to the 5' stem-loop of U1 RNA and interacts with Sm domain proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(3):747–751. doi: 10.1073/pnas.85.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikielny C. W., Bindereif A., Green M. R. In vitro reconstitution of snRNPs: a reconstituted U4/U6 snRNP participates in splicing complex formation. Genes Dev. 1989 Apr;3(4):479–487. doi: 10.1101/gad.3.4.479. [DOI] [PubMed] [Google Scholar]
- Reveillaud I., Lelay-Taha M. N., Sri-Widada J., Brunel C., Jeanteur P. Mg2+ induces a sharp and reversible transition in U1 and U2 small nuclear ribonucleoprotein configurations. Mol Cell Biol. 1984 Sep;4(9):1890–1899. doi: 10.1128/mcb.4.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Brunel C., Jeanteur P. Interaction of snRNAs with rapidly sedimenting nuclear sub-structures (hnRNPs) from HeLa cells. Nucleic Acids Res. 1983 Oct 11;11(19):6631–6646. doi: 10.1093/nar/11.19.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben E. D., Madore S. J., Pederson T. Protein binding sites are conserved in U1 small nuclear RNA from insects and mammals. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1217–1220. doi: 10.1073/pnas.80.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben E. D., Madore S. J., Pederson T. U1 small nuclear ribonucleoprotein studied by in vitro assembly. J Cell Biol. 1983 Jun;96(6):1751–1755. doi: 10.1083/jcb.96.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben E. D., Nenninger J. M., Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXXII. U2 small nuclear RNA precursors and their accurate 3' processing in vitro as ribonucleoprotein particles. J Mol Biol. 1985 May 5;183(1):69–78. doi: 10.1016/0022-2836(85)90281-5. [DOI] [PubMed] [Google Scholar]
- Wieben E. D., Pederson T. Small nuclear ribonucleoproteins of Drosophila: identification of U1 RNA-associated proteins and their behavior during heat shock. Mol Cell Biol. 1982 Aug;2(8):914–920. doi: 10.1128/mcb.2.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R., Nyffenegger T., De Robertis E. M. Nucleocytoplasmic distribution of snRNPs and stockpiled snRNA-binding proteins during oogenesis and early development in Xenopus laevis. Cell. 1983 Feb;32(2):425–434. doi: 10.1016/0092-8674(83)90462-2. [DOI] [PubMed] [Google Scholar]