Abstract
Microglia, the primary resident immune cell population in the CNS, react to signals of injury or infection and produce inflammatory cytokines, chemokines, and reactive oxygen species, many of which can be neurotoxic in large quantities. Indeed microglial hyperactivation is thought to contribute to the pathology of many neurodegenerative disorders as well as ischemic and traumatic brain injuries, suggesting that agents with the capacity to target microglial activities may be beneficial for treating neuronal injury. In this review, we discuss two seemingly unrelated microglial receptor signaling systems that potently modulate many microglial properties; purinergic P2 and estrogen receptors. Purinergic receptors regulate key microglial functions, including their production of pro-inflammatory cytokines, neurotrophic factors, migration, phagocytosis and chemotaxis. Many of these same endpoints are also altered by estrogen receptor signaling in microglia. Here we summarize the current microglial research in both receptor areas, particularly as it relates to ischemic and traumatic CNS injuries. We provide evidence from our own laboratory of potential cross-talk between these receptor systems and discuss evidence indicating that both purinergic and estrogen receptors may represent useful therapeutic targets for the treatment of CNS disorders.
Introduction
Microglia comprise 5–20% of total cells in the brain [1, 2]. While first identified in the very early days of neuroanatomy, and described by Nissl, Ramón Y Cajal, and Rio-Hortega in the late 1800s and early 1900s, microglia were largely ignored until the last two decades [2, 3]. Distributed throughout the parenchyma, microglia are the only resident immune cells in the CNS. Although they are stationary in the healthy CNS, they are not inactive [4, 5]. Acting as sentinels, they continuously monitor the CNS and make frequent and direct contact with neuronal synapses by extending and retracting their processes [6]. As a group, microglia can potentially survey the entire brain several times a day [7]. Microglia also function to phagocytically remove debris from normal neuronal remodeling processes, and they are the first responders to pathogens and molecular signals of tissue injury or trauma. Their transformation from the resting (ramified) state to the activated state begins within seconds of exposure to stimulus, and can be completed within a few hours, after even just brief contact [7].
Properties of activated microglia typically include: proliferation, increased phagocytic activities, migration towards the site of injury/damage, and production/release of inflammatory cytokines and chemokines. These inflammatory signals can have several effects. Some of these autocrine and paracrine effects cause increased diapedesis by inducing endothelial cells to express adhesion molecules necessary for other leukocytes, including T cells, to enter the CNS parenchyma [2, 8, 9]. Microglia also produce reactive oxygen species (ROS) including nitric oxide (NO), superoxide, hydroxyl radicals and hydrogen peroxide, which are directly antimicrobial. ROS can be neurotoxic, causing DNA damage and injury to oligodendrocytes by lipid peroxidation [1]. Many of the cytokines released by activated microglia are associated with astrocyte proliferation, altered neuronal neurotransmitter release, and oligodendrocyte toxicity [9]. Because these molecules produced by microglia can be neurotoxic, their inflammatory activities are predominantly viewed as harmful. However, it should be noted that microglia are not the only CNS cell type capable of responding to brain injury and producing these inflammatory mediators; astrocytes also have significant activity in this regard (reviewed in [10]).
The precise contributions of microglia to neuronal damage following head trauma, ischemia and neurodegenerative disease remain controversial. Although studies describing detrimental roles of microglia in all of these processes are numerous, recent studies in a number of disease models are beginning to support the idea that microglia do indeed perform important and critical support functions in the CNS. For example, microglial ablation worsens ischemic brain injury [11], and increases glioma growth [12], suggesting that at least some of their immune properties are beneficial. Much research has focused on compounds that can decrease microglial inflammatory activities following ischemic and traumatic brain injuries. In the present article, we will summarize the current literature on two receptor signaling systems in microglia, estrogen receptors and purinergic receptors, which among many other things, function to control inflammatory gene expression. We will review the major effects of both receptor systems in microglia and discuss some novel information from our own laboratory suggesting cross-talk between these pathways.
Purinergic Receptors
Extracellular nucleotides
The ligands for purinergic receptors are typically extracellular adenine nucleotides such as ATP, but some receptors bind pyrimidines as well. Nucleotides are released extracellularly in the healthy CNS from a number of sources [13, 14], including co-release with neurotransmitters from neurons [15–19] and release from nearby cells through membrane channels or gap junctions [16, 20]. In this regard, ATP is released from astrocytes during propagation of calcium waves [21–26]. Importantly, intracellular ATP concentrations average 3–5mM in most CNS cells [27], and its release into the extracellular space from lysed and damaged cells both within and surrounding the ischemic lesion or injury can further elevate nucleotide levels in the extracellular microenvironment. In addition, the levels and activities of ectonucleotidases (the extracellular enzymes that degrade nucleotides into molecules such as adenosine, an endogenous CNS neuroprotectant (reviewed in [28])), are also strongly regulated by pathological processes; their expression is greatly suppressed in infarcted tissue following embolic ischemia [29]. ATP is also released from platelets [30] and erythrocytes [31, 32] that infiltrate the infarct, further increasing nucleotide levels and their durations of action in the CNS. Once in the extracellular space, these nucleotides can then alter microglial function by binding to and activating cell surface receptors of the P2 purinergic receptor type.
Purinergic receptor families
Purinergic receptors are classified into two families: P1 receptors and P2 receptors. P1 receptors (also called adenosine receptors) have seven transmembrane spanning domains, are G protein-coupled and bind adenosine and adenosine monophosphate. There are four groups of P1 receptors based on ligand affinity, the G protein type to which they couple, and their biological effects: A1, A2a, A2b, and A3 (reviewed in [33–36].) These receptors will not be considered further here.
P2 receptors on the other hand, bind di- and tri-phosphate-containing nucleotides, and are further divided into two classes, P2X and P2Y, based on predicted membrane topology and intracellular signaling pathways. P2X receptor (P2XR) subunits have two transmembrane domains, and trimerize to form ligand-gated cation channels. P2Y receptors (P2YR) are metabotropic, have seven transmembrane domains and are G protein-coupled (Table 1). There are currently seven known P2XRs (P2X1-P2X7), and eight known mammalian P2YRs (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14). P2Y11 is found in humans and a few other species, but is not present in the rodent genome [37] and will not be discussed further here.
Table 1.
P2R ligands, heteromers, and G-protein associations.
| P2R | Select agonists | Select antagonists | Known heteromers (P2X) Signaling pathway (P2Y) |
|---|---|---|---|
| P2X1 | ATP, 2meSATP, α,β–meATP | TNP-ATP, NF 023, IP5I | P2X1/X2, P2X1/X4, P2X1/X5 |
| P2X2 | ATP ≥ ATPγS ≥ 2meSATP> α,β–meATP | Suramin, PPADS, isoPPADS, Reactive Blue 2 | P2X2/X1, P2X2/X3, P2X2/X6 |
| P2X3 | 2meSATP ≥ ATP ≥ α,β-meATP | TNP-ATP, PPADS | P2X3/X2 |
| P2X4 | ATP, α,β-meATP > CTP | TNP-ATP, Brilliant Blue G (both only weakly) | P2X4/X1, P2X4/X6, P2X4/X7 |
| P2X5 | ATP, α,β-meATP, ATPγS | Suramin, PPADS | P2X5/X1 |
| P2X6 | N/A (not known to form homomultimers) | N/A | P2X6/X2, P2X6/X4 |
| P2X7 | BzATP > ATP (mM) ≥ 2meSATP > α,β-meATP | KN62, Brilliant Blue G | P2X7/X4 |
| P2Y1 | 2meSADP > ADP > ATP | Suramin, PPADS, MRS2179, Reactive Blue 2 | Gq/11 |
| P2Y2 | UTP = ATP | Suramin, Reactive Blue 2 | Gq/11, possibly Gi |
| P2Y4 | UTP | PPADS, Reactive Blue 2 | Gq/11, possibly Gi |
| P2Y6 | UDP > UTP | PPADS, Reactive Blue 2, MRS2758 | Gq/11 |
| P2Y12 | 2meSADP > ADP > ATP | Suramin, Reactive Blue 2, Cangrelor (AR-C69931MX), MRS2395 | Gi |
| P2Y13 | ADP = 2meSADP | Suramin, Cangrelor, MRS2211 | Gi |
| P2Y14 | UDP-glucose, UDP-galactose | None known | Gi |
Note: Descriptions of agonist affinity and efficacy of antagonists vary by publication. This may be due to species differences, the system used for testing, or results of further experimentation. Agonist and antagonists frequently used are included here; order of agonists affinities are not noted unless they were listed in the same order in the majority of references [37, 39, 41, 87, 196, 197].
Evidence that P2XRs are multimeric, and that these configurations could be either homo- or heteromeric came from studies on P2X2/P2X3 receptors [38]. Since this first description, confirmation of the heteromeric nature of other P2XRs has emerged (summarized in Table 1). It was long thought that G protein-coupled receptors, including the P2YRs, operated as monomers. Although evidence has surfaced in the past decade that serpentine receptors may also form dimers or possibly even oligomers, examining such interactions is experimentally difficult and evidence is still sparse. However, with regard to purinergic receptors, there is evidence that P2Y2 receptors can homo-dimerize, and that P2Y1 receptors can hetero-dimerize with P1 receptors (reviewed in [37]), opening interesting possibilities for cross-talk between many purinergic receptor subtypes.
P2 receptor pharmacology
Studying the functions of a specific P2Rs or identifying the receptor responsible for a given nucleotide effect can be complex. All known P2 receptors respond to multiple nucleotide agonists (Table 1), and with the exception of the sugar-conjugated nucleotides, a single nucleotide can bind to multiple P2Rs. In addition, multiple P2Rs are usually expressed within a given cell type, and as noted above, many P2XR trimers can be heteromeric, further complicating the pharmacology. Likewise, many P2R antagonists also act on multiple receptors (Table 1). This complex matrix of receptors, oligomers, and ligand affinities means identifying the function of a single P2R in a cell or tissue of interest often requires using a combination of agonists and antagonists. (Although not always practical, P2XR multimers can often be identified by electrophysiological studies.) P2X7 is unique in its requirement for millimolar ATP concentrations for activation, whereas micromolar doses are sufficient for the activation of other P2Rs. (For a comprehensive review of specific P2R ligands and affinities, please see [39].) One caveat that should be noted regarding P2R ligand affinities is that much of this information is obtained through recombinant expression of receptors in heterologous expression systems, so the properties observed may not necessarily be representative of native receptors in situ. Therefore, identification of specific P2 receptor subtypes that can be targeted for therapeutic purposes using only pharmacologic approaches will not be simple. The development of novel, more selective agonists and antagonists will be crucial for advancement in this regard.
P2R gene clusters
In addition to sharing agonists and antagonists, many P2Rs also share chromosomes. There are four chromosomes that contain multiple P2 genes, and though the chromosome designations vary by species, the P2R genes found on a single chromosome are identical. P2X1 and P2X5 are located on mouse chromosome 11 (chromosome 17 in humans, and chromosome 10 in rat); P2Y2 and P2Y6 are both located on mouse chromosome 7 (human -11, rat -1); and P2X2, P2X4, and P2X7 are located on mouse chromosome 5 (human and rat chromosome 12). The largest gene cluster, likely arising from a gene duplication event [40] is found on human and mouse chromosome 3 (rat chromosome 2), containing P2Y1, P2Y12, P2Y13, and P2Y14. Of these, three genes are found in close proximity on the same strand (reverse) and incidentally, are arranged in the same order as their numbers: P2Y12 (mouse GeneID 70839, C57/Bl6 nucleotides 59066744–59020194), P2Y13 (mouse GeneID 74191, C57/Bl6 nucleotides 59014804–59011828) and P2Y14 (mouse GeneID 140795, C57/Bl6 nucleotides 58934546–58918547). It is likely that these latter genes are regulated by common mechanisms.
