Abstract
The HA protein of the 2009 pandemic H1N1viruses (H1N1pdm) is antigenically closely related to the HA of classical North American swine H1N1 influenza viruses (cH1N1). Since 1998, through mutation and reassortment of HA genes from human H3N2 and H1N1 influenza viruses, swine influenza strains are undergoing substantial antigenic drift and shift. In this report we describe the development of a novel monoclonal antibody (S-OIV-3B2) that shows high hemagglutination inhibition (HI) and neutralization titers against not only H1N1pdm, but also against representatives of the α, β, and γ clusters of swine-lineage H1 influenza viruses. Mice that received a single intranasal dose of S-OIV-3B2 were protected against lethal challenge with either H1N1pdm or cH1N1 virus. These studies highlight the potential use of S-OIV-3B2 as effective intranasal prophylactic or therapeutic antiviral treatment for swine-lineage H1 influenza virus infections.
Keywords: monoclonal antibody, H1N1pdm, protection, treatment, swine, influenza
Introduction
Influenza A viruses (IAVs) belong to the family Orthomyxoviridae and have a segmented, negative sense single strand RNA genome (Lamb, 1989). In addition to wild aquatic birds, which are considered the natural hosts, IAVs have been isolated from many animal species including humans, pigs, horses, dogs, cats, minks, marine mammals and a wide range of domestic birds (Webster et al., 1997). The segmented genome of IAVs allows for reassortment and production of novel strains with pandemic potential. In the 20thcentury, humans experienced three influenza pandemics: the Spanish flu of 1918 (H1N1), the Asian flu of 1957 (H2N2) and the Hong Kong flu of 1968 (H3N2) (Webster, 1997). These pandemic viruses carried genes derived from avian and human IAVs. In April 2009, swine-origin influenza H1N1 virus (H1N1pdm) caused the first influenza pandemic of the 21st century (Donaldson et al., 2009; Jain et al., 2009; Libster et al., 2010; Louie et al., 2010). H1N1pdm viruses are triple reassortant viruses whose genome contains genes derived from avian (PB2 and PA), human (PB1), North American swine (HA, NP and NS) and Eurasian swine (NA and M) influenza lineages (Garten et al., 2009). The HA of H1N1pdm strains are much more antigenically related to North American swine H1 strains than to contemporary human seasonal H1 strains. Currently, four clusters (α, β, γ, δ) of swine H1 viruses are found endemic in the North American swine population (Ma et al., 2010; Vincent et al., 2010; Vincent et al., 2009a; Vincent et al., 2009b). The α, β, γ clusters are derived from the classical swine H1 lineage, whereas cluster δ is derived from contemporary human H1 viruses. Phylogenetic analysis has shown that the HA of the H1N1pdm strains is more closely related to the swine-origin γ cluster (Garten et al., 2009; Smith et al., 2009). H1 viruses of the γ cluster, including H1N1pdm, showed substantial antigenic drift compared to the prototypical classical swine H1 viruses. Serological analysis using HI assays revealed that sera against the classical swine H1 viruses showed either limited or no cross-reaction to the H1N1pdm viruses (Garten et al., 2009). Sera against current swine-lineage α, β, γ clusters and commercial vaccine strains in the North American swine population had limited cross-reaction to H1N1pdm strains (Vincent et al., 2010). There is a constant risk of two-way influenza transmission events between pigs and humans that may lead to novel strains. Indeed, more than ten human cases of infection with swine influenza viruses were reported prior to the emergence of the H1N1pdm virus (Shinde et al., 2009). Although the progenitor of the H1N1pdm virus was never isolated in pigs prior to the emergence of the H1N1pdm virus itself, infection of pigs has been documented recurrently since the pandemic virus emerged in humans. In addition, the H1N1pdm has occasionally transferred to other animal species such as turkeys, cats, ferrets, cheetahs and dogs (Berhane et al., 2010; Howden et al., 2009; Weingartl, 2010; Weingartl et al., 2010). H1N1pdm virus infection in swine have been reported in Canada, Argentina, Australia, Singapore, Northern Ireland, Finland, Iceland, England, United States, Japan and China (Berhane et al., 2010; Maines et al., 2009; Pereda et al., 2010; Smith et al., 2009; Vijaykrishna et al., 2010).
