A few species of Adonis are the only land plants known to produce the valuable red carotenoid astaxanthin in abundance. The pathway to astaxanthin in the flowers of Adonis aestivalis was ascertained and shown to function efficiently when transferred into a bacterium. This work will enable the metabolic engineering of organisms better-suited than Adonis for the biological production of astaxanthin.
Abstract
A few species in the genus Adonis are the only land plants known to produce the valuable red ketocarotenoid astaxanthin in abundance. Here, we ascertain the pathway that leads from the β-rings of β-carotene, a carotenoid ubiquitous in plants, to the 3-hydroxy-4-keto-β-rings of astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4’-dione) in the blood-red flowers of Adonis aestivalis, an ornamental and medicinal plant commonly known as summer pheasant’s eye. Two gene products were found to catalyze three distinct reactions, with the first and third reactions of the pathway catalyzed by the same enzyme. The pathway commences with the activation of the number 4 carbon of a β-ring in a reaction catalyzed by a carotenoid β-ring 4-dehydrogenase (CBFD), continues with the further dehydrogenation of this carbon to yield a carbonyl in a reaction catalyzed by a carotenoid 4-hydroxy-β-ring 4-dehydrogenase, and concludes with the addition of an hydroxyl group at the number 3 carbon in a reaction catalyzed by the erstwhile CBFD enzyme. The A. aestivalis pathway is both portable and robust, functioning efficiently in a simple bacterial host. Our elucidation of the pathway to astaxanthin in A. aestivalis provides enabling technology for development of a biological production process and reveals the evolutionary origin of this unusual plant pathway, one unrelated to and distinctly different from those used by bacteria, green algae, and fungi to synthesize astaxanthin.
INTRODUCTION
The red carotenoid astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) is of abiding natural interest as the pigment that imparts an attractive red color to the feathers and skin of many birds (e.g., flamingo, scarlet ibis, and roseate spoonbill; Brush, 1981; Goodwin, 1984), lends a blue to reddish hue to the carapace of lobster, shrimp, krill, crabs, and other crustaceans (Goodwin, 1984; Matsuno, 2001), and affords an appetizing orange to red color to the flesh of salmon, red snapper, and other fish (Goodwin, 1984; Matsuno, 2001). This lipid-soluble pigment has found use as a colorant in foods and cosmetics (Klaüi and Bauernfeind, 1981; Münzel, 1981) and a pigmenting agent in poultry feeds (Bauernfeind et al., 1981; Marusich and Bauernfeind, 1981), and it has become of significant economic importance as a feed additive for the aquaculture industry (Simpson et al., 1981; Higuera-Ciapara et al., 2006). More recently, astaxanthin has generated considerable interest as a potential therapeutic agent for the treatment of oxidative stress, inflammation, and cardiovascular diseases in humans and animals (Hussein et al., 2006; Pashkow et al., 2008; Goswami et al., 2010; Fassett and Coombes, 2011).
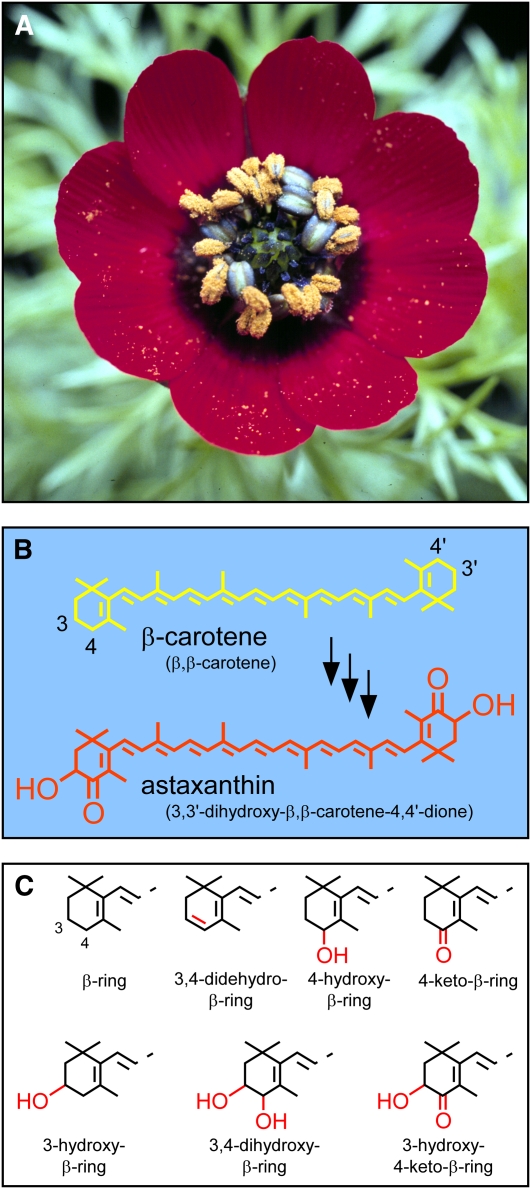
Astaxanthin is synthesized by certain bacteria (Johnson and An, 1991; Misawa et al., 1995b; Tao et al., 2006), several fungi (Johnson and An, 1991; Lukács et al., 2006), some green algae (Margalith, 1999), and a few species of the flowering plant genus Adonis (Figure 1A; Seybold and Goodwin, 1959; Egger, 1965; Neamţu et al., 1966; Renstrøm et al., 1981). The formation of astaxanthin from β-carotene, a carotenoid omnipresent in oxygenic photosynthetic organisms (Goodwin, 1980), requires only the addition of a hydroxyl and a carbonyl at the adjacent number 3 and number 4 carbons, respectively, of each β-ring (Figure 1B). Because plants already have enzymes able to add a hydroxyl group to the number 3 carbon of carotenoid β-rings (reviewed in Kim et al., 2009), it would seem that Adonis need only have acquired an ability to introduce a carbonyl at the number 4 carbon to produce astaxanthin.
Figure 1.
A. aestivalis in Flower, Chemical Structure of the Pigment Responsible for the Resplendent Red Color of the Flower Petals, and Structures of Carotenoid Rings Relevant to This Work.
(A) Mature flower of A. aestivalis.
(B) Structure of astaxanthin and of the carotenoid from which it is presumed to be derived, β-carotene. Colors of the structures approximate the colors of the carotenoids they represent. Semisystematic names for the carotenoids (Weedon and Moss, 1995) are in parentheses. Numbering of the carbon atoms is indicated for two positions of interest in the two β-rings of β-carotene.
(C) Structures of carotenoid rings relevant to this work. Carbon numbering for two positions of interest is indicated for the unmodified β-ring (top left). Features of interest are highlighted in red.
Enzymes able to catalyze the introduction of a carbonyl at the number 4 carbon of unmodified carotenoid β-rings have been identified in bacteria (Misawa et al., 1995a), green algae (Kajiwara et al., 1995; Lotan and Hirschberg, 1995), and cyanobacteria (Fernández-González et al., 1997). We previously attempted to identify cDNAs that might encode such a 4-ketolase enzyme in the flowers of Adonis aestivalis, an ornamental and medicinal plant commonly known as summer pheasant’s eye (Figure 1A). A complementation strategy that had been used to identify cDNAs for this enzyme in the green alga Haematococcus pluvialis (Kajiwara et al., 1995; Lotan and Hirschberg, 1995) was employed in this earlier effort (Cunningham and Gantt, 2005). Contrary to expectation, the products of the two closely related A. aestivalis cDNAs that were identified using this strategy were found to convert β-carotene into a mixture of carotenoids with 4-hydroxy-β-rings and/or 3,4-didehydro-β-rings rather than 4-keto-β-rings (Cunningham and Gantt, 2005; see Figure 1C for the structures of these rings).
We first speculated that 4-keto-β-rings might be produced in the flowers of A. aestivalis by keto-enol isomerization of 3,4-didehydro-4-hydroxy-β-rings (Cunningham and Gantt, 2005), but later came to consider it more likely that a second enzyme was needed to introduce a carbonyl at the now-activated number 4 carbon of a 4-hydroxy-β-ring and/or a 3,4-didehydro-β-ring. Here, we report the identification of cDNAs that encode a second A. aestivalis enzyme that, in combination with the enzyme specified by the two earlier identified cDNAs, efficiently converts β-carotene into astaxanthin in cells of Escherichia coli. The biochemical route to the 3-hydroxy-4-keto-β-rings of astaxanthin in A. aestivalis is unusual and unexpected, with the very same enzyme found to catalyze both the initial and terminal steps of a three-step pathway that is unrelated to and unlike the pathways that lead to astaxanthin in bacteria, fungi, and green algae. The portability and efficiency of the A. aestivalis pathway described in this article make the cDNAs that encode the requisite enzymes a promising resource for the metabolic engineering of bacteria, yeasts, and plants more well suited than Adonis for the biological production of astaxanthin.
RESULTS
The Enzymes That Convert β-Carotene into Astaxanthin in Adonis Reveal Themselves
We earlier introduced an A. aestivalis flower cDNA library into a β-carotene–accumulating strain of E. coli (cells contained plasmid pAC-BETA; see Table 1 for a description of this and other plasmids used in this work) and visually screened for transformants that produced orange colonies, rather than yellow ones, on agar plates (Cunningham and Gantt, 2005). This color complementation screening protocol was designed to exploit the red shift in the visible absorption spectrum that accompanies the introduction of a carbonyl at the number 4 carbon of one or both of the β-rings of β-carotene and was predicated on an assumption that, as in bacteria and green algae, a single gene product catalyzed the introduction of this carbonyl in the pathway to astaxanthin in A. aestivalis. Orange colonies were indeed obtained using this screen (Figure 2A). However, cultures inoculated with these relatively rare orange colonies (~1 in 20,000) were not found to produce the 4-keto-carotenoids that had been expected [i.e., echinenone (β,β-caroten-4-one) and canthaxanthin (β,β-carotene-4,4′-dione)] but rather accumulated a complex mixture of red, orange, and yellow carotenoids (lane 3 of Figure 2C) with 4-hydroxy-β-rings and/or 3,4-didehydro-β-rings (Cunningham and Gantt, 2005). Two closely related cDNAs were present in the library plasmids recovered from these colonies. Because the enzymes encoded by these cDNAs convert carotenoid β-rings into 4-hydroxy-β-rings and 3,4-didehydro-β-rings, and therefore act as both a 3,4-desaturase and a 4-hydroxylase, they will be designated as carotenoid β-ring 4-dehydrogenases (CBFDs) and the two cDNAs, previously known as AdKeto1 and AdKeto2 (Cunningham and Gantt, 2005), will henceforth be referred to as cbfd1 and cbfd2.
Table 1.