P2R expression in the CNS
Purinergic receptors have been extensively studied in the CNS. Indeed P2X3, P2X4, P2X5 and P2X6 were cloned from nervous system cDNA libraries, chosen because many purinergic effects were observed in the CNS despite the apparent limited expression of P2X1 and P2X2 in those tissues. Although the levels of P2R expression vary across the CNS, comprehensive studies of CNS P2R distribution have not been completed for all receptors. A list of references to studies examining distribution of P2R expression in the CNS is included in Table 2. Many studies (reviewed in [39, 41]) demonstrate the detection of a particular P2R in a specific neuronal structure. The references included in Table 2 are only those which examine multiple CNS areas at the same time, thereby allowing direct comparison of expression levels between regions. The frequent contradiction in reported results is most likely due to species differences, the assays used (Northern blot, RT-PCR, ISH, IHC) and their sensitivity, and the frequency of non-specific staining within the CNS using commercially available P2R antibodies [42–44]. Therefore, the presence of the receptor in the CNS in general, is noted in the table unless there is strong and consistent evidence that CNS expression is restricted to a particular area(s).
Table 2.
P2R expression in the CNS and microglia.
| P2R | CNS expression | Expression in resting microglia (in vivo, primary cultures, and cell lines) |
|---|---|---|
| P2X1 | Several areas; cerebellum most noted [198–200] | Yes [74, 82, 84, 201, 202] |
| P2X2 | Throughout [199, 200, 203, 204] | Yes [82, 99] (in cell lines) |
| P2X3 | Throughout [200, 205] | Yes [74, 84, 99] |
| P2X4 | Throughout [200, 206–210] | Yes [67, 75, 84, 99] |
| P2X5 | Primarily trigeminal mesencephalic nucleus [200, 211] | Possibly [99] |
| P2X6 | Throughout [200] | Possibly [99] |
| P2X7 | * Yes [212, 213] | Yes [75, 82, 84, 99, 212] |
| P2Y1 | Throughout [214–217] | Yes [78, 82, 99] |
| P2Y2 | Several areas [215] | Yes [78, 82, 99] |
| P2Y4 | Several areas; hippocampus and spinal cord most noted [215] | Yes [74, 99] (possibly [50, 85] by activity) |
| P2Y6 | hippocampus and spinal cord most noted [215] | Yes [75, 78, 82, 99] |
| P2Y12 | Several areas; spinal cord most noted [215, 218–221] | Yes [75, 84, 99] |
| P2Y13 | Uncertain | Yes [99, 222] (in culture; in vivo**) |
| P2Y14 | Uncertain [215] | Yes [74, 99] (in culture) |
Still controversial; antibody problems have contributed to conflicting reports of highly restricted and widespread expression.
Many studies of P2Y12 use techniques that could detect P2Y13 action as well: both receptors have the same agonists, signal through Gi, and most P2Y12 antagonists also inhibit P2Y13 [87].
P2R expression in microglia
While purinergic signaling has been studied for decades, investigation of P2R expression and function in immune cells has only become a focus in roughly the last fifteen years. Although the best-studied P2 receptor in microglia (and in immune cells in general) is P2X7, information on the function of other P2Rs in microglia is now beginning to emerge. Indeed, studies examining the expression of all known P2X and P2Y receptors in microglia have only been published in the last six years. Whether due to the availability of more sensitive assays or better reagents, the number of receptors reported to be expressed in microglia has steadily increased. Early studies in microglia typically detected only P2X1, P2X4, P2X7, and P2Y6, but more recent studies indicate the presence of additional receptors as well (Table 2). For example, microglial expression of P2Y12 is now well-established, and its role in chemotaxis is central to normal microglial function [45, 46]. The contradiction among reports about which receptors are expressed in microglia likely results from the use of different microglial sources: immortalized cell lines, primary cultures (many from different CNS regions), or in vivo examination. Genetic drift within microglial cell lines may also contribute to conflicting results from different labs. Additional studies within each system are needed to verify the presence or absence of the receptors detected least often, or at very low levels in microglia, namely that of P2X2, P2X5, P2X6, P2Y4, P2Y13, and P2Y14. Recent studies from our laboratory using murine microglia freshly isolated from whole brain demonstrate the presence of mRNA for P2X6, P2Y13 and P2Y14 at all ages examined, whereas that for P2X2 was not detected at all, and P2X5 and P2Y4 expression was observed only at specific ages [47]. It should be noted that P2R expression profiles in primary microglia were also significantly different from their freshly-isolated microglial counterparts (from animals of the same age) [47], suggesting that purinergic receptor study in vitro may not necessarily reflect the in vivo situation accurately.
P2 receptor function in microglia
Information on P2X7 function in microglia is the most plentiful, and to date, its role in modulating cytokine release has received the most focus. Maturation and release of interleukin-1 (IL-1)β by P2X7 engagement from lipopolysaccharide (LPS)-primed microglia (the result of caspase-1 activation), is the best-studied of these cytokine effects [48–52]. In macrophage-like cells, caspase-1 activation is primarily regulated by the assembly of a multi-protein complex called the inflammasome [53], of which the P2X7 receptor is an integral component [54, 55].
P2X7 signaling also promotes the production and release of other cytokines including tumor necrosis factor (TNF)-α via activation of several mitogen-activated protein (MAP) kinase family members [50, 56, 57]. P2X7 activation increases TNF-α release in LPS-treated microglia [58], whereas its antagonism decreases TNF-α expression [59]. The mechanisms of P2X7 receptor regulation of LPS-induced IL-6 production are however, somewhat less clear: P2X7 activation decreases IL-6 release [58] while its inhibition decreases IL-6 mRNA levels [59]. P2X7 receptor antagonism also decreases IL-12 and cyclooxygenase (COX)-2 expression in LPS-treated microglial cultures [59]. Consistent with observations in other cell types, prolonged P2X7 activation in microglia leads to pore formation [48, 60] and eventually apoptosis through activation of caspases -1, -3, and -8 [61, 62]. P2X7 receptor activation also promotes plasminogen release [63] and activation of the transcription factors NFAT (nuclear factor of activated T cells) and NF-κB (nuclear factor of kappaB), key regulators of many pro-inflammatory genes [64, 65]. A more complete list of the reported functions of P2X7 and other purinergic receptors in microglia can be found in Table 3. It should be noted that other P2X receptors may also play a role in some effects attributed to P2X7 by virtue of the use of benzoyl-benzoic ATP (BzATP), often regarded as a “selective” P2X7 receptor agonist. BzATP is a more potent agonist of P2X1 and P2X4 receptors than it is for P2X7, but it has affinity for all P2X receptors except for P2X6 [39].
Table 3.
P2R function in microglia and association with inflammatory CNS conditions.
| P2R | Function in/through microglia | Association with CNS disease, injury, or inflammation (in microglia) |
|---|---|---|
| P2X1 | Unknown | Unknown |
| P2X2 | Unknown | Unknown |
| P2X3 | Unknown | Unknown |
| P2X4 | Chemotaxis, pain (allodynia), BDNF production and release Possibly decreased COX-2 following LPS |
Expression increased in/following: OGD, ischemia, peripheral nerve injury, LPS treatment, inflammatory pain (formalin injection), spinal cord injury, brain injury, experimental autoimmune neuritis, TLR activation, morphine treatment, IFNγ treatment [46, 66–68, 84, 97, 98, 100, 101, 106, 107, 110, 223, 224] |
| P2X5 | Unknown | Unknown |
| P2X6 | Unknown | Unknown |
| P2X7 | Microglial activation, proliferation, pore formation, apoptosis, protection of neurons from glutamate toxicity Activation of: NF-κB; caspases -1, -3, -8; NFAT; ERK; p38 Increases: iNOS, 2-AG, LC3-II; IL-1β, ROS following LPS Release of: ATP, plasminogen, TNF-α, superoxide, NO, IL-18, CCL3 (MIP- 1), MRF-1 Decreases: MRF-1 synthesis, glutamate uptake, lysosomal function IL-6, IL-12, TNF-α, COX-2, IL-1β following LPS treatment |
Expression increased in/following: seizures, OGD, ischemia, MS lesions, ALS, AD plaques, AB, i.v. anesthesia, LPS, scrapies [48–52, 56–65, 80–82, 225–230] Presence is associated with higher EAE incidence [59, 93–95, 99, 101–103, 109, 111, 231, 232] |
| P2Y1 | Leukotriene release, possible decrease in iNOS following LPS treatment | Unknown [84, 233] |
| P2Y2 | Possibly Mitosis | Unknown [50] |
| P2Y4 | Possibly Mitosis | Unknown [50] |
| P2Y6 | Phagocytosis |
Expression increased in/following: seizures, LPS [75, 93, 99] |
| P2Y12 | Chemotaxis, membrane ruffling, process extension, neuropathic pain Decreases: proliferation |
Expression increased in/following: seizures, neuropathic pain [45, 46, 70–73] Expression decreased in/following: Microglial activation [72, 73, 93, 116] |
| P2Y13 | Possibly chemotaxis, membrane ruffling, decreased proliferation* | Unknown [45, 70] |
| P2Y14 | Unknown |
Expression decreased in/following: LPS [99] |
Many studies of P2Y12 function use techniques that may detect P2Y13 action as well.
P2X4 receptors in microglia are mainly associated with pain; their expression is specifically increased in spinal cord microglia following peripheral nerve injury which directly contributes to tactile allodynia [66]. It is thought that P2X4 contributes to pain by leading to increased production and release of brain-derived neurotrophic factor (BDNF) [67, 68], a neurotrophin increased in pain models which modulates neurotransmitter release and interneuron activity [69]. A recent study has also described a role for P2X4 in microglial chemotaxis [46], which prior, had been primarily associated with P2Y12 [70].
P2Y12 was first described as involved in microglial membrane ruffling and chemotaxis [70], and its role in migration has since been reinforced [45] and shown to involve the activation of the PI-3 kinase [46] and Akt [71] signaling pathway. P2Y12 is also important in directed microglial process extension and migration toward injury [72]. Interestingly, just as P2X4 is beginning to be associated with microglial movement, P2Y12 has recently been shown to have a role in neuropathic pain [73]. Whereas some of the studies described above have made use of genetic manipulations such as P2Y12 knockout mice to verify P2Y12 involvement, it is important to keep in mind that some antagonists such as cangrelor, previously believed to be P2Y12-specific, also antagonize P2Y13 [37]. Since both receptors also have common agonists, and both are Gi-coupled, a role for P2Y13 may not have been ruled out in these pharmacologic studies. Knowledge of P2 receptor agonist and antagonist specificity continues to grow, so verifying receptor activity through the use of multiple receptor ligands and/or a combination of pharmacologic and genetic manipulations remains ideal.
Although less studied, information on the function of other P2Rs in microglia is beginning to emerge. P2Y1 activation was recently reported to promote IL-10 release [74]; P2Y2 or P2Y4 activation may lead to mitosis in retinal microglia [50]; and there is great interest in the involvement of P2Y6 in phagocytosis [75]. In addition, there are some effects of nucleotide signaling which have not yet been attributed to a specific P2R: ATP induction of a ramified morphology in primary cultures [76] and amoeboid morphology in N9 cells [77], IL-6 release through activation of a receptor thought to be of the P2Y type [78], and inhibition of both TNF-α and IL-6 release from LPS-treated microglia through the actions of one or more P2Y receptors [79].