Vaccines to novel influenza viruses take several months to produce and its efficacy is limited in high-risk populations such as the young, the elderly, and the immunosuppressed. Passive immunotherapy represents a plausible anti-influenza strategy. In the past, neutralizing mAbs against influenza virus have been developed and shown to be effective for passive protection in animal models (Hanson et al., 2006; Prabhu et al., 2009; Simmons et al., 2007; Sui et al., 2009; Throsby et al., 2008). In this study, we developed a monoclonal antibody, S-OIV-3B2, that reacted against the HA of H1N1pdm viruses. Interestingly, S-OIV-3B2 had high HI and neutralization titers against swine influenza viruses of the α, β, γ clusters. Furthermore, protection against lethal H1N1pdm and prototypic swine H1 challenge was achieved after a single dose of S-OIV-3B2 administered by the intranasal route either preceding or following virus inoculation.
Materials and Methods
Cells and Virus
s/p20 myeloma cells (ATCC, Manassas, VA, USA) were cultured in modified Eagle’s medium (MEM) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA). Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA, USA) were maintained in MEM containing 5% FBS. A/California/04/2009 (H1N1) virus (Ca/04) was kindly provided by the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA. A/swine/Iowa/15/1930 (H1N1) virus (Sw30) was obtained from the ATCC (Manassas, VA, USA). Mouse-adapted A/California/04/2009 (H1N1) virus (ma-Ca/04) was developed in our laboratory (Ye et al., 2010b). Viruses were propagated in MDCK cells and stored at −70°C until use. All viruses were titrated by the Reed-Muench method to determine the TCID50 and MLD50 (Reed and Muench, 1938).
Immunization
Eight-week old female Balb/c mice were immunized by intraperitoneal injection with Ca/04. Doses consisted of 200 μl of supernatant containing 2 × 105 TCID50 of virus. Boost immunizations were given at 10, 20 and 30 days post-vaccination. Animal studies using the Ca/04 virus were conducted under BSL-3 conditions and performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland-College Park.
Production of mAbs
Mouse spleen cells collected at 4 days after the 3rd immunization boost with Ca/04 were fused to the s/p20 myeloma cells as previously described (Yang et al., 2008). After selection of the hybridomas in hypoxanthine aminopterin thymidine media (HAT) (Invitrogen, Carlsbad, CA, USA), antibody producing cells were screened by the hemagglutination inhibition (HI) method (Webster et al., 2005) and sub-cloned by limiting dilution. Positive clones were checked for isotype by using Iso-Gold™ Rapid Mouse-Monoclonal Isotyping Test Kit (BioAssay Works, Ijamsville, MD, USA) as described in the manufacturer’s protocol. The mAb’s ascites were generated as previously described (Yang et al., 2008). Total protein concentration in the acites was quantified by two independent methods: bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA) and protein absorbance at 280 nm using the NanoDrop ND-1000 photometer (Thermo Scientific, Wilmington, DE, USA). Total IgG concentration in the ascites was quantified using the mouse IgG ELISA kit (Immunology Consultants Laboratory, Inc., Newberg, OR, USA).
Neutralization assays
For microneutralization (MN) assays, serially diluted mAbs were first incubated with 100 TCID50 of viruses for 1 h at 37° C (Table 2). The virus-mAb mixture was then absorbed to MDCK cells for 1 h at 37° C. Infected cells were washed twice with Phosphate Buffered Saline (PBS) and replenished with Opti-MEM (Gibco, Grand Island, NY, USA). The infected cell supernatants were harvested at 4 days post-infection (dpi) and were analyzed by HA assay (Webster et al., 2005).
Table 2.
