Abstract
The Factor V Leiden mutation is associated with ischemic stroke in children, but not in adults. Whether it is associated with ischemic stroke in young adults, however, is uncertain. To address this issue, we performed a meta-analysis of 18 case-control studies of ischemic stroke in adults ≤ 50 years of age published before June 2009. Across all studies, Factor V Leiden was detected in 154 of 2,045 cases (7.5%) and 217 of 5,307 controls (4.1%), yielding a fixed effect odds ratio of 2.00 (95% CI: 1.59–2.51). However, further analyses revealed substantial heterogeneity among these studies (p=0.005 for Q-test of heterogeneity). Hypothesizing that this heterogeneity could be related to differences among studies in case selection criteria, we stratified the meta-analysis into studies for which case samples were enriched or not enriched to include cases having an increased likelihood of prothrombotic genetic involvement (“selected” ischemic stroke studies, n = 9) and those that recruited cases from consecutive neurology referrals or hospitalizations (“unselected” ischemic stroke studies, n = 8). Among the nine “selected” ischemic stroke studies, Factor V Leiden was more strongly associated with stroke [OR = 2.73 (95% CI: 1.98–3.75)], whereas among the eight “unselected” ischemic stroke studies, the association between Factor V Leiden and stroke was substantially weaker [OR=1.40 (95% CI: 0.998–1.95)]. This difference was found to be statistically significant (p = 0.003 for Woolf’s test for heterogeneity). We conclude that Factor V Leiden is associated with ischemic stroke in young adults, particularly in patient populations where there is an increased clinical suspicion of prothrombotic state.
Keywords: meta-analysis, Factor V Leiden, stroke, risk factor, young adults
INTRODUCTION
Because the majority of strokes occur in individuals over the age of 65, genetic association studies of ischemic stroke have tended to focus on the elderly. Nevertheless, 5–10% of strokes occur in individuals under the age of 45 [1]. Given the high prevalence of strokes in the general population, this translates into a significant number of young adult stroke patients. Clinical outcomes typically differ between early- and mature-onset strokes, and the etiologies may also differ by age of onset, including a more prominent role for genetic factors in early-onset compared to mature-onset disease [2]. A number of candidate genes have been investigated as potential risk factors for ischemic stroke in young adults. Of these, one of the most thoroughly studied polymorphisms is Factor V Leiden.
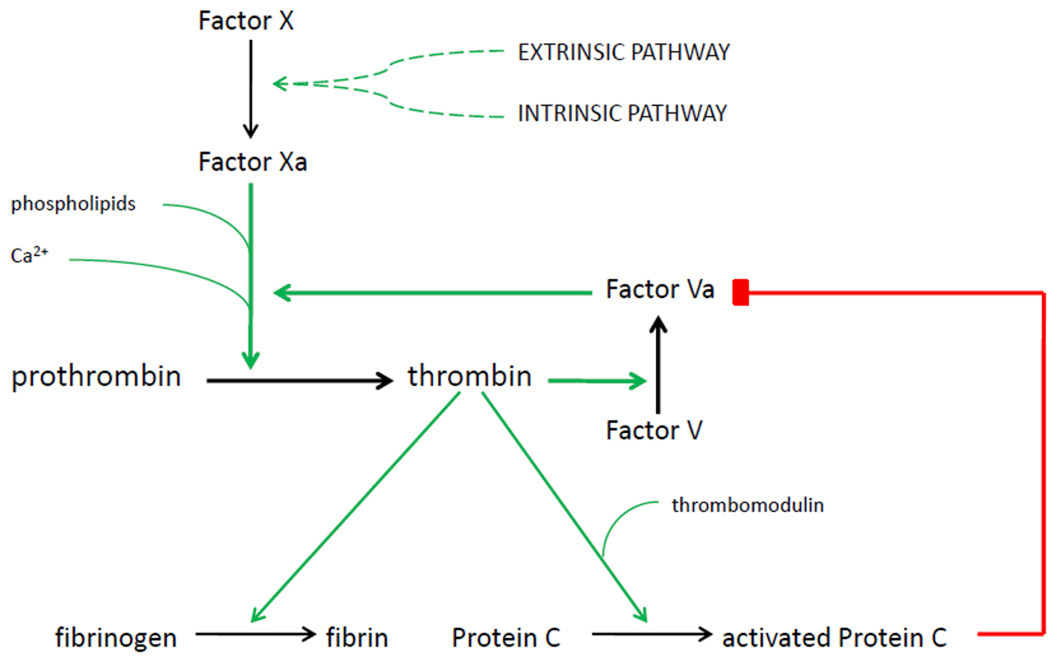
Factor V Leiden is the most common prothrombotic genetic predisposition. It is present in 5.2% of Caucasian Americans and 1.2% of African Americans, though it is more common in certain European populations and is least common in East Asian, African, and indigenous Australian populations [3]. Figure 1 illustrates the role that Factor V plays in the coagulation cascade. The Factor V Leiden mutation is characterized by a substitution of glutamine for arginine at position 506, which prevents Factor V from being inactivated by activated protein C, thereby leading to a state of hypercoagulability [4].