Finally, an interesting and complicated role for P2Rs in the regulation of inducible nitric oxide synthase (iNOS) and reactive oxygen species has been observed. Both ROS and iNOS have been shown to be regulated by nucleotide treatment of microglia, but the results are mixed and sometimes stimulus-dependent. For example, some studies have observed induction of iNOS or ROS production following treatment with nucleotides alone [80, 81], or potentiation following treatment with IFNγ [82]; and likewise, treatment with P2X7 antagonists decreased LPS- or beta-amyloid (Aβ)-stimulated iNOS and ROS production [59, 83]. However, some studies have observed no effect of ATP on ROS levels following microglial activation [63, 79], and studies from our laboratory and others have found that nucleotide treatment decreases iNOS and NO in LPS-stimulated microglia [84, 85]. The complexity of these responses likely involves the engagement of different P2 receptors in the context of different microglial stimuli.
Microglial P2Rs in neurodegenerative disease, ischemia and traumatic brain injury
It is generally well-accepted that microglia, and their production of inflammatory and cytotoxic molecules in particular, are associated with neuronal damage resulting from neurodegenerative disease processes as well as ischemic and traumatic brain injury. As a result, many studies report on compounds and signaling pathways that serve to decrease or constrain microglial pro-inflammatory activities, and thereby presumably, neuron damage. In this regard, purinergic receptor signaling in microglia alters their release of neurotoxic molecules as discussed above, and evidence of associations between P2 receptor function and CNS pathology is growing (reviewed in [86–88]). Therefore, it is not surprising that in the past five years there has been increasing interest in how P2R expression in microglia is changed in various CNS conditions. Indeed, P2 receptors, such as P2X7, have recently become the target of several novel drugs currently in clinical trials to treat inflammatory disorders in the periphery (reviewed in [89–92]); their use in treatment of CNS inflammation to reduce neuronal damage is sure to be forthcoming.
There is increased expression of P2X4 and P2X7 in conditions such as status epilepticus [93], amyotrophic lateral sclerosis (ALS) [94], multiple sclerosis (MS) [94], Alzheimer’s disease (AD) [95], and prion infection [96]. These receptors are also found to be increased and/or to contribute to neuronal damage in several models of CNS injury, including: infection as modeled by treatment with LPS or other Toll-like receptor (TLR) agonists [59, 97, 98], though one study found P2X7 levels to be decreased [99]; ischemia as modeled by middle cerebral artery occlusion (MCAO) or oxygen-glucose deprivation (OGD) [100–104]; peripheral or inflammatory nerve injury [66, 105]; traumatic spinal cord or brain injury [106, 107]; autoimmune neuritis or experimental autoimmune encephalomyelitis (EAE) [108, 109]; IFNγ treatment [110]; and β-amyloid treatment [95, 111]. However, the function of this up-regulation in these many conditions is not yet known. It is reasonable to surmise that P2X4 and P2X7 receptors may indeed play a role in some of the above mentioned pathologies; however, infarct size was not reduced in P2X7 receptor knockout mice which had been exposed to MCAO [112]. These data suggest that even though P2X7 receptor expression is increased in microglia following ischemia, P2X7 up-regulation does not appear to measurably exacerbate brain damage despite pharmacologic evidence that infarct size is reduced by P2X7 receptor antagonists in some studies [103, 113]. The protection conferred by some P2X7 antagonists may be the result of non-specific antagonism of other non-P2X7 receptors, as discussed previously. In addition, P2X7 receptor antagonists have demonstrated neuroprotective effects in animal models of traumatic spinal cord injury [114, 115], although these effects are not postulated to occur solely via modulation of microglial activities. Rather, P2X7 antagonism is thought to directly decrease neuronal apoptosis [115] while also perhaps decreasing glial cell activation and neutrophil recruitment to the CNS [114].
Although less studied, expression of P2Y6 and P2Y12 receptors has also been found to be increased following epileptic seizures [93] and in neuropathic pain models [73, 116]. Interestingly, microglial P2Y6 expression is increased whereas that of P2Y12 and P2Y14 is decreased following LPS treatment [99]. Studies using targeted gene knockouts for these receptors have not yet been reported.
While there are strong correlations between P2R expression in microglia and many different CNS pathologies, there is still very little information available about their regulation under these conditions, or even under normal conditions. Despite studies demonstrating amelioration of damage following P2R knock down or antagonism, it is not known if microglial P2 receptor expression is changed as a result of the conditions, or if the conditions develop as a result of altered P2R expression and signaling. This distinction is very important as it will have tremendous impact on any potential treatments or preventative therapies utilizing purinergic receptors. A particularly intriguing recent article showed that expression of additional P2X7 receptors in microglia was sufficient to drive microglial activation and proliferation [117], suggesting that aberrant P2R regulation in microglia may very well contribute to pathology. Additional studies are necessary to better understand the role of microglial P2Rs in the healthy and injured/diseased CNS to validate them as useful therapeutic targets.
Estrogen Receptors
Estrogen in neurodegenerative disease
Similar to purinergic receptors, estrogen receptor signaling has also been associated with CNS disease, and the modulation of microglial inflammatory activity. However, unlike the majority of P2R evidence thus far, estrogen signaling is negatively correlated with disease incidence and pathology; it is thought to play a protective role. Sexual dimorphisms in the incidence of many neurodegenerative disorders have been observed. Men have a higher incidence of Parkinson’s disease and ALS, while women have a higher incidence of MS and AD [118–120]. However, it should be mentioned that there remains debate about the role of sex specifically, versus longer lifespan, in the development of late-onset AD. While there are many factors that likely contribute to the sexual dimorphisms observed in neurodegenerative diseases, the role of estrogen is of particular interest. Among women who have MS, symptom severity is reduced when estrogen levels are high (such as during late pregnancy, or with the use of oral contraceptives), symptoms are greatly exacerbated upon parturition when estrogen levels precipitously decline, and relapses are more common during the low-estrogen points in the menstrual cycle [121]. In some studies, post-menopausal estrogen replacement is correlated with lower incidence and delayed onset of AD (reviewed in [118–120, 122]). Estrogen also has beneficial effects both in human and animal models of PD and MS [123, 124]. In addition, damage from ischemic injury is greater in animals that have undergone ovariectomy or which are reproductively senescent (reviewed in [118, 120, 122]), further suggesting a protective role for gonadal hormones such as estrogens. However, not all studies with estrogens have proved beneficial in rodents or humans; these results will be discussed further below.
Estrogen neuroprotection and ischemic/traumatic brain injury
There are numerous studies which have evaluated the beneficial role of estrogens in ischemic and traumatic CNS injury (reviewed in [147, 164–166]). Estrogens exert multiple effects in the CNS, including performing anti-oxidant activities, decreasing glutamate receptor function (thereby reducing neuronal excitotoxicity), synthesizing neurotrophins/growth factors and increasing expression of cell survival genes (ultimately providing neurotrophic support to damaged neurons), reducing neuronal apoptosis, stimulating axonal remyelination, and promoting synaptogenesis and dendritic arborization to maintain or promote new functionality in damaged CNS areas [119, 120, 122, 127, 167]. Also, as mentioned above, estrogens also attenuate CNS inflammatory processes. Thus it seems that estrogens may have the relatively unique potential to alter the activity of both neurons and inflammatory cells concurrently, and as such, exert pleiotropic, beneficial effects in the CNS. Information which is currently lacking in the literature however, is how quantitatively important estrogen anti-inflammatory effects (as opposed to its neuronal effects) are for the treatment of ischemic and traumatic brain injury, the goal of therapeutics that would target this receptor system in microglia.
Most estrogen studies in microglia make the assumption that reduced microglial inflammatory activities will result in neuroprotection, but few studies actually test this. Estrogen has been shown to be neuroprotective in models of CNS disease which involve inflammation, it is thought primarily, by decreasing microglial production of neurotoxic chemokines and cytokines such as interferon (IFN)-γ, TNF-α, IL-1 and IL-6, thereby reducing leukocyte infiltration into the parenchyma [118, 119], further decreasing production of these cytotoxins. But a direct link between specific inhibition of microglial inflammatory activities and neuronal protection has not yet been demonstrated in vivo because of the inability to selectively inhibit neuronal estrogen effects. It should be noted that estrogen also has anti-inflammatory effects on circulating immune cells, but its effects on CNS-resident immune cells, as opposed to its effects on peripheral immune cells, seem to be necessary for estrogen-induced neuroprotection in some CNS pathologies including EAE [168, 169].
One in vitro study has begun to address the question of estrogen-induced neuroprotection by inhibiting microglial inflammatory functions using conditioned medium experiments from estrogen-treated microglial cultures stimulated with LPS [170]. Although a specific role for inflammatory molecules per se in promoting neuronal death was not demonstrated, LPS-conditioned medium from estrogen treated microglia caused less death to neuronal cultures than conditioned medium from non-estrogen treated cultures, suggesting that at least in vitro, estrogen alters microglial parameters that are functionally beneficial to neurons. The fundamental question is however, whether this is the result of inhibition of inflammatory molecules, and if this is functionally significant. Also, it is not yet clear if reduction of microglial inflammatory activities is sufficient to confer neuroprotection following an ischemic or traumatic brain injury. However, a very elegant study in this regard was recently performed [11]. Ablation of proliferating microglia (and therefore, microglial production of inflammatory substances) in a mouse MCAO model demonstrated that infarct size was greatly increased in the absence of microglia. Moreover, it was shown that microglial production of neuroprotective substances such as insulin-like growth factor (IGF)-1 early after ischemic injury may contribute to the beneficial effects of microglia. Since ablation of microglial activities in general is deleterious following ischemic injury, then the effects of estrogen (if neuroprotection is conferred by its microglial actions alone), must not eliminate the beneficial activities of microglia. It will be important to better characterize the effects of estrogen not only on attenuating inflammatory microglial gene expression, but also on altering the expression of and cross-talk between other critical neurotrophins/growth factors and their receptors in microglia. Much is recognized on these topics in neurons [171–173], but little is known of such interactions in microglia.
Complexity of human estrogen studies
Whereas estrogen therapy is beneficial in many CNS pathologies, results from clinical studies have not been uniformly positive. Some clinical studies even indicate negative effects of estrogens on AD symptoms, and the speeding of ALS onset [125, 126]. Some of these contradictory results may be due to use of equine-derived hormones that are not well characterized. The pharmaceuticals used in the Women’s Health Initiative contained high levels of estrone, as opposed to 17β-estradiol, which is the predominant form circulating in pre-menopausal women, and the major estrogen form used in the rodent research studies upon which the clinical trials were based. Other variables potentially related to the range of clinical outcomes are the inclusion of progestin or progesterone in the drug formulations used, the route of administration (differential metabolism when given intraperitoneally or subcutaneously in rodents, versus digestive tract when given orally in women), and the timing of the initiation of treatment in relation to the onset of menopause [127, 128]. In many inflammatory conditions and in AD in particular, estrogen treatment appears only to be protective, rather than therapeutic after the inflammation has begun. While some hypothesize that this is because damage began as soon as estrogen was removed (due to ovariectomy or menopause), the reason for this remains poorly understood and more research is needed to unveil the mechanisms by which estrogen is beneficial.
It is also interesting to note that while estrogen is considered protective in MS, and it decreases symptom severity, women have a higher risk of developing the condition. One potential explanation is the cycling of estrogen throughout the estrous cycle, and its biphasic effects on the immune system; high estrogen concentrations promote Th2 (anti-inflammatory) responses, while low levels favor the Th1 (pro-inflammatory) immune response which predominates in MS [120, 129]. Another suggested mechanism involves the differential ability of female microglia (but not male microglia) to activate neuroantigen primed T cells [130]. It is likely that this gender predisposition is multi-factorial, and that many mechanisms contribute. Indeed the astrocyte response to brain injury has also recently been shown to vary significantly between males and females, as well as throughout the estrus cycle [131], so other cell types may also influence gender predisposition in any number of CNS disorders in which they are involved.