Neutralization titer (NT) of S-OIV-3B2 against different viruses
| Virus | Subtype | NT titer |
|---|---|---|
| A/California//04/2009 | H1N1 | 12800 |
| A/swine/Iowa/15/1930(classical) | H1N1 | 6400 |
| A/swine/Minnesota/02053/2008(α) | H1N1 | 25600 |
| A/swine/Nebraska/02013/2008(β) | H1N1 | 3200 |
| A/swine/Missouri/02060/2008(γ) | H1N1 | 12800 |
| A/swine/Texas/01976/2008(δ) | H1N2 | <100 |
Protection studies in mice
The ascites were filtered using a 0.22μm filter and then heat inactivated at 56°C for 30min. Six-week old mice (n=5/group) were treated via the intranasal route with a single dose of 50 μl PBS per mouse (divided in equal parts in each nostril) containing 12.5 mg/kg of total IgG containing the S-OIV-3B2 mAb (as indicated above). The treatment was performed at different time points before or after challenge as indicated in the results section. Mock-treated mice received 14 mg/kg of total IgG containing the control 2B9 mAb (against the NP viral protein, prepared in our laboratory). Two different strains were used for challenge, ma-Ca/04 and SW30, at doses of 10 MLD50 of ma-Ca/04 and 250 TCID50 of SW30, respectively. Dose dependence evaluation was performed with mice receiving 12.5 mg/kg, 6.25 mg/kg and 1.25 mg/kg of total IgG containing the S-OIV-3B2 mAb at 24 h before challenge. Specificity to H1N1pdm antiviral activity was evaluated with mice treated with the high dose of S-OIV-3B2 mAb (12.5 mg/kg total IgG) administered 24 h before challenge with 10MLD50 of influenza A/Puerto Rico/8/34 (H1N1) (PR8), which does not react to S-OIV-3B2 mAb. Body weight changes, morbidity and mortality were monitored daily. Mice showing body weight losses ≥ 25% of pre-infection values were euthanized for ethical reasons. Challenge studies were conducted under BSL-3 conditions approved by USDA and performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland, College Park.
Results and Discussion
Characterization of mAbS-OIV-3B2 against HA of H1N1pdm
Spleen cells, obtained from a mouse immunized with Ca/04 virus, were fused to s/p20 myeloma cells. Four monoclonal antibodies were isolated that showed HI activity against the H1N1pdm strains. One monoclonal antibody, mAb S-OIV-3B2 (IgG2a), showed significant HI activity not only against different H1N1pdm strains (A/Netherlands/602/2009, A/Mexico/4108/2009, and A/New York/18/2009), but also against the prototypical swine H1 strain SW30 (Table 1). This result suggested that mAb S-OIV-3B2 recognized an epitope that is conserved between the pandemic and the classical swine strains. mAb S-OIV-3B2 showed no HI activity against human seasonal H1 and H3 influenza viruses or avian H5, H7 and H9 influenza viruses. We expanded the HI profile of mAb S-OIV-3B2 against a panel of swine H1 influenza viruses of clusters α, β, γ, and δ (Table 1). Microneutralization assays (MN) showed that mAbS-OIV-3B2 could react with viruses from clusters α, β, γ, but showed no reaction with viruses from cluster δ, consistent with the observation that the latter is derived from seasonal human H1 influenza strains (Table 2). Thus, mAb S-OIV-3B2 displays a broad HI and MN profile against swine-lineage H1 influenza strains.
Table 1.
HI reaction profile of S-OIV-3B2 against different influenza viruses
| Virus | Subtype | HI titer |
|---|---|---|
| A/Brisbane/59/2007 | H1N1 | <10 |
| A/Puerto Rico/8/1934 | H1N1 | <10 |
| A/NewCaledonia/20/1999 | H1N1 | <10 |
| A/Malaya/302/1954 | H1N1 | <10 |
| A/Brisbane/10/2007 | H3N2 | <10 |
| A/Viet Nam/1203/2004 | H5N1 | <10 |
| A/chicken/Delaware/VIVA/2004 | H7N2 | <10 |