Plasmids Used in This Work
| Plasmid | Description | Source or Reference |
| pBluescript SK− | cDNA library vector; pUC origin; Ampr | Stratagene |
| pSTBlue-1 | Blunt cloning vector; pUC origin; Ampr; Kanr | Novagen |
| pBAD/His A | Expression vector; arabinose inducible; pBR322 origin; Ampr | Invitrogen |
| pBAD/His B | Expression vector; arabinose inducible; pBR322 origin; Ampr | Invitrogen |
| pCBFD1 | Previously called pAdKeto1, pUC origin; Ampr | Cunningham and Gantt (2005) |
| pCBFD2 | Previously called pAdKeto2, pUC origin; Ampr | Cunningham and Gantt (2005) |
| pCBFD1/CBFD2 | Previously called pAdKeto1/2, contains A. aestivalis cDNAs cbfd1 (AdKeto1) and cbfd2 (AdKeto2), pUC origin; Ampr | Cunningham and Gantt (2005) |
| pCBFD2Bad | Previously referred to as pAdKeto2Bad; contains the A. aestivalis cbfd2 cDNA with expression under control of the araBAD promoter; pBR322 origin; Ampr | Cunningham and Gantt (2005) |
| pAC-BETA | Produces β-carotene in E. coli; p15A origin; Capr | Cunningham et al. (1996) |
| pAC-BETAipi | pAC-BETA with an Erwinia herbicola ipi gene added to improve yield; p15A origin; Capr | Cunningham and Gantt (2005) |
| pAC-BETA-CBFD1/2 | pAC-BETA with the A. aestivalis cbfd1 and cbfd2 cDNAs inserted; ~13.6 kb; p15A origin; Capr | This work |
| pAC-CANTHipi | Produces canthaxanthin in E. coli; some echinenone and β-carotene also accumulate; p15A origin; Capr | Cunningham and Gantt (2007) |
| pHPK | H. pluvialis cDNA library plasmid; the cDNA encodes a carotenoid β-ring 4-ketolase (BKT); pUC origin; Ampr | Lotan and Hirschberg (1995) |
| pHBFD1 | A. aestivalis cDNA library plasmid, pUC origin; Ampr | This work |
| pHBFD2 | A. aestivalis cDNA library plasmid, pUC origin; Ampr | This work |
| pHBFD1Bad | hbfd1 cDNA cloned, in frame, in pBAD/His B; expression under control of the araBAD promoter; ~5.3 kb; pBR322 origin; Ampr | This work |
| pCBFD1/HBFD1Bad | cbfd1 cDNA inserted in pHBFD1Bad; ~6.9 kb; pBR322 origin; Ampr | This work |
| pCBFD2Bad/HBFD1 | A. aestivalis cbfd2 cDNA, fused to the araBAD promoter, and araC gene were together inserted into pHBFD1; ~6.7 kb; pUC origin; Ampr | This work |
| pNaHBFD-likeBad | N. advena cDNA encoding a polypeptide similar to the A. aestivalis HBFD polypeptides cloned in frame in pBAD/His A; expression under control of the araBAD promoter; ~5.6 kb; pBR322 origin; Ampr | This work |
| pCBFD1/NaHBFD-likeBad | cbfd1 cDNA inserted in pNaHBFD-likeBad; ~7.2 kb; pBR322 origin; Ampr | This work |
Ampr, ampicillin resistance; Capr, chloramphenicol resistance; Kanr, kanamycin resistance.
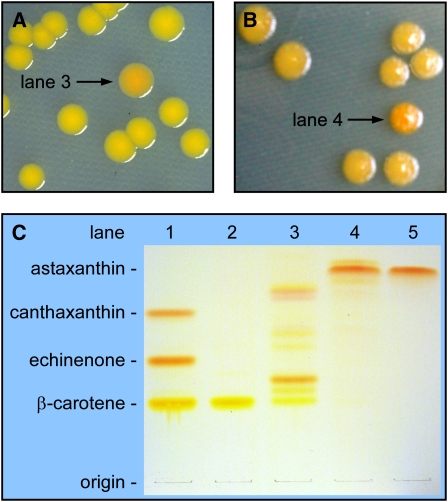
Figure 2.
Color Complementation Screening of an A. aestivalis cDNA Library Enabled the Identification of cDNAs That Encode the Enzymes Needed to Convert β-Carotene into Astaxanthin.
(A) An A. aestivalis cDNA library was introduced into a strain of E. coli that had been engineered to produce β-carotene (cells contained plasmid pAC-BETA). Cultures inoculated with the rare orange colonies (e.g., that indicated with an arrow) selected from among a multitude of yellow colonies were found to produce a complex mixture of carotenoids (lane 3 of [C]) with 4-hydroxy and/or 3,4-didehydro-β-rings (Cunningham and Gantt, 2005).
(B) A second screening of the A. aestivalis cDNA library was performed in E. coli engineered to produce those pigments found in orange colonies selected in the initial screen (cells contained plasmid pAC-BETA-CBFD1/2, constructed by inserting the two cDNAs recovered from library plasmids selected in the initial screen, cbfd1 and cbfd2, into pAC-BETA). Colonies a darker orange to red in color (e.g., indicated with an arrow) were selected for analysis.
(C) Reverse-phase TLC separation of pigments extracted from E. coli containing the plasmids listed below (lanes 1 to 4) and of a synthetic astaxanthin standard (lane 5). Lane 1, pAC-BETA + pHPK (contains an H. pluvialis cDNA that encodes an enzyme that converts β-carotene into echinenone and canthaxanthin); lane 2, pAC-BETA; lane 3, pAC-BETA and pCBFD1 (a cDNA library plasmid recovered from the orange colony indicated with an arrow in [A]); lane 4, pAC-BETA-CBFD1/2 and pHBFD1 (a cDNA library plasmid recovered from that colony indicated with an arrow in [B]); lane 5, astaxanthin standard. Note: The apparent colors of carotenoids on TLC plates are very much affected by the concentrations of the pigments, with higher concentrations of yellow carotenoids appearing more orange to red in color.
We reasoned that a second enzyme might be required to introduce a carbonyl at the now-activated number 4 carbon of a 4-hydroxy-β-ring and/or a 3,4-didehydro-β-ring in the pathway to astaxanthin in the flowers of A. aestivalis. A second screening of the A. aestivalis cDNA library was therefore performed in E. coli engineered to produce those pigments found in the orange colonies selected in the initial screen. Transformants again were spread on agar plates, and colonies that were a darker orange in color were selected for further analysis (Figure 2B). Such colonies were observed at a surprisingly high frequency (~1 in 200), but only a scant few of the many that were analyzed were found to differ from the parent E. coli strain in their carotenoid composition (see Supplemental Table 1 online), with astaxanthin the predominant pigment in cultures inoculated with these colonies (Figure 2C, compare lanes 4 and 5). One or the other of two closely related cDNAs, initially referred to as AdKC17 and AdKC28 (because they were the 17th and 28th cDNAs selected in this screen for Adonis cDNAs encoding polypeptides that might be involved in ketocarotenoid biosynthesis) were present in the library plasmids recovered from these colonies. These cDNAs, for reasons that will become clear, will hereafter be referred to as hbfd1 and hbfd2.
A Three-Step Pathway for Conversion of Carotenoid β-Rings into 3-Hydroxy-4-Keto-β-Rings
An accumulation of astaxanthin, rather than canthaxanthin and/or echinenone, in certain of the darker orange colonies selected in the second A. aestivalis cDNA library screen was very much unexpected. This finding indicated that, as anticipated, the products of two A. aestivalis cDNAs were required to produce a 4-keto-β-ring. In addition, however, it implied that the product of one of these two A. aestivalis cDNAs also served to catalyze the introduction of a hydroxyl group at the number 3 carbon of each ring. At what point in the pathway does this 3-hydroxylation reaction occur? What exactly is the sequence of the biochemical reactions that convert the β-rings of β-carotene into the 3-hydroxy-4-keto-β-rings of astaxanthin in the flowers of A. aestivalis?
The polypeptides encoded by the A. aestivalis hbfd1 and hbfd2 cDNAs were not found able to use either β-carotene (cf. Figures 3A and 3C) or zeaxanthin (β,β-carotene-3,3′-diol; see Supplemental Figure 1 online), a carotenoid with 3-hydroxy-β-rings, as substrates in cells of E. coli. The initial reaction of the pathway that leads from the β-rings of β-carotene to the 3-hydroxy-4-keto-β-rings of astaxanthin, therefore, is one that is catalyzed by the carotenoid β-ring 4-dehydrogenase enzyme encoded by the earlier-identified cbfd1 and cbfd2 cDNAs: the conversion of β-rings into 4-hydroxy-β-rings and/or 3,4-didehydro-β-rings (Figure 3B; Cunningham and Gantt, 2005).
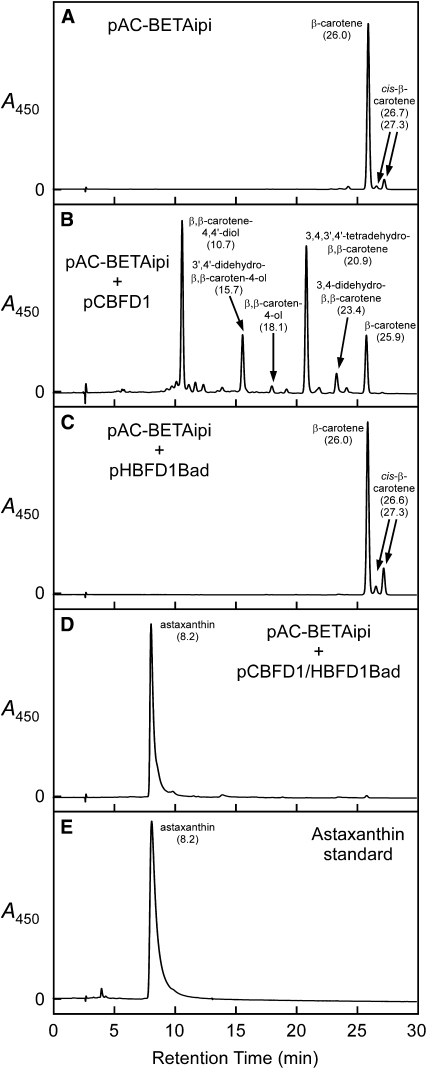
Figure 3.
The Products of A. aestivalis cDNAs cbfd1 and hbfd1 Together Catalyze a Near Complete Conversion of β-Carotene into Astaxanthin in Cells of E. coli, with the Initial Reaction Catalyzed by the Product of cbfd1.
