Abstract
Background
The mechanism of epithelial to mesenchymal transition (EMT) could be adopted by tumor cells for migration and invasion. We have reported that ARCaPE human prostate cancer cells undergo EMT-like changes during xenograft growth in athymic mice.
Methods
In this report, we assessed the extent of EMT by tracking changes in cloned ARCaPE cells expressing red fluorescence protein during successive orthotopic prostate tumor formation. Cancer cells with stromal-like morphology were isolated and examined for EMT-like changes.
Results
EMT-like morphologic and expression changes were detected after one round of in vivo tumor formation. Importantly, when recovered tumor cells were used in second round xenograft tumor formation, a large fraction of ARCaPE cells showed drastic EMT-like changes, with markedly enlarged cell size and divergent cell shapes similar to those of mesenchymal stromal cells. The morphologic change was accompanied by increased growth and metastasis, as tumor incidence increased while red fluorescent tumor cells could be detected from circulating blood, bone marrow, peritoneal ascites, and lung of the tumor-bearing mice. Recovered clones from these samples had lost epithelial markers but many showed activated stromal marker vimentin expression. The EMT appeared permanent since the newly acquired morphology was sustained after continuous passages.
Conclusions
Results from this study demonstrate that through interaction with the host tumor microenvironment, cancer cells acquire cellular plasticity. During xenograft tumor formation and metastasis, a single clone of cancer cells could yield a heterogeneous population, with a substantial number of tumor cells adopting mesenchymal stroma-like phenotypes.
Keywords: Epithelial to mesenchymal transition, orthotopic tumor formation, prostate cancer progression and metastasis, red fluorescence protein
Introduction
Cellular heterogeneity is a common histopathologic feature in prostate cancer. Cancer cells undertake progressive morphologic and behavioral changes during disease progression and metastasis (1-3). Because these changes are often correlated with the malignant state of the disease, investigating the morphologic and behavioral alterations may reveal clues to the mechanism by which tumor cells acquire more aggressive phenotype and metastatic potential.
Epithelial to mesenchymal transition (EMT) is both a morphologic and a behavioral change and is frequently observed in clinical prostate tumor specimens (4). In normal human prostate, for example, luminal epithelial cells unanimously express typical epithelial markers, including E-cadherin (E-cad) (5-11). At the primary site, prostate cancer cells can be stained for the same markers but show an abnormal and sporadic pattern. In metastatic sites, the intensity of immunostaining for epithelial markers is further reduced, and many cancer cells completely lose epithelial expression. Instead, some cancer cells start to express mesenchymal stromal cell markers, such as vimentin (12). As the stromal features may provide cancer cells with migratory and invasive advantages, EMT in tumor cells is a sign of poor prognosis (13,14).
We have used human prostate cancer cell lines in an established mouse prostate xenograft tumor progression model to study the role of EMT in prostate cancer progression and metastasis (15,16). The ARCaPE human prostate cancer cell line (17) was inoculated intracardially into athymic mice for tumor formation. The initial ARCaPE cells display typical epithelial cell morphology and have only limited tumorigenic potential. When recovered by cloning from xenograft tumors and used in another round of inoculation, these cells show dramatically increased tumorigenicity, forming multiple tumors in a shorter period. Importantly, cells recovered from these tumors showed signs of EMT, as detected by loss of epithelial marker expression (15,16). These results indicate that the host tumor microenvironment plays an important role in promoting tumor progression and metastasis, while increased tumorigenic potential is accompanied by EMT of the tumor cells.
In the present study, we used a similar animal model to further examine the involvement of EMT in prostate cancer progression and metastasis, with attention focused on the extent to which ARCaPE tumor cells have undergone EMT. By tracking cancer cells tagged with red fluorescence protein in tumor-bearing mice, we found that after successive xenograft inoculation, a substantial number of tumor cells acquired a mesenchymal stromal phenotype, and these cells could be detected in distant sites from the tumor, and could be cloned for studying the mechanism of cancer progression.
Materials and Methods
Cell culture conditions
All the cells used in this study were cultured in T-medium (Invitrogen, Carlsbad, CA) containing penicillin (100 unites/ml) and streptomycin (100 μg/ml) and 5% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), in a humidified incubator at 37°C with atmospheric air supplemented with 5% CO2.
Establishment of cell lines
Isolation and characterization of the human prostate cancer ARCaPE cell line has been reported (17). To establish ARCaPE clones stably expressing a red fluorescence protein, a eukaryotic expression vector, pAsRed2, encoding a variant AsRed2 Anemonia sulcata red fluorescence protein, was purchased from Clontech (Mountain View, CA). The vector was linearized at the backbone with FspI restriction enzyme, and the blunt ends were destroyed by a 10-minute treatment with exonuclease III. DNA of the treated vector (2 μg) was used for transfection to ARCaPE cells with the GenePORTER reagent following the manufacturer’s recommended protocol (Gene Therapy Systems, San Diego, CA). To select for stably transfected clones, G418 (350 μg/ml) was added 48 hours later for 3 weeks. Cells that survived the selection were subjected to another cloning by limited dilution. Transfected single-cell colonies were identified with red fluorescence microscopy, and amplified for further characterization.
Orthotopic inoculation for in vivo tumor formation and metastasis
Establishment of the mouse model for human prostate cancer progression and metastasis has been reported (15,16). To perform orthotopic inoculation, male athymic mice (NCR nu/nu) between 6 and 8 weeks of age were subjected to anesthesia. After surgical preparation, an abdominal incision was made to expose the mouse prostate. The dorsolateral lobe of the prostate gland was inoculated with 5 × 105 cancer cells in 15 μl of PBS in a calibrated syringe delivered by a 28g needle. After wound closure and recovery from anesthesia, the animals were maintained with ad libitum access to standard lab chow and water.