| A/guinea fowl/Hong Kong/WF10/1999 | H9N2 | <10 |
| A/swine/Minnesota/02053/2008 (α) | H1N1 | 20480 |
| A/swine/Minnesota/02093/2008 (α) | H1N1 | 20480 |
| A/swine/North Carolina/02084/2008 (β) | H1N1 | 20480 |
| A/swine/Kentucky/02086/2008 (β) | H1N1 | 20480 |
| A/swine/Nebraska/02013/2008 (β) | H1N1 | 10240 |
| A/swine/Ohio/02026/2008 (γ) | H1N1 | 20480 |
| A/swine/Missouri/02060/2008 (γ) | H1N1 | 20480 |
| A/swine/Iowa/02096/2008 (γ) | H1N1 | 20480 |
| A/swine/Ohio/511445/2007 (γ) | H1N1 | 20480 |
| A/swine/North Carolina/02023/2008 (γ) | H1N1 | 20480 |
| A/swine/Texas/01976/2008 (δ) | H1N2 | <10 |
| A/swine/Iowa/02039/2008 (δ) | H1N2 | <10 |
| A/swine/Minnesota/02011/2008 (δ) | H1N2 | <10 |
| A/swine/Iowa/15/1930 (classical) | H1N1 | 20480 |
| A/swine/Tennessee/25/1977 (classical) | H1N1 | 20480 |
| A/Netherlands/602/2009 (H1N1pdm) | H1N1 | 20480 |
| A/California//04/2009 (H1N1pdm) | H1N1 | 20480 |
| A/Mexico/4108/2009 (H1N1pdm) | H1N1 | 20480 |
| Rg-NY/18HA1: 7NL/602 (H1N1pdm)a | H1N1 | 20480 |
Rg-NY/18HA1:7NL/602, reverse genetic recombinant carrying the HA gene from A/New York/18/2009 (H1N1) and remaining 7 genes from A/Netherland/602/2009/H1N1
Intranasal administration of mAbS-OIV-3B2 protects against lethal challenge with H1N1pdm and classical swine H1N1 strains
To evaluate whether mAb S-OIV-3B2 could be used as an anti-viral agent against swine-lineage H1 viruses, prophylactic and therapeutic treatments were implemented in the mouse model of influenza infection. Previously, it was shown that a mAb against the HA of H5 influenza subtypes could provide protection against highly pathogenic H5N1 viruses using a single intranasal administration dose (Ye et al., 2010a). The same approach was utilized to ascertain whether S-OIV-3B2 could protect against swine-lineage H1 strains.
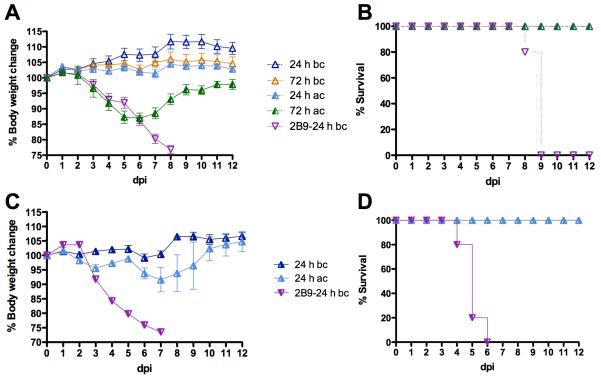
S-OIV-3B2 was delivered via intranasal droplets to 6-week old Balb/c mice at 24 or 72 h before or after challenge with 10 MLD50 of ma-Ca/04. The S-OIV-3B2 was not purified from the ascites; therefore treatment doses referred herein correspond to total amount of IgG in the ascites. Pre-treatment with 12.5 mg/kg of S-OIV-3B2 at 24 or 72 h before challenge resulted in no signs of disease or weight loss and 100% survival, (Fig 1A and B), suggesting that the S-OIV-3B2 could be stably maintained for at least 72 h prior to challenge. In contrast, mock-treated mice showed exacerbated signs of disease, significant body weight loss and succumbed to the challenge by 10 dpi. These results show that S-OIV-3B2 not only increases the chances of survival, but also prevents the disease associated with H1N1pdm. Mice that received S-OIV-3B2 at 24 or 72 h after challenge also showed 100% survival rates (Fig 1A). More importantly, S-OIV-3B2 administration at 24 h after challenge resulted in neither signs of disease nor body weight loss. When mice received S-OIV-3B2 at 72 h after challenge, less than 15% bodyweight loss was observed and mice started to recover by 7 dpi (Fig 1A and B).
Fig 1. Treatment effect of S-OIV-3B2 against lethal challenge of swine-lineage H1.
A,B) 6-week old mice (n=5/group) were treated with 12.5 mg/kg doses of S-OIV-3B2 via the intranasal route at 24 h and 72 h before (bc) or after challenge (ac) with 10 MLD50 of ma-Ca/04; C,D) 6-week old mice (n=5/group) were treated with 12.5mg/kg doses of S-OIV-3B2 via the intranasal route at 24 h before or after challenge with 250 TCID50 of SW30. Mock-treated mice received 14 mg/kg doses of control IgG mAb (2B9) at 24 h before challenge. A, C) Percent body weight change; B, D) Percent survival.