Shown are HPLC elution profiles for extracts of E. coli cultures wherein cells contained the following plasmids: (A) pAC-BETAipi; (B) pAC-BETAipi and pCBFD1; (C) pAC-BETAipi and pHBFD1Bad; (D) pAC-BETAipi and pCBFD1/HBFD1Bad. (E) displays an HPLC elution profile for a synthetic astaxanthin standard. Absorption spectra for the peaks in (A), (D), and (E) are displayed in Supplemental Figure 6 online. Absorption spectra for the peaks in (B) are shown in Supplemental Figure 7 online. Arabinose (0.05% [w/v]) was included in the growth medium of cultures wherein cells contained pHBFD1Bad or pCBFD1/HBFD1Bad to induce production of the HBFD1 polypeptide. Numbers in parentheses below carotenoid names are HPLC retention times in minutes.
Time-course studies of the conversion of β-carotene into astaxanthin in cells of E. coli (Figure 4B) revealed that carotenoids with 4-keto-β-rings [echinenone, canthaxanthin, and adonirubin (3-hydroxy-β,β-carotene-4,4′-dione)] are bona fide intermediates in the pathway. Because carotenoids with 3-hydroxy-β-rings or with 3,4-dihydroxy-β-rings were not detected in these experiments, it would appear that hydroxylation of the number 3 carbon does not occur until after the 4-keto group has been introduced. The second reaction of the pathway, a reaction that requires the product of the hbfd1 or hbfd2 cDNA, is then the conversion of a 4-hydroxy-β-ring and/or a 3,4-didehydro-β-ring into a 4-keto-β-ring. The hydroxylation of the number 3 carbon of a 4-keto-β-ring, consequently, is the third and final step of the pathway. But which A. aestivalis gene product catalyzes this terminal pathway reaction?
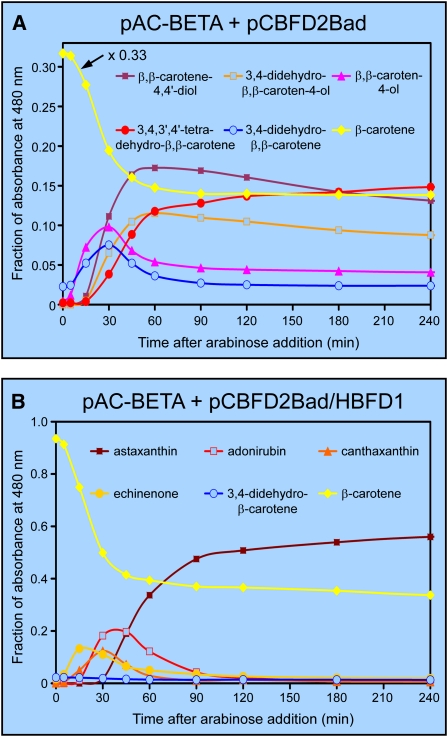
Figure 4.
Time-Course Studies of the Conversion of Carotenoid β-Rings into 4-Hydroxy-β-Rings and 3-Hydroxy-4-Keto-β-Rings, as Catalyzed by the Enzymes of A. aestivalis in Cells of E. coli.
(A) Time-course study for an E. coli culture wherein cells contained plasmids pAC-BETA and pCBFD2Bad.
(B) Time-course study wherein cells contained pAC-BETA and pCBFD2Bad/HBFD1. Cultures were grown overnight on a rotary shaker at 28°C, diphenylamine (200 μM) was then added to block further synthesis of colored carotenoids (Cunningham and Gantt, 2007), and arabinose (0.2% [w/v]) was added 2 h later (at time = 0) to induce production of the polypeptide encoded by the A. aestivalis cbfd2 cDNA. Samples were taken at the indicated times for analysis by HPLC (see Supplemental Figure 8 online), the fraction of the total integrated area (detector set to 480 nm) was ascertained for individual peaks, and the data were plotted as a function of time after the addition of arabinose. An abbreviated scale was used for the y axis in (A) to more clearly illustrate changes in the amounts of the various intermediates over time. Accordingly, the amount of β-carotene at each time point was multiplied by 0.33 to keep on scale.
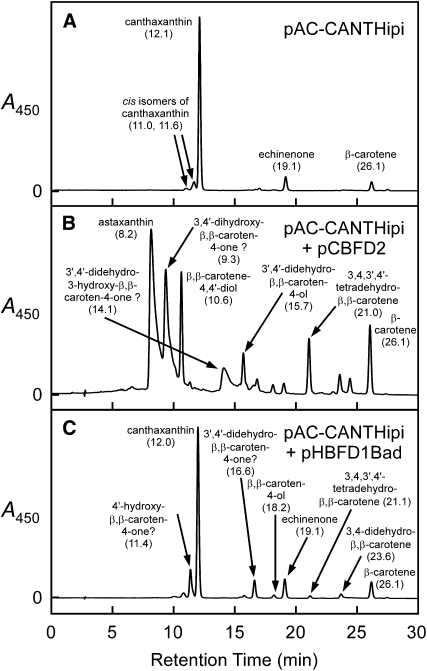
Plasmids containing either the cbfd2 cDNA or the hbfd1 cDNA were separately introduced into a strain of E. coli that had been engineered to produce canthaxanthin, a carotenoid with 4-keto-β-rings (β,β-carotene-4,4′-dione; cells contained the plasmid pAC-CANTHipi). An accumulation of astaxanthin was observed in those cultures wherein cells contained the plasmid with the cbfd2 cDNA (Figure 5B) but not in those wherein cells contained the plasmid with the hbfd1 cDNA (Figure 5C). Considerable amounts of carotenoids with 4-hydroxy-β-rings and 3,4-didehydro-β-rings also were found in those cultures with the cbfd2 cDNA, but carotenoids with 4-keto-β-rings were not observed in extracts of these cultures (Figure 5B), indicating that the product of the cbfd2 cDNA is able to catalyze the conversion of 4-keto-β-rings into 3-hydroxy-4-keto-β-rings, whereas the 4-ketolase enzyme encoded by the H. pluvialis bkt cDNA (in plasmid pAC-CANTHipi) is much less adept (if able to do so at all) at modifying 4-hydroxy-β-rings or 3,4-didehydro-β-rings. From these observations, we conclude that the product of the A. aestivalis cbfd2 cDNA, which functions as a 4-hydroxylase/3,4-desaturase when provided with carotenoid β-rings as the substrate in E. coli (Figure 3B; Cunningham and Gantt, 2005), acts as a 3-hydroxylase when presented with carotenoid 4-keto-β-rings.
Figure 5.
The Product of the A. aestivalis cbfd2 cDNA, Earlier Shown to Add a Hydroxyl to the Number 4 Carbon of Carotenoid β-Rings (Cunningham and Gantt, 2005), Adds a Hydroxyl to the Number 3 Carbon of 4-Keto-β-Rings.
Shown are HPLC elution profiles for extracts of E. coli cultures wherein cells contained the following: (A) pAC-CANTHipi; (B) pAC-CANTHipi and pCBFD2; and (C) pAC-CANTHipi and pHBFD1Bad. Absorption spectra for the peaks in (A) are displayed in Supplemental Figure 6 online. Absorption spectra for the peaks in (B) and (C) are shown in Supplemental Figure 7 online. Arabinose (0.05% [w/v]) was included in the growth medium of the E. coli culture wherein cells contained pHBFD1Bad. Numbers in parentheses are HPLC retention times in minutes. Those peak identifications followed by question marks in (B) and (C) are speculative: They are consistent with absorption spectra, HPLC retention times, and the known catalytic capabilities of the carotenoid pathway enzymes that are present in the E. coli cells, but the requisite standards were not available for comparison.
Canthaxanthin was the predominant pigment in cultures of E. coli wherein cells contained the plasmids pAC-CANTHipi and pHBFD1Bad (Figure 5C), much as in control cultures where cells contained only the pAC-CANTHipi plasmid (Figure 5A). Those cultures with cells containing both the pAC-CANTHipi and pHBFD1Bad plasmids, however, also accumulated small amounts of several carotenoids with 4-hydroxy-β-rings and/or 3,4-didehydro-β-rings (Figure 5C). From this result, together with the earlier finding that β-carotene was not a substrate for the HBFD1 enzyme in E. coli (Figure 3C), we infer that the reaction catalyzed by the product of the hbfd1 cDNA is, to some degree at least, reversible.
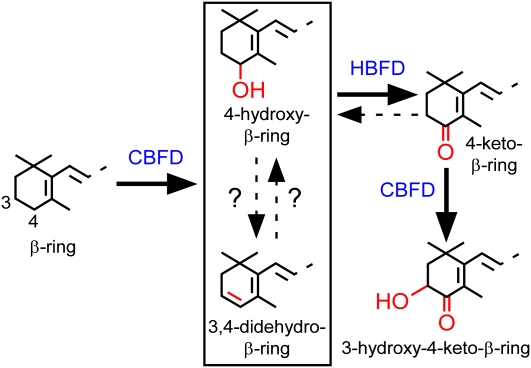
The route to a 3-hydroxy-4-keto-β-ring, as catalyzed by the enzymes of A. aestivalis in E. coli, is a linear three-step pathway that commences with the activation of the number 4 carbon of a β-ring through the action of the products of the cbfd1 and cbfd2 cDNAs, continues with the further dehydrogenation of this carbon to yield a carbonyl in a reaction catalyzed by an enzyme encoded by the hbfd1 and hbfd2 cDNAs, and concludes with the addition of an hydroxyl group at the number 3 carbon in a reaction catalyzed by the products of the cbfd1 and cbfd2 cDNAs (Figure 6). Two aspects of the pathway remain uncertain: the substrate for the enzyme encoded by the hbfd1 and hbfd2 cDNAs (whether a 4-hydroxy-β-ring or a 3,4-didehydro-β-ring) and the interconvertibility of 4-hydroxy-β-rings and 3,4-didehydro-β-rings.
Figure 6.
The Pathway from Carotenoid β-Rings to 3-Hydroxy-4-Keto-β-Rings as Catalyzed by the Products of the cbfd and hbfd cDNAs of A. aestivalis in Cells of E. coli.
The specific substrate for the enzyme encoded by the A. aestivalis hbfd cDNAs, whether a 3,4-didehydro-β-ring or a 4-hydroxy-β-ring, and the interconvertibility of these two rings remain uncertain.
[See online article for color version of this figure.]
What Is the Substrate for the Enzyme Encoded by the hbfd1 and hbfd2 cDNAs?