Tissue specimen collection and tumor cell recovery
Animals bearing orthotopic prostate tumors were euthanized 3 months after the inoculation, or prematurely when tumor burden reached the allowed tumor size set by the IACUC (2 cm in the longest dimension). After aseptic preparation, the animal was subjected to thorough necropsy to inspect tissue and organs for metastasis.
Orthotopic tumor was dissected under sterile conditions. A cube of the tumor (≈ 0.125 cm3) was used for preparation of single cell suspensions by dicing with a razor blade and Dispase II digest (Roche Applied Science, Indianapolis, IN) at 37°C for 1 hour. A fraction (5 × 105) of the cells were plated to a 15 cm culture dish for colony formation. To culture metastatic cancer cells from bone marrow, tibias were dissected and tibial bone marrow cells were collected by marrow cavity flushing with 5 ml PBS. Peritoneal ascites were collected and immediately diluted into 5 ml PBS before coagulation took place. The cell samples were washed 3 times in PBS and were subjected to cell culture. To culture circulating tumor cells, left ventricle blood (≈ 250 μl) was collected under sterile conditions without anticoagulant. The blood was dripped immediately onto a 6 cm culture dish to form individual droplets. After the blood was coagulated at room temperature for 10 minutes, cell culture medium was added to the dish to cover the droplets. Alternatively, the blood was washed in PBS for three times, and whole blood cells were subjected to culture. To detect and recover cancer cells in the lung, the three right lobes were dissected and cut into small dice with a sterile razor blade. The sample was washed three times in PBS and subjected to Dispase II digestion. The preparation was washed three times in PBS and cells were subjected to cell culture. In all the ex vivo cultures G418 (150 μg/ml) was added to remove cells of the mouse host.
Intracardiac inoculation for in vivo tumor formation and metastasis
The protocol used for intracardiac inoculation has been reported (15). Briefly, 5 × 105 cells in 50 μl PBS was injected to left ventricle of male athymic mice. After recovery from anesthesia, the animals were monitored for tumor formation for 6 months. Cancer-related death was used as end point of the study. X-ray radiography was used to detect skeletal lesion. After euthanasia, thorough necropsy was performed and left ventricle blood (250 μl) was cultured for detection of tumor cells under red fluorescence microscope. Tissues and organs suspected for tumor growth were dissected and fixed in paraformaldehyde for histopathologic confirmation.
Cell proliferation assay
The protocol used for assaying growth rate has been reported (15). Briefly, 100 μl cells (1 ×106/ml) were seeded to 96-well plates in triplicate. After cultured for 48 hours, the cells were subjected to MTT assay using the reported protocol.
Western blotting
Whole cell lysates were prepared from 1 × 106 tumor cells on a 10 cm culture dish in 0.5 ml Triple Detergent cell lysis buffer (18), containing 1x protease inhibitor cocktail (Roche Applied Science) and 100 μM phenylmethanesulphonyl fluoride (PMSF). From each sample, 20 μg total protein was fractionated on a 4-20% gradient gel (BioRad, Hercules, CA). The proteins were blotted onto nitrocellulose membrane and subjected to antibody detection using our reported protocol (19). Antibodies to E-cad, vimentin, cytokeratin 18, β-actin, and all the secondary antibodies conjugated with horseradish-peroxidase were purchased (Santa Cruz Biotechnology, Santa Cruz, CA). Specific signals were detected with the ECL plus western blotting detection kit (GE Healthcare Bio-Sciences, Piscataway, NJ).
Fluorescence microscopy and microphotography
All fluorescence observations were carried out with a Nikon E300 inverted fluorescence microscope, and images were captured with a MagnaFire digital microscope camera (Optronics, Goleta, CA). To facilitate comparison, all the images were taken at least 10 minutes after the excitation source was ignited, and all the images were taken with the following settings: 40x magnification with 8.3 seconds exposure, 100x magnification with 4.15 seconds exposure, and 200x magnification with 2.075 seconds exposure.
Results
Appearance of stromal cell-like clones in ARCaPE tumors
We previously reported that although ARCaPE human prostate cancer cells initially had limited ability for in vivo tumor formation, these cells could acquire additional malignant potential through successive implanting and in vivo xenograft growth (15,16). The increased malignancy was accompanied by EMT, as the recovered clones from xenograft tumors showed mesenchymal stromal morphology, decreased expression of epithelial markers (i.e., E-cad, CK-18, and CK-19) and increased expression of a mesenchymal stromal marker (i.e., vimentin). EMT seemed to signal the increased malignant potential of cancer cells.
In these studies, the recovered clones were isolated from xenograft tumors based on morphologic similarity to the parental cells, to ensure that the clones were of the ARCaPE lineage and not contaminants of the mouse host. We noticed during the isolation that a substantial number of clones formed in the ex vivo culture showed atypical morphologies, divergent from both the parental ARCaPE and normal cells of the mouse host, but with similarity to mesenchymal stromal cells. When clones recovered from a xenograft tumor were implanted orthotopically to six athymic mice, for instance, atypical cells with stromal morphology were seen in all the prostate tumors by ex vivo culture (Figure 1). Repeated experiments confirmed that the appearance of atypical cells was a common phenomenon in ARCaPE xenograft tumors.
Figure 1. Atypical cells in ARCaPE xenograft tumors.