To test whether S-OIV-3B2 could provide protection against other swine-lineage H1 viruses, the antibody was delivered to 6-week old DBA/J2 mice via intranasal droplets 24 h before or after challenge with 250 TCID50 of SW30. DBA/J2 mice were used instead of Balb/c mice because they are more susceptible to infection with influenza viruses and, left untreated, succumbed to a 250 TCID50 dose of SW30 virus chosen in this study. Our data showed that treatment with 12.5 mg/kg of S-OIV-3B2 at 24 h before or after challenge resulted in 100% survival (Fig 1D). Administration of S-OIV-3B2 before challenge resulted in neither signs of disease nor body weight losses (Fig 1C). Mice treated 24 h after challenge showed slight body weight loss but eventually recovered from challenge. These observations are in sharp contrast with the mock-treated mice that showed exacerbated signs of disease, significant body weight losses, and died by 7 dpi (Fig 1). These results highlight the potential use of S-OIV-3B2 as an intranasal anti-viral treatment not only for H1N1pdm infections but also for other swine-lineage H1 strains.
Protective effect of S-OIV-3B2 shows dose dependence and specificity to H1N1pdm
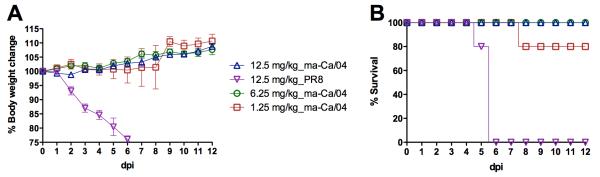
To further evaluate the antiviral activity and specificity to H1N1pdm, we performed a dose escalation study and set up a challenge control with the PR8 virus, to which S-OIV-3B2 does not cross-react. Doses of 12.5 and 6.25 mg/kg administered at 24 h before challenge resulted in 100% survival (n=5/group) and no clinical signs. In the group treated with lowest dose (1.25 mg/kg, n=5), one mouse showed rapid bodyweight loss and died by 8 dpi, whereas the rest of the mice survived with no clinical signs (Fig 2). This study shows a dose-dependent effect of S-OIV-3B2 against lethal H1N1pdm challenge in mice. It also shows the high antiviral activity of the mAb because administration of a single dose at just 1.25 mg/kg resulted in 80% protection. Specificity of S-OIV-3B2 was achieved by showing lack of protection against the PR8 strain even at the 12.5 mg/kg dose (Fig 2). S-OIV-3B2-treated mice challenged with 10 MLD50 of PR8, succumbed to the infection by 6 dpi.
Fig 2. Protective effect of S-OIV-3B2 shows dose dependence and H1N1pdm antigenic specificity.
6-week old mice (n=5/group) were treated with 12.5 mg/kg, 6.25 mg/kg and 1.25 mg/kg doses of S-OIV-3B2 via the intranasal route at 24 h before challenge with 10 MLD50 of ma-Ca/04 or PR8. A) Percent body weight change; B) Percent survival.
S-OIV-3B2 significantly decreases virus replication in mouse lungs
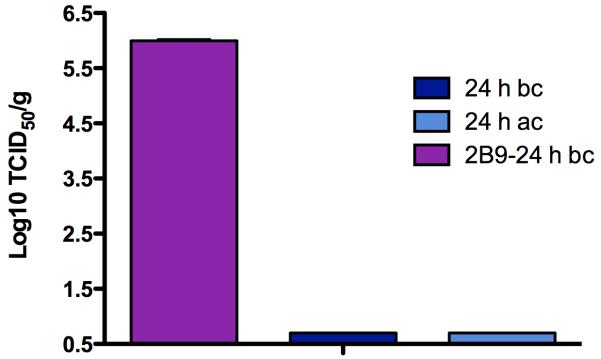
To determine whether treatment with S-OIV-3B2 resulted in reduced virus replication, 6-week old Balb/c mice inoculated with 12.5 mg/kg of S-OIV-3B2 at 24 h before or after challenge with 10 MLD50 of ma-Ca/04 were sacrificed at 3 dpi, lungs collected and virus titers in lung homogenates determined. Virus titers in the lungs were negligible in mice that received S-OIV-3B2 either before or after challenge, indicating complete block of virus replication. Mock-treated mice showed virus lung titers in the order of 106 TCID50 (Fig 3). These results strongly support the data described in Figs 1 and 2, and suggest that the mechanism of protective action of S-OIV-3B2 in vivo is by binding and neutralizing the virus.
Fig 3. S-OIV-3B2 inhibits lung virus replication in challenged mice.
