Summary
Some pathogenic microbes utilize homologous recombination to generate antigenic variability in targets of immune surveillance. These specialized systems rely on the cellular recombination machinery to catalyze dedicated, high-frequency reactions that provide extensive diversity in the genes encoding surface antigens. A description of the specific mechanisms that allow unusually high rates of recombination without deleterious effects on the genome in the well characterized pilin antigenic variation systems of Neisseria gonorrhoeae and Neisseria meningitidis is presented. We will also draw parallels to selected bacterial and eukaryotic antigenic variation systems, and suggest the most pressing unanswered questions related to understanding these important processes.
Keywords: Neisseria, pili, antigenic variation, recombination, gene conversion
Introduction
Antigenic variation is the process by which pathogenic organisms provide multiple versions of a surface antigen to evade host immune selection. It is often grouped with the related process of phase variation, which generates two phases of expression, either between two forms of an antigen or between a phase ON and phase OFF state. Site specific recombination between inverted recognition sites is the most common mechanism to switch between two forms of an antigen, while ON/OFF phase variation generally occurs by changes in repetitive DNA sequence repeat numbers in the promoter or within the phase variable gene itself (Bayliss, 2009). We will not be focusing on phase variation in this review.
The bacteria Neisseria gonorrhoeae and Neisseria meningitidis are strict human pathogens that colonize mucosal surfaces. N. gonorrhoeae generally colonizes the urogenital tract and is the causative agent of the sexually transmitted infection, gonorrhea which usually presents as urethritis in men and cervicitis in women. N. meningitidis usually colonizes the nasopharynx without incidence but can cause a serious septicemia and/or bacterial meningitis. N. gonorrhoeae and N. meningitidis express three major variable surface antigens that promote bacterial survival. These antigenic variation systems alter the lipooligosaccharide (LOS), the opacity (Opa) family of outer membrane proteins, and the pilus. LOS variants can selectively subvert host immune defense mechanisms and have been found to escape monoclonal antibody bactericidal killing (van Vliet et al., 2009, Bayliss et al., 2008). Opa outer membrane proteins mediate adherence to host cells and certain variants can promote cellular invasion additionally Opa protein expression can increase resistance to complement-mediated bacteriolysis (Makino et al., 1991, Bos et al., 1997). The LOS and Opa outer membrane protein each rely on ON/OFF phase variation of multiple genes to generate a more diverse repertoire of antigenic variants (Stern et al., 1986, Danaher et al., 1995, Jennings et al., 1995).
In contrast, pilin antigenic variation occurs as a result of non-reciprocal DNA recombination between one of multiple pilS silent storage loci and the pilin expression locus, pilE (Hagblom et al., 1985). Pilin is the major subunit of the Neisserial type IV pilus apparatus and pili are essential for establishing infection (Cohen & Cannon, 1999, Exley et al., 2009, Lauer et al., 1993, Robertson et al., 1977). The pilus assists in cellular adherence, bacterial aggregation and can mediate twitching motility and natural DNA transformation (Rudel et al., 1992, Sparling, 1966, Wolfgang et al., 1998, Park et al., 2001). A great deal of mechanistic insight into bacterial antigenic variation has been derived from study of the pathogenic Neisseria species (reviewed in Kline et al., 2003) and this review will highlight the unique molecular processes required for pilin antigenic variation in the pathogenic Neisseria, while drawing parallels with other pathogenesis-associated antigenic variation systems.
Recombination-based Antigenic Variation Systems
Recombination-associated antigenic variation systems have been identified in both prokaryotic and eukaryotic pathogens, and genomic sequencing suggests there may be many other organisms that encode antigenic variation systems (Table 1). Yet with the exception of Neisseria, Borrelia, and Trypanosoma, very little is known about the molecular factors and mechanisms required to generate antigenic variability by DNA recombination and less is known about these systems than has been determined in the pathogenic Neisseria. Members of the bacterial genus Borrelia are the causative agents of the multi-system disorder Lyme disease which is transmitted to humans through infected ticks during a blood meal. Members of the Trypanosoma genus are eukaryotic parasites transmitted through insect vectors that can cause fatal human diseases such as sleeping sickness and Chagas disease. The pathogenic Neisseria, Borrelia and Trypanosoma genus all evade the host immune response by high frequency antigenic variation of surface structures.