Figure 1.
Regulation of Factor V activity by thrombin and activated protein C. The extrinsic and intrinsic coagulation pathways converge on the activation of Factor X. During the initial phase of clot formation, Factor X catalyzes the conversion of prothrombin into thrombin at a relatively low rate. Thrombin activates Factor V, which forms a complex with Factor X and increases its prothrombinase activity by five orders of magnitude. Thrombin also activates protein C, which degrades Factor V and provides balance to the coagulation system. In the Factor V Leiden mutation, a single point mutation prevents the protein from being inactivated by activated protein C. This leads to a state of hypercoagulability.
Across multiple studies, Factor V Leiden has been associated with venous thromboembolism in adults and with ischemic stroke in children [5,6], though it is not associated with ischemic stroke in the general adult population [7]. However, studies of Factor V Leiden and ischemic stroke in young adults are equivocal; many are based on relatively small sample sizes, limiting their ability to consistently detect associations of modest effect. Here, we report a systematic review and meta-analysis of association studies of Factor V Leiden and ischemic stroke in young adults.
METHODS
Using the keywords Factor V Leiden, ischemic stroke, and young adults, we searched PubMed and MEDLINE databases for case-control studies of ischemic stroke in adults ≤ 50 years of age published before June 2009. Any identified articles were then hand-searched for references to additional relevant studies. Studies were included in the meta-analysis if: (1) neuroimaging was used to confirm clinical diagnoses of ischemic stroke; (2) controls were derived from the same population as cases; (3) Factor V genotypes were available for all participants; (4) the numbers of cases and controls with and without the Factor V Leiden were provided in the article; and (5) the study included only cases with first stroke less than 50 years of age, or the number of cases in this age group with and without Factor V Leiden could be clearly obtained from the study [8]. Studies that used activated protein C resistance as a surrogate marker for Factor V Leiden or selected only for patients with patent foramen ovale were excluded. We did not include unpublished data in this review.
Data-analysis was performed using Comprehensive Meta-Analysis version 2.0 by Biostat (http://www.meta-analysis.com). For each study, Factor V Leiden carrier frequencies and sample sizes for both case and control populations were extracted, and crude odds ratios were calculated. A pooled odds ratio was calculated under both fixed-effects and random-effects models. The random-effects model generally has less power than the fixed effect model since it allows for the true effect to differ across studies. It gives more weight to smaller studies and less weight to larger studies than does a fixed-effects model and produces a larger confidence interval. The calculation for the fixed-effects model was repeated with each of the studies individually removed from the analysis to confirm that no single study was principally responsible for the findings. This option was not available for the random-effects model. Between-study heterogeneity was assessed using the Q-test, which is based on comparing the estimated study-specific treatment effects to the estimated overall treatment effect. We additionally computed the I2 statistic, which describes the proportion of variation across studies due to heterogeneity rather than due to chance [9].