Time-course experiments showed that the β-rings of β-carotene are converted into 4-hydroxy-β-rings and 3,4-didehydro-β-rings with similar kinetics and comparable yields in cells of E. coli containing the cbfd2 cDNA (Figure 4A). However, neither carotenoids with 4-hydroxy-β-rings nor those with 3,4-didehydro-β-rings were observed to accumulate to any appreciable degree in cells of E. coli that also contained and expressed the hbfd1 cDNA (Figures 3D and 4B). It would appear, then, that either both ring types are substrates for the enzyme encoded by the hbfd1 cDNA or, if only one ring type is a substrate for the enzyme, then the other ring type is readily converted into it.
Biochemical assays of carotenoid pathway reactions in vitro are made difficult by the unavailability and lipophilic nature of most substrates and the membrane-associated if not membrane-integral location of most enzymes. To ascertain the identity of the substrate for the HBFD1 enzyme, therefore, the hbfd1 cDNA was placed under the control of the arabinose-inducible araBAD promoter and expression was induced in cultures of E. coli that had been allowed to accumulate carotenoids with 4-hydroxy-β-rings and 3,4-didehydro-β-rings (cells contained the plasmids pAC-BETA-CBFD1/2 and pHBFD1Bad or the plasmids pAC-BETAipi and pCBFD1/HBFD1Bad; see Table 1), with the protocol for these experiments essentially the same as that used for the time course studies shown in Figure 4. Experiments were performed both with and without the addition of the carotenoid pathway inhibitor diphenylamine (as described in the legend to Figure 4), but metabolism of the accumulated carotenoids was not observed in any case: carotenoid compositions of the induced cultures did not differ from those of uninduced control cultures when extracted 1 to 3 h after the addition of arabinose. These negative results were not likely due to an insufficiency of the enzyme encoded by the hbfd1 cDNA because astaxanthin became the predominant pigment if arabinose was added at the time of inoculation. We speculate that the 4-hydroxy-carotenoids and 3,4-didehydro-carotenoids that accumulated before expression of the hbfd1 cDNA was induced were inaccessible to the newly made HBFD1 enzyme. Even for successful time-course experiments, such as those illustrated in Figure 4, as much as one-third of the accumulated substrate (i.e., β-carotene in the case of the experiments shown in Figure 4) was not metabolized during the course of the experiment.
Carotenoids with 4-hydroxy-β-rings are probably the usual and customary substrates for the enzyme encoded by the hbfd1 and hbfd2 cDNAs when this enzyme functions in its natural milieu: the membranes of the chromoplasts of A. aestivalis. The accumulation of carotenoids with 3,4-didehydro-β-rings in cells of E. coli containing pAC-BETA and one or both of the cbfd cDNAs (Figure 3B) may simply be a consequence of the non-native environment. Carotenoids with 4-hydroxy-β-rings are the predominant pigments in the flowers of three species of Adonis with yellow petals (Czygan, 1969), and small amounts of such carotenoids have been detected in the red, ketocarotenoid-containing flower petals of A. aestivalis (Neamţu et al., 1966; Kamata et al., 1990) as well, but carotenoids with 3,4-didehydro-β-rings have not been reported to be present in the flowers of any species of Adonis. The enzymes encoded by the hbfd1 and hbfd2 cDNAs will therefore be designated as carotenoid 4-hydroxy-β-ring 4-dehydrogenases (HBFDs).
DISCUSSION
Several and Sundry Routes to Astaxanthin
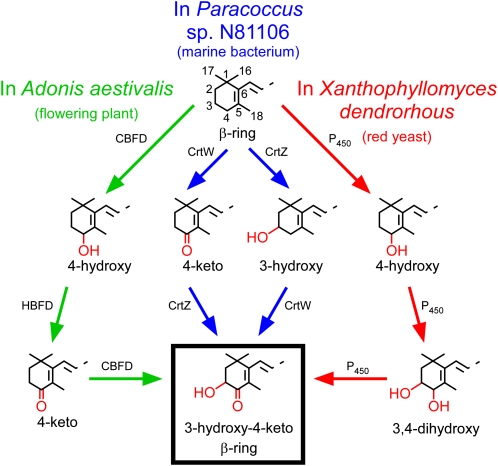
This work was undertaken with an assumption that, as in bacteria, cyanobacteria, and green algae, a single enzyme served to catalyze the introduction of a carbonyl at the number four carbon of carotenoid β-rings in the pathway to astaxanthin in the flowers of A. aestivalis. Instead, it was discovered that A. aestivalis employs a strikingly different way, one that requires two gene products and entails two separate biochemical reactions, to achieve this same result. An ability to add a carbonyl to the number 4 carbon of carotenoid β-rings and to produce the 3-hydroxy-4-keto-β-rings of astaxanthin has arisen several times in nature. Figure 7 illustrates pathways that lead to carotenoid 3-hydroxy-4-keto-β-rings in three quite disparate organisms: a marine bacterium, a basidiomycetous yeast, and A. aestivalis. Each of these pathways requires two different gene products to convert a β-ring into a 3-hydroxy-4-keto-β-ring, but otherwise they are dissimilar, both in the sequence of the biochemical reactions and in the evolutionary origin of the enzymes that catalyze these reactions. There are, so far, four different ways known to introduce a 4-keto group to carotenoid β-rings, and four different enzymes have been identified, as well, that introduce a 3-hydroxyl group (see legend to Figure 7). However, additional enzymes that catalyze these reactions likely still remain to be discovered, given that certain insects, fish, and birds have been found able to convert ingested β-carotene and/or zeaxanthin into astaxanthin and other 4-ketocarotenoids (Thommen, 1971; Goodwin, 1986).
Figure 7.
Distinctly Different Biosynthetic Pathways Serve to Convert β-Rings into 3-Hydroxy-4-Keto-β-Rings in Carotenoids of the Flowering Plant A. aestivalis, the Marine Bacterium Paracoccus sp N81106, and the Red Yeast X. dendrorhous.
The pathway in Paracoccus sp N81106 (previously known as Agrobacterium aurantiacum) was ascertained by Misawa et al. (1995b). The pathway in X. dendrorhous (also known as Phaffia rhodozyma) has not been definitively established but is thought to operate using a single cytochrome P450 enzyme (with a cytochrome P450 reductase also needed to donate electrons; Alcaíno et al., 2008; Ukibe et al., 2009) to sequentially oxidize the number 3 and number 4 carbons of the two β-rings of β-carotene (Ojima et al., 2006; Álvarez et al., 2006; Martín et al., 2008). Note that the biosynthesis of carotenoids with 3-hydroxy-4-keto-β-rings in the green alga H. pluvialis entails the use of an enzyme similar in amino acid sequence to the CrtW of Paracoccus sp N81106 to catalyze the addition of the carbonyl (Kajiwara et al., 1995; Lotan and Hirschberg, 1995), but hydroxylation of the number 3 carbon may be catalyzed by a cytochrome P450 enzyme (Schoefs et al., 2001) rather than a CrtZ (or the related CHYb) enzyme. Note also that certain bacteria and cyanobacteria employ a fourth way, using an enzyme referred to as CrtO, to add a carbonyl to the number 4 carbon of carotenoid β-rings (Fernández-González et al., 1997; Tao and Cheng, 2004). A fourth way is available, as well, to add a 3-hydroxyl group to carotenoid β-rings: A carotenoid β-ring 3-hydroxylase enzyme of a type referred to as CrtR is present in certain cyanobacteria (Masamoto et al., 1998). CBFD, the carotenoid β-ring 4-dehydrogenase enzyme encoded by the A. aestivalis cbfd1 and cbfd2 cDNAs; HBFD, the carotenoid 4-hydroxy-β-ring 4-dehydrogenase enzyme encoded by the A. aestivalis hbfd1 and hbfd2 cDNAs.
Origin of the Pathway to Astaxanthin in Adonis
The biosynthesis and accumulation of astaxanthin in the flower petals of A. aestivalis is very much an anomaly. In the more than 50 years that have passed since the report of Seybold and Goodwin (1959) that astaxanthin is the predominant pigment in the flower petals of Adonis annua, no flowering plants other than a few species in the genus Adonis have been found to produce this valuable red pigment in abundance, if in any amount at all. (Astaxanthin was reported by Czeczuga [1987] to be a constituent of the autumn carotenoids of Metasequoia glyptostroboides, the dawn redwood, but a later examination of the autumn carotenoids in this same species by others [Ida et al., 1991] did not detect astaxanthin.) Whence came the ability of Adonis to synthesize astaxanthin?
The duplication of a gene encoding a preexisting carotenoid pathway enzyme, the CHYb-type carotenoid β-ring 3-hydroxylase (EC 1.14.13.129) that converts carotenoid β-rings into 3-hydroxy-β-rings (and thereby converts β-carotene into zeaxanthin) almost certainly provided the raw material for the initial and key enzyme of the pathway that leads from β-carotene to astaxanthin in the flowers of A. aestivalis: the carotenoid β-ring 4-dehydrogenase encoded by the cbfd1 and cbfd2 cDNAs. Of all the plant and algal CHYb sequences available, the two A. aestivalis CBFD polypeptides are most similar to that of A. aestivalis itself (Figure 8A). It is noteworthy that the CBFD enzyme was not able to hydroxylate the number 3 carbon of unmodified carotenoid β-rings or 4-hydroxy-β-rings in cells of E. coli (Figure 3B; Cunningham and Gantt, 2005), yet readily catalyzed this reaction when presented with carotenoid 4-keto-β-rings (Figure 5B). The inability of the CBFD to hydroxylate the number 3 carbon of unmodified carotenoid β-rings or 4-hydroxy-β-rings may be of important advantage in preventing a premature (i.e., prior to addition of the carbonyl) 3-hydroxylation and thereby maximizing the yield of astaxanthin. In accord, the conversion of β-carotene into astaxanthin through the action of the A. aestivalis enzymes in E. coli was found to be very much impaired by the presence of a functional bacterial 3-hydroxylase enzyme (see Supplemental Figure 1 online).