Estrogen effects on microglia
In microglia, estrogen treatment exerts several anti-inflammatory effects. It decreases superoxide production, iNOS expression, and NO release from activated cells [132–135]. Estrogen pretreatment also decreases morphological changes characteristic of microglial activation, as well as their production of prostaglandin (PG) E2, matrix metalloprotease (MMP)-9, and TNF-α in activated microglia [133–135]. Increased proteosome activity [136], uptake of β–amyloid [137], and viability of microglia treated with β–amyloid [138] following estrogen exposure has also been reported. In addition, estrogen treatment lowers microglial numbers in the hippocampus of very elderly mice [139], and reduces the number of activated microglia following MPTP treatment [140] and intracerebroventricular injection of LPS [141], suggesting that it can attenuate the microglial inflammatory response following diverse stimuli.
However, not all rodent estrogen studies have produced beneficial results. Estrogen replacement therapy did not reduce microglial activation in one study using ovariectomized, LPS-treated rats; indeed memory impairment was worsened in the presence of chronic hormone replacement [142]. Estrogen treatment of gonadally intact male mice did not reduce basal microglial number [143], nor did it reduce microglial production of TNF-α or IL-6 following LPS treatment [144]. In another study, pretreatment with selective estrogen receptor modulators (SERMs), but not 17β-estradiol, resulted in decreased NO and IL-6 production from LPS-treated microglia in vitro [145], whereas both SERMs and 17β-estradiol were found to be efficacious in reducing major histocompatability complex II expression in microglia in LPS-treated rats [146].
It may be that beneficial estrogen effects are stimulus-specific, and that estrogen has more consistent effects when microglia are activated by more CNS relevant stimuli. For example, estrogen has clear benefits in animal models of ischemia [147], Parkinson’s disease [123] and MS [148]. However, studies utilizing LPS as the microglial or inflammatory stimulus are less consistent; some reports indicate beneficial effects [141, 146] and others do not [142, 144]. Although additional complexities in the effects of estrogen are also likely to be involved, the route of LPS delivery (intraperitoneal vs. intracerebroventricular vs. intraparenchymal), the type of LPS used (E. coli serotype (011:B4 vs. 055:B5) vs. Salmonella, for example), as well as the genetic strain of the rodent model (rat vs. mouse; inbred vs. outbred) differ among LPS studies, so these variables may also contribute to the heterogeneity of estrogen anti-inflammatory/neuroprotective effects reported in the literature with regard to LPS stimulation.
Estrogen receptor signaling
Estrogens are steroid hormones, and they are found in humans in three main forms– estradiol, estriol, and estrone, with 17β-estradiol having the highest affinity for estrogen receptors [149, 150]. Exogenous estrogens, both naturally occurring (such as soy phytoestrogens) and synthetic, can produce biological effects via interactions with estrogen receptors [151]. Estrogens exert both estrogen receptor (ER)-dependent and ER-independent effects in many tissues, including microglia. There are two known nuclear estrogen receptors, ERα and ERβ. A conformational change is induced in these ligand-activated transcription factors upon estrogen binding that favors dimerization and promotes recruitment of transcriptional cofactors, ultimately altering gene transcription [152].
Estrogen receptors can also exert effects independent of their own DNA binding domains (also called “non-genomic” effects). In this mechanism, estrogen receptors can indirectly influence gene transcription either by physically interacting with: 1) other transcription factors (such as AP-1 [153] or NF-κB [154]) which alters their ability to promote transcription; or 2) kinases at the plasma membrane or within the cytoplasm that initiate kinase cascades such as the MAPKs [155], key intracellular signaling pathways involved in regulating inflammatory gene expression [156]. Estrogens can also exert receptor-mediated effects by interacting with transmembrane receptors on the cell surface. One example is the orphan G protein-coupled receptor GPR30, which has a high affinity for estrogen [157]. Other plasma membrane receptors for estrogen have also been proposed in the CNS, but they have not yet been identified. Finally, when given at supraphysiological concentrations, estrogen can produce estrogen receptor-independent effects by functioning as a phenolic antioxidant [158]. Thus, there are many mechanisms whereby estrogens can promote neuroprotection, but in healthy microglia in vivo, estrogen effects likely involve interactions with ERα, since ERα is expressed in microglia in the healthy CNS [144, 159], and to our knowledge there are no reports of estrogen exerting effects in microglia via interactions with GPR30 or as a phenolic anti-oxidant.
ERβ expression and/or function in healthy microglia in vivo on the other hand is a little less clear. It has been detected in microglia [160–163], but the microglia used in these studies were either immortalized cell lines or primary cultured microglia; strong differences in microglial gene expression in vivo and in vitro have been discussed above in relation to P2 receptors. However, it should be noted that ERβ expression in microglia has been reported in vivo, but this was done in post-ischemic animals [163]. In this regard, recent studies of steroid receptors in microglia isolated from healthy, uninjured mice also did not detect ERβ expression in microglia [144, 159], suggesting that ERβ is likely not expressed in healthy murine microglia. It is not yet clear if ERβ expression is induced in vivo when microglia become activated, or what microglial stimuli may induce its expression. Further research is needed to verify ERβ expression and function in microglia in the healthy CNS in vivo.
Potential regulation of P2Rs by estrogen
Both purinergic and estrogen receptor signaling have been shown to produce dramatic effects on microglia, modulating their morphology, migration, phagocytosis, and production of cytokines, ROS, and neurotrophic factors. Despite their overlapping spheres of influence, information on possible regulation of P2Rs by estrogen is extremely sparse [174, 175]. Our laboratory observed that treatment of N9 microglia with estrogen altered expression of certain P2Rs (data not shown), but these results were rather inconsistent. Moreover, consistency of estrogen effects was not conferred by culturing the cells is charcoal-stripped serum or by serum starving the cells for various lengths of time prior to harvest. To determine the cause of these sporadic results, N9 cell samples were harvested with each passage and ER expression was examined by qRT-PCR. Surprisingly, the levels of estrogen receptor expression varied greatly from passage to passage; sometimes ERα was not detectable at all, and ERβ, which was absent at most times, was suddenly detectable at others (Figure 1). This monitoring was repeated with three other cell batches, and similar, unsystematic results were obtained: the patterns were not the same among batches nor was there any discernable correlation with passage number, time between passages, basal expression of other genes, or the age of the cell stock. The underlying cause of this sporadic estrogen receptor expression is still unknown, but in our hands, there does seem to be some instability in ER expression in N9 cells, a commonly used microglial model for estrogen studies (Table 4). Although genomic instability has been reported in many other cell lines immortalized using the v-myc oncogene [176–178], there have been no such reports to our knowledge of this occurring in N9 microglia, which were also immortalized using the v-myc or v-mil oncogenes [179].
Figure 1. ERα (A) and ERβ (B) mRNA variation with passage number in N9 microglial cells.
N9 cells were harvested at different passages as indicated in the figure, RNA was extracted, reverse transcribed and ER expression examined by qRT-PCR. Expression was normalized to β-tubulin and is graphed in arbitrary units. N.D. = not detected. Results are representative of four independent experiments.
Table 4.
Summary: Culturing methods and treatment conditions for the study of microglial estrogen responses in vitro.
| Microglial source | Culturing methods | E2 dose/treatment time |
|---|---|---|
| Primary neonatal cultures (F344 rat cortex) [234] |
Mixed cultures maintained in: DMEM/F12, 10% FBS, P/Sa Microglia harvested by: shakingb Plating surface coating: poly-D-lysine Nonadherent cells removed after: 30min Media for treatments: serum-free, phenol red-free DMEM/F12 (changed prior to shaking) |
0.1nM E2 for 24hrs |
| Primary neonatal cultures (rat cortexc) [235] |
Mixed cultures maintained in: DMEM, 10%FBS, 1.4mM glutamine Microglia harvested by: shaking overnight Plating surface coating: none Nonadherent cells removed after: 2hr Media for treatments: same as maintenance media |
50μM estriol, 1hr before stimulating treatment |
| Primary neonatal cultures (albino rat cortexc) [133] |
Mixed cultures maintained in: MEM, 0.6% glucose, 10% FBS, 1% nonessential amino acids, P/S Microglia harvested by: shaking vigorously for 15hrs Plating surface coating: none Nonadherent cells removed after: 1–3hrs, then adherent cells lifted by trypsin and replated Media for treatments: phenol-red free, serum-free DMEM 24hrs after plating |
1nM E2, 4hr before stimulating treatment |
| Primary neonatal cultures (Sprague Dawley rat cortex) [134] |
Mixed cultures maintained in: MEM, 0.6% glucose, 20% FBS, 1% nonessential amino acids, P/S Microglia harvested by: shaking Plating surface coating: none Nonadherent cells removed after: N/A Media for treatments: MEM, 10% FBS; 24hr before steroid treatments switched to serum-free, phenol red-free DMEM, 5mM L-glutamine, P/S |
10pM–10nM E2, 0–4hrs before stimulating treatment |
| Primary neonatal cultures (Sprague Dawley rat cortex) [236] |
Mixed cultures maintained in: MEM, 0.6% glucose, 20% FBS, 1% nonessential amino acids, P/S Microglia harvested by: shaking Plating surface coating: none Nonadherent cells removed after: N/A Media for treatments: MEM, 10% FBS for 24hrs; switched to MEM with 10% charcoal-stripped FBS for 24hrs before treatment |
10nM E2, 4hrs before stimulating treatment |
| Primary neonatal cultures (Wistar rat forebrain) [237] |
Mixed cultures maintained in: DMEM, 10% FCS, gentamycin, amphotericin B Microglia harvested by: shaking Plating surface coating: poly-L-lysine Nonadherent cells removed after: 10min and then adherent cells collected by scraping and replated Media for treatments: DMEM, 3% FCS or serum-free DMEM |
100nM E2, 72hrs before stimulating treatment |
| Primary neonatal cultures (Sprague Dawley rat forebrain) [132] |
Mixed cultures maintained in: MEM, 10% heat-inactivated-FCS, 2mM glutamine Microglia harvested by: shaking Plating surface coating: polyethylenimine Nonadherent cells removed after: 1–2hrs Media for treatments: serum-free MEM after 24hrs |
1nM E2, 30min alone or 24hrs before stimulating treatment |
| Primary neonatal cultures (Wistar rat whole brain) [238] |
Mixed cultures maintained in: DMEM 10% FBS Microglia harvested by: collecting media Plating surface coating: none Nonadherent cells removed after: N/A Media for treatments: same as maintenance media |
0.1–10μM E2, 30 min before stimulating treatment |
| Primary adult cultures (Sprague Dawley rat olfactory bulb) [239] |
Mixed cultures maintained in: DMEM/F12, 0.5% glucose, 10% FBS, P/S Microglia harvested by: shaking Plating surface coating: poly-D-lysine Nonadherent cells removed after: 3hrs Media for treatments: Optimem N2 and DMEM/F12 (1:1) for 24hrs then 100% Optimem N2 |
2nM E2, 4hrs before media change that included 2nM E2 and stimulating treatment |
| Primary adult cultures (Sprague Dawley rat olfactory bulb) [161] |
Mixed cultures maintained in: DMEM/F12, 0.5% glucose, 10%FBS, P/S Microglia harvested by: shaking Plating surface coating: poly-D-lysine Nonadherent cells removed after: 3hrs Media for treatments: Optimem N2 and DMEM/F12 (1:1) for 24hrs then100% Optimem N2 |
Media change with 2nM E2, 4hrs before stimulating treatment |
| Primary neonatal cultures (mouse cortexc) [240] |