In addition, we performed a second meta-analysis that stratified studies into those for which the case set was enriched with individuals whose stroke was likely to be related to a prothrombotic genetic condition (‘selected’ ischemic stroke studies) and those for which cases that recruited cases from consecutive neurology referrals or hospitalizations (‘unselected’ ischemic stroke studies). Among the 18 studies included in the meta-analysis, 9 were considered “selected” studies [10–18], and 8 were considered ‘unselected’ studies [20–27]; one study was not included in either category because half of the cases were referred for thrombophilic work-up and the other half were consecutive hospital admissions [19]. The ‘selected’ studies included two studies that included only cases explicitly defined as having cryptogenic ischemic stroke [10,11], two studies that included only cases suggestive of having cryptogenic stroke (e.g. absence of a cardioembolic source or other conventional risk factors) [12–13], and five studies that recruited cases from a subset of patients referred for thrombophilic work-up [14–18]. A Woolf’s test for heterogeneity was used to compare the odds ratio for the pooled set of “selected” studies to that of the pooled set of “unselected” studies.
RESULTS
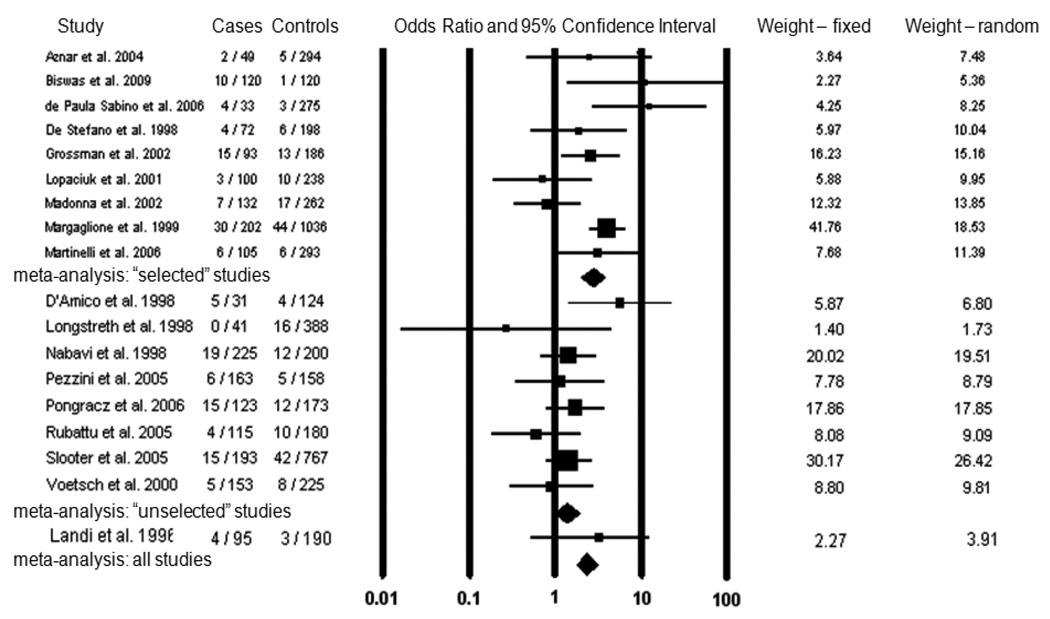
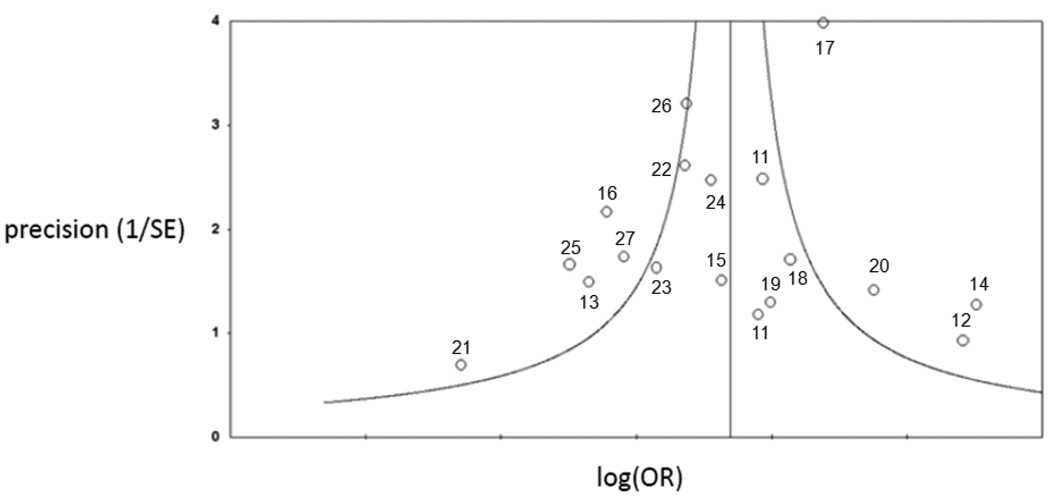
Eighteen studies matched our selection criteria and were included in the meta-analysis Table 1 shows the demographic characteristics of the studies included in the meta-analysis. The results of our preliminary meta-analysis are shown in Figure 2. One study included two pediatric stroke cases that could not be separated from the rest of the study sample, though Factor V Leiden was not significantly associated with stroke in this study [19]. The studies were conducted in several European countries, the United States, Brazil, and India, and the majority of participants were of Caucasian ancestry. In thirteen of these studies, the reported odds ratio for Factor V Leiden and