Table 1.
Controlled DNA Recombination Associated Antigenic Variation Systems
| Species | Gene | # of Expression Sites | # of Storage Loci | Reference | |
|---|---|---|---|---|---|
| Prokaryotic Pathogens | Neisseria gonorrhoeae | pilE | 1 | 19 | (Hamrick et al., 2001) |
| Neisseria meningitidis | pilE | 1 | 8 | (Tettelin et al., 2000) | |
| Borrelia burgdorferi | vlsE | 1 | 15 | (Zhang et al., 1997) | |
| Borrelia hermsii | vsp/vlp | 1 | ~59 | (Dai et al., 2006) | |
| Anaplasma marginale | msp2/msp3 | 1 | ~10 | (Brayton et al., 2001) | |
| Mycoplamsa genitalium | mgpB/mgpC | 1 | 9 | (Iverson-Cabral et al., 2007) | |
| Treponema pallidum | tprK | 1 | 47 | (Centurion-Lara et al., 2004) | |
| Eukaryotic Pathogens | Trypanosoma brucei | vsg | 14–23 | ~2000 | (McCulloch & Horn, 2009) |
| Babesia bovis | ves1 | 150 | ~24 | (Brayton et al., 2007) | |
| Pneumocystis carinii | msg | 73 | 1 | (Keely & Stringer, 2009, Edman et al., 1996) |
The pathogenic Neisseria, and Borrelia species each encode one expression locus, pilE and vlsE, respectively; whereas the eukaryotic Trypanosoma species encode 14–23 telomeric vsg expression sites (McCulloch & Horn, 2009). The expression loci share regions of conserved homology with the silent loci which are themselves flanked by regions of variability. In N. gonorrohoeae, an antigenic variation frequency of 0.13 recombination events per cell with a rate of 4×10−3 events per cell per generation has been reported, whereas N. meningitidis displays an antigenic variation frequency and rate of 0.03 events per cell and 1.6×10−3 events per cell per generation, respectively (Criss et al., 2005, Helm & Seifert, 2010). Similarly, Trypanosoma brucei shows a rate of 1×10−3 events per cell per generation (Turner & Barry, 1989). The mechanism by which the silent donor copy is chosen for recombination at the expression locus remains unknown. In N. gonorrohoeae there appears to be a preference for certain pilS donor copies more than others while in T. brucei there is a hierarchy of preference where telomeric vsg copies are activated first, followed by subtelomeric and lastly pseudogene vsg copies (Criss et al., 2005, Morrison et al., 2005).