Mice (n=3/group) were treated with 12.5 mg/kg of S-OIV-3B2 at 24 h before (bc) or after challenge (ac) with 10 MLD50 of ma-Ca/04. The mock-treated mice received 14 mg/kg doses of control IgG mAb (2B9) at 24 h before challenge. At day 3 post-challenge, lungs were homogenized and viruses in homogenates were titrated in MDCK cells. BLD, below the limit detection.
In summary, in this study we developed a monoclonal antibody- S-OIV-3B2- against the HA of H1N1pdm. Our results showed that S-OIV-3B2 had high HI and neutralization titers, not only against multiple H1N1pdm strains, but also against classical swine and other H1 strains that belong to clusters α, β, and γ. To our knowledge, this is the first report demonstrating a monoclonal antibody with broad cross-reaction against multiple swine H1 influenza lineages. In contrast, another mAb S-OIV-5H7 developed in our laboratory showed only reaction with H1N1pdm strains, not against other swine H1 strains (not shown). More recently, Wrammert et al (2011) generated several monoclonal antibodies against H1N1pdm but their antiviral activity or reaction profile against other swine H1 strains was not provided.
Using S-OIV-3B2 in vitro in tissue culture cells or embryonated chicken eggs, we could not obtain H1N1pdm escape mutant virus (data not shown); however, it may be that escape mutants would readily emerge if tested under in vivo conditions. Nevertheless, despite the significant antigenic drift that has occurred between the classical swine H1 strains and current endemic swine-lineage H1 in the North American swine population, it is tempting to speculate that S-OIV-3B2 targets a highly conserved epitope. The high neutralization titer of S-OIV-3B2 against swine-lineage H1 further confirms the HI profile and also indicates its potential immune passive therapy against swine-lineage H1 influenza viruses.
Since development of vaccines against a pandemic strain usually takes several months and strains resistant to available antiviral drugs can readily occur (de Jong et al., 2005; Le et al., 2005; Reece, 2007), passive antibody immune therapy represents an attractive alternative antiviral strategy. It is the strategy of choice for the prevention and treatment of respiratory syncytial virus (RSV) infections in high-risk infants where a large dose of a “humanized” IgG mAb is injected intravenously or intramuscularly (Hu and Robinson, 2010). Recently, monoclonal antibodies against highly pathogenic H5N1 influenza virus have been reported as potential antiviral treatment of H5N1 infection in the mouse model (Prabhu et al., 2009; Simmons et al., 2007; Sui et al., 2009; Throsby et al., 2008). These studies showed the administration of passive antibodies via the intraperitoneal, intramuscular or intravenous routes. More recently, we developed a monoclonal antibody that, when administered as single dose intranasally, provided protection against highly pathogenic H5N1 (Ye et al., 2010a). Here intranasal administration was again used to show that S-OIV-3B2 could provide protection against lethal challenge with classical swine H1N1 and H1N1pdm influenza strains. It should be noted that S-OIV-3B2 maintained virus neutralization activity even when given 72 h prior to challenge. This result further confirms our previous finding that the neutralization activity of a monoclonal antibody is retained at least for three days in the mouse respiratory tract (Ye et al., 2010a). S-OIV-3B2 was also effective when given at 72 h post-challenge and significantly reduced the morbidity in Balb/c mice. Previous studies showed a human monoclonal antibody (A06) derived from a survivor of highly pathogenic H5N1 infection that could also protect mice from H1N1pdm infection if the antibody was administered intraperitoneally (Kashyap et al., 2010). However, these studies did not determine whether A06 was effective if administered 48 or 72 h prior to infection. Similarly, A06 treatment resulted in 100% and 50% survival when given at 24 h or 72 h post challenge, respectively. It should also be noted the challenge dose (25 MLD50) used by Kashyap et al. (2010) is slightly higher than in our study (10 MLD50) in Balb/c mice. Considering the limited efficacy of current antiviral drugs when given beyond the 48 h window after infection, antibody-mediated therapy should be considered a plausible strategy against influenza (Cram et al., 2001; Gillissen and Hoffken, 2002). Future studies are needed to determine whether S-OIV-3B2 can be “humanized” while maintaining its neutralizing antiviral activity.