ischemic stroke was greater than one, and in five of these the odds ratio was significantly greater than one (p<0.05). Across all studies combined, Factor V Leiden was detected in 154 of 2,045 cases (7.5%) and 217 of 5,307 controls (4.1%), yielding a pooled odds ratio of 2.00 (95% CI: 1.59–2.51) under a fixed-effects model and 1.89 (95% CI: 1.31–2.72) under a random-effects model. Repeating the fixed effects meta-analysis with each of the studies individually removed did not significantly alter the calculated odds ratio (data not shown). To assess publication bias, we created a funnel plot of all the studies used in the meta-analysis (Figure 3), which conformed to the expected shape of the curve and demonstrated overall left-right symmetry [28].
Table 1.
Demographic characteristics of studies included in meta-analysis
| Study | N | Males/Females | Ethnicity | Mean Age (case/control) |
|---|---|---|---|---|
| Aznar et al. 2004 | 343 | 105/238 | Caucasian | <50 |
| Biswas et al. 2009 | 240 | NP | Asian | <40 |
| de Paula Sabino et al. 2006 | 308 | 72/256 | Caucasian | 31/34 |
| De Stefano et al. 1998 | 270 | 113/157 | Caucasian | 34/49 |
| Grossman et al. 2002 | 279 | 138/141 | Caucasian | 36/33 (median) |
| Lopaciuk et al. 2001 | 338 | 207/131 | Caucasian | 38/33 |
| Madonna et al. 2002 | 394 | 183/211 | Caucasian | 38/36 |
| Margaglione et al. 1999 | 1238 | 545/693 | Caucasian | 39 (median, cases) |
| Martinelli et al. 2006 | 398 | 0/398 | Caucasian | 34/34 |
| D'Amico et al. 1998 | 155 | 60/95 | Caucasian | 34/33 |
| Longstreth et al. 1998 | 429 | 0/429 | Caucasian / African-American | 37/38 |
| Nabavi et al. 1998 | 425 | NP | Caucasian | 35/<45 |
| Pezzini et al. 2005 | 321 | 169/152 | Caucasian | 35/35 |
| Pongracz et al. 2006 | 451 | NP | Caucasian | <50/<49 |
| Rubattu et al. 2005 | 295 | 149/146 | Caucasian | <45 |
| Slooter et al. 2005 | 960 | 0/906 | Caucasian | 39/40 |
| Voetsch et al. 2000 | 378 | 161/217 | Caucasian | <45 |
| Landi et al. 1996 | 285 | NP | Caucasian | 33/<45 |
NP = not provided
Figure 2.