Novel Aspects of Neisseria pilin antigenic variation
How the DNA recombination events leading to pilin antigenic variation are initiated in the pathogenic Neisseria was unknown until recently. A transposon-based genetic screen identified insertions in a region of DNA upstream of the expressed pilE locus that was required for pilin antigenic variation, but did not alter pilin expression (Sechman et al., 2005, Kline et al., 2007). A subsequent targeted genetic screen identified 11 GC base pairs in this pilE upstream region that when individually mutated absolutely inhibited pilin antigenic variation and a 12th GC base pair (designated G3) that when mutated retained a residual level of pilin antigenic variation (Figure 1A&C) (Cahoon & Seifert, 2009). Interestingly, a mutation of a neighboring GC base pair (designated G0) alone had no effect on pilin antigenic variation but mutating G0 in addition to G3 resulted in a complete loss-of-function, suggesting that G0 can partially substitute for G3 (Figure 1A&C) (Cahoon & Seifert, 2009). The organization of these 12 GC base pairs conforms to a guanine quartet or guanine quadruplex (G4) forming sequence (Figure 1A&B) (Cahoon & Seifert, 2009). Biophysical studies confirmed that the G-rich sequence forms a G4 structure in vitro and that point mutations that disrupt pilin antigenic variation, also disrupt the structure (Cahoon & Seifert, 2009). Growth of N. gonorrhoeae on N-methyl mesoporphyrin IX (NMM), which interacts specifically with G4 structures but not with double stranded or single stranded DNA (Ren & Chaires, 1999), inhibited the frequency of pilin antigenic variation by specifically depressing the recombination of some but not all pilS copies (Cahoon & Seifert, 2009). The molecular basis for this selective inhibition is not known. Additionally, point mutations that interfere with pilin antigenic variation and the G4 structure prevented single stranded nicks from being detected in the G4 forming sequence and the opposite C-rich strand, while treatment with NMM decreased the nicks detected specifically on the G-rich strand but not the C-rich strand (Cahoon & Seifert, 2009). We concluded from this study that for antigenic variation to occur this G4 structure must form and this was the first instance of a G4 structure being implicated in a prokaryotic biological process. We predict that other molecular processes of prokaryotes will also be influenced by G4 structures. It is yet to be determined when and how the pilE G4 structure forms, whether formation is regulated, and if proteins required for pilin antigenic variation bind and process the structure (Figure 2). Since the pilE G4 forming sequence is only localized upstream of the pilE but not near any pilS loci, and since the pilE G4 forming sequence is located a few hundred base pairs away from where recombination occurs, it is likely that there is considerable processing of DNA after the G4 structure forms to yield recombination at pilE.
Figure 1. The pilin expression locus guanine quartet (pilE G4).
A. The pilE G4 motif and surrounding DNA sequence. Mutation of GC bps (boxed in black) completely blocks pilin antigenic variation while mutation of G3 (boxed in grey) has some residual activity. The DNA element underlined in black forms a guanine quartet (G4 or G-quadruplex) motif. When G3 is mutated a second G4 motif can form (underlined in grey) using the G0 base instead (See Figure 1C)). B. Cartoon of a guanine quartet structure. An all parallel G4 structure composed of the pilE G4 sequence is shown where guanines are numbered as indicated in Figure 1A. C. Kinetic pilus-dependent colony phase variation assay: This assay measures the average number of visible pilus-dependent colony morphology changes occurring over time and is a surrogate measure of antigenic variation. Mutation of G0 has no effect on phase variation while mutation of G3 shows some residual activity. Mutation of both G0 and G3 shows a loss of phase variation comparable to the G4 mutant strain (G4mt, G12→A). Error bars represent the standard error of the mean of 10 colonies. Asterisk indicates P<0.05 as measured by two-tailed Student’s T test.
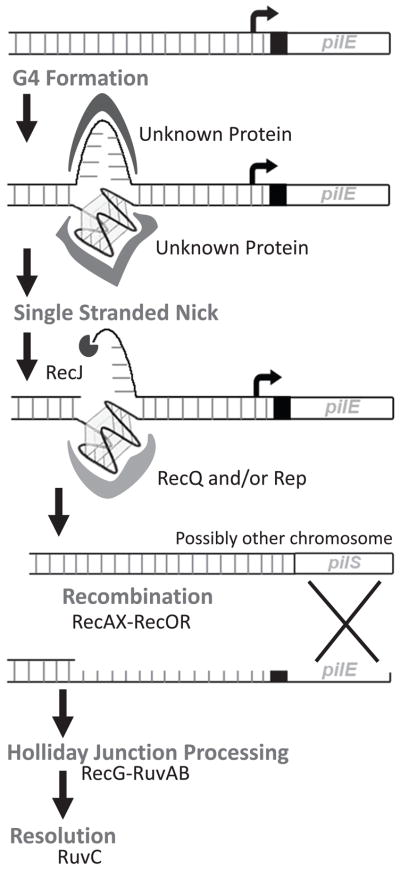
Figure 2. A working model for N. gonorrhoeae pilin antigenic variation.