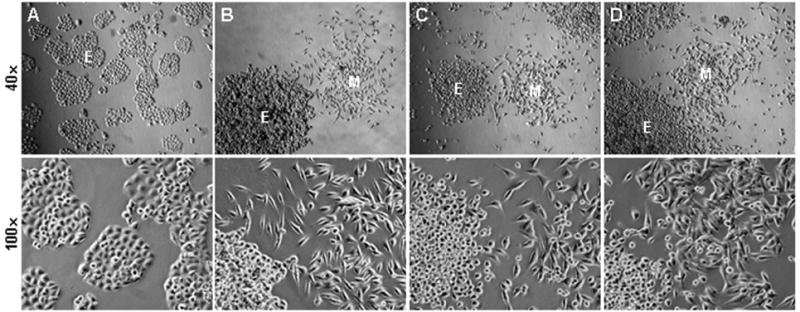
Panel A shows the morphology of the ARCaPEBM clone, which was isolated from an ARCaPE tibial bone metastasis (15), based on morphologic similarity to the parental cells. In this experiment, ARCaPEBM cells were implanted to mouse prostate for orthotopic tumor formation. When the orthotopic tumors were examined in low density ex vivo culture, many colonies displayed atypical morphologies, divergent from parental ARCaPEBM cells (A) and mouse host cells (data not shown), but similar to mesenchymal stromal cells. Representative morphologies of the colonies are shown in panels B, C, and D. Colonies with epithelia-like morphologies are marked with E, and colonies with mesenchymal stroma-like morphologies are marked with M. In each example, a low magnification image (40x) is used to show differences between colonies, and a higher magnification (100x) image to show differences in cellular morphology and cell size.
Tracking ARCaPE tumor cells in vivo in athymic mice
To determine the origin of these cells and assess the extent of EMT of the tumor cells, we tagged the ARCaPE used in xenograft tumor formation with an AsRed2 red fluorescence protein to distinguish the cancer from the host cells. Two measures were taken to ensure that the red fluorescence protein was stably expressed in the ARCaPE cells. Firstly, the DNA construct used in the transfection was linearized and both ends destroyed by exonuclease III. Used in a ligation reaction and transformation to competent E. coli cells, the treated DNA did not yield any colonies (data not shown), confirming that the treated DNA vector could not be recircularized. Secondly, after introducing the DNA by transfection, ARCaPE clones were subjected to a vigorous antibiotic selection (G418, 400 μg/ml, for 8 passages). Subsequently, red fluorescent ARCaPE clones were cultured continuously for more than 30 passages without antibiotic selection, and no loss of the AsRed2 expression was observed. Following the validation, a representative clone, ARCaPE-R1 at passage 5 (Figure 2A), was used in this study.
Figure 2. EMT-like changes in ARCaPE cells after one round of orthotopic tumor formation.
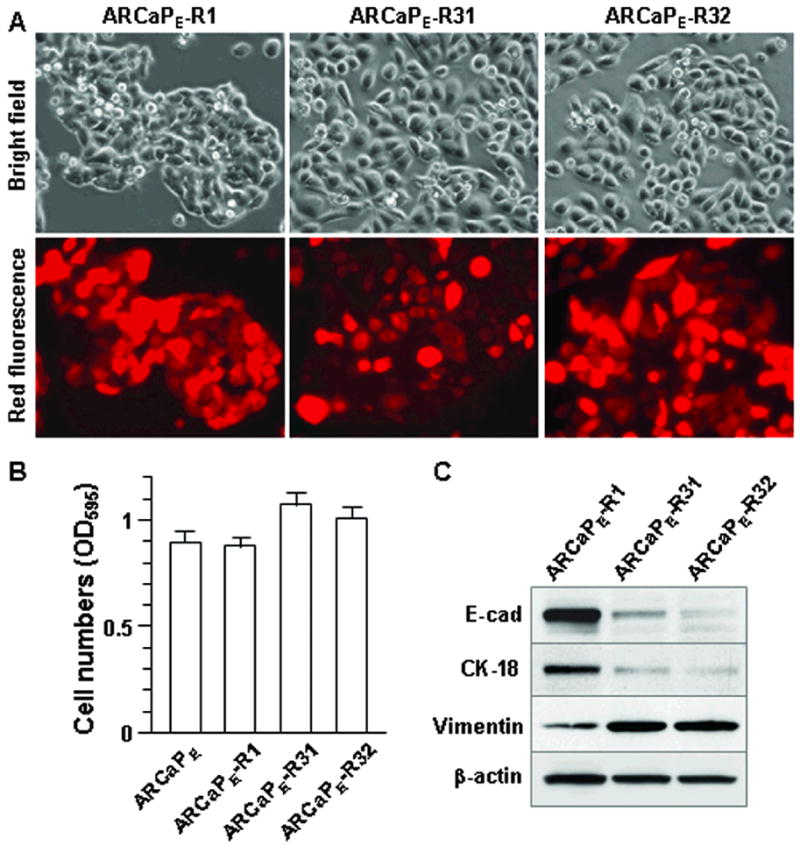
Following orthotopic tumor formation with red fluorescent ARCaPE-R1 cells, prostate tumors from athymic mice were subjected to ex vivo culture, and representative clones ARCaPE-R31 and ARCaPE-R32 were recovered. A, morphologic changes in cultured tumor cells. Compared to parental ARCaPE-R1, the recovered tumor cells display increased size with few intercellular junctions. Microphotographs were taken at 200x magnification. For the same view, bright field (upper) and red fluorescence (lower) photographs are shown. B, increased growth rate of the cultured tumor cells as determined by MTT assay. The result is representative of two experiments with passages 4 and 7 of the cloned cells, respectively. C, signs of EMT as determined by western blotting. The result is representative of two experiments with passages 4 and 7 of the cloned cells.