Meta-analysis of Factor V Leiden and ischemic stroke in young adults. For each study, the number of individuals with Factor V Leiden and the total number of individuals are indicated for both case and control populations. The odds ratio for each study is given by a square, the size of which is proportional to the sample size, and a horizontal line denotes the 95% confidence interval. The pooled odds ratio for “selected” studies, “unselected” studies, and all studies under a fixed effects model is represented by a diamond.
Figure 3.
Funnel plot of studies included in meta-analysis of Factor V Leiden and ischemic stroke in young adults. Each point on the graph represents an individual study in the meta-analysis. The number beside each point corresponds to the reference number for that study. The curved lines represent the expected distribution of studies, and the vertical line indicates the log of the pooled odds ratio.
Significant evidence for between-study heterogeneity was detected in our meta-analysis (p = 0.005 for Q-test of heterogeneity, I2 = 52%). Hypothesizing that this heterogeneity was due to differences in case selection criteria among the studies, we analyzed the nine “selected” and eight “unselected” studies separately. Among “selected” ischemic stroke studies, Factor V Leiden demonstrated a stronger association than in the original meta-analysis [OR = 2.73 (95% CI: 1.98–3.75) under a fixed-effects model and OR = 2.54 (95% CI: 1.45–4.46) under a random-effects model], whereas “unselected” ischemic stroke studies demonstrated a weaker, non-significant association [OR = 1.40 (95% CI: 0.998–1.95) under a fixed-effects model and OR = 1.38 (95% CI: 0.95–2.01) under a random-effects model]. The difference between these odds ratios was statistically significant (p = 0.003 for Woolf’s test for heterogeneity). Moreover, between-study heterogeneity was reduced in “unselected” ischemic stroke studies (p = 0.318 for Q-test of heterogeneity, I2 = 14%) and increased in “selected” ischemic stroke studies (p = 0.014 for Q-test of heterogeneity, I2 = 58%).
DISCUSSION
In this meta-analysis, Factor V Leiden demonstrated a significant association with ischemic stroke in young adults. Repeat meta-analyses showed that the results were not skewed by any single study. Left-right symmetry in the funnel plot indicates that studies with large and small effect sizes were equally represented in the literature, and concordance with the expected shape of the curve shows that, as expected, larger studies had odds ratios closer to the overall odds ratio than smaller studies. These observations argue against the presence of publication bias. However, there was strong statistical evidence for between-study heterogeneity, suggesting that the association between Factor V Leiden and ischemic stroke differed across populations. Stratification of the studies by case selection criteria supported this conclusion. The magnitude of the association between Factor V Leiden and ischemic stroke in young adults was elevated in “selected” ischemic stroke studies and was diminished to a non-significant level in “unselected” ischemic stroke studies. This difference was found to be statistically significant, and the fact that heterogeneity was also increased in “selected” ischemic stroke studies and reduced in “unselected” ischemic stroke studies further supports the hypothesis that differences in study design are likely responsible for the heterogeneity observed in the initial meta-analysis.
To date, one previous meta-analysis of Factor V Leiden and ischemic stroke in young adults has been published [7]. In that study, the odds ratio for Factor V Leiden was 1.37 (95% CI: 0.96–1.97). This is consistent with the odds ratio that we calculated for “unselected” ischemic stroke studies, but it is notably smaller than the odds ratio for all ischemic stroke studies in our meta-analysis. The previous meta-analysis was based on seven independent studies, all of which were included in our meta-analysis [13,15,16,17,21,22,27]. Interestingly, of these seven studies, four were defined as “selected” ischemic stroke studies in our stratified meta-analysis [13,15–17]. Thus, despite being somewhat enriched for genetic susceptibility, the previous meta-analysis detected less of an effect than ours did.