Shown is the pilin expression locus (pilE) and upstream region. We propose that initiation of pilin antigenic variation begins with the formation of the pilE G4 structure which may be mediated by structure-specific binding proteins. Once a single stranded nick occurs either by a G4 specific nuclease or by a replication stall, (the latter is shown), RecQ and/or Rep unwind the pilE G4 structure and RecJ degrades the single stranded DNA substrate. Then homologous pairing occurs between pilE and a pilS donor. Next, RecOR assists RecA-mediated strand exchange that is modulated by RecX and/or RdgC. Then the Holliday junction DNA recombination intermediate is processed by RecG and RuvAB which is then resolved by RuvC.
For a G4 structure to form duplex DNA must first be melted, and therefore it is possible that the pilE G4 structure forms either during transcription and/or replication. Moreover, since the activation energy of G4 formation provides a barrier to formation it is also likely that proteins aid in the formation of the structure. Interestingly, G4 structure formation has been suggested to be a prerequisite to recombination in immunoglobin class-switching another high frequency genetic diversification system (Dempsey et al., 1999). In contrast, T. brucei vsg antigenic variation appears to be initiated by a double strand break adjacent to the 70 base pair repeats in the active expression site (Boothroyd et al., 2009). However, this may not be the only mechanism of initiation in T. brucei since mutation of Mre11, an enzyme involved in double strand break repair, impairs homologous recombination but not vsg switching (Tan et al., 2002, Robinson et al., 2002).
In the pathogenic Neisseria, the homologous recombination events leading to pilin antigenic variation are non-reciprocal. The pilS donor copy is never lost while the pilE locus undergoes gene conversion. Models which account for both gene conversion at the pilE locus and genomic conservation at the pilS loci include the unequal crossing over model, the hybrid intermediate model, and the half cross over model (reviewed in Kline et al., 2003). The ability to perform high frequency gene conversion in the context of a bacterial chromosome and to conserve the pilS sequences is proposed to be facilitated by the polyploid nature the bacteriam, which suggests that the pathogenic Neisseria may have evolved polypoidy to accomplish non-reciprocal high frequency DNA recombination at pilE without genetic loss of the pilS donor sequences (Tobiason & Seifert, 2006, Tobiason & Seifert, 2010). N. gonorrhoeae has a single copy of pilE and 19 unique pilS cassettes located in six discrete chromosomal loci while N. meningitidis possess 8 pilS cassettes in a single locus upstream of pilE (Tettelin et al., 2000, Hamrick et al., 2001). The divergence in genomic organization and increased number of pilS cassettes in N. gonorrhoeae compared to N. meningitidis may account for the higher frequency and rate of pilin antigenic variation observed in N. gonorrhoeae or may be due to a need for more antigenic diversity within a sexually transmitted infection.
Iron Availability and Pilin Antigenic Variation
The pathogenic Neisseria undergo pilin antigenic variation under both laboratory conditions (Gould et al., 1944, Scherp & Fitting, 1949) and within the human host but whether differing environmental factors that are encountered during infection might influence the frequency of pilin antigenic variation has not been explored (Seifert et al., 1994, Rytkonen et al., 2004). In contrast, B. burgdorferi vlsE antigenic variation only occurs during mammalian infections which suggest that host factors and/or environmental cues are required for the process (Ohnishi et al., 2003). In N. gonorrhoeae, the frequency of pilin antigenic variation has been measured under several environmental conditions that are predicted to fluctuate during the course of infection or have been shown to influence N. gonorrhoeae cellular processes (Serkin & Seifert, 2000). Of the conditions tested (carbon source, temperature, aromatic amino acid availability, oxygen availability and iron availability), only iron limitation was found to significantly alter the frequency of pilin antigenic variation suggesting that iron is limited in some locations in the host (Serkin & Seifert, 2000). Under iron limiting conditions, a 5–9 fold increase in pilin antigenic variation was observed suggesting low iron availability in the host environment signals when an increase in pilin antigenic variation might be advantageous (Serkin & Seifert, 2000). We speculate that a stimulation of the frequency of pilin antigenic variation would most likely occur when N. gonorrhoeae are about to transit to a new host because a new repertoire of antigenic variants would not be recognized by a previously elicited host response. It is also possible that pilin antigenic variation is stimulated to provide pilus phase variants for functional purposes in the host.