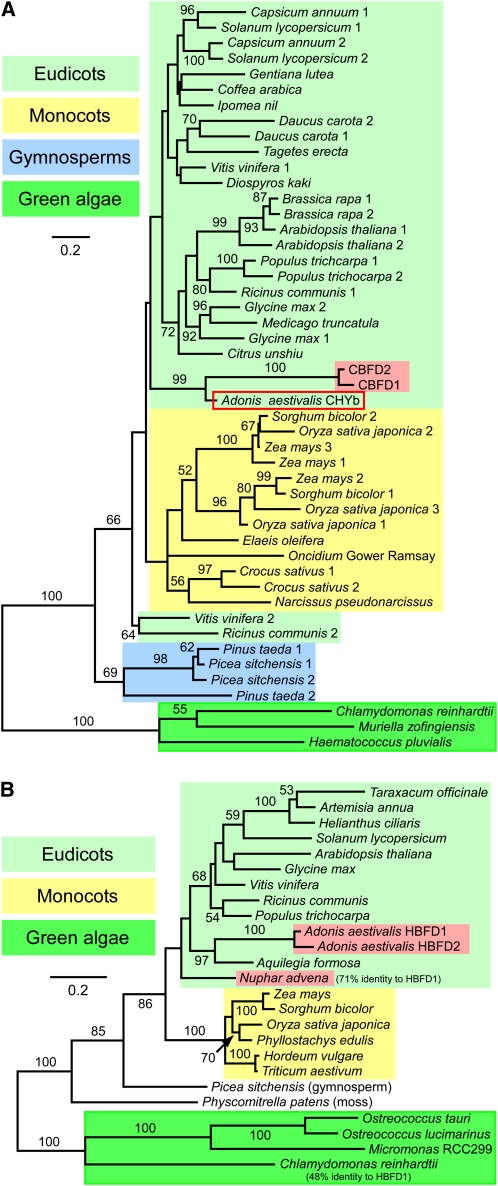
Figure 8.
Maximum Likelihood Trees for the CBFD and HBFD Enzymes of A. aestivalis and Related Polypeptides Encoded by cDNAs and Genes of Other Plants and Green Algae.
(A) Tree for plant and green algal carotenoid β-ring 3-hydroxylase enzymes of the membrane-integral, diiron, nonheme oxygenase type (CHYb-type) together with the A. aestivalis CBFD1 and CBFD2 polypeptides. CBFD1 and CBFD2 are highlighted with a red background. The A. aestivalis CHYb is enclosed in a red box. The amino acid sequences of CBFD1 and CBFD2 are ~90% identical to each other and ~58 to 60% identical overall to the A. aestivalis CHYb.
(B) Tree for the A. aestivalis HBFD1 and HBFD2 enzymes together with related polypeptides encoded by cDNAs and genes of other plants and green algae. HBFD1 and HBFD2 are highlighted with a red background, as is a N. advena polypeptide also shown to have 4-hydroxy-β-ring 4-dehydrogenase activity (Figure 9). Green algal sequences served as an outgroup for each tree. One thousand bootstrap trials were conducted, with bootstrap values >50% indicated. Alignments used to construct these trees are shown in Supplemental Figures 3 (for the CBFD and CHYb enzymes) and 4 (for HBFD and related polypeptides) online and are available in FASTA format as Supplemental Data Sets 1 and 2 online, respectively.
The CHYb and CBFD enzymes are members of a vast, varied, and versatile superfamily of membrane-integral, diiron, nonheme oxygenases that also includes the H. pluvialis carotenoid β-ring 4-ketolase (Kajiwara et al., 1995; Lotan and Hirschberg, 1995) and a plethora of membrane-associated fatty acid desaturases, hydroxylases, epoxidases, acetylenases, and ketolases in plants, animals, fungi, and bacteria (reviewed in Shanklin and Cahoon, 1998). No member of this important enzyme family has yet been crystallized, but, even so, much has been learned of the structure and function of many family members. Of particular relevance to the origin of the A. aestivalis CBFD, it has been reported that substrate specificity, regioselectivity, and catalytic specificity of certain fatty acid desaturases can be altered by changing only a few amino acids. Thus, for example, a fatty acid desaturase can quite easily be converted into a hydroxylase (Broadwater et al., 2002) or an acetylenase (Gagné et al., 2009) and vice versa. Indeed, it has been argued that desaturation and hydroxylation are alternative outcomes of the same catalytic event, with hydroxylation the default option (Broadwater et al., 2002).
The second enzyme of the pathway to astaxanthin in A. aestivalis, the carotenoid 4-hydroxy-β-ring 4-dehydrogenase encoded by the hbfd1 and hbfd2 cDNAs, also appears to have been derived from a preexisting, plastid-targeted dehydrogenase (Figure 8B). The specific function of the ancestor of the HBFD enzyme is unknown, but a role in the biosynthesis of carotenoids appears unlikely (see Supplemental Figure 2 online). The two A. aestivalis HBFD and the various plant and algal polypeptides that are closely related to these polypeptides (Figure 8B) each have at their N terminus, immediately following a putative chloroplast transit peptide, sequences indicative of an NADP binding Rossmann fold (Rao and Rossmann, 1973; predictions of coenzyme specificity were as described in Kallberg and Persson, 2006; http://bioinfo6.limbo.ifm.liu.se/coenzyme/), thus indicating the involvement of NADP+ in the reactions catalyzed by these enzymes.
When aligned with closely related plant and algal polypeptides, it can be seen that the A. aestivalis HBFD1 and HBFD2 lack 18 to 20 amino acids at the C terminus that are specified by the C-terminal exon of the genes in many plants (see Supplemental Figure 3 online). The absence of the amino acids specified by this exon, whether resulting from alternative splicing or the absence of this exon in the hbfd1 and hbfd2 genes, may be important to the specific function of the A. aestivalis gene products, but the full-length ancestor of this enzyme in Adonis most likely already was endowed with at least some ability to convert carotenoid 4-hydroxy-β-rings (and/or 3,4-didehydro-β-rings) into 4-keto-β-rings. An HBFD-like polypeptide encoded by a Nuphar advena cDNA (this included the C-terminal exon) was found able to catalyze this reaction in E. coli (Figure 9). N. advena (spatterdock, of the water lily family Nymphaeaceae) produces yellow flowers, is not known to produce carotenoids with 4-keto-β-rings, and is not a particularly close relative of A. aestivalis (family Ranunculaceae).
Figure 9.
An N. advena cDNA That Encodes a Polypeptide (NaHBFD-Like) Similar in Amino Acid Sequence to the Products of the A. aestivalis hbfd1 and hbfd2 cDNAs, When Combined with the A. aestivalis cbfd2 cDNA, Leads to the Conversion of β-Carotene into Astaxanthin in Cells of E. coli.
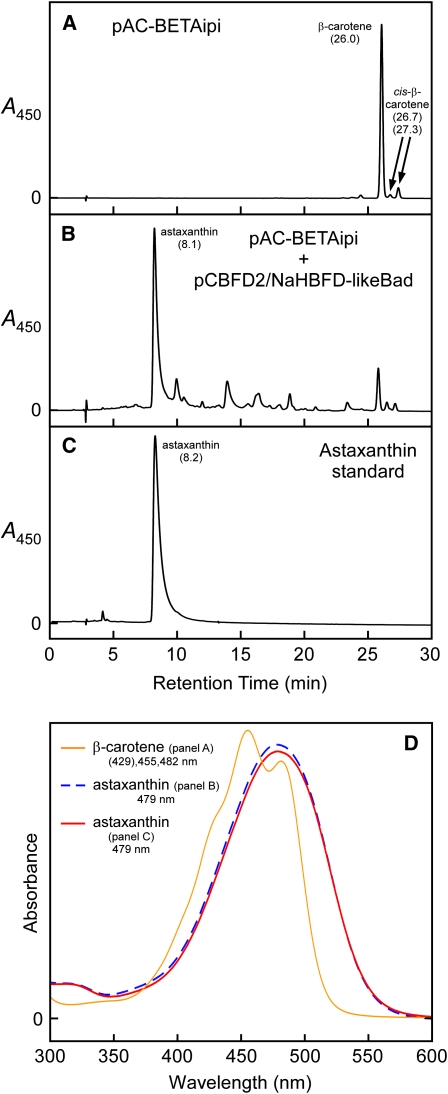
Shown are HPLC elution profiles for extracts of E. coli cultures wherein cells contained the following plasmids: (A) pAC-BETAipi and (B) pAC-BETAipi and pCBFD2/NaHBFD-likeBad. (C) displays an HPLC elution profile for a synthetic astaxanthin standard, and (D) shows absorption spectra for the major peaks of (A) to (C). Note: The N. advena cDNA was fused, in frame, to the arabinose-inducible araBAD promoter to ensure high-level expression. Arabinose (0.2% [w/v]) was included in the growth medium for that culture containing pCBFD2/NaHBFD-likeBad. Numbers in parentheses below carotenoid names are HPLC retention times in minutes.
[See online article for color version of this figure.]
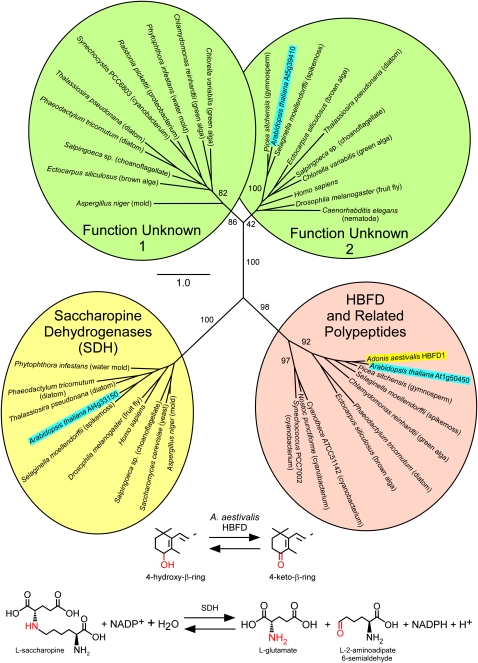
Many of the HBFD-related polypeptides in plants and algae have mistakenly been annotated as saccharopine dehydrogenase (SDH) enzymes. Three very different genes in Arabidopsis thaliana, for example, have been annotated as encoding SDH, but only one of these, At4g33150, has actually been shown to produce a polypeptide with SDH activity (Tang et al., 1997). The resemblance of the other two putative SDH-encoding genes, At5g39410 and At1g50450 (the latter one is closely related to HBFD; ~67% amino acid sequence identity overall) to the At4g33150 polypeptide or to other known SDH is quite remote (Figure 10), and the three Arabidopsis gene products apparently have different locations within the plant cell, with the authentic SDH (At4g33150) residing in the cytosol, the HBFD-related polypeptide (At1g50450) targeted to the plastid, and the third gene product (At5g39410) found in mitochondria and elsewhere. The three Arabidopsis genes have very different phylogenetic distribution patterns as well (Figure 10). Genes that specify polypeptides similar in amino acid sequence to HBFD are found in plants, algae, many cyanobacteria, and certain bacteria, whereas genes related to At4g33150 (encoding SDH) are present in animals and fungi (including yeasts) as well as in land plants, but not in cyanobacteria or in green algae, and genes related to At5g39410 (function unknown) are in plants, algae, animals, and fungi (not including yeasts), but not in cyanobacteria.
Figure 10.
Maximum Likelihood Tree for Selected Members of the Extended Saccharopine Dehydrogenase Family of Enzymes.