Cells of the ARCaPE-R1 clone were inoculated orthotopically to the prostates of athymic mice. In the first round of orthotopic inoculation, only 2 of the 6 subject mice presented with palpable tumors at 3 months. Necropsy revealed that these animals bore prostate tumors in sizes of 1.4 cm and 1.7 cm in the longest dimension. Neither animal showed formation of peritoneal ascites. A culture of left ventricular blood and tibial bone marrow did not yield any red fluorescent colonies. The lung, examined under fluorescence microscope and cultured ex vivo, showed no red fluorescent cells. Based on these results, we concluded that similar to our previous report on the parental ARCaPE (15,16), the ARCaPE-R1 cells had limited tumorigenic and metastatic potential.
EMT-like changes in ARCaPE cells after one round of xenograft tumor formation
We recovered red fluorescent cells in the orthotopic tumor by low density ex vivo culture. Red fluorescent clones were isolated from cells of the mouse host by treatment with G418, which removed cells that were not transfected with AsRed2 red fluorescence protein. The red fluorescent cells showed relatively uniform cell size and morphology, but were distinguishable from the parental red fluorescent ARCaPE-R1 cells (Figure 2A). Different from the pentagonal cell shape, tight intercellular junction, and cobblestone-like organization of parental cells, recovered tumor cells exhibited a flattened and enlarged shape with longitudinal extension. Intercellular junctions were reduced, as most cells appeared separated from each other. For further analyses, two representative clones, ARCaPE-R31 and ARCaPE-R32, were picked respectively from ex vivo cultures of the two orthotopic tumors (Figure 2A). The growth rate of ARCaPE-R31 and ARCaPE-R32 was increased (Figure 2B), but there was little change in the capability of migration and invasion compared to parental ARCaPE-R1 cells (data not shown). In western blotting, these clones were found to express much less E-cad and CK-18, but increased vimentin expression (Figure 2C), suggesting EMT. These results indicated that after one round of in vivo tumor formation, most of the ARCaPE cells had undergone EMT.
Drastic EMT-like changes in ARCaPE cells after second round xenograft tumor formation
The two representative clones, ARCaPE-R31 and ARCaPE-R32, were used for a second round of orthotopic inoculation. Each of the clones was re-inoculated in 6 athymic mice. The second round of inoculation resulted in markedly increased tumor formation. In both cases, all the 6 animals were found to bear orthotopic tumors. The tumor formation took a significantly shorter period than the first round inoculation. Following the second round of inoculation, for example, ARCaPE-R31 tumor burden reached 2 cm in the longest dimension in an average of 66.1±7 days. Importantly, when examined for metastasis in distant sites, all the animals were found to have red fluorescent cells in the blood, bone marrow, and lung (Figure 3A). In addition, all the animals developed peritoneal ascites, with the fluid volume ranging from 0.6 ml to 3.7 ml, and all the ascites samples contained red fluorescent cells (Figure 3A).
Figure 3. Cellular heterogeneity and metastasis of ARCaPE orthotopic tumors after re-inoculation.
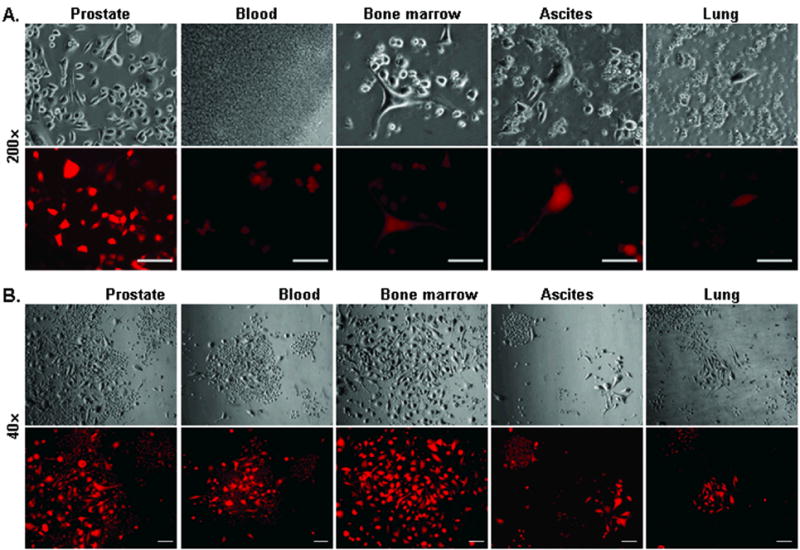
Following the first round orthotopic tumor formation, cloned ARCaPE-R31 cells were re-inoculated orthotopically into athymic mice. A, detection of metastatic tumor cells. After euthanasia due to tumor burden, samples of the prostate, blood, bone marrow, ascites, and lung were used in ex vivo culture of tumor cells. Similar results were obtained when animals bearing ARCaPE-R32 tumors were examined. All microphotographs were taken at 200x magnification, 24 hours into the culture. For each field, photos of the bright field (upper) and red fluorescence (lower) are shown. Bar = 100 μm. B, heterogeneity of the tumor cells. In this presentation, microphotographs of ARCaPE-R31 tumor cells from orthotopic tumor and metastasis sites were taken after 2 weeks of ex vivo culture. All the microphotographs are at 40x magnification to show cell size differences between colonies and between cells within the same colony. For each field, photos of the bright field (upper) and red fluorescence (lower) are shown. Bar = 100 μm.
In order to assess the changes in the ARCaPE cells, we performed ex vivo culture of red fluorescent cells from the orthotopic tumor, left ventricular blood, tibial bone marrow, ascites, and lung, using samples from two mice inoculated with ARCaPE-R31 and ARCaPE-R32 cells, respectively. G418 (150 μg/ml) was added to remove cells of the mouse host.