We conclude that Factor V Leiden is associated with ischemic stroke in young adults, but that this association is predominantly seen in patients who are referred for thrombophilic work-up. Unfortunately, none of the ‘selected’ studies included in our meta-analysis reported their clinical criteria for patient referral. If patients were referred for thrombophilic evaluation only if they had a personal history of deep venous thrombosis, then the observed association between Factor V Leiden and ischemic stroke is perhaps explained by the fact that Factor V Leiden is known to be a significant risk factor for deep venous thrombosis. On the other hand, if patients were considered to be at risk for a prothrombotic condition solely by virtue of having had an early-onset ischemic stroke, then the study would effectively be an “unselected” study. In reality, the different ‘selected’ study populations were likely “enriched” to varying degrees, as evidenced by the between-study heterogeneity in this group.
Our findings do not support the conclusion that Factor V Leiden is a risk factor for ischemic stroke in “unselected” populations of young adults. In fact, the odds ratio for unselected ischemic stroke studies may have been driven by the cryptogenic stroke patients in those groups. Had the studies been limited to strokes of known etiology, the association between Factor V Leiden and ischemic stroke in young adults would probably have been even less.
In addition to systematic differences in patient populations, there are other potential sources of confounding in this meta-analysis [29,30]. Although the vast majority of studies were conducted in European populations, racial homogeneity does not always guarantee genetic homogeneity. This is exemplified by the fact that the prevalence of Factor V Leiden is known to vary across different European countries. Therefore, admixture cannot be ruled out as a confounding factor. Furthermore, if a disease-causing allele is more frequent in one population than in another, it will be easier to detect an association in that population, especially with a limited sample size. For example, one study of ischemic stroke in young Chinese adults had to be excluded because Factor V Leiden was not detected in any of the cases or controls [31]. Another potential source of confounding is survival bias. If a disease-causing allele produces significant mortality, especially in the short term, case-control association studies may underestimate its effect because some cases will have died before they can be included in a study. Finally, linkage disequilibrium could potentially cause a spurious association between Factor V Leiden and ischemic stroke in young adults. This is unlikely, however, given that Factor V is a candidate gene known to be involved in clot formation, Factor V Leiden has been implicated in other forms of thrombosis, and a positive association between Factor V Leiden and ischemic stroke in young adults was identified in multiple populations.
It should be noted that Factor V Leiden has been more strongly associated with venous thromboembolic events – namely pulmonary emboli (PE) and deep venous thromboses (DVT) – than with arterial ischemic stroke. For this reason, an important potential mechanism for the association of Factor V Leiden with ischemic stroke is a paradoxical venous-to-arterial embolus via a patent foramen ovale (PFO). We specifically excluded studies that considered only stroke patients with PFO, and among the studies included in our meta-analyses, the absence or presence of a PFO was not consistently reported. Nevertheless, paradoxical embolism may have played a significant role in the pathogenesis of cryptogenic strokes occurring in those studies. A recent meta-analysis of studies examining the association between Factor V Leiden and PFO-positive stroke found a trend towards a positive association, though it did not meet the threshold for significance [32].
All of the studies included in this meta-analysis were case-control association studies. Because stroke in young adults is a rare outcome with a potentially long latency period, the case-control approach is an efficient design for studying genetic risk factors for early onset stroke. While case-control studies can be prone to biases related to assessment of exposures, this is an unlikely source of bias in our study because the exposure is genotype and cases and controls were plated together for genotyping. However, unlike cohort studies in which study subjects are enrolled prior to an event, case-control studies may be subject to survival bias to the extent that cases characterized by high fatality rates following the event are less likely to be included in the study sample. Thus, while cohort studies have certain advantages over case-control studies, they are highly cost-prohibitive for the study of the genetics of early onset stroke. As a result, the literature is largely restricted to case-control studies.
In conclusion, we report that Factor V Leiden is a risk factor for ischemic stroke in young adults in selected patient populations. Meta-analysis is not a substitute for large studies conducted in single, homogeneous populations, and further research is needed to confirm these findings and characterize the nature of the association in greater detail.