Acknowledgments
We are indebted to Yonas Araya, Theresa Wolter-Marth, and Ivan Gomez-Osorio for their excellent laboratory techniques and animal handling assistance. This research was possible through funding by the USDA-ARS Specific Cooperative Agreement No. 58-3625-0-611, CSREES-USDA grant (1865-05523), and NIAID-NIH contract (HHSN266186700010C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berhane Y, Ojkic D, Neufeld J, Leith M, Hisanaga T, Kehler H, Ferencz A, Wojcinski H, Cottam-Birt C, Suderman M, Handel K, Alexandersen S, Pasick J. Molecular characterization of pandemic H1N1 influenza viruses isolated from turkeys and pathogenicity of a human pH1N1 isolate in turkeys. Avian Dis. 2010;54:1275–1285. doi: 10.1637/9422-061410-Reg.1. [DOI] [PubMed] [Google Scholar]
- Cram P, Blitz SG, Monto A, Fendrick AM. Influenza. Cost of illness and considerations in the economic evaluation of new and emerging therapies. Pharmacoeconomics. 2001;19:223–230. doi: 10.2165/00019053-200119030-00001. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, Yardley IE. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. Bmj. 2009;339:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr., Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen A, Hoffken G. Early therapy with the neuraminidase inhibitor oseltamivir maximizes its efficacy in influenza treatment. Med Microbiol Immunol. 2002;191:165–168. doi: 10.1007/s00430-002-0139-9. [DOI] [PubMed] [Google Scholar]
- Hanson BJ, Boon AC, Lim AP, Webb A, Ooi EE, Webby RJ. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir Res. 2006;7:126. doi: 10.1186/1465-9921-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden KJ, Brockhoff EJ, Caya FD, McLeod LJ, Lavoie M, Ing JD, Bystrom JM, Alexandersen S, Pasick JM, Berhane Y, Morrison ME, Keenliside JM, Laurendeau S, Rohonczy EB. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can Vet J. 2009;50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- Hu J, Robinson JL. Treatment of respiratory syncytial virus with palivizumab: a systematic review. World J Pediatr. 2010;6:296–300. doi: 10.1007/s12519-010-0230-z. [DOI] [PubMed] [Google Scholar]
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- Kashyap AK, Steel J, Rubrum A, Estelles A, Briante R, Ilyushina NA, Xu L, Swale RE, Faynboym AM, Foreman PK, Horowitz M, Horowitz L, Webby R, Palese P, Lerner RA, Bhatt RR. Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS pathogens. 2010;6:e1000990. doi: 10.1371/journal.ppat.1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA. Genes and proteins of the influenza viruses. In: Krug RM, editor. The influenza viruses. 1st ed Plenum Press; New York: 1989. [Google Scholar]
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, Pham ND, Ngyen HH, Yamada S, Muramoto Y, Horimoto T, Takada A, Goto H, Suzuki T, Suzuki Y, Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, Cavalieri ML, Guglielmo MC, Areso MS, Gilligan T, Santucho F, Cabral G, Gregorio GL, Moreno R, Lutz MI, Panigasi AL, Saligari L, Caballero MT, Almeida R.M. Egues, Meyer M.E. Gutierrez, Neder MD, Davenport MC, Del Valle MP, Santidrian VS, Mosca G, Dominguez M. Garcia, Alvarez L, Landa P, Pota A, Bolonati N, Dalamon R, Mercol V.I. Sanchez, Espinoza M, Peuchot JC, Karolinski A, Bruno M, Borsa A, Ferrero F, Bonina A, Ramonet M, Albano LC, Luedicke N, Alterman E, Savy V, Baumeister E, Chappell JD, Edwards KM, Melendi GA, Polack FP. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- Ma W, Vincent AL, Lager KM, Janke BH, Henry SC, Rowland RR, Hesse RA, Richt JA. Identification and characterization of a highly virulent triple reassortant H1N1 swine influenza virus in the United States. Virus Genes. 2010;40:28–36. doi: 10.1007/s11262-009-0413-7. [DOI] [PubMed] [Google Scholar]
- Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Cappuccio J, Quiroga MA, Baumeister E, Insarralde L, Ibar M, Sanguinetti R, Cannilla ML, Franzese D, Cabrera O.E. Escobar, Craig MI, Rimondi A, Machuca M, Debenedetti RT, Zenobi C, Barral L, Balzano R, Capalbo S, Risso A, Perfumo CJ. Pandemic (H1N1) 2009 outbreak on pig farm, Argentina. Emerg Infect Dis. 2010;16:304–307. doi: 10.3201/eid1602.091230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu N, Prabakaran M, Hongliang Q, He F, Ho HT, Qiang J, Goutama M, Lim AP, Hanson BJ, Kwang J. Prophylactic and therapeutic efficacy of a chimeric monoclonal antibody specific for H5 haemagglutinin against lethal H5N1 influenza. Antivir Ther. 2009;14:911–921. doi: 10.3851/IMP1413. [DOI] [PubMed] [Google Scholar]