Mixed cultures maintained in: MEM, 0.6% glucose, 20% FBS, 1% nonessential amino acids, P/S Microglia harvested by: shaking Plating surface coating: none Nonadherent cells removed after: 3hrs Media for treatments: MEM, 10% FBS for 24hrs, then DMEM, 10% charcoal-stripped FBS for 24hrs, then serum-free DMEM for 6hr before treatment |
1nM E2, 10min before stimulating treatment |
| Primary neonatal cultures (C57Bl/6J mouse whole brain) [241] |
Mixed cultures maintained in: DMEM, 10% FBS, 1% L-glutamine, P/S, 0.5% fungizone, then new media was used DMEM, 10% horse serum, 1% L-glutamine, P/S after five days Microglia harvested by: shaking Plating surface coating: none Nonadherent cells removed after: N/A Media for treatments: phenol red-free, serum-free DMEM for 48hrs |
0.1nM–1mM E2, for 15hrs before stimulating treatment |
| Primary neonatal cultures (BALB/c mouse whole brain) [144] |
Mixed cultures maintained in: DMEM, 10% FCS, 5ng/mL GM-CSF Microglia harvested by: shaking Plating surface coating: none Nonadherent cells removed after: 1hr Media for treatments: same as maintenance, with or without phenol red |
10pM–1μM E2 (in media replacement), 10–30min before stimulating treatment |
| Primary neonatal cultures (human frontal cortex from autopsy) [137] |
Mixed cultures maintained in: N/S Microglia harvested by: Percoll centrifugation Plating surface coating: none Nonadherent cells removed after: 18–24hrs later Media for treatments: DMEM, 2% HEPES, 1% sodium pyruvate, 10% FBS, gentamicin (media changed weekly, cells used for experiments two weeks later) |
10–100nM E2, for 1.5 or 48hrs before stimulating treatment |
| BV-2 [160] |
Cultures maintained in: DMEM, 5%FBS, P/S Media for treatments: changed to phenol-red free DMEM, 0.25% charcoal-stripped FBS the next day; changed to phenol red-free DMEM, 5% charcoal-stripped FBS 16 hours later |
1fM–10nM E2, 30min–24hrs before stimulating treatment |
| BV-2 [236] |
Cultures maintained in: DMEM/F12, 10% FBS 2g/L sodium carbonate, 0.11g/L sodium pyruvate Media for treatments: plated in DMEM, 10% FBS; changed to phenol red-free DMEM, 10% charcoal-stripped FBS 24hr later, media replaced with serum-free DMEM at treatment |
10nM E2, 4hrs before stimulating treatment |
| HAPI [242] |
Cultures maintained in: DMEM, 5%FBS, 20mM L-glutamine, P/S Media for treatments: replaced with maintenance media containing treatments |
1–20nM E2, with stimulating treatment |
| N9 [132] |
Cultures maintained in: RPMI, 5% heat-inactivated-FCS, 25μM β-mercaptoethanol Media for treatments: phenol red-free RPMI at least 12hrs before treatments |
10pM–1μM, 24 hrs before stimulating treatment |
| N9 [235] |
Cultures maintained in: MEM, 10% FBS, 1.4mM glutamine, 20μM β-mercaptoethanol Media for treatments: same as maintenance |
1–100μM E2 or 0.1–50μM estriol, 1hr before stimulating treatment |
| N9 [138] |
Cultures maintained in: MEM, 10% FBS, 1.4mM glutamine, 20μM β-mercaptoethanol Media for treatments: serum-free N2 24hrs after plating |
10–250pg/mL, concurrent with stimulating treatment |
| N9 [243] |
Cultures maintained in: RPMI, 5% heat-inactivated-FCS, 25μM β-mercaptoethanol Media for treatments: serum-free, phenol-red free RPMI 12hrs before treatment |
1nM E2, with or 24hrs before stimulating treatment |
| N9 [136] |
Cultures maintained in: RPMI, 10% FCS, P/S Media for treatments: phenol red-free RPMI, 10% charcoal-stripped serum for 24hrs before treatment |
1pm–1μM E2, for 3–48hrs |
| N9 [244] |
Cultures maintained in: IMDM, 10% heat inactivated-FBS, 25μM β-mercaptoethanol Media for treatments: plated in IMDM, 10% heat-inactivated-FBS for 24- 48hrs; switched phenol red-free IMDM, 10% charcoal-stripped FBS 24hrs before treatment |
1nM E2, for 0–72 hrs before stimulating treatment |
| N11 [245] |
Cultures maintained in: DMEM, 10% FCS, P/S, 2mM L-glutamine Media for treatments: DMEM, 1% FCS, P/S, 2mM L-glutamine for 24hrs, then replaced with this same media immediately before treatments |
2nM–2mM E2, 30min before stimulating treatment |
P/S (penicillin/streptomycin)
“shaking” refers to gentle shaking for 5hrs or less, unless otherwise noted.
specific rodent strain was not provided
BV2 cell line derived from C57Bl/6 neonatal mouse whole brain primary cultures [181]
HAPI cell line derived from neonatal rat whole brain[246]
N9 an N11 cell lines derived from ICR-CD1 neonatal mouse cortex primary cultures [179]
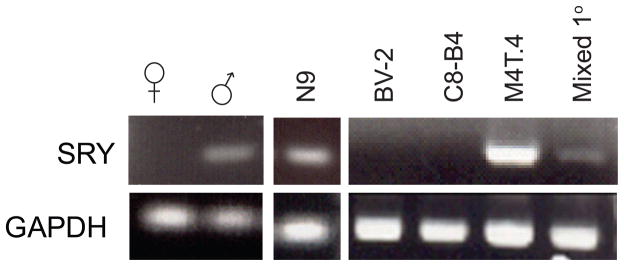
Given the variability of estrogen responses in immortalized microglia, the use of primary microglia seems a reasonable alternative. However, there is a lack of consensus in the field regarding best practices for in vitro examination of estrogen function in microglia in general, and primary cultures are equally complex. Table 4 provides a summary of the range of methods employed for examining estrogen effects in microglia, both in immortalized and primary cultures. In addition to the wide variety of experimental protocols, most primary neonatal microglial cultures are prepared from mixed sex litters, which are not always 50% male and 50% female. Indeed the majority of studies do not report attention to sex in this regard at all, which for most immune function studies is probably irrelevant. However, for studies of estrogen action, additional variability in estrogen responses may come from microglial preparations that are skewed towards one sex or the other, given that cells in the CNS can be highly sexually dimorphic. Our own recent studies have addressed the potential for sexual dimorphism in microglia freshly isolated from murine brain, and not surprisingly, there are sex differences in a number of microglial genes which may influence cellular function [47, 159, 180], although estrogen receptor expression itself is not different between the sexes [159, 180]. Differences in the fundamental responses of male and female astrocytes and microglia have been previously documented [130, 131], suggesting that the sex composition of primary cultures may strongly influence estrogen responses observed in vitro. In this regard, we have evaluated the sex of some commonly used murine microglial cell lines N9, BV-2 and M4T.4, reportedly derived from single cell clones [179, 181, 182], and one commercially available microglial cell line C8-B4 [183] as shown in figure 2. Assessing expression of the Y chromosome-specific gene SRY by RT-PCR, we find that N9 and M4T.4 cells are male and that BV-2 and C8-B4 cells are female. Differences in culturing conditions, treatment parameters, sex of the microglia, genetic strain of microglial sources and the harvesting methods used for primary microglial cultures (and thereby perhaps also the subpopulation of cells examined), makes it difficult to identify the optimal procedures for studying microglial estrogen responses, and further complicates the comparison of results among the published studies discussed previously.
Figure 2. SRY genotype in common murine microglial cell lines.
N9, BV-2, C8-B4, M4T.4 and mixed sex primary neonatal cultures were cultured for at least 7 days prior to harvesting of genomic DNA. The SRY gene was amplified as previously described [47]. GAPDH amplification served as a positive control for DNA integrity in female samples. Results are representative of at least 2 independent cell thaws.
Given the difficulties of estrogen studies in vitro, we have focused on in vivo investigations of potential cross-talk between P2Rs and ERs. Because the expression of certain purinergic receptors is sexually dimorphic [159, 180], we hypothesized that estrogen would have differential effects on P2X receptor expression in microglia freshly isolated from gonadectomized, estrogen-treated mice. As summarized in Table 5, we find that estrogen increases P2X1 and P2X7 mRNA levels in male microglia, but not in female microglia, and P2X4 expression is not altered in microglia from either sex (Crain, Zitzer and Watters, unpublished observations). Although gonads are often the main source of differences in many traits between males and females, our data suggests that microglia from males and females are fundamentally different, and that these differences cannot be compensated for by manipulation of the gonadal hormone environment. They also underscore the need for a better understanding of how purinergic and estrogen receptors contribute to microglial function in both the normal and diseased CNS.
Table 5.
Effect of estrogen on expression of select P2X receptors in freshly-isolated adult murine microglia
| Receptor | Male | Female |
|---|---|---|
| P2X1 | ↑ | - |
| P2X4 | - | - |
| P2X7 | ↑ | - |
↑ gene expression is increased
- gene expression is unchanged
Closing remarks
Neuroinflammation is a hallmark of almost all CNS disorders and injuries. As a result, microglia, and their production of neurotoxic inflammatory substances represent an important therapeutic target to alleviate neuronal damage. Purinergic receptors regulate key microglial properties, including their production of pro-inflammatory cytokines, migration and chemotaxis. If specific P2 receptor subtypes involved in microglial recruitment to sites of neuronal injury could be partially antagonized for example, perhaps less toxic inflammatory mediators would be synthesized in the immediate area of the damaged neurons. Likewise, antagonizing the P2 receptors involved in production of neurotoxic IL-1β may also decrease neuronal damage. However, therapeutics in this regard should be equally balanced, and should not prevent all P2R-regulated microglial activities, as many are beneficial. Similarly, agonizing those P2 receptors whose activities increase neurotrophin or growth factor production by microglia may serve to promote neuron survival and health. Further studies to fully elucidate the role of each P2 receptor in microglia before therapeutically targeting these receptors would be prudent. Lastly, as discussed above, the P2 receptor field in general, suffers from the lack of available, highly selective ligands that allow discrimination of particular receptor subtypes. Additional medicinal chemistry studies are necessary to address this issue.
Given the wide variations in how microglial estrogen studies in vitro are experimentally performed, it is not surprising that the literature on estrogen neuroprotection is inconsistent. A resolution will likely not be reached unless some attempt is made to standardize experimental procedures and protocols, at least within a given cell line or primary cell preparation. We propose that more attention should be paid to the genetic strain of the animals used for primary cell culture, the relative representation of male and female pups in mixed sex neonatal primary cultures, and the regional source of microglia used for the primary cultures. In this regard, the number of primary microglial studies referenced on PubMed that do not acknowledge the rat or mouse strain used for the preparation of neonatal primary cultures (many of which are highly cited) was surprising. Differences in peripheral and even microglial immune responses based on rat and mouse strain are well-documented [184–190], but this issue is not often addressed in the estrogen-microglia literature. In addition, although it is established that neurons from different brain regions exhibit region-specific properties, it is less clear if the same is true of microglia from different CNS regions. It has been suggested that the unique local environments created by neurons could influence microglia and thereby cause regional differences in their characteristics. A few studies provide evidence for regional differences in microglia: their activities are different in different brain regions following various challenges [191–193]. There are also reports of differences in inflammatory gene expression and proliferation rates of cultured microglia derived from different CNS regions [194, 195]. Many studies using primary microglia in culture isolate cells from the whole brain, but microglia from cortex and olfactory bulb are also common sources for primary cultures. When utilizing microglia from the whole brain, there are likely many different microglial populations within a given culture, and the relative abundance of each of these “sub-populations” may influence overall responses to nucleotides and estrogens. Results from our laboratory on differences in microglial gene expression based on CNS region are forthcoming.
