Abstract
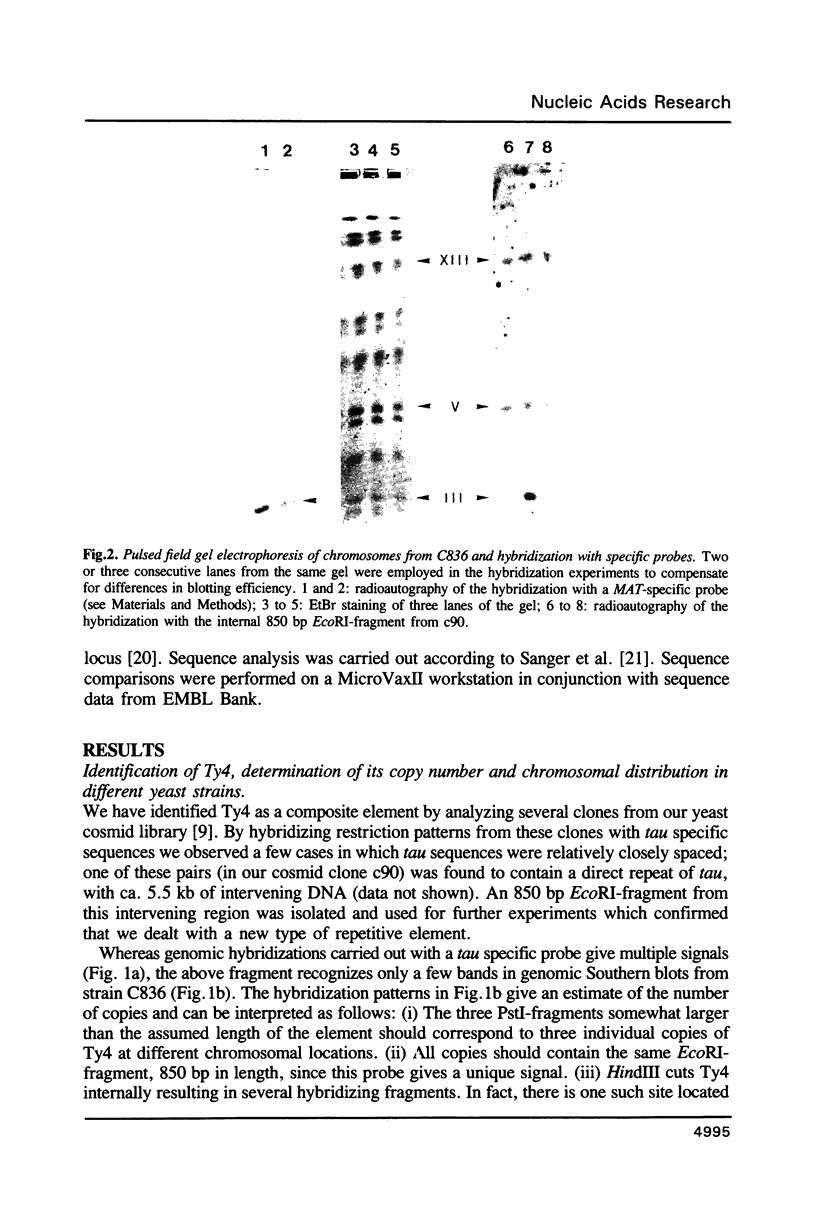
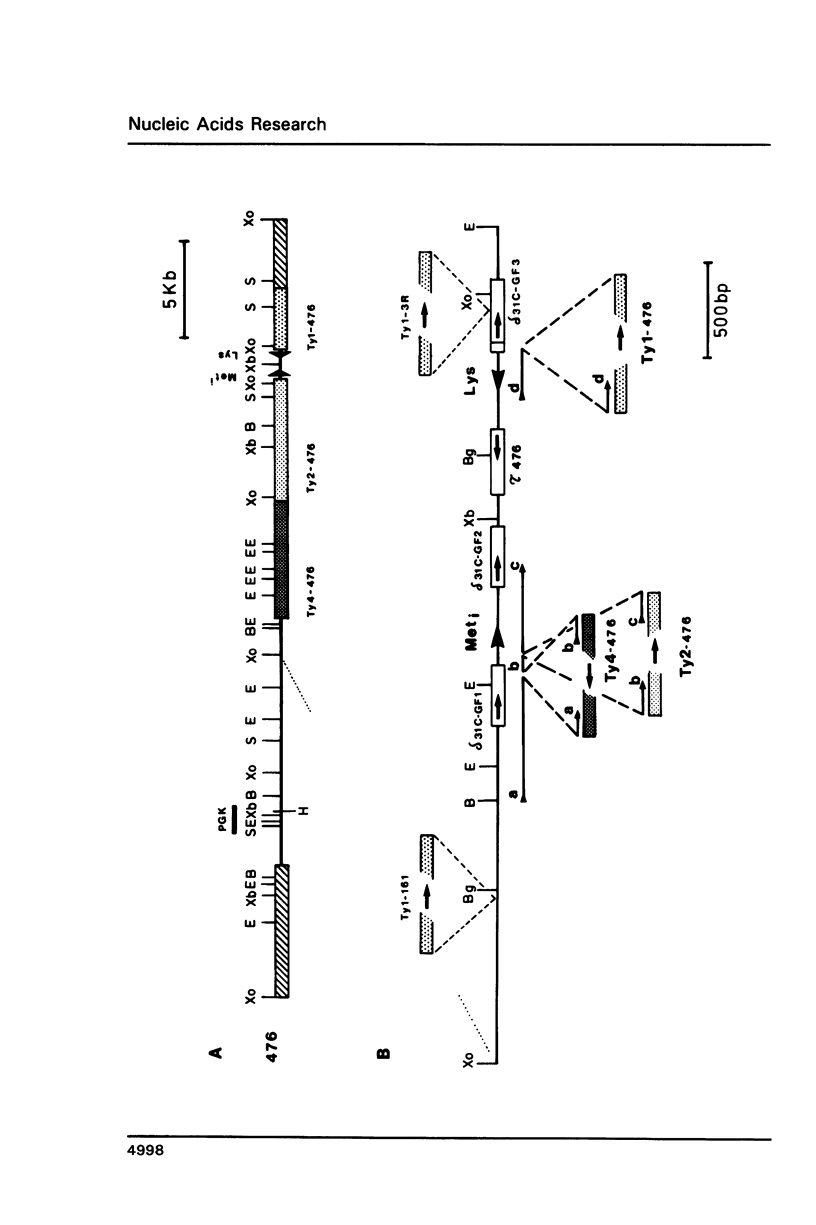
We have identified a composite element, Ty4, in S. cerevisiae that is ca 6.3 kb in length and contains two tau sequences as long terminal repeats. According to hybridization analyses, Ty4 occurs in low but varying copy number (one to four copies) in different yeast strains. By several criteria, Ty4 is a novel type of retroelement which is similar but not related to the other Ty elements in yeast. Two cosmid clones from strain C836 (c90 and c476) carrying individual copies of Ty4 were isolated. By restriction analysis and nucleotide sequence we show that c476 derives from the 'transposition right arm hot spot' of chromosome III [1]. The analysis of c476 revealed that an initiator tRNA(Met) gene is present at this locus and that an unusual concentration of different Ty elements has occurred: in addition to the Ty4, a Ty1 and a Ty2 element were detected in this region, confirming its highly polymorphic character.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Ahlstrom-Jonasson L., Smith M., Tatchell K., Nasmyth K. A., Hall B. D. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981 Nov;27(1 Pt 2):15–23. doi: 10.1016/0092-8674(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G. E., Genbauffe F. S., Cooper T. G. tau, a repeated DNA sequence in yeast. Proc Natl Acad Sci U S A. 1984 May;81(10):2965–2969. doi: 10.1073/pnas.81.10.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J., Bilanchone V. W., Haywood L. J., Dildine S. L., Sandmeyer S. B. A yeast sigma composite element, TY3, has properties of a retrotransposon. J Biol Chem. 1988 Jan 25;263(3):1413–1423. [PubMed] [Google Scholar]