A prominent feature of all the samples cultured was the appearance of marked heterogeneity in tumor cell morphology. We found that in each culture there were frequent colonies with very large red fluorescent cells (Figure 3B). Whereas an average parental ARCaPE cell measured 34 μm in diameter, large cells of the ex vivo culture could reach 68 μm ×280 μm, similar to large stromal cells in shape and size. Most of these cells were seen as individual cells, not forming intercellular junctions with adjacent cells. Equally prominent was heterogeneity of cellular morphology within a single colony, where cells could have dramatically different sizes (Figure 3B). We tried to isolate clones with a homologous cellular morphology from two of such colonies by limited dilution. In each case, resultant clones always contained cells with heterogeneous cellular morphology and varied cell sizes. Although periodical observation suggested that cells in these clones were continuously changing their morphology, detailed investigation remains to be carried out to confirm this observation. Based on these observations, we concluded that metastatic tumor cells displayed high levels of cellular plasticity (20), and the plasticity was acquired through interacting with the host tumor microenvironment.
This conclusion was supported by control experiments, in which we examined both the parental ARCaPE cells and the red fluorescent ARCaPE-R1 clone used in the initial inoculation to see whether these cells spontaneously change their morphology during the extended cloning process. In experiments to clone 50 individual cells with limited dilution, all the single colonies from both cell lines retained their small cell size and cobblestone-like organization, and none of the colonies contained large stromal-like cells (data not shown). The appearance of large stroma-like cells in the ARCaPE cell lineage is thus a phenomenon specific to xenograft tumor formation.
To estimate the frequency of colonies of large cells, we examined the two cultures of orthotopic tumors by counting colonies of large cells in 6 randomly chosen 1 cm2 areas on a 10 cm dish under low magnification (40x). In the ARCaPE-R31 orthotopic culture, 214 of the 1260 colonies (17%) contained large cells, and in ARCaPE-R32 orthotopic culture, 192 of the 800 colonies (24%) contained large cells. Frequent large cell colonies were also seen in ex vivo cultures of the blood, bone marrow, ascites and lung. These observations indicate that after a second round of orthotopic inoculation, many of the tumor cells have acquired a new morphology completely different from parental ARCaPE and from cells isolated after the first round of inoculation.
Isolation and characterization of stromal cell-like ARCaPE clones
To isolate the fluorescently red tumor cells for further analysis, we picked 3 clones from each ex vivo culture of the five samples from the mouse bearing an ARCaPE-R31 orthotopic tumor. Cells from a single colony of less than 100 cells were picked with the cloning disk method, placed in 40 ml of culture medium, and cloned by limited dilution. A representative clone was then picked from each cloning for further study.
The resultant clones from each sample showed diversified morphologies (Figure 4). Two of the three clones from the orthotopic prostate tumor (clones 1 and 3), for example, were long fibroblast-like cells, while the other one was much smaller (clone 2). The three clones derived from the blood all contained large cells of spindle-like shape (clones 4, 5 and 6), as did clones from bone marrow (clones 7 and 9) and ascites (clones 10 and 11). Distinct from all of the above, other clones were seen with large, round, and flat cell shapes, including ascites clone 12 and two isolated from mouse lung (clones 13 and 14), which were different from clone 15 from the same organ. Importantly, even though these cells were all giving out red fluorescence, indicating their ARCaPE origin, none of the clones kept morphology reminiscent of the parental cell. We concluded that after repeated inoculation, many ARCaPE cells had undergone substantial EMT.
Figure 4. Mesenchymal stroma-like clones recovered from re-inoculated xenograft tumors.
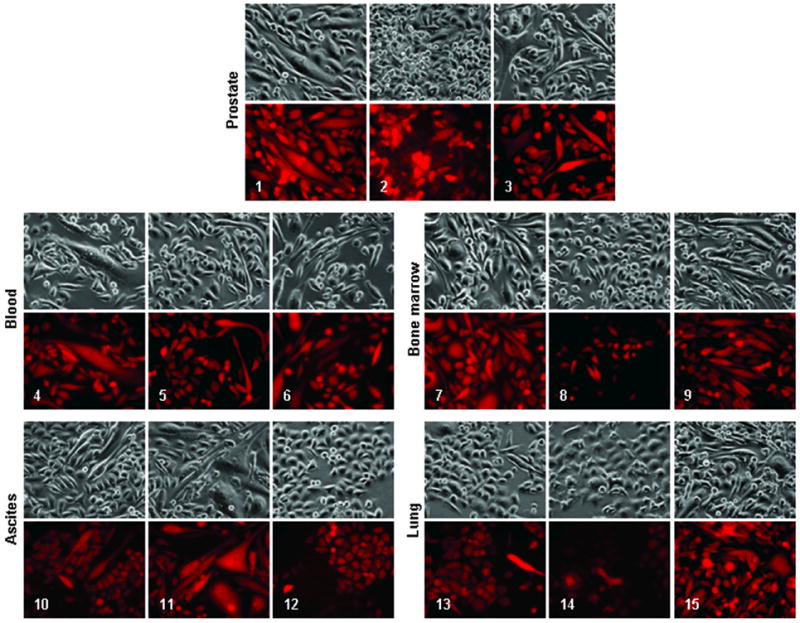
In this experiment, ARCaPE-R31 xenograft tumor cells were cloned from ex vivo culture. These clones were isolated from cultures of the orthotopic tumor (clones 1, 2, and 3), left ventricle blood (clones 4, 5, and 6), tibial bone marrow (clones 7, 8, and 9), peritoneal ascites (clones 10, 11, and 12), and lung (clones 13, 14, and 15). All the microphotographs are at 200x magnification. For each field, photos of the bright field (upper) and red fluorescence (lower) are shown.