Each of the four clades encompasses polypeptides from a great diversity of species. One clade contains polypeptides that, in several cases at least, have been shown to function as SDHs (saccharopine dehydrogenase [NADP(+), l-glutamate-forming]; EC 1.5.1.10). Note: This enzyme, often referred to as saccharopine reductase, is unrelated to the l-lysine–forming saccharopine dehydrogenase (EC 1.5.1.7). A second clade contains the A. aestivalis HBFD1 enzyme and related polypeptides of unknown function from Arabidopsis and other plants, algae, and cyanobacteria. Two additional clades, labeled “Function Unknown 1” and “Function Unknown 2,” contain polypeptides whose functions have not been ascertained. The A. aestivalis HBFD1 is highlighted with a yellow background. Three Arabidopsis polypeptides are each highlighted with a bright-blue background. The reactions catalyzed by SDH and by the A. aestivalis HBFD are illustrated below the tree. Like that catalyzed by SDH, the reaction catalyzed by HBFD probably requires the participation of NADP+, but this has not yet been demonstrated. One thousand bootstrap trials were conducted, with bootstrap support values indicated for the major branches. The alignment used to construct this tree is shown in Supplemental Figure 5 online and is available in FASTA format as Supplemental Data Set 3 online.
The reaction catalyzed by SDH, an oxidative deamination, bears some resemblance to that catalyzed by the HBFD enzyme, except that the reaction occurs at a C-N bond rather than a C-O bond (Figure 10). Crystal structures have been obtained for SDH from Magnaporthe grisea (rice blast fungus; Johansson et al., 2000) and Saccharomyces cereviseae (Andi et al., 2006), and reaction mechanisms involving a Schiff base intermediate have been proposed (Johansson et al., 2000; Vashishtha et al., 2009). Given the considerable evolutionary distance between HBFD and SDH (Figure 10), however, it is an open question as to whether or to what degree the mechanisms of these two enzymes might be similar.
The phylogenetic occurrence pattern for hbfd-related genes is in accordance with an origin in the ancestral cyanobacterial endosymbiont that gave rise to chloroplasts, but the occurrence of related genes in many bacteria suggests a role not specifically related to photosynthesis. What might be the function of HBFD-related polypeptides in plants? We can only speculate, but it is likely that these polypeptides catalyze the conversion of an allylic secondary hydroxyl group (as in a 4-hydroxy-β-ring; Figure 1C), into a carbonyl (or, alternatively, the reverse of this reaction) for some class of lipophilic compounds within the chloroplast. It is probably, in other words, an allyl-alcohol:NADP+ oxidoreductase, substrate unknown.
Transcripts for Enzymes of the Pathway to Astaxanthin Are Abundant in Adonis Flowers
The amount of astaxanthin that accumulates in the flower petals of Adonis is substantial (~1% of the dry weight in the petals of A. annua according to Renstrøm et al. [1981]) and very much more than the ~0.2% of dry weight typically found for total carotenoids in green tissues of flowering plants such as, for instance, Arabidopsis (Maass et al., 2009). The regulation of the pathway leading to astaxanthin in A. aestivalis is beyond the scope of this article, and we have, as yet, no data relevant to this matter, but information on the expression of genes encoding some of the pathway enzymes is available from an analysis by Li et al. (2008) of a collection of 4189 ESTs from an A. aestivalis cDNA library constructed using mRNA extracted from fully opened flowers. The CBFD enzyme is represented by 17 ESTs, with nine for cbfd1 and eight for cbfd2. The HBFD is less well represented, but the number of ESTs for this enzyme also is relatively high for a biochemical pathway enzyme, with two for hbfd1 and four for hbfd2. If one includes ESTs for other enzymes of the carotenoid pathway (Li et al., 2008), >1% of the A. aestivalis flower ESTs are of cDNAs for enzymes of the pathway leading to astaxanthin. Transcripts for another key enzyme, 1-deoxy-d-xylulose 5-phosphate synthase, the gateway enzyme for the nonmevalonate isoprenoid pathway that provides substrates for the carotenoid pathway in plant plastids (reviewed in Rodríguez-Concepción, 2010), also are plentiful (13 of the 4189 ESTs). We conclude that an abundance of transcripts, abetted by duplications that have given rise to at least two genes for both CBFD and HBFD, helps to ensure that the enzymes of the pathway leading to astaxanthin are produced in amounts sufficient to enable an ample flow of carbon into the carotenoid pathway and an efficient conversion of carotenoid precursors into astaxanthin.
Prospects for the Biological Production of Astaxanthin
The very high cost of astaxanthin, the bulk of which is produced by chemical synthesis and consumed by the aquaculture industry, has led to intense interest in the development of potentially less costly biological sources of this pigment. The red yeast Xanthophyllomyces dendrorhous (Lukács et al., 2006) and the green alga H. pluvialis (Del Campo et al., 2007) currently serve as the principal biological sources, but astaxanthin obtained from X. dendrorhous and H. pluvialis is considerably more costly to produce than is the synthetic pigment (Lukács et al., 2006; Schmidt et al., 2011), and strain improvements and/or modifications of culture conditions seem likely to yield only incremental reductions in the cost of production. A. aestivalis has itself received some attention as a prospective biological source of astaxanthin (Kamata et al., 1990), with efforts made to develop improved strains (Rodney, 1995; Rayton et al., 2006), but the relatively small flowers of this plant render it ill suited for commercial production.
Rather than attempt to improve the production of astaxanthin in X. dendrorhous or H. pluvialis or A. aestivalis, a more attractive strategy, one that could conceivably lead to an order of magnitude reduction in the cost of production, would be to move the pathway to an industrial microorganism. The requisite genes from H. pluvialis, X. dendrorhous, and several bacteria have been introduced into E. coli (Wang et al., 1999; Tao et al., 2006; Harada et al., 2009; Scaife et al., 2009), Candida utilis (Miura et al., 1998), and S. cerevisiae (Ukibe et al., 2009), with particularly promising results for E. coli, where remarkable success has been achieved in modifying central metabolism to redirect a massive amount of carbon into the isoprenoid pathways that provide the substrates needed to make astaxanthin (reviewed in Kirby and Keasling, 2008; Harada and Misawa, 2009).
A genetically modified bacterium likely will soon provide the most economical way to make astaxanthin, biologically or otherwise. However, a crop plant that already is included as a constituent of fish feed (e.g., maize [Zea mays], canola [Brassica napus], soybean [Glycine max], and pea [Pisum sativum]) may, in the end, provide the most cost-effective platform for the manufacture of astaxanthin for the aquaculture industry. A number of attempts have been made to engineer plants to produce astaxanthin in nongreen plant tissues such as roots, seeds, fruits, and tubers (reviewed in Misawa, 2009; Zhu et al., 2009). A recurrent problem has been a relatively low yield of astaxanthin, both in absolute terms and as a percentage of the total carotenoid pigment, with intermediates in the pathway between β-carotene and astaxanthin typically the predominant ketocarotenoids. This problem has been overcome in large part by a judicious choice of pathway genes (Hasunuma et al., 2008; Zhong et al., 2011) and by incorporation of the introduced genes into the plastid rather than the nuclear genome (Hasunuma et al., 2008). A plastid localization for the introduced genes, however, comes with the disadvantage that they are expressed constitutively (i.e., in chloroplasts as well as in chromoplasts) and could therefore have a detrimental effect on photosynthesis and crop yield.
The conversion of β-carotene into astaxanthin as catalyzed by the enzymes of A. aestivalis in E. coli is near to complete (Figure 3D), making the A. aestivalis genes a suitable choice for use in the bioengineering of E. coli and other industrial microorganisms. However, the A. aestivalis pathway genes are perhaps most valuable for their potential use in engineering crop plants to manufacture astaxanthin. The enzymes encoded by the A. aestivalis genes are already well adapted to operate in plant plastids and should enable the production of astaxanthin with high yield and efficiency in a tissue-specific manner in plants better suited than Adonis for the biological production of astaxanthin.
METHODS
Color Complementation Screening
Color complementation screening of an Adonis aestivalis (summer pheasant’s eye) cDNA library in carotenoid-producing Escherichia coli was performed essentially as described earlier (Cunningham et al., 1996; Cunningham and Gantt, 2005). Agar plates (1.5% Bacto agar) used for screening contained Luria-Bertani medium supplemented with ampicillin (150 mg/L) and chloramphenicol (30 mg/L). The cDNA library (Cunningham et al., 2000) was constructed using mRNA extracted from immature and developing flower buds of an A. aestivalis cultivar known as Loders Red.
Plasmids
Table 1 lists plasmids used in this work. Those plasmids not previously described were constructed as follows: To make pAC-BETA-CBFD1/2, the cbfd1 and cbfd2 cDNAs (note: these cDNAs were previously referred to as AdKeto1 and AdKeto2; Cunningham and Gantt, 2005) were excised together from pCBFD1/CBFD2 as a 3.0-kb PvuII fragment and ligated into HindIII-digested, Klenow-blunted pAC-BETA. pHBFD1Bad was made by amplifying the hbfd1 cDNA in pHBFD1 with the oligonucleotide primers HBFD1Nco-N (5′-CACACCATGGCTCCTGTTCTCCTTG-3′) and HBFD1-C (5′-CTGGGCTACATAATGAATAATCCAATC-3′). The PCR product was blunt cloned in pSTBlue1, excised as an NcoI-XhoI fragment (the XhoI site was in the multiple cloning site of pSTBlue1), and then ligated into NcoI + XhoI digested pBAD/His B. To make pCBFD1/HBFD1Bad, the cbfd1 cDNA was excised from pCBFD1 as a PvuII-XhoI fragment and ligated into pHBFD1Bad that had been digested with KpnI, blunted with the Klenow enzyme, purified by phenol/chloroform extraction, and then digested with XhoI and gel purified. pCBFD2Bad/HBFD1 was made by digesting pCBFD2Bad with SphI, blunting with mung bean nuclease followed by phenol/chloroform extraction, and then digesting with XhoI and agarose gel-purifying to yield a 2.43-kb fragment containing the cbfd2 cDNA fused to the araBAD promoter and including the araC gene. This was ligated into pHBFD1 that had been digested with KpnI, blunted with mung bean nuclease, purified by phenol/chloroform extraction, digested with XhoI, and gel purified. To make pNaHBFD-likeBad, a Nuphar advena cDNA (GenBank EU348740) was excised from a cDNA library plasmid with PstI (restriction site near the beginning of the open reading frame) and KpnI (within the multiple cloning site immediately following the 3′ end of the cDNA). The resulting fragment was ligated in the corresponding sites of plasmid pBAD/His A to give an in-frame fusion under the control of the arabinose-inducible araBAD promoter. Plasmid pCBFD1/NaHBFD-likeBad was made by excising the cbfd1 cDNA from pCBFD1 with PvuII and XhoI, blunting this 1.6-kb fragment with mung bean nuclease, and then ligating it in pNaHBFD-likeBad that had been digested with KpnI and blunted with mung bean nuclease.