Protein Factors Required for Pilin Antigenic Variation
N. gonorrhoeae pilin antigenic variation is a specialized recombination system that occurs via a RecF-like pathway of homologous recombination, which utilizes enzymes that participate in general recombination and repair pathways and enzymes that are non-pathway specific (Skaar et al., 2002, Kline & Seifert, 2005b, Mehr et al., 2000, Mehr & Seifert, 1998, Mehr & Seifert, 1997, Sechman et al., 2005, Stohl & Seifert, 2001, Koomey et al., 1987) (Table 2). In N. gonorrhoeae, pilin antigenic variation is totally dependent on RecA (Koomey et al., 1987), whereas B. burgdorferi vslE antigenic variation is RecA independent (Liveris et al., 2008) and T. brucei encodes five RecA orthologues (Rad51 and four Rad51 paralogues) but only some Rad51 paralogues participate in vsg antigenic variation (Conway et al., 2002, Robinson et al., 2002, McCulloch & Barry, 1999, Proudfoot & McCulloch, 2005, Proudfoot & McCulloch, 2006). In N. gonorrhoeae, RecA is tempered by the RecA modulator RecX which enhances pilin antigenic variation, and T. brucei Rad51 is mediated by BRCA2 during vsg antigenic variation (Stohl & Seifert, 2001, Gruenig et al., 2010, Hartley & McCulloch, 2008). Interestingly, the Neisseria RecX protein is a potent inhibitor of the RecA/DNA nucleoprotein filment, even though recX mutants show decreased frequencies of pilin antigenic variation (Gruenig et al., 2010, Stohl et al., 2003). These findings suggest that for RecA to be effective in promoting pilin antigenic variation it must limit its ability to promote heteroduplex formation. The reason why there is a constraint on RecA filament length is unknown.
Table 2.
Protein Factors Required For Pilin Antigenic Variation
| Protein | Associated Function | Extent of pilin antigenic variation deficiency | Reference |
|---|---|---|---|
| RecA | recombinase | complete loss | (Koomey et al., 1987) |
| RecX | RecA modulator | some residual activity | (Stohl & Seifert, 2001) |
| RdgC | RecA modulator | some residual activity | (Mehr et al., 2000) |
| RecOR | recombinase, assists in RecA- mediated strand exchange | complete loss | (Mehr & Seifert, 1998, Sechman et al., 2005) |
| RecJ | 5′→3′ single strand exonuclease | partial loss | (Hill & Grant, 2002, Skaar et al., 2002) |
| RecQ | 3′→5′ helicase | partial loss | (Mehr & Seifert, 1998) |
| Rep | 3′→5′ helicase, replication restart | partial loss | (Kline & Seifert, 2005b) |
| RecG | 3′→5′ helicase, branch migration of Holliday junctions | some residual activity | (Sechman et al., 2006, Sechman et al., 2005) |
| RuvAB | 5′→3′ helicase, branch migration of Holliday junctions | some residual activity | (Sechman et al., 2006) |
| RuvC | resolvase, resolution of Holliday junctions | some residual activity | (Sechman et al., 2006) |
Although unlike N. gonorrhoeae, B. burgdorferi does not require RecA for vslE antigenic variation, both organisms require the Holliday junction helicase RuvAB to accomplish antigenic variation (Dresser et al., 2009, Lin et al., 2009, Sechman et al., 2006). There may be other functional similarities between N. gonorrhoeae, B. burgdorferi, and T. brucei antigenic variation systems but further investigation is needed to define additional protein factors involved in the process in the latter two species.