- Eigel A., Feldmann H. Ty1 and delta elements occur adjacent to several tRNA genes in yeast. EMBO J. 1982;1(10):1245–1250. doi: 10.1002/j.1460-2075.1982.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafner J., Robertis E. M., Philippsen P. Delta sequences in the 5' non-coding region of yeast tRNA genes. EMBO J. 1983;2(4):583–591. doi: 10.1002/j.1460-2075.1983.tb01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbauffe F. S., Chisholm G. E., Cooper T. G. Tau, sigma, and delta. A family of repeated elements in yeast. J Biol Chem. 1984 Aug 25;259(16):10518–10525. [PubMed] [Google Scholar]
- Hansen L. J., Chalker D. L., Sandmeyer S. B. Ty3, a yeast retrotransposon associated with tRNA genes, has homology to animal retroviruses. Mol Cell Biol. 1988 Dec;8(12):5245–5256. doi: 10.1128/mcb.8.12.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Nelböck-Hochstetter P., Feldmann H. Nucleotide sequence and characteristics of a Ty element from yeast. Nucleic Acids Res. 1985 Apr 25;13(8):2745–2758. doi: 10.1093/nar/13.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Stucka R., Krieg R., Feldmann H. Analysis of yeast chromosomal regions carrying members of the glutamate tRNA gene family: various transposable elements are associated with them. Nucleic Acids Res. 1988 Nov 25;16(22):10623–10634. doi: 10.1093/nar/16.22.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Hayflick J. S., Chen C. Y., Seeburg P. H., Derynck R. The primary structure of the Saccharomyces cerevisiae gene for 3-phosphoglycerate kinase. Nucleic Acids Res. 1982 Dec 11;10(23):7791–7808. doi: 10.1093/nar/10.23.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsman A. J., Kingsman S. M. Ty: a retroelement moving forward. Cell. 1988 May 6;53(3):333–335. doi: 10.1016/0092-8674(88)90151-1. [DOI] [PubMed] [Google Scholar]
- Nelböck P., Stucka R., Feldmann H. Different patterns of transposable elements in the vicinity of tRNA genes in yeast: a possible clue to transcriptional modulation. Biol Chem Hoppe Seyler. 1985 Nov;366(11):1041–1051. doi: 10.1515/bchm3.1985.366.2.1041. [DOI] [PubMed] [Google Scholar]