Here we have reviewed the literature on the role of microglia in multiple CNS disorders including ischemic and traumatic brain injury, as well as the known functions of purinergic and estrogen receptors in this important cell type. If the purinergic and estrogen receptor systems in microglia are to become viable therapeutic targets for the treatment of neuronal damage resulting from ischemic and traumatic brain injuries (or any other disorder involving neuroinflammation), additional studies are necessary to better delineate specific effects of individual purinergic P2 and estrogen receptor types. The effects of these receptor systems (not only on inflammatory gene expression endpoints, but also on gene expression for microglial neurotrophins, growth factors and other molecules equally important for governing net microglial function in the CNS), are critical for informing effective therapeutic strategies.
It is interesting to speculate that with regard to the most promising therapeutic microglial targets for estrogens, ligands that can selectively activate ERβ may have clinical benefit, pending confirmatory studies that ERβ is upregulated in activated microglia. However, given the beneficial effects of estrogens on neurons and astrocytes as well as microglia, it may not be necessary to activate ERs in specific cell types, or even a specific estrogen receptor subtype. The neuroprotective effects of estrogen may be augmented if mediated by several cell types and receptors concurrently. In contrast, we predict that cell type selectivity will be more important for P2 receptor ligands, as will the identification of specific P2 receptor subtypes that mediate opposing inflammatory and neurotrophic responses of microglia. Before multifaceted studies addressing the interactions of both P2 and estrogen receptor systems can be designed and relevant drugs developed, additional studies will be necessary to better understand the complexities of each receptor system individually in microglia.
Acknowledgments
This work was supported by the NIH/NINDS R01NS049033 and the NIH Training Grant R25GM083252 (JMC).
Footnotes
Conflict of interest: The authors have no conflict of interest.
References
- 1.Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem (Tokyo) 2001;130(2):169–75. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 2.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85(3):352–70. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 3.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 4.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 5.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 6.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 8.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 9.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 10.Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006;1088:219–29. doi: 10.1196/annals.1366.012. [DOI] [PubMed] [Google Scholar]
- 11.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective Ablation of Proliferating Microglial Cells Exacerbates Ischemic Injury in the Brain. J Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galarneau H, Villeneuve J, Gowing G, Julien JP, Vallieres L. Increased Glioma Growth in Mice Depleted of Macrophages. Cancer Res. 2007;67(18):8874–8881. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
- 13.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. American Journal of Physiology. 1993;265(3 Pt 1):C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 14.Lutz PL, Kabler S. Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Research. 1997;769(2):281–6. doi: 10.1016/s0006-8993(97)00719-1. [DOI] [PubMed] [Google Scholar]
- 15.Dunant Y, Israel M. Neurotransmitter release at rapid synapses. Biochimie. 2000;82(4):289–302. doi: 10.1016/s0300-9084(00)00194-2. [DOI] [PubMed] [Google Scholar]
- 16.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochemical Research. 2001;26(8–9):959–69. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 17.Kasai Y, Ohta T, Nakazato Y, Ito S. Release of dopamine and ATP from PC12 cells treated with dexamethasone, reserpine and bafilomycin A1. Journal of Veterinary Medical Science. 2001;63(4):367–72. doi: 10.1292/jvms.63.367. [DOI] [PubMed] [Google Scholar]
- 18.Matsuka Y, Neubert JK, Maidment NT, Spigelman I. Concurrent release of ATP and substance P within guinea pig trigeminal ganglia in vivo. Brain Research. 2001;915(2):248–55. doi: 10.1016/s0006-8993(01)02888-8. [DOI] [PubMed] [Google Scholar]
- 19.Poelchen W, Sieler D, Wirkner K, Illes P. Co-transmitter function of ATP in central catecholaminergic neurons of the rat. Neuroscience. 2001;102(3):593–602. doi: 10.1016/s0306-4522(00)00529-7. [DOI] [PubMed] [Google Scholar]
- 20.Cantiello HF. Nucleotide transport through the cystic fibrosis transmembrane conductance regulator. Bioscience Reports. 1997;17(2):147–71. doi: 10.1023/a:1027381412574. [DOI] [PubMed] [Google Scholar]
- 21.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15735–40. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. Journal of Neuroscience. 2000;20(8):2835–44. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. Journal of Neuroscience. 1998;18(21):8794–804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. Journal of Neuroscience. 1999;19(2):520–8. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. Journal of Neuroscience. 2001;21(7):2215–23. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiga H, Tojima T, Ito E. Ca2+ signaling regulated by an ATP-dependent autocrine mechanism in astrocytes. Neuroreport. 2001;12(12):2619–22. doi: 10.1097/00001756-200108280-00007. [DOI] [PubMed] [Google Scholar]
- 27.Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc. 2007;118:199–208. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de Mendonça A. Adenosine A2A receptors and brain injury: Broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Progress in Neurobiology. 2007;83(5):310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi H, Yokofujita J, Murakami K, Okada A, Kuroda M. Microglial ecto-Ca2+-ATPase activity in a rat model of focal homologous blood clot embolic cerebral ischemia: an enzyme histochemical study. Brain Research Bulletin. 2003;60(1–2):93–104. doi: 10.1016/s0361-9230(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 30.Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. American Journal of Physiology. 1999;276(1 Pt 1):C267–78. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 31.Edwards J, Sprung R, Sprague R, Spence D. Chemiluminescence detection of ATP release from red blood cells upon passage through microbore tubing. Analyst. 2001;126(8):1257–60. doi: 10.1039/b100519g. [DOI] [PubMed] [Google Scholar]
- 32.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Medical Science Monitor. 2001;7(4):669–74. [PubMed] [Google Scholar]
- 33.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredholm BB. Adenosine receptors as targets for drug development. Drug News Perspect. 2003;16(5):283–9. doi: 10.1358/dnp.2003.16.5.829316. [DOI] [PubMed] [Google Scholar]
- 36.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–52. [PMC free article] [PubMed] [Google Scholar]
- 37.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377(6548):432–5. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 39.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 40.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends in Pharmacological Sciences. 2003;24(2):52. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 42.Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24(28):6307–14. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashour F, Atterbury-Thomas M, Deuchars J, Evans RJ. An evaluation of antibody detection of the P2X1 receptor subunit in the CNS of wild type and P2X1-knockout mice. Neurosci Lett. 2006;397(1–2):120–5. doi: 10.1016/j.neulet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CM, Nedergaard M. Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci. 2006;29(5):257–62. doi: 10.1016/j.tins.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Nasu-Tada K, Koizumi S, Inoue K. Involvement of beta1 integrin in microglial chemotaxis and proliferation on fibronectin: different regulations by ADP through PKA. Glia. 2005;52(2):98–107. doi: 10.1002/glia.20224. [DOI] [PubMed] [Google Scholar]
- 46.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55(6):604–16. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 47.Crain J, Nikodemova M, Watters J. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. Journal of Neuroinflammation. 2009;6(1):24. doi: 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156(4):1531–9. [PubMed] [Google Scholar]
- 49.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185(3):579–82. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 Purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci Lett. 2000;282(3):153–6. doi: 10.1016/s0304-3940(00)00887-9. [DOI] [PubMed] [Google Scholar]
- 51.Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol. 2000;164(9):4893–8. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- 52.Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22(2):234–44. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 54.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176(7):3877–83. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 55.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180(11):7147–57. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 56.Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75(3):965–72. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rampe D, Wang L, Ringheim GE. P2X7 receptor modulation of beta-amyloid- and LPS-induced cytokine secretion from human macrophages and microglia. J Neuroimmunol. 2004;147(1–2):56–61. doi: 10.1016/j.jneuroim.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27(18):4957–68. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chessell IP, Michel AD, Humphrey PP. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol. 1997;121(7):1429–37. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Di Virgilio F. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36(9):1295–301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447(1):71–5. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 63.Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, Kohsaka S. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. Br J Pharmacol. 1998;123(7):1304–10. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139(7):1635–43. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274(19):13205–10. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 67.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–8. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29(11):3518–28. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–82. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Irino Y, Nakamura Y, Inoue K, Kohsaka S, Ohsawa K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J Neurosci Res. 2008;86(7):1511–9. doi: 10.1002/jnr.21610. [DOI] [PubMed] [Google Scholar]
- 72.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 73.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28(19):4949–56. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seo DR, Kim SY, Kim KY, Lee HG, Moon JH, Lee JS, Lee SH, Kim SU, Lee YB. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp Mol Med. 2008;40(1):19–26. doi: 10.3858/emm.2008.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–5. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wollmer MA, Lucius R, Wilms H, Held-Feindt J, Sievers J, Mentlein R. ATP and adenosine induce ramification of microglia in vitro. J Neuroimmunol. 2001;115(1–2):19–27. doi: 10.1016/s0165-5728(01)00257-0. [DOI] [PubMed] [Google Scholar]
- 77.Xiang Z, Chen M, Ping J, Dunn P, Lv J, Jiao B, Burnstock G. Microglial morphology and its transformation after challenge by extracellular ATP in vitro. J Neurosci Res. 2006;83(1):91–101. doi: 10.1002/jnr.20709. [DOI] [PubMed] [Google Scholar]
- 78.Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78(6):1339–49. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- 79.Ogata T, Chuai M, Morino T, Yamamoto H, Nakamura Y, Schubert P. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Res. 2003;981(1–2):174–83. doi: 10.1016/s0006-8993(03)03028-2. [DOI] [PubMed] [Google Scholar]
- 80.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278(15):13309–17. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 81.Skaper SD, Facci L, Culbert AA, Evans NA, Chessell I, Davis JB, Richardson JC. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia. 2006;54(3):234–42. doi: 10.1002/glia.20379. [DOI] [PubMed] [Google Scholar]
- 82.Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, Gonzalez FA, Weisman GA, Sun GY. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87(2):344–52. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 83.Kim SY, Moon JH, Lee HG, Kim SU, Lee YB. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med. 2007;39(6):820–7. doi: 10.1038/emm.2007.89. [DOI] [PubMed] [Google Scholar]
- 84.Brautigam VM, Frasier C, Nikodemova M, Watters JJ. Purinergic receptor modulation of BV-2 microglial cell activity: potential involvement of p38 MAP kinase and CREB. J Neuroimmunol. 2005;166(1–2):113–25. doi: 10.1016/j.jneuroim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17(11):2267–76. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 86.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32(2):79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7(7):575–90. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 89.Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5(1):63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151(5):571–9. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friedle SA, Curet MA, Watters JJ. Recent Patents on Novel P2X(7) Receptor Antagonists and Their Potential for Reducing Central Nervous System Inflammation. Recent Pat CNS Drug Discov. doi: 10.2174/157488910789753530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guile SD, Alcaraz L, Birkinshaw TN, Bowers KC, Ebden MR, Furber M, Stocks MJ. Antagonists of the P2X7 Receptor. From Lead Identification to Drug Development. Journal of Medicinal Chemistry. 2009;52(10):3123–3141. doi: 10.1021/jm801528x. [DOI] [PubMed] [Google Scholar]