Analysis of Carotenoid Pigments in E. coli
Detailed descriptions of E. coli culture and carotenoid analysis are given by Cunningham and Gantt (2005). In brief, liquid cultures of E. coli strain TOP10 were grown in Luria-Bertani medium for 1 d at 28°C on a rotary platform shaker at ~325 cycles per min in darkness. Antibiotics were included in the medium, as appropriate, and arabinose (0.05 or 0.2% [w/v]) was added as needed. Liquid cultures were harvested by centrifugation, residual liquid was removed from above the pellet with a pipettor, and pellets were extracted with acetone. For thin layer chromatography (TLC), acetone extracts were spotted onto reverse-phase TLC plates (Whatman KC18 Silica Gel 60 Å), and the pigments were separated using a mobile phase of acetone/methanol/water (50/50/4 by volume). HPLC analyses were performed as earlier described (Cunningham and Gantt, 2005, 2007) with two exceptions. First, pigment extracts (after transfer to ethyl acetate) were evaporated to dryness under a stream of nitrogen and resolubilized in the initial HPLC mobile phase prior to injection. Second, a mobile phase gradient of 10 to 50% B (ethyl acetate) in A (acetonitrile/water/triethylamine, 90/10/0.1 by volume) over the course of 30 min was used to separate the pigments. Carotenoid identifications were based on absorption spectra, HPLC retention times, and molecular masses in comparison with those of standard compounds. HPLC peak areas were ascertained using HP ChemStation for LC Rev. A.06.03 [509]. Peaks were examined individually and adjusted using an advanced baseline, drop shoulders, and/or tangents, as appropriate. Mass spectrometry was performed as previously described (Cunningham and Gantt, 2005). Synthetic astaxanthin was obtained from Sigma-Aldrich (stock number A9335). HPLC retention times and absorption maxima for a number of carotenoids are given in Supplemental Table 2 online.
Phylogenetic Analysis
Amino acid sequences were aligned using MAFFT version 6 (Katoh and Toh, 2008; http://mafft.cbrc.jp/alignment/server/). Maximum likelihood trees were created using RAxML version 7.0.4 (Stamatakis, 2006), with the PROTGAMMAWAG model of evolution (WAG substitution matrix and gamma rate categories = 4). Branch support was estimated with 1000 rapid bootstraps. Trees were plotted using FigTree version 1.3.1 (program available at http://tree.bio.ed.ac.uk/software/figtree/).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ABK41044 (A. aestivalis hbfd1 [AdKC28] cDNA), ABK41045 (A. aestivalis hbfd2 [AdKC17] cDNA), and EU348740 (an N. advena cDNA that encodes a polypeptide similar in amino acid sequence to the products of the A. aestivalis hbfd1 and hbfd2 cDNAs). Accession numbers for sequences used to create the trees displayed in Figures 8 and 10 are given in the supplemental data (see the legends to Supplemental Figures 3 to 5 online).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The A. aestivalis HBFD1 Does Not Modify Carotenoid 3-Hydroxy-β-Rings, and an Active β-Ring 3-Hydroxylase Enzyme Impairs the Conversion of β-Carotene into Astaxanthin as Catalyzed by the Enzymes of A. aestivalis in E. coli.
Supplemental Figure 2. Arabidopsis T-DNA Lines with Insertions in Gene At1g50450, Which Encodes a Polypeptide Similar in Sequence to the HBFD Enzymes of A. aestivalis, Do Not Differ from Wild-Type Plants in their Carotenoid Composition.
Supplemental Figure 3. Alignment of Plant and Green Algal CHYb-Type Carotenoid β-Ring 3-Hydroxylase Enzymes and Polypeptides Encoded by the A. aestivalis cbfd1 and cbfd2 cDNAs.
Supplemental Figure 4. Alignment of the Polypeptides Encoded by the A. aestivalis hbfd1 and hbfd2 cDNAs and Related Polypeptides Encoded by cDNAs or Genes of Other Plants and Green Algae.
Supplemental Figure 5. Alignment of Selected Members of the Extended Saccharopine Dehydrogenase Family of Enzymes.
Supplemental Figure 6. Absorption Spectra for Peaks in the HPLC Elution Profiles Displayed in Figures 3A, 3D, 3E, and 5A.
Supplemental Figure 7. Absorption Spectra for Peaks in the HPLC Elution Profiles Displayed in Figures 3B, 5B, and 5C.
Supplemental Figure 8. HPLC Elution Profiles, with Absorption Spectra of Selected Peaks, for Two of the Pigment Extracts that Provided Data for the Time-Course Studies Shown in Figure 4.
Supplemental Table 1. Adonis aestivalis cDNAs Selected in a Color Complementation Screen Performed Using E. coli Containing the Plasmid pAC-BETA-CBFD1/2 as Host.
Supplemental Table 2. HPLC Retention Times and Absorption Peaks for Known Carotenoids.
Supplemental Data Set 1. Text File of the Alignment Used to Generate the Tree Shown in Figure 8A.
Supplemental Data Set 2. Text File of the Alignment Used to Generate the Tree Shown in Figure 8B.
Supplemental Data Set 3. Text File of the Alignment Used to Generate the Tree Shown in Figure 10.
Supplementary Material
Acknowledgments
We thank Noel Whitaker (University of Maryland) for mass spectrometry analyses, Ruth Timme (University of Maryland) for advice and assistance with the phylogenetic analyses, Joseph Hirschberg (The Hebrew University of Jerusalem, Israel) for providing a plasmid containing an H. pluvialis cDNA that encodes a carotenoid β-ring 4-ketolase enzyme, and Claude dePamphilis (Pennsylvania State University) for the gift of a plasmid with an N. advena cDNA. This work was supported in part by funding from Quest International (Naarden, The Netherlands) and by a grant from the National Science Foundation (MCB-0316448).
AUTHOR CONTRIBUTIONS
F.X.C. conceived, designed, and performed the research, analyzed the data, and wrote the article. E.G. consulted on the experimental design, assisted in interpretation of the results, and helped write the article.
References
- Alcaíno J., Barahona S., Carmona M., Lozano C., Marcoleta A., Niklitschek M., Sepúlveda D., Baeza M., Cifuentes V. (2008). Cloning of the cytochrome p450 reductase (crtR) gene and its involvement in the astaxanthin biosynthesis of Xanthophyllomyces dendrorhous. BMC Microbiol. 8: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez V., Rodríguez-Sáiz M., de la Fuente J.L., Gudiña E.J., Godio R.P., Martín J.F., Barredo J.L. (2006). The crtS gene of Xanthophyllomyces dendrorhous encodes a novel cytochrome-P450 hydroxylase involved in the conversion of β-carotene into astaxanthin and other xanthophylls. Fungal Genet. Biol. 43: 261–272 [DOI] [PubMed] [Google Scholar]
- Andi B., Cook P.F., West A.H. (2006). Crystal structure of the his-tagged saccharopine reductase from Saccharomyces cerevisiae at 1.7-Å resolution. Cell Biochem. Biophys. 46: 17–26 [DOI] [PubMed] [Google Scholar]
- Bauernfeind J.C., Adams C.R., Marusich W.L. (1981). Carotenes and other vitamin A precursors in animal feed. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications, Bauernfeind J.C., (New York: Academic Press; ), pp. 563–743 [Google Scholar]
- Broadwater J.A., Whittle E., Shanklin J. (2002). Desaturation and hydroxylation. Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J. Biol. Chem. 277: 15613–15620 [DOI] [PubMed] [Google Scholar]
- Brush A.H. (1981). Carotenoids in wild and captive birds. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications, Bauernfeind J.C., (New York: Academic Press; ), pp. 539–562 [Google Scholar]
- Cunningham F.X., Jr, Gantt E. (2005). A study in scarlet: Enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant J. 41: 478–492 [DOI] [PubMed] [Google Scholar]
- Cunningham F.X., Jr, Gantt E. (2007). A portfolio of plasmids for identification and analysis of carotenoid pathway enzymes: Adonis aestivalis as a case study. Photosynth. Res. 92: 245–259 [DOI] [PubMed] [Google Scholar]
- Cunningham F.X., Jr, Lafond T.P., Gantt E. (2000). Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J. Bacteriol. 182: 5841–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F.X., Jr, Pogson B., Sun Z., McDonald K.A., DellaPenna D., Gantt E. (1996). Functional analysis of the β and ε lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8: 1613–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeczuga B. (1987). Ketocarotenoids — Autumn carotenoids in Metasequoia glyptostroboides. Biochem. Syst. Ecol. 15: 303–306 [Google Scholar]
- Czygan F.-C. (1969). Untersuchungen über den Stoffwechsel der Ketocarotinoide in Adonis-Arten. Planta 85: 35–41 [DOI] [PubMed] [Google Scholar]
- Del Campo J.A., García-González M., Guerrero M.G. (2007). Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 74: 1163–1174 [DOI] [PubMed] [Google Scholar]
- Egger K. (1965). Die Ketocarotenoide in Adonis annua L. Phytochemistry 4: 609–618 [Google Scholar]
- Fassett R.G., Coombes J.S. (2011). Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar. Drugs 9: 447–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-González B., Sandmann G., Vioque A. (1997). A new type of asymmetrically acting β-carotene ketolase is required for the synthesis of echinenone in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 272: 9728–9733 [DOI] [PubMed] [Google Scholar]
- Gagné S.J., Reed D.W., Gray G.R., Covello P.S. (2009). Structural control of chemoselectivity, stereoselectivity, and substrate specificity in membrane-bound fatty acid acetylenases and desaturases. Biochemistry 48: 12298–12304 [DOI] [PubMed] [Google Scholar]
- Goodwin T.W. (1980). The Biochemistry of the Carotenoids, Vol. 1, Plants, 2nd ed (London: Chapman & Hall; ). [Google Scholar]
- Goodwin T.W. (1984). The Biochemistry of the Carotenoids, Vol. 2, Animals, 2nd ed (London: Chapman & Hall; ). [Google Scholar]
- Goodwin T.W. (1986). Metabolism, nutrition, and function of carotenoids. Annu. Rev. Nutr. 6: 273–297 [DOI] [PubMed] [Google Scholar]
- Goswami G., Chaudhuri S., Dutta D. (2010). The present perspective of astaxanthin with reference to biosynthesis and pharmacological importance. World J. Microbiol. Biotechnol. 26: 1925–1939 [Google Scholar]