Acknowledgments
This work was supported in part by an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship; an American Medical Association Foundation Seed Grant; an American Heart Association Grant-in-Aid; the Office of Research and Development, Medical Research Service, and the Baltimore Geriatrics Research, Education, and Clinical Center of the Department of Veterans Affairs; the National Institute of Neurological Disorders and Stroke (NINDS) and the NIH Office of Research on Women's Health (ORWH) (Grant R01 NS45012); the National Human Genome Research Institute (NHGRI) (Grant U01 HG004436)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lethbridge-Cejku M, Schiller JS, Bernadel L. Summary health statistics for U.S. Adults: National Health Interview Survey, 2002. 2004 [PubMed] [Google Scholar]
- 2.Markus HS, editor. Stroke Genetics. Oxford: Oxford University Press; 2003. pp. 165–166. [Google Scholar]
- 3.Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women: implications for venous thromboembolism screening. JAMA. 1997;277:1305–1307. [PubMed] [Google Scholar]
- 4.Mann K, Kalafatis M. Factor V: a combination of Dr. Jekyll and Mr. Hyde. Blood. 2003;101:20–30. doi: 10.1182/blood-2002-01-0290. [DOI] [PubMed] [Google Scholar]
- 5.Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance) Blood. 1995;85:1504–1508. [PubMed] [Google Scholar]
- 6.Barnes C, Deveber G. Prothrombotic abnormalities in childhood ischaemic stroke. Thromb Res. 2006;118:67–74. doi: 10.1016/j.thromres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am. Heart J. 2003;146:948–957. doi: 10.1016/S0002-8703(03)00519-2. [DOI] [PubMed] [Google Scholar]
- 8.Dichgans M, Markus HS. Genetic association studies in stroke: Methodological issues and proposed standard criteria. Stroke. 2005;36:2027–2031. doi: 10.1161/01.STR.0000177498.21594.9e. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aznar J, Mira Y, Vaya A, et al. Factor V leiden and prothrombin G20210A mutations in young adults with cryptogenic ischemic stroke. Thromb Haemost. 2004;91:1031–1034. doi: 10.1160/TH03-11-0690. [DOI] [PubMed] [Google Scholar]
- 11.Grossmann R, Geisen U, Merati G, et al. Genetic risk factors in young adults with 'cryptogenic' ischemic cerebrovascular disease. Blood Coagul Fibrinolysis. 2002;13:583–590. doi: 10.1097/00001721-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Biswas A, Ranjan R, Meena A, et al. Prothrombotic factors and the risk of acute onset non-cardioembolic stroke in young Asian Indians. Thromb Res. 2009 doi: 10.1016/j.thromres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Lopaciuk S, Bykowska K, Kwiecinski H, et al. Factor V leiden, prothrombin gene G20210A variant, and methylenetetrahydrofolate reductase C677T genotype in young adults with ischemic stroke. Clin Appl Thromb Hemost. 2001;7:346–350. doi: 10.1177/107602960100700418. [DOI] [PubMed] [Google Scholar]
- 14.de Paula Sabino A, Ribeiro DD, Carvalho MG, Cardoso J, Dusse LM, Fernandes AP. Factor V leiden and increased risk for arterial thrombotic disease in young Brazilian patients. Blood Coagul Fibrinolysis. 2006;17:271–275. doi: 10.1097/01.mbc.0000224846.35001.64. [DOI] [PubMed] [Google Scholar]
- 15.De Stefano V, Chiusolo P, Paciaroni K, et al. Prothrombin G20210A mutant genotype is a risk factor for cerebrovascular ischemic disease in young patients. Blood. 1998;91:3562–3565. [PubMed] [Google Scholar]
- 16.Madonna P, de Stefano V, Coppola A, et al. Hyperhomocysteinemia and other inherited prothrombotic conditions in young adults with a history of ischemic stroke. Stroke. 2002;33:51–56. doi: 10.1161/hs0102.100483. [DOI] [PubMed] [Google Scholar]