Until recently, whether the RecBCD pathway is involved in pilin antigenic variation was controversial. Initially recB, recC, and recD mutants were shown to have no difference in pilin antigenic variation when compared to the parental strain by a colony based PCR assay and by colony phase variation assay (Mehr & Seifert, 1998). Then two reports measuring pilin antigenic variation in recB and recD mutants yielded conflicting results showing either an inhibition or reliance on the RecBCD pathway in two different N. gonorrhoeae strains. However, the conclusions of these studies were based on assays that may have been influenced by the drastically reduced growth rate of the recB and recD mutants (Chaussee et al., 1999, Hill et al., 2007). Therefore, to definitively determine whether the RecBCD pathway plays a role in pilin antigenic variation, the rate and frequency of pilin antigenic variation was directly measured in the same two strains by sequencing the pilE locus in randomly selected piliated progeny of recB and recD mutants (Helm & Seifert, 2009). The recB and recD mutants showed similar rates and frequencies of pilin antigenic variation as the parental strains, conclusively demonstrating that N. gonorrhoeae pilin antigenic variation is absolutely independent of the RecBCD pathway of recombination (Helm & Seifert, 2009).
A Working Model
A working model for N. gonorrhoeae pilin antigenic variation can be proposed based on the knowledge we have gained about the processes and molecules required for this genetic diversification system (Figure 2). We propose that pilin antigenic variation begins with the formation of the G4 structure after the DNA duplex is melted, and that this formation is mediated by one or more unknown structure specific binding proteins. Since N. gonorrhoeae is polyploid, recombination may be initiated at pilE only on the chromosome carrying a formed pilE G4 structure, but this hypothesis remains to be directly tested. Once formed, the G4 structure could then be nicked by an endonuclease. Alternatively, a nick could be produced by a stalled replication fork on the leading strand since the pilE G4 structure is located on the lagging strand during DNA replication, inversion of the pilE G4 sequence orientation caused a complete loss of pilin antigenic variation, and DNA polymerases cannot replicate through a formed pilE G4 structure (Cahoon & Seifert, 2009). However, since the replication restart helicase PriA has no role in pilin antigenic variation (Kline & Seifert, 2005a), if the replication fork collapses, it is not restored through PriA. This nicked substrate could then be processed by the RecJ exonuclease and RecQ and/or Rep helicases (Cahoon & Seifert, 2009, Kline & Seifert, 2005b, Mehr & Seifert, 1998, Sechman et al., 2005, Skaar et al., 2002). Since Escherichia coli RecQ helicase has been shown to unwind G4 structures in vitro (Wu & Maizels, 2001), it is likely that the gonococcal RecQ helicase resolves the pilE G4 structure during or after antigenic variation. Then homologous pairing occurs between the pilS donor sequence and pilE, and RecOR assists RecA-mediated strand exchange which is modulated by RecX (Koomey et al., 1987, Mehr & Seifert, 1998, Sechman et al., 2005, Stohl & Seifert, 2001). RdgC, a DNA binding protein which is associated with recombination and replication fork repair may also temper RecA (Briggs et al., 2007, Mehr et al., 2000). The resultant DNA recombination intermediate is likely processed by the RecG and RuvAB helicases and then RuvC Holliday junction can resolve the structure (Sechman et al., 2006, Sechman et al., 2005). While these steps are all reasonable based on what we presently know about pilin antigenic variation, the actual detailed steps are likely to be more complex, and await further characterization for both the pathogenic Neisseria pilin antigenic variation system and other types of recombination-associated antigenic variation systems.
Acknowledgments
We would like to thank Adrienne Chen for careful reading of the manuscript, Vitaly Kuryavyi for modeling of the pilE G4 nucleotide orientation, and Ryan Smith for assistance with Adobe Photoshop. Our work is funded by NIH Grants RO1 AI044239 and R37 AI033493.