- Picologlou S., Dicig M. E., Kovarik P., Liebman S. W. The same configuration of Ty elements promotes different types and frequencies of rearrangements in different yeast strains. Mol Gen Genet. 1988 Feb;211(2):272–281. doi: 10.1007/BF00330604. [DOI] [PubMed] [Google Scholar]
- Rothstein R., Helms C., Rosenberg N. Concerted deletions and inversions are caused by mitotic recombination between delta sequences in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Mar;7(3):1198–1207. doi: 10.1128/mcb.7.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S. B., Bilanchone V. W., Clark D. J., Morcos P., Carle G. F., Brodeur G. M. Sigma elements are position-specific for many different yeast tRNA genes. Nucleic Acids Res. 1988 Feb 25;16(4):1499–1515. doi: 10.1093/nar/16.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Hauber J., Feldmann H. Conserved and non-conserved features among the yeast Ty elements. Curr Genet. 1986;11(3):193–200. doi: 10.1007/BF00420606. [DOI] [PubMed] [Google Scholar]
- Stucka R., Hauber J., Feldmann H. One member of the tRNA(Glu) gene family in yeast codes for a minor GAGtRNA(Glu) species and is associated with several short transposable elements. Curr Genet. 1987;12(5):323–328. doi: 10.1007/BF00405754. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Delta sequences and double symmetry in a yeast chromosomal replicator region. J Mol Biol. 1982 Apr 5;156(2):293–307. doi: 10.1016/0022-2836(82)90330-8. [DOI] [PubMed] [Google Scholar]
- Venegas A., Gonzalez E., Bull P., Valenzuela P. Isolation and structure of a yeast initiator tRNAmet gene. Nucleic Acids Res. 1982 Feb 11;10(3):1093–1096. doi: 10.1093/nar/10.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmington J. R., Green R. P., Newlon C. S., Oliver S. G. Polymorphisms on the right arm of yeast chromosome III associated with Ty transposition and recombination events. Nucleic Acids Res. 1987 Nov 11;15(21):8963–8982. doi: 10.1093/nar/15.21.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]