Abstract
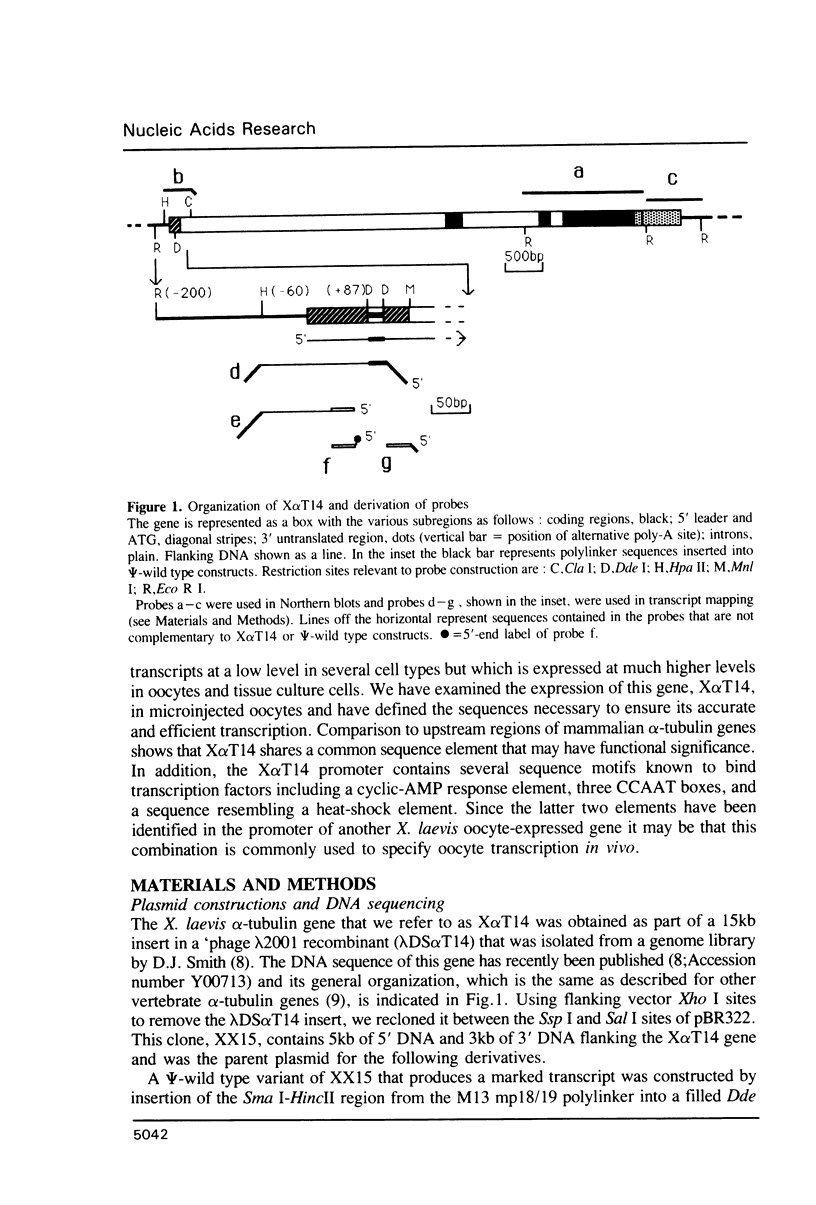
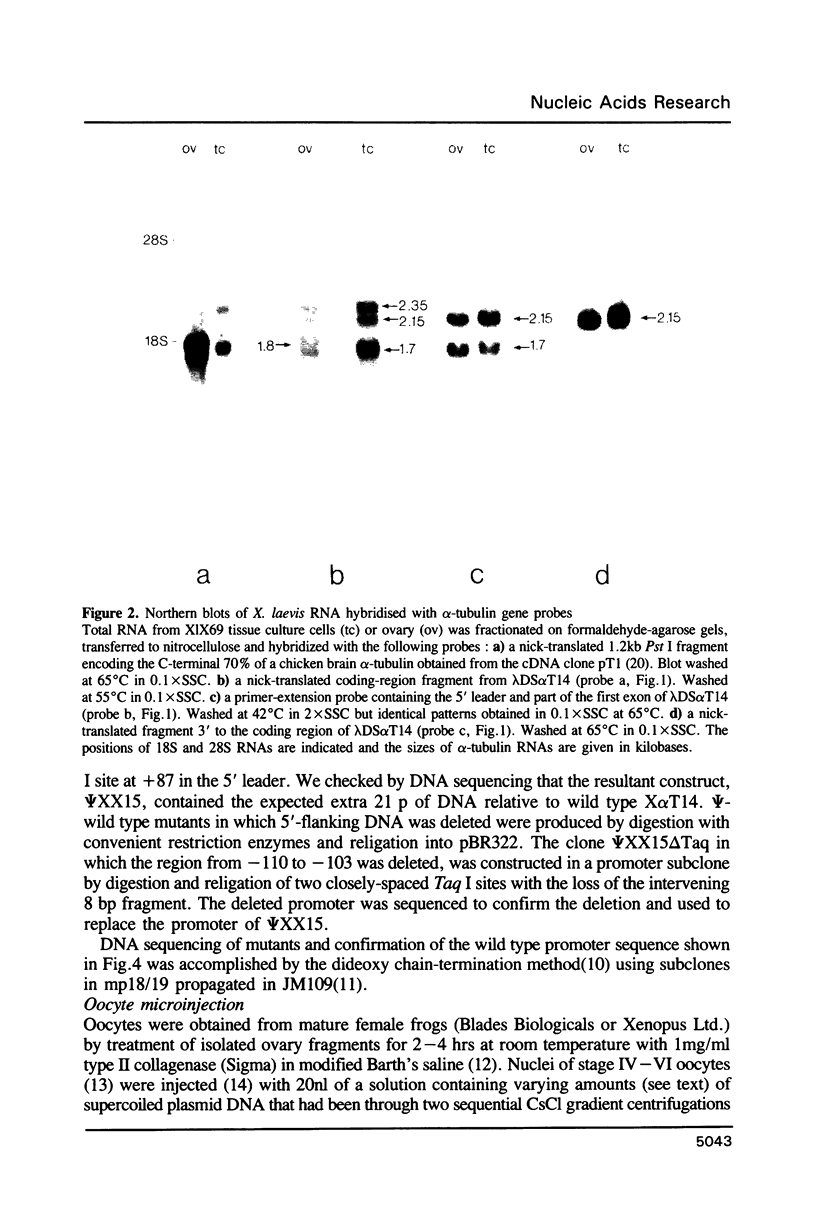
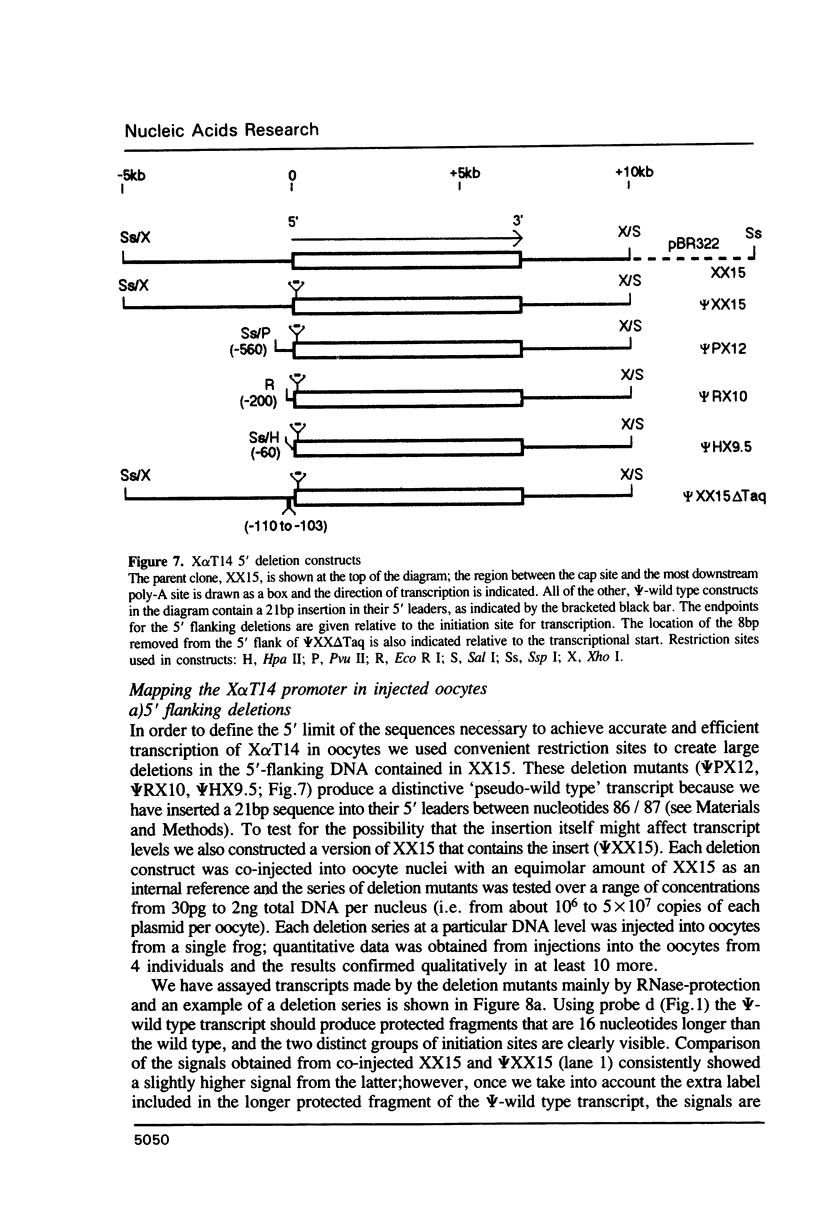
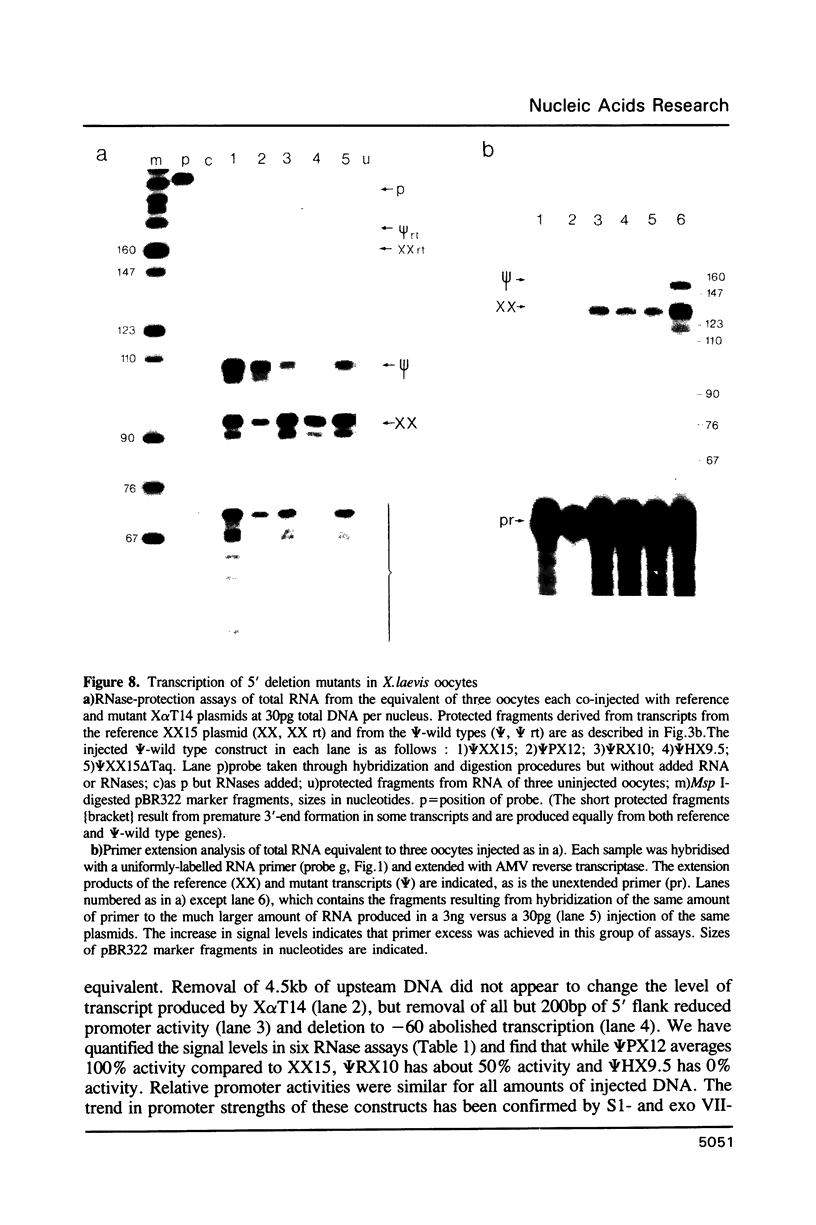
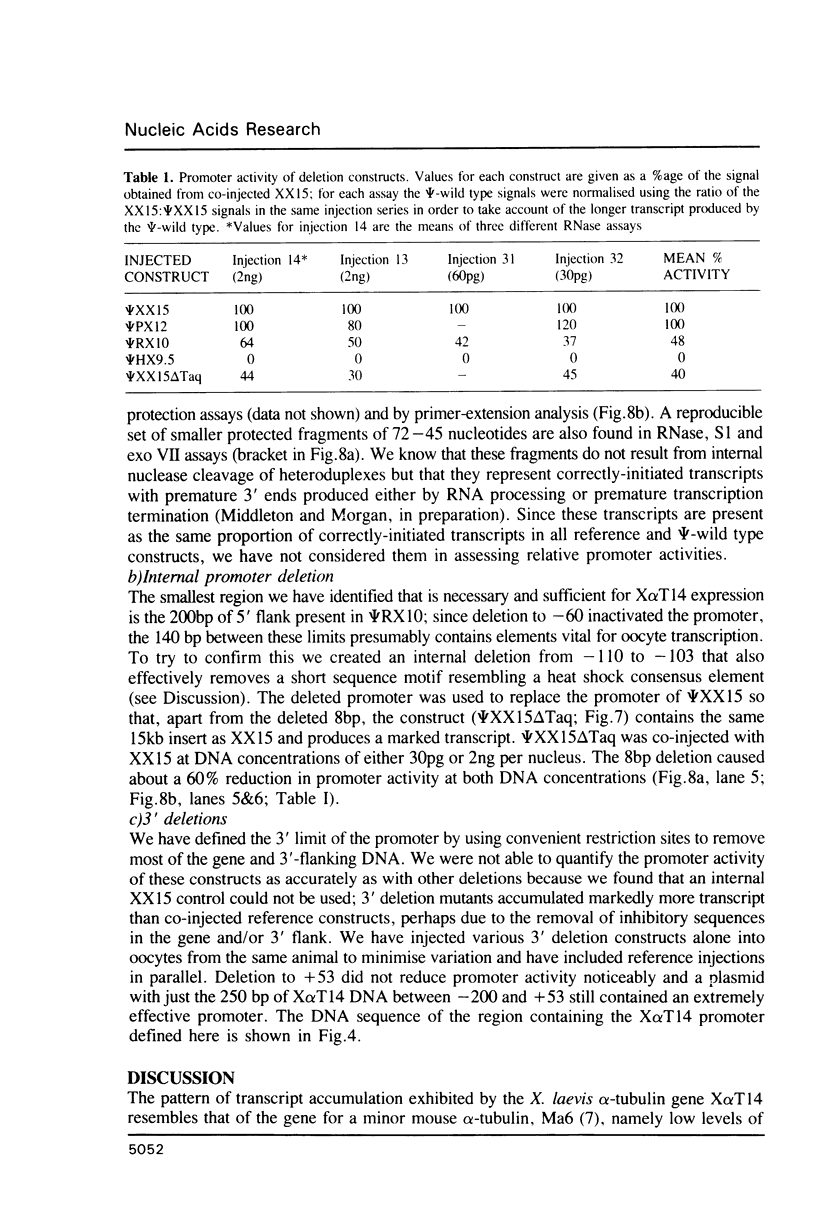
We have studied the expression of X alpha T14, a member of the alpha-tubulin multigene family in Xenopus laevis. Small amounts of X alpha T14 RNA are detectable in a range of cell types, but much higher levels are present in ovary and tissue culture cells. In oocytes X alpha T14 transcripts accumulate during early vitellogenesis but their level declines in more advanced stages. Faithful and efficient initiation of transcription occurred on cloned X alpha T14 injected into oocytes even at low template levels. We have examined the amount of transcript produced by various deletion mutants relative to a co-injected control gene. The presence of 200bp of DNA 5' and 53bp of DNA 3' to the initiation site sufficed for high levels of promoter