Literature Cited
- Bayliss CD. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol Rev. 2009;33:504–520. doi: 10.1111/j.1574-6976.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- Bayliss CD, Hoe JC, Makepeace K, Martin P, Hood DW, Moxon ER. Neisseria meningitidis escape from the bactericidal activity of a monoclonal antibody is mediated by phase variation of lgtG and enhanced by a mutator phenotype. Infection and immunity. 2008;76:5038–5048. doi: 10.1128/IAI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd CE, Dreesen O, Leonova T, Ly KI, Figueiredo LM, Cross GA, Papavasiliou FN. A yeast-endonuclease-generated DNA break induces antigenic switching in Trypanosoma brucei. Nature. 2009;459:278–281. doi: 10.1038/nature07982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Hogan D, Belland RJ. Selection of Opa+ Neisseria gonorrhoeae by limited availability of normal human serum. Infection and immunity. 1997;65:645–650. doi: 10.1128/iai.65.2.645-650.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Knowles DP, McGuire TC, Palmer GH. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4130–4135. doi: 10.1073/pnas.071056298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, Bidwell SL, Brown WC, Crabtree J, Fadrosh D, Feldblum T, Forberger HA, Haas BJ, Howell JM, Khouri H, Koo H, Mann DJ, Norimine J, Paulsen IT, Radune D, Ren Q, Smith RK, Jr, Suarez CE, White O, Wortman JR, Knowles DP, Jr, McElwain TF, Nene VM. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–1413. doi: 10.1371/journal.ppat.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs GS, McEwan PA, Yu J, Moore T, Emsley J, Lloyd RG. Ring structure of the Escherichia coli DNA-binding protein RdgC associated with recombination and replication fork repair. The Journal of biological chemistry. 2007;282:12353–12357. doi: 10.1074/jbc.C700023200. [DOI] [PubMed] [Google Scholar]
- Cahoon LA, Seifert HS. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science. 2009;325:764–767. doi: 10.1126/science.1175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurion-Lara A, LaFond RE, Hevner K, Godornes C, Molini BJ, Van Voorhis WC, Lukehart SA. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Molecular microbiology. 2004;52:1579–1596. doi: 10.1111/j.1365-2958.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- Chaussee MS, Wilson J, Hill SA. Characterization of the recD gene of Neisseria gonorrhoeae MS11 and the effect of recD inactivation on pilin variation and DNA transformation. Microbiology. 1999;145(Pt 2):389–400. doi: 10.1099/13500872-145-2-389. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Cannon JG. Human experimentation with Neisseria gonorrhoeae: progress and goals. J Infect Dis. 1999;179(Suppl 2):S375–379. doi: 10.1086/513847. [DOI] [PubMed] [Google Scholar]
- Conway C, Proudfoot C, Burton P, Barry JD, McCulloch R. Two pathways of homologous recombination in Trypanosoma brucei. Molecular microbiology. 2002;45:1687–1700. doi: 10.1046/j.1365-2958.2002.03122.x. [DOI] [PubMed] [Google Scholar]
- Criss AK, Kline KA, Seifert HS. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol Microbiol. 2005;58:510–519. doi: 10.1111/j.1365-2958.2005.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Restrepo BI, Porcella SF, Raffel SJ, Schwan TG, Barbour AG. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Molecular microbiology. 2006;60:1329–1343. doi: 10.1111/j.1365-2958.2006.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher RJ, Levin JC, Arking D, Burch CL, Sandlin R, Stein DC. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. Journal of bacteriology. 1995;177:7275–7279. doi: 10.1128/jb.177.24.7275-7279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey LA, Sun H, Hanakahi LA, Maizels N. G4 DNA binding by LR1 and its subunits, nucleolin and hnRNP D, A role for G-G pairing in immunoglobulin switch recombination. The Journal of biological chemistry. 1999;274:1066–1071. doi: 10.1074/jbc.274.2.1066. [DOI] [PubMed] [Google Scholar]