To assess whether the EMT observed is transient or permanent and irreversible, we cultured these clones continuously for 20 passages. This experiment demonstrated that for each isolated clone, the cellular morphology in each passage was similar to the initial morphology of the clone, and none of the clones had adapted the epithelial morphology of the parental ARCaPE cells (data not shown). It appeared that the EMT in ARCaPE clones isolated from in vivo xenograft tumors is permanent and irreversible.
Phenotypic characterization of the stromal cell-like ARCaPE clones
We performed western blotting to determine whether the isolated clones maintained EMT-like gene expressions like the parental ARCaPE-R31 cells. Most of the clones expectedly showed markedly reduced expressions of epithelial markers E-cad and CK-18, and most expressed higher levels of mesenchymal stromal marker vimentin (Figure 5), confirming that these cells have retained properties of EMT. On the other hand, two clones (clones 2 and 10) were found unexpectedly expressing higher levels of E-cad than the other clones (Figure 5) or the parental ARCaPE-R31 cells (Figure 2C). Additional investigation is needed to determine whether these cells have undertaken mesenchymal to epithelial transition (MET). Another two clones (clones 13 and 14) isolated from the mouse lung lost vimentin expression (Figure 5), even though these cells did not express epithelial markers either. As this finding may be explained by increased heterogeneity of the tumor cells, further investigation is needed to determine whether these clones have undergone EMT.
Figure 5. EMT marker expression in clones recovered from re-inoculated xenograft tumors.
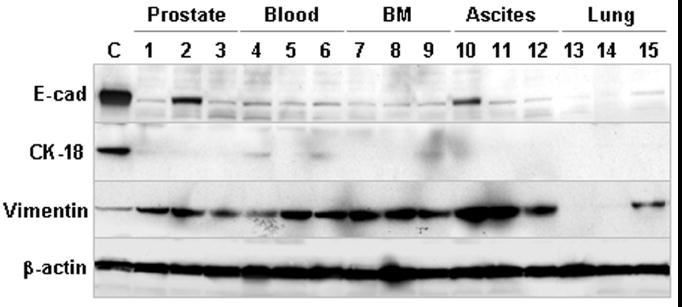
Established clones from ARCaPE-R31 orthotopic tumor and metastases were subjected to western blotting analyses. Tissue origins of the clones are depicted on the top (BM, bone marrow). ARCaPE-R1 cells were used as control (C). The results are representative of two experiments with passages 5 and 12 of the clones, respectively.
Increased tumorigenic potential in the stromal cell-like ARCaPE clones
We evaluated increased malignancy of the isolated ARCaPE clones. Cells from representative clones (clones 1, 3, 6, and 7, Figure 4) were inoculated intracardially to athymic mice, and cancer-related death was used as a measure of the malignancy. The four clones all showed increased malignancies (Table 1). Whereas inoculation of the parental ARCaPE-R1 cells caused a 16.7% incidence of cancer-related death within 180 days, inoculation of the stroma-like clones resulted in 100% death in less than 80 days (Table 1). The moribund animals all displayed signs of severe cachexia. Necropsy revealed scattered tumor lesions in skeleton, lymph nodes, and kidney, while most animals have splenomegaly. In addition, red fluorescent tumor cells were detected in cultures of left ventricle blood (data not shown). These findings suggested that the mice died of multiple metastases.
Table 1.
Increased metastatic potential of the stromal cell-like ARCaPE clones.
| ARCaPE-R1 | Clone 1 | Clone 3 | Clone 6 | Clone 7 | |
|---|---|---|---|---|---|
| Group size | 12 mice | 6 mice | 6 mice | 6 mice | 6 mice |
| Cancer-related death | 2/12* | 6/6 | 6/6 | 6/6 | 6/6 |
| Days of survival (Mean±SD) | 136±11.3** | 72.5±2.3 | 79.5±5.4 | 77.5±6.0 | 70.3±4.1 |
Animals were monitored for tumor-related death for 6 months.
Calculated using data from the two cancer-related deaths.
Discussion
Human prostate cancer ARCaPE is a unique cell line for studying EMT (16). Because of the tight correlation between EMT and tumor malignant potential, detailed investigation of ARCaPE cells may lead to a mechanistic elucidation of molecular events underlying EMT in prostate cancer progression and metastasis.
In the present study, we used the previously described mouse model of human prostate cancer progression and metastasis (15,16) to assess the extent of EMT in an ARCaPE clone following successive orthotopic inoculation of ARCaPE cells. We tagged the cancer cells with red fluorescence protein to sensitively detect tumor cell metastasis in the mouse host, and to identify cancer cells that adopted significant morphologic changes. This study revealed that through successive in vivo tumor formation and metastasis, substantial numbers of ARCaPE cells underwent drastic changes, adopting a mesenchymal stromal morphology and expression profile (Figures 3, 4 and 5). Assisted by red fluorescence tracking, we isolated representative clones and demonstrated that ARCaPE cells with stromal morphology have altered gene expression and increased malignancy. The study has potentially significant implications for the role of EMT in prostate cancer progression and metastasis.