- Reece PA. Neuraminidase inhibitor resistance in influenza viruses. J Med Virol. 2007;79:1577–1586. doi: 10.1002/jmv.20951. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, Lindstrom S, Gubareva LV, Deyde V, Garten RJ, Harris M, Gerber S, Vagasky S, Smith F, Pascoe N, Martin K, Dufficy D, Ritger K, Conover C, Quinlisk P, Klimov A, Bresee JS, Finelli L. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- Simmons CP, Bernasconi NL, Suguitan AL, Mills K, Ward JM, Chau NV, Hien TT, Sallusto F, Ha do Q, Farrar J, de Jong MD, Lanzavecchia A, Subbarao K. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4:e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, Cinatl J, ter Meulen J, Lasters I, Carsetti R, Peiris M, de Kruif J, Goudsmit J. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, Smith GJ, Peiris JS, Guan Y. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science. 2010;328:1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AL, Lager KM, Faaberg KS, Harland M, Zanella EL, Ciacci-Zanella JR, Kehrli ME, Jr., Janke BH, Klimov A. Experimental inoculation of pigs with pandemic H1N1 2009 virus and HI cross-reactivity with contemporary swine influenza virus antisera. Influenza Other Respi Viruses. 2010;4:53–60. doi: 10.1111/j.1750-2659.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AL, Ma W, Lager KM, Gramer MR, Richt JA, Janke BH. Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus Genes. 2009a;39:176–185. doi: 10.1007/s11262-009-0386-6. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Swenson SL, Lager KM, Gauger PC, Loiacono C, Zhang Y. Characterization of an influenza A virus isolated from pigs during an outbreak of respiratory disease in swine and people during a county fair in the United States. Vet Microbiol. 2009b;137:51–59. doi: 10.1016/j.vetmic.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Webster R, Cox N, Stohr K. WHO Manual on Animal Influenza Diagnosis and Surveillance. World Health Organization Department of Communicable Disease Surveillance and Response; 2005. [Google Scholar]
- Webster RG. Predictions for future human influenza pandemics. J Infect Dis. 1997;176:S14–19. doi: 10.1086/514168. [DOI] [PubMed] [Google Scholar]
- Webster RG, Shortridge KF, Kawaoka Y. Influenza: interspecies transmission and emergence of new pandemics. FEMS Immunol Med Microbiol. 1997;18:275–279. doi: 10.1111/j.1574-695X.1997.tb01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM. Did the 2009 pandemic influenza virus originate in humans? Future microbiology. 2010;5:989–991. doi: 10.2217/fmb.10.62. [DOI] [PubMed] [Google Scholar]
- Weingartl HM, Berhane Y, Hisanaga T, Neufeld J, Kehler H, Emburry-Hyatt C, Hooper-McGreevy K, Kasloff S, Dalman B, Bystrom J, Alexandersen S, Li Y, Pasick J. Genetic and pathobiologic characterization of pandemic H1N1 2009 influenza viruses from a naturally infected swine herd. J Virol. 2010;84:2245–2256. doi: 10.1128/JVI.02118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Berhane Y, Salo T, Li M, Hole K, Clavijo A. Development and application of monoclonal antibodies against avian influenza virus nucleoprotein. J Virol Methods. 2008;147:265–274. doi: 10.1016/j.jviromet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Ye J, Shao H, Hickman D, Angel M, Xu K, Cai Y, Song H, Fouchier RA, Qin A, Perez DR. Intranasal delivery of an IgA monoclonal antibody effective against sublethal H5N1 influenza virus infection in mice. Clin Vaccine Immunol. 2010a;17:1363–1370. doi: 10.1128/CVI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Sorrell EM, Cai Y, Shao H, Xu K, Pena L, Hickman D, Song H, Angel M, Medina RA, Manicassamy B, Garcia-Sastre A, Perez DR. Variations in the hemagglutinin of the 2009 H1N1 pandemic virus: potential for strains with altered virulence phenotype? PLoS Pathog. 2010b;6:e1001145. doi: 10.1371/journal.ppat.1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]