- 17.Margaglione M, D'Andrea G, Giuliani N, et al. Inherited prothrombotic conditions and premature ischemic stroke: Sex difference in the association with factor V Leiden. Arterioscler Thromb Vasc Biol. 1999;19:1751–1756. doi: 10.1161/01.atv.19.7.1751. [DOI] [PubMed] [Google Scholar]
- 18.Martinelli I, Battaglioli T, Burgo I, Di Domenico S, Mannucci PM. Oral contraceptive use, thrombophilia and their interaction in young women with ischemic stroke. Haematologica. 2006;91:844–847. [PubMed] [Google Scholar]
- 19.Landi G, Cella E, Martinelli I, Tagliabue L, Mannucci PM, Zerbi D. Arg506Gln factor V mutation and cerebral ischemia in the young. Stroke. 1996;27:1697–1698. [PubMed] [Google Scholar]
- 20.D'Amico D, Moschiano F, Leone M, et al. Genetic abnormalities of the protein C system: Shared risk factors in young adults with migraine with aura and with ischemic stroke? Cephalalgia. 1998;18:618–621. doi: 10.1046/j.1468-2982.1998.1809618.x. discussion 591. [DOI] [PubMed] [Google Scholar]
- 21.Longstreth WT, Jr, Rosendaal FR, Siscovick DS, et al. Risk of stroke in young women and two prothrombotic mutations: Factor V leiden and prothrombin gene variant (G20210A) Stroke. 1998;29:577–580. doi: 10.1161/01.str.29.3.577. [DOI] [PubMed] [Google Scholar]
- 22.Nabavi DG, Junker R, Wolff E, et al. Prevalence of factor V leiden mutation in young adults with cerebral ischaemia: A case-control study on 225 patients. J Neurol. 1998;245:653–658. doi: 10.1007/s004150050262. [DOI] [PubMed] [Google Scholar]
- 23.Pezzini A, Grassi M, Del Zotto E, et al. Cumulative effect of predisposing genotypes and their interaction with modifiable factors on the risk of ischemic stroke in young adults. Stroke. 2005;36:533–539. doi: 10.1161/01.STR.0000155741.31499.c2. [DOI] [PubMed] [Google Scholar]
- 24.Pongracz E, Andrikovics H, Csornai M, Bernat IS, Nagy Z, et al. Contribution of the −455G/A polymorphism at beta-fibrinogen gene and of the leiden mutation to hemorheological parameters in ischemic stroke patients. Clin Hemorheol Microcirc. 2006;35:75–82. [PubMed] [Google Scholar]
- 25.Rubattu S, Speranza R, Ferrari M, et al. A role of TNF-alpha gene variant on juvenile ischemic stroke: A case-control study. Eur J Neurol. 2005;12:989–993. doi: 10.1111/j.1468-1331.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 26.Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213–1217. doi: 10.1111/j.1538-7836.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 27.Voetsch B, Damasceno BP, Camargo EC, et al. Inherited thrombophilia as a risk factor for the development of ischemic stroke in young adults. Thromb Haemost. 2000;83:229–233. [PubMed] [Google Scholar]
- 28.Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More than numbers: The power of graphs in meta-analysis. Am J Epidemiol. 2009;169:249–255. doi: 10.1093/aje/kwn340. [DOI] [PubMed] [Google Scholar]
- 29.Cordell HJ, Clayton DG. Genetic association studies. Lancet. 2005;366:1121–1131. doi: 10.1016/S0140-6736(05)67424-7. [DOI] [PubMed] [Google Scholar]
- 30.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 31.Shi C, Kang X, Wang Y, Zhou Y. The coagulation factor V Leiden, MTHFRC677T variant and eNOS 4ab polymorphism in young Chinese population with ischemic stroke. Clin Chim Acta. 2008;396(1–2):7–9. doi: 10.1016/j.cca.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Pezzini A, Grassi M, Zotto ED, et al. Do common prothrombotic mutations influence the risk of cerebral ischaemia in patients with patent foramen ovale? systematic review and meta-analysis. Thromb Haemost. 2009;101:813–817. [PubMed] [Google Scholar]