This study revealed that EMT is a general phenomenon of ARCaPE tumor cells during in vivo xenograft growth. In a previous study (15), we noticed the presence of large cells bearing stromal morphologies in ARCaPE tumors (Figure 1), but were not sure whether those cells were derived from the ARCaPE lineage, since cells of the mouse host can be transformed by tumor cells (21). In the present study, we used red fluorescence protein tagging to confirm that these cells are descendents of the ARCaPE cells. Moreover, red fluorescence protein tagging revealed that substantial numbers of tumor cells have undergone EMT. Especially after a second round of inoculation, from 17% to 24% of the tumor cells were seen with mesenchymal stromal features (Figure 4). EMT is thus a trend for ARCaPE tumor cells when subjected to in vivo growth and metastasis.
In clinical specimens, prostate cancer cells in the transition state of EMT are difficult to identify and the relevance of EMT to prostate cancer is controversial (14). On the other hand, histopathologic analyses demonstrate that prostate tumor cells often lose epithelial properties and adapt mesenchymal stromal gene expression. Loss of the epithelial marker E-cad, for example, is a common observation in prostate cancer specimens (5-11), while enhanced expression of the stromal cell intermediate filament protein vimentin is frequently seen (12,13). Loss of E-cad and activated vimentin expressions are the two most informative markers of EMT (22). Moreover, the EMT-like phenotype is correlated to progression and metastasis (14-16,19), suggesting that EMT is an integral aspect of prostate cancer progression. In this study, we documented the progressive conversion of a clone of ARCaPE human prostate cancer cells from epithelial to mesenchymal stroma-like cells (Figures 2, 3, and 4). Results from this study confirmed that prostate cancer cells have the capability of undertaking an EMT-like process. Most importantly, by evaluating isolated clones in athymic mice upon intracardiac inoculation, we demonstrated that cancer cells with EMT-like properties had increased tumorigenic potency (Table 1).
The tumor microenvironment plays a dominant role in determining the fate of tumor cells (23-31). During prostate cancer progression and metastasis, infiltrating tumor cells come into direct contact with mesenchymal stromal cells. Tumor cells may have to be assisted by stromal cells for growth and survival by cancer-stromal interaction, and adopting stromal properties through EMT may provide tumor cells with advantages in migration, invasion and metastasis. The red fluorescent ARCaPE-R1 cells used in this study were from a recently established clone, and were mostly morphologically homogeneous (Figure 2). When subjected to cloning again in vitro, all the clones maintained the epithelial morphology. In contrast, during xenograft tumor formation and metastasis in vivo, these cells would frequently adopt stromal morphologies, which became more pronounced after successive inoculation. This study indicated an obligatory role of the host tumor microenvironment in promoting EMT-like changes. Further investigation is needed to define how interactions with the tumor microenvironment could result in such drastic morphologic and behavioral changes in cancer cells.
Results from this study suggest that mesenchymal stromal features are fixed permanently to xenograft tumor cells. ARCaPE cells have the tendency to undergo EMT following a variety of extracellular and intracellular cues. We have reported that soluble factors, including EGF, IGF-1, TGFβ1, and β2-microglubulin, could induce EMT in ARCaPE cells by receptor-mediated signal transduction (16,32,33). The factor-induced EMT, however, was mostly transient and reversible, because the treated cells would resume epithelial morphology once the inducing factor was removed. ARCaPE cells can also commit to EMT by endogenous and genetic changes. We have reported that stable overexpression of a constitutively active SNAIL mutant leads to EMT in ARCaPE cells (19), and the same is true with stable overexpression of LIV-1 (16). Importantly in both cases, the genetic manipulations resulted in permanent and irreversible EMT in these cells. Since cancer cells can acquire genomic and genetic changes through interaction with mesenchymal stromal cells (34-36), it is likely that the permanent EMT observed in this study is caused by genetic changes. The representative clones have been isolated from ARCaPE tumors and identification of the causal factors is currently underway.
Finally, this study demonstrated that fluorescence protein tagging is a sensitive technique to track xenograft tumor cells metastasis. Following a second inoculation, orthotopic tumors showed increased growth rates and distant metastasis. Due to tumor burden, the animals were sacrificed before metastatic bone lesions could be detected by conventional methods (data not shown). Nonetheless, with red fluorescence protein tagging, metastatic tumor cells could be detected and cloned from blood, bone marrow, ascites, and lung because tumor cells could be detected and distinguished from mouse host cells by red fluorescence (Figures 3 and 4). Similarly, we expect that red fluorescence may facilitate quantification and comparison of metastatic cells in these tissues. We are currently testing to quantify these cells with flow cytometric methods.
Conclusions
By tracking the fate of cancer cells with red fluorescence protein, this work demonstrated that human ARCaPE prostate cancer cells undergo EMT-like changes, which in turn render cancer cells with increased metastatic potential. This work established an in vivo animal model for elucidating the role of cancer-stromal interaction in promoting EMT during prostate cancer progression and metastasis.
Acknowledgments
This work is supported by research grants R21CA112330, PC040578, CA132388 (RXW), and CA98912-02 (LWKC).