activity, although maximum activity required 560 bp of 5' flanking DNA. The DNA between -200 and -60 was necessary for transcription in oocytes and contains several sequence motifs implicated in transcriptional regulation including three CCAAT boxes and a sequence resembling a heat shock element. An 8 bp deletion that removed the latter element from 5kb of 5'-flanking DNA reduced promoter activity by 60%.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. L. Most kappa immunoglobulin mRNA in human lymphocytes is homologous to a small family of germ-line V genes. Nature. 1984 Jan 5;307(5946):77–80. doi: 10.1038/307077a0. [DOI] [PubMed] [Google Scholar]
- Bienz M. A CCAAT box confers cell-type-specific regulation on the Xenopus hsp70 gene in oocytes. Cell. 1986 Sep 26;46(7):1037–1042. doi: 10.1016/0092-8674(86)90703-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. The multitubulin hypothesis revisited: what have we learned? J Cell Biol. 1987 Mar;104(3):381–383. doi: 10.1083/jcb.104.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J. Tubulin genes and the diversity of microtubule function. Oxf Surv Eukaryot Genes. 1984;1:36–60. [PubMed] [Google Scholar]
- Diaz M. O., Gall J. G. Giant readthrough transcription units at the histone loci on lampbrush chromosomes of the newt Notophthalmus. Chromosoma. 1985;92(4):243–253. doi: 10.1007/BF00329807. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Dworkin-Rastl E. Changes in RNA titers and polyadenylation during oogenesis and oocyte maturation in Xenopus laevis. Dev Biol. 1985 Dec;112(2):451–457. doi: 10.1016/0012-1606(85)90417-8. [DOI] [PubMed] [Google Scholar]
- Elliott E. M., Henderson G., Sarangi F., Ling V. Complete sequence of three alpha-tubulin cDNAs in Chinese hamster ovary cells: each encodes a distinct alpha-tubulin isoprotein. Mol Cell Biol. 1986 Mar;6(3):906–913. doi: 10.1128/mcb.6.