- Harada H., Misawa N. (2009). Novel approaches and achievements in biosynthesis of functional isoprenoids in Escherichia coli. Appl. Microbiol. Biotechnol. 84: 1021–1031 [DOI] [PubMed] [Google Scholar]
- Harada H., Yu F., Okamoto S., Kuzuyama T., Utsumi R., Misawa N. (2009). Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl. Microbiol. Biotechnol. 81: 915–925 [DOI] [PubMed] [Google Scholar]
- Hasunuma T., Miyazawa S., Yoshimura S., Shinzaki Y., Tomizawa K., Shindo K., Choi S.K., Misawa N., Miyake C. (2008). Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J. 55: 857–868 [DOI] [PubMed] [Google Scholar]
- Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F.M. (2006). Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 46: 185–196 [DOI] [PubMed] [Google Scholar]
- Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. (2006). Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 69: 443–449 [DOI] [PubMed] [Google Scholar]
- Ida K., Saito F., Takeda S. (1991). Isomers of rhodoxanthin in reddish brown leaves of gymnosperms and effect of daylight intensity on the contents of pigments during autumnal coloration. J. Plant Res. 104: 157–169 [Google Scholar]
- Johansson E., Steffens J.J., Lindqvist Y., Schneider G. (2000). Crystal structure of saccharopine reductase from Magnaporthe grisea, an enzyme of the α-aminoadipate pathway of lysine biosynthesis. Structure 8: 1037–1047 [DOI] [PubMed] [Google Scholar]
- Johnson E.A., An G.H. (1991). Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 11: 297–326 [Google Scholar]
- Kajiwara S., Kakizono T., Saito T., Kondo K., Ohtani T., Nishio N., Nagai S., Misawa N. (1995). Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol. Biol. 29: 343–352 [DOI] [PubMed] [Google Scholar]
- Kallberg Y., Persson B. (2006). Prediction of coenzyme specificity in dehydrogenases/reductases. A hidden Markov model-based method and its application on complete genomes. FEBS J. 273: 1177–1184 [DOI] [PubMed] [Google Scholar]
- Kamata T., Neamtu G., Tanaka Y., Sameshima M., Simpson K.L. (1990). Utilization of Adonis aestivalis as a dietary pigment source for rainbow trout Salmo gairdneri. Nippon Suisan Gakkaishi 56: 783–788 [Google Scholar]
- Katoh K., Toh H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9: 286–298 [DOI] [PubMed] [Google Scholar]
- Kim J., Smith J.J., Tian L., Dellapenna D. (2009). The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol. 50: 463–479 [DOI] [PubMed] [Google Scholar]
- Kirby J., Keasling J.D. (2008). Metabolic engineering of microorganisms for isoprenoid production. Nat. Prod. Rep. 25: 656–661 [DOI] [PubMed] [Google Scholar]
- Klaüi H., Bauernfeind J.C. (1981). Carotenoids as food color. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications, Bauernfeind J.C., (New York: Academic Press; ), pp. 47–317 [Google Scholar]
- Li R., Links M.G., Gjetvaj B., Sharpe A., Hannoufa A. (2008). Development of an Adonis aestivalis expressed sequence tag population as a resource for genes of the carotenoid pathway. Genome 51: 888–896 [DOI] [PubMed] [Google Scholar]
- Lotan T., Hirschberg J. (1995). Cloning and expression in Escherichia coli of the gene encoding β-C-4-oxygenase, that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett. 364: 125–128 [DOI] [PubMed] [Google Scholar]
- Lukács G., Linka B., Nyilasi I. (2006). Phaffia rhodozyma and Xanthophyllomyces dendrorhous: Astaxanthin-producing yeasts of biotechnological importance. Acta Alimentaria 35: 99–107 [Google Scholar]
- Maass D., Arango J., Wüst F., Beyer P., Welsch R. (2009). Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS ONE 4: e6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalith P.Z. (1999). Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 51: 431–438 [DOI] [PubMed] [Google Scholar]
- Martín J.F., Gudiña E., Barredo J.L. (2008). Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microb. Cell Fact. 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich W.L., Bauernfeind J.C. (1981). Oxycarotenoids in poultry feed. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications, Bauernfeind J.C., (New York: Academic Press; ), pp. 319–462 [Google Scholar]
- Masamoto K., Misawa N., Kaneko T., Kikuno R., Toh H. (1998). β-Carotene hydroxylase gene from the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 39: 560–564 [DOI] [PubMed] [Google Scholar]
- Matsuno T. (2001). Aquatic animal carotenoids. Fish. Sci. 67: 771–783 [Google Scholar]
- Misawa N. (2009). Pathway engineering of plants toward astaxanthin production. Plant Biotechnol. 26: 93–99 [Google Scholar]
- Misawa N., Kajiwara S., Kondo K., Yokoyama A., Satomi Y., Saito T., Miki W., Ohtani T. (1995a). Canthaxanthin biosynthesis by the conversion of methylene to keto groups in a hydrocarbon β-carotene by a single gene. Biochem. Biophys. Res. Commun. 209: 867–876 [DOI] [PubMed] [Google Scholar]
- Misawa N., Satomi Y., Kondo K., Yokoyama A., Kajiwara S., Saito T., Ohtani T., Miki W. (1995b). Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 177: 6575–6584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Kondo K., Saito T., Shimada H., Fraser P.D., Misawa N. (1998). Production of the carotenoids lycopene, β-carotene, and astaxanthin in the food yeast Candida utilis. Appl. Environ. Microbiol. 64: 1226–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel K. (1981). Carotenoids in pharmaceutical and cosmetic products. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications, Bauernfeind J.C., (New York: Academic Press; ), pp. 745–754 [Google Scholar]
- Neamţu G., Tǎmaş V., Bodea C. (1966). Die Carotinoide aus einigen Adonis-arten. Rev. Roum. Biochim. 3: 305–310 [Google Scholar]
- Ojima K., Breitenbach J., Visser H., Setoguchi Y., Tabata K., Hoshino T., van den Berg J., Sandmann G. (2006). Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a β-carotene 3-hydroxylase/4-ketolase. Mol. Genet. Genomics 275: 148–158 [DOI] [PubMed] [Google Scholar]
- Pashkow F.J., Watumull D.G., Campbell C.L. (2008). Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 101(10A): 58D–68D [DOI] [PubMed] [Google Scholar]
- Rao S.T., Rossmann M.G. (1973). Comparison of super-secondary structures in proteins. J. Mol. Biol. 76: 241–256 [DOI] [PubMed] [Google Scholar]
- Rayton S., Rayton J., Foley L., Jones P.W. (2006). Ketocarotenoids from Adonis palaestina. International patent application WO/2006/119346 [Google Scholar]
- Renstrøm B., Berger H., Liaaen-Jensen S. (1981). Esterified, optically pure (3S, 3′S)-astaxanthin from flowers of Adonis annua. Biochem. Syst. Ecol. 9: 249–250 [Google Scholar]
- Rodney M. (1995). Astaxanthin from flowers of the genus Adonis. US Patent 5453565 [Google Scholar]
- Rodríguez-Concepción M. (2010). Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 504: 118–122 [DOI] [PubMed] [Google Scholar]
- Seybold A., Goodwin T.W. (1959). Occurrence of astaxanthin in the flower petals of Adonis annua L. Nature 184(suppl. 22): 1714–1715 [DOI] [PubMed] [Google Scholar]
- Scaife M.A., Burja A.M., Wright P.C. (2009). Characterization of cyanobacterial β-carotene ketolase and hydroxylase genes in Escherichia coli, and their application for astaxanthin biosynthesis. Biotechnol. Bioeng. 103: 944–955 [DOI] [PubMed] [Google Scholar]
- Schmidt I., Schewe H., Gassel S., Jin C., Buckingham J., Hümbelin M., Sandmann G., Schrader J. (2011). Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 89: 555–571 [DOI] [PubMed] [Google Scholar]
- Schoefs B., Rmiki N., Rachadi J., Lemoine Y. (2001). Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 500: 125–128 [DOI] [PubMed] [Google Scholar]
- Shanklin J., Cahoon E.B. (1998). Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 611–641 [DOI] [PubMed] [Google Scholar]
- Simpson K.L., Katayama T., Chichester C.O. (1981). Carotenoids in fish feeds. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications, Bauernfeind J.C., (New York: Academic Press: ), pp. 463–538 [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Tang G., Miron D., Zhu-Shimoni J.X., Galili G. (1997). Regulation of lysine catabolism through lysine-ketoglutarate reductase and saccharopine dehydrogenase in Arabidopsis. Plant Cell 9: 1305–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Cheng Q. (2004). Novel β-carotene ketolases from non-photosynthetic bacteria for canthaxanthin synthesis. Mol. Genet. Genomics 272: 530–537 [DOI] [PubMed] [Google Scholar]
- Tao L., Rouvière P.E., Cheng Q. (2006). A carotenoid synthesis gene cluster from a non-marine Brevundimonas that synthesizes hydroxylated astaxanthin. Gene 379: 101–108 [DOI] [PubMed] [Google Scholar]
- Thommen H. (1971). Metabolism. In Carotenoids, Isler O., Guttman H., Solms U., (Basel, Switzerland: Birkhäuser Verlag; ), pp. 637–668 [Google Scholar]
- Ukibe K., Hashida K., Yoshida N., Takagi H. (2009). Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl. Environ. Microbiol. 75: 7205–7211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishtha A.K., West A.H., Cook P.F. (2009). Chemical mechanism of saccharopine reductase from Saccharomyces cerevisiae. Biochemistry 48: 5899–5907 [DOI] [PubMed] [Google Scholar]
- Wang C.W., Oh M.K., Liao J.C. (1999). Engineered isoprenoid pathway enhances astaxanthin production in Escherichia coli. Biotechnol. Bioeng. 62: 235–241 [DOI] [PubMed] [Google Scholar]
- Weedon B.C.L., Moss G.P. (1995). Structure and nomenclature. In Carotenoids: Isolation and Analysis, Vol. 1A, Britton G., Liaaen-Jensen S., Pfander H., (Basel, Switzerland: Birkhäuser Verlag; ), pp. 27–70 [Google Scholar]
- Zhong Y.J., Huang J.C., Liu J., Li Y., Jiang Y., Xu Z.F., Sandmann G., Chen F. (2011). Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis. J. Exp. Bot. 62: 3659–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Naqvi S., Capell T., Christou P. (2009). Metabolic engineering of ketocarotenoid biosynthesis in higher plants. Arch. Biochem. Biophys. 483: 182–190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.