References
- 1.Poste G, Greig R. On the genesis and regulation of cellular heterogeneity in malignant tumors. Invasion Metastasis. 1982;2(3):137–176. [PubMed] [Google Scholar]
- 2.Rubin H. The significance of biological heterogeneity. Cancer Metastasis Rev. 1990;9(1):1–20. doi: 10.1007/BF00047585. [DOI] [PubMed] [Google Scholar]
- 3.Smith HS, Wolman SR, Hackett AJ. The biology of breast cancer at the cellular level. Biochim Biophys Acta. 1984;738(3):103–123. doi: 10.1016/0304-419x(84)90009-x. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 5.De Marzo AM, Knudsen B, Chan-Tack K, Epstein JI. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology. 1999;53(4):707–713. doi: 10.1016/s0090-4295(98)00577-9. [DOI] [PubMed] [Google Scholar]
- 6.Giroldi LA, Schalken JA. Decreased expression of the intercellular adhesion molecule E-cadherin in prostate cancer: biological significance and clinical implications. Cancer Metastasis Rev. 1993;12(1):29–37. doi: 10.1007/BF00689788. [DOI] [PubMed] [Google Scholar]
- 7.Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Isaacs WB. Reduction of E-cadherin levels and deletion of the alpha-catenin gene in human prostate cancer cells. Cancer Res. 1993;53(15):3585–3590. [PubMed] [Google Scholar]
- 8.Otto T, Rembrink K, Goepel M, Meyer-Schwickerath M, Rubben H. E-cadherin: a marker for differentiation and invasiveness in prostatic carcinoma. Urol Res. 1993;21(5):359–362. doi: 10.1007/BF00296837. [DOI] [PubMed] [Google Scholar]
- 9.Rembrink K, Otto T, Goepel M, Enzawi SM, Rubben H. E-cadherin: expression of the epithelial cell-cell-adhesion molecule in prostatic carcinoma and normal prostate. Investig Urol (Berl) 1994;5:24–27. [PubMed] [Google Scholar]
- 10.Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, Debruyne FM, Schalken JA. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54(14):3929–3933. [PubMed] [Google Scholar]
- 11.Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, Debruyne FM, Isaacs WB. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52(18):5104–5109. [PubMed] [Google Scholar]
- 12.Wernert N, Seitz G, Achtstatter T. Immunohistochemical investigation of different cytokeratins and vimentin in the prostate from the fetal period up to adulthood and in prostate carcinoma. Pathol Res Pract. 1987;182(5):617–626. [PubMed] [Google Scholar]
- 13.Zhau HE, Pisters LL, Hall MC, Zhao LS, Troncoso P, Pollack A, Chung LW. Biomarkers associated with prostate cancer progression. J Cell Biochem Suppl. 1994;19:208–216. [PubMed] [Google Scholar]
- 14.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, Chung LW, Zhau HE. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66(15):1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 16.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu ZR, Zhou BP, Huang WC, Chung LW. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25(6):601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang H, Kao C, Sang QA, Pathak SJ, Chung LW. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A. 1996;93(26):15152–15157. doi: 10.1073/pnas.93.26.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan C, editor. Molecular Cloning, A Laboratory Manual. 2. Vol. 3. Cold Spring Harbor Laboratory Press; 1989. p. 18.32. [Google Scholar]
- 19.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, Marshall FF, Zhau HE, Chung LW. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18(8):858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 20.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4(8):657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 21.Pathak S, Nemeth MA, Multani AS, Thalmann GN, von Eschenbach AC, Chung LW. Can cancer cells transform normal host cells into malignant cells? Br J Cancer. 1997;76(9):1134–1138. doi: 10.1038/bjc.1997.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie NC, Raz E. Found in translation: A new player in EMT. Dev Cell. 2006;11(4):434–436. doi: 10.1016/j.devcel.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Caligaris-Cappio F, Gregoretti MG, Merico F, Gottardi D, Ghia P, Parvis G, Bergui L. Bone marrow microenvironment and the progression of multiple myeloma. Leuk Lymphoma. 1992;8(1-2):15–22. doi: 10.3109/10428199209049813. [DOI] [PubMed] [Google Scholar]
- 24.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173(1):10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 25.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264(1):169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 26.Heppner GH, Miller FR. The cellular basis of tumor progression. Int Rev Cytol. 1998;177:1–56. doi: 10.1016/s0074-7696(08)62230-5. [DOI] [PubMed] [Google Scholar]
- 27.Nicolson GL. Generation of phenotypic diversity and progression in metastatic tumor cells. Cancer Metastasis Rev. 1984;3(1):25–42. doi: 10.1007/BF00047691. [DOI] [PubMed] [Google Scholar]
- 28.Price JE. Host-tumor interactions in the progression of breast cancer metastasis. In Vivo. 1994;8(1):145–154. [PubMed] [Google Scholar]
- 29.Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70(9-10):506–521. doi: 10.1046/j.1432-0436.2002.700905.x. [DOI] [PubMed] [Google Scholar]
- 30.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11(2):97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 31.Yuan J, Glazer PM. Mutagenesis induced by the tumor microenvironment. Mutat Res. 1998;400(1-2):439–446. doi: 10.1016/s0027-5107(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 32.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O’Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68(7):2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 33.Wu D, Zhau HE, Huang WC, Iqbal S, Habib FK, Sartor O, Cvitanovic L, Marshall FF, Xu Z, Chung LW. cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: implication in human prostate cancer bone metastasis. Oncogene. 2007;26(35):5070–5077. doi: 10.1038/sj.onc.1210316. [DOI] [PubMed] [Google Scholar]
- 34.Chung LW, Huang WC, Sung SY, Wu D, Odero-Marah V, Nomura T, Shigemura K, Miyagi T, Seo S, Shi C, Molitierno J, Elmore J, Anderson C, Isotani S, Edlund M, Hsieh CL, Wang R, Shehata B, Zhau HE. Stromal-epithelial interaction in prostate cancer progression. Clin Genitourin Cancer. 2006;5(2):162–170. doi: 10.3816/CGC.2006.n.034. [DOI] [PubMed] [Google Scholar]
- 35.Rhee HW, Zhau HE, Pathak S, Multani AS, Pennanen S, Visakorpi T, Chung LW. Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cell Dev Biol Anim. 2001;37(3):127–140. doi: 10.1290/1071-2690(2001)037<0127:PPAGCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, Lee JK, Amin MB, Lyles R, Johnstone PA, Marshall FF, Chung LW. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68(23):9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]