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E. M., Okayama H., Sarangi F., Henderson G., Ling V. Differential expression of three alpha-tubulin genes in Chinese hamster ovary cells. Mol Cell Biol. 1985 Jan;5(1):236–241. doi: 10.1128/mcb.5.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Cowan N. J. Structural features and restricted expression of a human alpha-tubulin gene. Nucleic Acids Res. 1985 Jan 11;13(1):207–223. doi: 10.1093/nar/13.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrell A., Shuttleworth J., Colman A. Transcript levels and translational control of hsp70 synthesis in Xenopus oocytes. Genes Dev. 1987 Jul;1(5):433–444. doi: 10.1101/gad.1.5.433. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Karin M. Complexities of gene regulation by cAMP. Trends Genet. 1989 Mar;5(3):65–67. doi: 10.1016/0168-9525(89)90027-9. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Korn L. J. Upstream sequences required for transcription of the TFIIIA gene in Xenopus oocytes. Nucleic Acids Res. 1988 May 11;16(9):3801–3814. doi: 10.1093/nar/16.9.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pestell R. Q. Microtubule protein synthesis during oogenesis and early embryogenesis in Xenopus laevis. Biochem J. 1975 Mar;145(3):527–534. doi: 10.1042/bj1450527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J. The complete sequence of a frog alpha-tubulin gene and its regulated expression in mouse L-cells. Biochem J. 1988 Jan 15;249(2):465–472. doi: 10.1042/bj2490465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasante A., Wang D., Dobner P., Dolph P., Lewis S. A., Cowan N. J. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986 Jul;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley M. E., Patient R. K. Highly efficient beta globin transcription in the absence of both a viral enhancer and erythroid factors. Development. 1987 Dec;101(4):815–827. doi: 10.1242/dev.101.4.815. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]