- 93.Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28(37):9133–44. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol. 2006;65(11):1090–7. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- 96.Takenouchi T, Iwamaru Y, Imamura M, Kato N, Sugama S, Fujita M, Hashimoto M, Sato M, Okada H, Yokoyama T, Mohri S, Kitani H. Prion infection correlates with hypersensitivity of P2X7 nucleotide receptor in a mouse microglial cell line. FEBS Lett. 2007;581(16):3019–26. doi: 10.1016/j.febslet.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 97.Raouf R, Chabot-Dore AJ, Ase AR, Blais D, Seguela P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology. 2007;53(4):496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Guo LH, Guo KT, Wendel HP, Schluesener HJ. Combinations of TLR and NOD2 ligands stimulate rat microglial P2X4R expression. Biochem Biophys Res Commun. 2006;349(3):1156–62. doi: 10.1016/j.bbrc.2006.08.146. [DOI] [PubMed] [Google Scholar]
- 99.Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48(2):144–56. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D’Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volonte C. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120(1):85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 101.Cavaliere F, Dinkel K, Reymann K. Microglia response and P2 receptor participation in oxygen/glucose deprivation-induced cortical damage. Neuroscience. 2005;136(3):615–23. doi: 10.1016/j.neuroscience.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 102.Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63(7):686–99. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 103.Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull. 2008;31(6):1121–30. doi: 10.1248/bpb.31.1121. [DOI] [PubMed] [Google Scholar]
- 104.Wixey JA, Reinebrant HE, Carty ML, Buller KM. Delayed P2X4R expression after hypoxia-ischemia is associated with microglia in the immature rat brain. J Neuroimmunol. 2009;212(1–2):35–43. doi: 10.1016/j.jneuroim.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 105.Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163(1–2):120–7. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 106.Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163(1–2):185–9. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Z, Artelt M, Burnet M, Trautmann K, Schluesener HJ. Lesional accumulation of P2X4 receptor+ monocytes following experimental traumatic brain injury. Exp Neurol. 2006;197(1):252–7. doi: 10.1016/j.expneurol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152(2):495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 109.Sharp AJ, Polak PE, Simonini V, Lin SX, Richardson JC, Bongarzone ER, Feinstein DL. P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2008;5:33. doi: 10.1186/1742-2094-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(19):8032–7. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Di Virgilio F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J Immunol. 2009;182(7):4378–85. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- 112.Le Feuvre RA, Brough D, Touzani O, Rothwell NJ. Role of P2X7 receptors in ischemic and excitotoxic brain injury in vivo. J Cereb Blood Flow Metab. 2003;23(3):381–4. doi: 10.1097/01.WCB.0000048519.34839.97. [DOI] [PubMed] [Google Scholar]
- 113.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26(7):974–82. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- 114.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106(30):12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–7. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 116.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28(11):2892–902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29(12):3781–91. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Estrogen and cytokines production - the possible cause of gender differences in neurological diseases. Curr Pharm Des. 2005;11(8):1017–30. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- 119.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci. 2006;1089:302–23. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 120.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29(4):507–19. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El-Etr M, Vukusic S, Gignoux L, Durand-Dubief F, Achiti I, Baulieu EE, Confavreux C. Steroid hormones in multiple sclerosis. Journal of the Neurological Sciences. 2005;233(1–2):49–54. doi: 10.1016/j.jns.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 122.Gandy S. Estrogen and neurodegeneration. Neurochem Res. 2003;28(7):1003–8. doi: 10.1023/a:1023246921127. [DOI] [PubMed] [Google Scholar]
- 123.Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson’s disease. Frontiers in Neuroendocrinology. 2009;30(2):142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 124.Singh MN, Martin-Hirsch PL, Martin FL. The multiple applications of tamoxifen: an example pointing to SERM modulation being the aspirin of the 21st century. Med Sci Monit. 2008;14(9):RA144–8. [PubMed] [Google Scholar]
- 125.Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. 2008;51(3):618–26. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rudnicki SA. Estrogen replacement therapy in women with amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 1999;169(1–2):126–127. doi: 10.1016/s0022-510x(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 127.Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28(3):235–42. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- 128.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone Therapy: Physiological Complexity Belies Therapeutic Simplicity. Science. 2004;304(5675):1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 129.Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3(1):97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- 130.Dasgupta S, Jana M, Liu X, Pahan K. Myelin Basic Protein-primed T Cells of Female but Not Male Mice Induce Nitric-oxide Synthase and Proinflammatory Cytokines in Microglia: IMPLICATIONS FOR GENDER BIAS IN MULTIPLE SCLEROSIS. J Biol Chem. 2005;280(38):32609–32617. doi: 10.1074/jbc.M500299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cordeau P, Jr, Lalancette-Hebert M, Weng YC, Kriz J. Live Imaging of Neuroinflammation Reveals Sex and Estrogen Effects on Astrocyte Response to Ischemic Injury. Stroke. 2008;39(3):935–942. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- 132.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–56. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 133.Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35(9–10):1309–16. doi: 10.1016/s0531-5565(00)00161-3. [DOI] [PubMed] [Google Scholar]
- 134.Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21(6):1809–18. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu X, Fan XL, Zhao Y, Luo GR, Li XP, Li R, Le WD. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J Neurosci Res. 2005;81(5):653–65. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- 136.Reed JL, Dimayuga FO, Davies LM, Keller JN, Bruce-Keller AJ. Estrogen increases proteasome activity in murine microglial cells. Neurosci Lett. 2004;367(1):60–5. doi: 10.1016/j.neulet.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 137.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75(4):1447–54. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- 138.Harris-White ME, Chu T, Miller SA, Simmons M, Teter B, Nash D, Cole GM, Frautschy SA. Estrogen (E2) and glucocorticoid (Gc) effects on microglia and A beta clearance in vitro and in vivo. Neurochem Int. 2001;39(5–6):435–48. doi: 10.1016/s0197-0186(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 139.Lei DL, Long JM, Hengemihle J, O’Neill J, Manaye KF, Ingram DK, Mouton PR. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003;121(3):659–66. doi: 10.1016/s0306-4522(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 140.Tripanichkul W, Sripanichkulchai K, Finkelstein DI. Estrogen down-regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication. Brain Res. 2006;1084(1):28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 141.Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147(5):2263–72. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- 142.Marriott LK, Hauss-Wegrzyniak B, Benton RS, Vraniak PD, Wenk GL. Long-term estrogen therapy worsens the behavioral and neuropathological consequences of chronic brain inflammation. Behav Neurosci. 2002;116(5):902–11. doi: 10.1037//0735-7044.116.5.902. [DOI] [PubMed] [Google Scholar]
- 143.Tripanichkul W, Jaroensuppaperch EO, Finkelstein DI. Estrogen enhances the number of nigral dopaminergic neurons of adult male mice without affecting nigral neuroglial number and morphology. Neurosci Lett. 2008;435(3):210–4. doi: 10.1016/j.neulet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 144.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–74. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 145.Suuronen T, Nuutinen T, Huuskonen J, Ojala J, Thornell A, Salminen A. Anti-inflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflamm Res. 2005;54(5):194–203. doi: 10.1007/s00011-005-1343-z. [DOI] [PubMed] [Google Scholar]
- 146.Tapia-Gonzalez S, Carrero P, Pernia O, Garcia-Segura LM, Diz-Chaves Y. Selective oestrogen receptor (ER) modulators reduce microglia reactivity in vivo after peripheral inflammation: potential role of microglial ERs. J Endocrinol. 2008;198(1):219–230. doi: 10.1677/JOE-07-0294. [DOI] [PubMed] [Google Scholar]
- 147.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Frontiers in Neuroendocrinology. 2009;30(2):201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Offner H, Polanczyk M. A Potential Role for Estrogen in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Annals of the New York Academy of Sciences. 2006;1089 doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]; Estrogens and Human Diseases. :343–372. [Google Scholar]
- 149.Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47(4):269–75. doi: 10.1016/j.maturitas.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 150.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 151.Alonso LC, Rosenfield RL. Oestrogens and puberty. Best Pract Res Clin Endocrinol Metab. 2002;16(1):13–30. doi: 10.1053/beem.2002.0177. [DOI] [PubMed] [Google Scholar]
- 152.Fowler AM, Alarid ET. Dynamic control of nuclear receptor transcription. Sci STKE. 2004;2004(256):pe51. doi: 10.1126/stke.2562004pe51. [DOI] [PubMed] [Google Scholar]
- 153.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41(5):263–275. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor [kappa]B. Oncogene. 25(51):6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- 155.Sheldahl LC, Marriott LK, Bryant DM, Shapiro RA, Dorsa DM. Neuroprotective effects of estrogen and selective estrogen receptor modulators begin at the plasma membrane. Minerva Endocrinol. 2007;32(2):87–94. [PubMed] [Google Scholar]
- 156.Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int. 2006;49(2):204–14. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 157.Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29(2):199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- 158.Manthey D, Behl C. From structural biochemistry to expression profiling: neuroprotective activities of estrogen. Neuroscience. 2006;138(3):845–50. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 159.Crain JM, Watters JJ. Society for Neuroscience. Washington D.C.: 2008. Age-dependent expression of cytokines in mouse microglia; p. 728.9. [Google Scholar]
- 160.Baker AE, V, Brautigam M, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–32. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 161.Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol. 2008;195(1–2):47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, Naftolin F. Estrogen and microglia: A regulatory system that affects the brain. J Neurobiol. 1999;40(4):484–96. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi N, Tonchev AB, Koike K, Murakami K, Yamada K, Yamashima T, Inoue M. Expression of estrogen receptor-beta in the postischemic monkey hippocampus. Neurosci Lett. 2004;369(1):9–13. doi: 10.1016/j.neulet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 164.Hoffman G, Merchenthaler I, Zup S. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29(2):217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 165.Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003;993:125–33. doi: 10.1111/j.1749-6632.2003.tb07521.x. discussion 159–60. [DOI] [PubMed] [Google Scholar]
- 166.Yang SH, Liu R, Perez EJ, Wang X, Simpkins JW. Estrogens as protectants of the neurovascular unit against ischemic stroke. Curr Drug Targets CNS Neurol Disord. 2005;4(2):169–77. doi: 10.2174/1568007053544174. [DOI] [PubMed] [Google Scholar]
- 167.Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6(1):13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- 168.Garidou L, Laffont S, Douin-Echinard V, Coureau C, Krust A, Chambon P, Guery JC. Estrogen Receptor {alpha} Signaling in Inflammatory Leukocytes Is Dispensable for 17{beta}-Estradiol-Mediated Inhibition of Experimental Autoimmune Encephalomyelitis. J Immunol. 2004;173(4):2435–2442. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- 169.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. Journal of Neuroimmunology. 2005;170(1–2):85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 170.Xuan L, Xiao-Lan F, Yan Z, Guang-Rui L, Xu-Ping L, Rui L, Wei-Dong L. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. Journal of Neuroscience Research. 2005;81(5):653–665. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- 171.Marin R, Díaz M, Alonso R, Sanz A, Arévalo MA, Garcia-Segura LM. Role of estrogen receptor [alpha] in membrane-initiated signaling in neural cells: Interaction with IGF-1 receptor. The Journal of Steroid Biochemistry and Molecular Biology. 2009;114(1–2):2–7. doi: 10.1016/j.jsbmb.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 172.Scharfman HE, MacLusky NJ. Estrogen-growth factor interactions and their contributions to neurological disorders. Headache. 2008;48(Suppl 2):S77–89. doi: 10.1111/j.1526-4610.2008.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ. Cross-Talk Between Membrane-Initiated and Nuclear-Initiated Oestrogen Signalling in the Hypothalamus. Journal of Neuroendocrinology. 2009;21(4):263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carley ME, Cliby WA, Spelsberg TC. P2X(3) receptor subunit messenger RNA expression in the female mouse bladder after oophorectomy with or without estrogen replacement. Am J Obstet Gynecol. 2002;187(1):103–6. doi: 10.1067/mob.2002.125705. [DOI] [PubMed] [Google Scholar]
- 175.Papka RE, Hafemeister J, Storey-Workley M. P2X receptors in the rat uterine cervix, lumbosacral dorsal root ganglia, and spinal cord during pregnancy. Cell Tissue Res. 2005;321(1):35–44. doi: 10.1007/s00441-005-1114-8. [DOI] [PubMed] [Google Scholar]
- 176.Felsher DW, Bishop JM. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci U S A. 1999;96(7):3940–4. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li Y, Lu J, Prochownik EV. c-Myc-mediated genomic instability proceeds via a megakaryocytic endomitosis pathway involving Gp1balpha. Proc Natl Acad Sci U S A. 2007;104(9):3490–5. doi: 10.1073/pnas.0610163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Neiman PE, Elsaesser K, Loring G, Kimmel R. Myc oncogene-induced genomic instability: DNA palindromes in bursal lymphomagenesis. PLoS Genet. 2008;4(7):e1000132. doi: 10.1371/journal.pgen.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. European Journal of Immunology. 1989;19(8):1443–8. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- 180.Crain JM, Watters JJ. Cytokine and BDNF expression vary with age and sex in mouse microglia. Journal of Neurochemistry. 2009;108(Suppl 1):138. [Google Scholar]
- 181.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 182.Olson JK, Zamvil SS, Miller SD. Efficient technique for immortalization of murine microglial cells relevant for studies in murine models of multiple sclerosis. Journal of Neuroscience Methods. 2003;128(1–2):33–43. doi: 10.1016/s0165-0270(03)00145-6. [DOI] [PubMed] [Google Scholar]
- 183.Alliot F, Marty MC, Cambier D, Pessac B. A spontaneously immortalized mouse microglial cell line expressing CD4. Developmental Brain Research. 1996;95(1):140–143. doi: 10.1016/0165-3806(96)00101-0. [DOI] [PubMed] [Google Scholar]
- 184.Chung IY, Norris JG, Benveniste EN. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991;173(4):801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sedgwick JD, Schwender S, Gregersen R, Dorries R, ter Meulen V. Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med. 1993;177(4):1145–1152. doi: 10.1084/jem.177.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sebastian J, Guido S. Strain-specific expression of microglial keratan sulfate proteoglycans in the normal rat central nervous system: Inverse correlation with constitutive expression of major histocompatibility complex class II antigens. Glia. 1996;18(3):255–260. doi: 10.1002/(SICI)1098-1136(199611)18:3<255::AID-GLIA9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 187.Lambertsen KL, Gregersen R, Finsen B. Microglial-Macrophage Synthesis of Tumor Necrosis Factor After Focal Cerebral Ischemia in Mice Is Strain Dependent. J Cereb Blood Flow Metab. 2002;22(7):785–797. doi: 10.1097/00004647-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 188.Piehl F, Olsson T. Inflammation and susceptibility to neurodegeneration: the use of unbiased genetics to decipher critical regulatory pathways. Neuroscience. 2009;158(3):1143–50. doi: 10.1016/j.neuroscience.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 189.Simmons DA, Broderick PA. Cytokines, stressors, and clinical depression: augmented adaptation responses underlie depression pathogenesis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):793–807. doi: 10.1016/j.pnpbp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 190.Phillip GP, Ping W, Bradford TS. Cellular inflammatory response after spinal cord injury in sprague-dawley and lewis rats. The Journal of Comparative Neurology. 1997;377(3):443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 191.Phillips LM, Simon PJ, Lampson LA. Site-specific immune regulation in the brain: differential modulation of major histocompatibility complex (MHC) proteins in brainstem vs. hippocampus. J Comp Neurol. 1999;405(3):322–33. doi: 10.1002/(sici)1096-9861(19990315)405:3<322::aid-cne3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 192.Kullberg S, Aldskogius H, Ulfhake B. Microglial activation, emergence of ED1-expressing cells and clusterin upregulation in the aging rat CNS, with special reference to the spinal cord. Brain Res. 2001;899(1–2):169–86. doi: 10.1016/s0006-8993(01)02222-3. [DOI] [PubMed] [Google Scholar]
- 193.Kanaan NM, Kordower JH, Collier TJ. Age and region-specific responses of microglia, but not astrocytes, suggest a role in selective vulnerability of dopamine neurons after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure in monkeys. Glia. 2008;56(11):1199–214. doi: 10.1002/glia.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ren L, Lubrich B, Biber K, Gebicke-Haerter PJ. Differential expression of inflammatory mediators in rat microglia cultured from different brain regions. Brain Res Mol Brain Res. 1999;65(2):198–205. doi: 10.1016/s0169-328x(99)00016-9. [DOI] [PubMed] [Google Scholar]
- 195.Marshall GP, 2nd, Demir M, Steindler DA, Laywell ED. Subventricular zone microglia possess a unique capacity for massive in vitro expansion. Glia. 2008;56(16):1799–808. doi: 10.1002/glia.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110(3):415–32. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 197.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7(6):423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Florenzano F, Carrive P, Viscomi MT, Ferrari F, Latini L, Conversi D, Cabib S, Bagni C, Molinari M. Cortical and subcortical distribution of ionotropic purinergic receptor subunit type 1 (P2X(1)R) immunoreactive neurons in the rat forebrain. Neuroscience. 2008;151(3):791–801. doi: 10.1016/j.neuroscience.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 199.Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PP. Localization of P2X purinoceptor transcripts in the rat nervous system. Mol Pharmacol. 1995;48(4):569–73. [PubMed] [Google Scholar]
- 200.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16(8):2495–507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xiang Z, Burnstock G. Changes in expression of P2X purinoceptors in rat cerebellum during postnatal development. Brain Res Dev Brain Res. 2005;156(2):147–57. doi: 10.1016/j.devbrainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 202.Xiang Z, Burnstock G. Expression of P2X receptors on rat microglial cells during early development. Glia. 2005;52(2):119–26. doi: 10.1002/glia.20227. [DOI] [PubMed] [Google Scholar]
- 203.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371(6497):519–23. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 204.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93(15):8063–7. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377(6548):428–31. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 206.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375(1–2):129–33. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- 207.Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. Embo J. 1996;15(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- 208.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci U S A. 1996;93(8):3684–8. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seguela P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2x ATP receptor ion channel with widespread distribution in the brain. J Neurosci. 1996;16(2):448–55. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Le KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Seguela P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83(1):177–90. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- 211.Guo W, Xu X, Gao X, Burnstock G, He C, Xiang Z. Expression of P2X5 receptors in the mouse CNS. Neuroscience. 2008;156(3):673–92. doi: 10.1016/j.neuroscience.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 212.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36(9):1277–83. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 213.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, Umemura A, Mase M, Yamada K, Shimada S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 214.Moran-Jimenez MJ, Matute C. Immunohistochemical localization of the P2Y(1) purinergic receptor in neurons and glial cells of the central nervous system. Brain Res Mol Brain Res. 2000;78(1–2):50–8. doi: 10.1016/s0169-328x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- 215.Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498(4):443–54. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- 216.Simon J, Webb TE, Barnard EA. Distribution of [35S]dATP alpha S binding sites in the adult rat neuraxis. Neuropharmacology. 1997;36(9):1243–51. doi: 10.1016/s0028-3908(97)00124-x. [DOI] [PubMed] [Google Scholar]
- 217.Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y(1) purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;421(3):374–84. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 218.Laitinen JT, Uri A, Raidaru G, Miettinen R. [(35)S]GTPgammaS autoradiography reveals a wide distribution of G(i/o)-linked ADP receptors in the nervous system: close similarities with the platelet P2Y(ADP) receptor. J Neurochem. 2001;77(2):505–18. doi: 10.1046/j.1471-4159.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- 219.Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44(3):242–50. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- 220.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–7. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 221.Amadio S, Tramini G, Martorana A, Viscomi MT, Sancesario G, Bernardi G, Volonte C. Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neuroscience. 2006;141(3):1171–80. doi: 10.1016/j.neuroscience.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 222.Light AR, Wu Y, Hughen RW, Guthrie PB. Purinergic receptors activating rapid intracellular Ca increases in microglia. Neuron Glia Biol. 2006;2(2):125–138. doi: 10.1017/S1740925X05000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Guo LH, Schluesener HJ. Acute but not chronic stimulation of glial cells in rat spinal cord by systemic injection of lipopolysaccharide is associated with hyperalgesia. Acta Neuropathol. 2006;112(6):703–13. doi: 10.1007/s00401-006-0135-z. [DOI] [PubMed] [Google Scholar]
- 224.Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29(4):998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di Virgilio F, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99(3):745–58. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- 226.Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108(1):115–25. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 227.Tanaka S, Koike T. Selective inflammatory stimulations enhance release of microglial response factor (MRF)-1 from cultured microglia. Glia. 2002;40(3):360–71. doi: 10.1002/glia.10142. [DOI] [PubMed] [Google Scholar]
- 228.Kaya N, Tanaka S, Koike T. ATP selectively suppresses the synthesis of the inflammatory protein microglial response factor (MRF)-1 through Ca(2+) influx via P2X(7) receptors in cultured microglia. Brain Res. 2002;952(1):86–97. doi: 10.1016/s0006-8993(02)03200-6. [DOI] [PubMed] [Google Scholar]
- 229.Witting A, Walter L, Wacker J, Moller T, Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci U S A. 2004;101(9):3214–9. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Morioka N, Abdin MJ, Kitayama T, Morita K, Nakata Y, Dohi T. P2X(7) receptor stimulation in primary cultures of rat spinal microglia induces downregulation of the activity for glutamate transport. Glia. 2008;56(5):528–38. doi: 10.1002/glia.20634. [DOI] [PubMed] [Google Scholar]
- 231.Nakanishi M, Mori T, Nishikawa K, Sawada M, Kuno M, Asada A. The effects of general anesthetics on P2X7 and P2Y receptors in a rat microglial cell line. Anesth Analg. 2007;104(5):1136–44. doi: 10.1213/01.ane.0000260615.12553.4e. tables of contents. [DOI] [PubMed] [Google Scholar]
- 232.Takenouchi T, Nakai M, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, Wei J, Sekigawa A, Sato M, Kojima S, Kitani H, Hashimoto M. The activation of P2X7 receptor impairs lysosomal functions and stimulates the release of autophagolysosomes in microglial cells. J Immunol. 2009;182(4):2051–62. doi: 10.4049/jimmunol.0802577. [DOI] [PubMed] [Google Scholar]
- 233.Ballerini P, Di Iorio P, Ciccarelli R, Caciagli F, Poli A, Beraudi A, Buccella S, D’Alimonte I, D’Auro M, Nargi E, Patricelli P, Visini D, Traversa U. P2Y1 and cysteinyl leukotriene receptors mediate purine and cysteinyl leukotriene co-release in primary cultures of rat microglia. Int J Immunopathol Pharmacol. 2005;18(2):255–68. doi: 10.1177/039463200501800208. [DOI] [PubMed] [Google Scholar]
- 234.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol. 1997;143(2):313–8. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- 235.Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111(1–2):77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 236.Vegeto E, Ghisletti S, Meda C, Etteri S, Belcredito S, Maggi A. Regulation of the lipopolysaccharide signal transduction pathway by 17beta-estradiol in macrophage cells. J Steroid Biochem Mol Biol. 2004;91(1–2):59–66. doi: 10.1016/j.jsbmb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 237.Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: the two receptors mediate differential effects of corticosteroids. Glia. 1997;20(1):23–37. [PubMed] [Google Scholar]
- 238.Lieb K, Engels S, Fiebich BL. Inhibition of LPS-induced iNOS and NO synthesis in primary rat microglial cells. Neurochem Int. 2003;42(2):131–7. doi: 10.1016/s0197-0186(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 239.Johnson AB, Sohrabji F. Estrogen’s effects on central and circulating immune cells vary with reproductive age. Neurobiol Aging. 2005;26(10):1365–74. doi: 10.1016/j.neurobiolaging.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 240.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25(8):2957–68. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Brown CM, Choi E, Xu Q, Vitek MP, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17beta-estradiol. Neurobiol Aging. 2008;29(12):1783–94. doi: 10.1016/j.neurobiolaging.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cheepsunthorn P, Mairaue N, Nasee K. Estrogen enhances the inhibitory effect of iron on microglial nitric oxide production. J Med Assoc Thai. 2006;89(6):840–5. [PubMed] [Google Scholar]
- 243.Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol. J Neurochem. 2001;78(6):1315–24. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- 244.Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161(1–2):123–36. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 245.Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, Munch G. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci. 2001;14(12):1961–7. doi: 10.1046/j.0953-816x.2001.01820.x. [DOI] [PubMed] [Google Scholar]
- 246.Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35(1):53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]