- Dresser AR, Hardy PO, Chaconas G. Investigation of the genes involved in antigenic switching at the vlsE locus in Borrelia burgdorferi: an essential role for the RuvAB branch migrase. PLoS Pathog. 2009;5:e1000680. doi: 10.1371/journal.ppat.1000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JC, Hatton TW, Nam M, Turner R, Mei Q, Angus CW, Kovacs JA. A single expression site with a conserved leader sequence regulates variation of expression of the Pneumocystis carinii family of major surface glycoprotein genes. DNA Cell Biol. 1996;15:989–999. doi: 10.1089/dna.1996.15.989. [DOI] [PubMed] [Google Scholar]
- Exley RM, Sim R, Goodwin L, Winterbotham M, Schneider MC, Read RC, Tang CM. Identification of meningococcal genes necessary for colonization of human upper airway tissue. Infection and immunity. 2009;77:45–51. doi: 10.1128/IAI.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RG, Kane LW, Mueller JH. On the Growth Requirements of Neisseria gonorrhoeae. Journal of bacteriology. 1944;47:287–292. doi: 10.1128/jb.47.3.287-292.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenig MC, Stohl EA, Chitteni-Pattu S, Seifert HS, Cox MM. Less is more: Neisseria gonorrhoeae RecX protein stimulates recombination by inhibiting RecA. The Journal of biological chemistry. 2010;285:37188–37197. doi: 10.1074/jbc.M110.171967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985;315:156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Hamrick TS, Dempsey JA, Cohen MS, Cannon JG. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology. 2001;147:839–849. doi: 10.1099/00221287-147-4-839. [DOI] [PubMed] [Google Scholar]
- Hartley CL, McCulloch R. Trypanosoma brucei BRCA2 acts in antigenic variation and has undergone a recent expansion in BRC repeat number that is important during homologous recombination. Molecular microbiology. 2008;68:1237–1251. doi: 10.1111/j.1365-2958.2008.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm RA, Seifert HS. Pilin antigenic variation occurs independently of the RecBCD pathway in Neisseria gonorrhoeae. Journal of bacteriology. 2009;191:5613–5621. doi: 10.1128/JB.00535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm RA, Seifert HS. Frequency and rate of pilin antigenic variation of Neisseria meningitidis. J Bacteriol. 2010;192:3822–3823. doi: 10.1128/JB.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SA, Grant CC. Recombinational error and deletion formation in Neisseria gonorrhoeae: a role for RecJ in the production of pilE (L) deletions. Mol Genet Genomics. 2002;266:962–972. doi: 10.1007/s00438-001-0618-5. [DOI] [PubMed] [Google Scholar]
- Hill SA, Woodward T, Reger A, Baker R, Dinse T. Role for the RecBCD recombination pathway for pilE gene variation in repair-proficient Neisseria gonorrhoeae. Journal of bacteriology. 2007;189:7983–7990. doi: 10.1128/JB.00980-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell-Adams B, Seifert HS. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Molecular microbiology. 2000;37:1146–1158. doi: 10.1046/j.1365-2958.2000.02067.x. [DOI] [PubMed] [Google Scholar]
- Iverson-Cabral SL, Astete SG, Cohen CR, Totten PA. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Molecular microbiology. 2007;66:55–73. doi: 10.1111/j.1365-2958.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- Jennings MP, Hood DW, Peak IR, Virji M, Moxon ER. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Molecular microbiology. 1995;18:729–740. doi: 10.1111/j.1365-2958.1995.mmi_18040729.x. [DOI] [PubMed] [Google Scholar]
- Keely SP, Stringer JR. Complexity of the MSG gene family of Pneumocystis carinii. BMC genomics. 2009;10:367. doi: 10.1186/1471-2164-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline KA, Criss AK, Wallace A, Seifert HS. Transposon mutagenesis identifies sites upstream of the Neisseria gonorrhoeae pilE gene that modulate pilin antigenic variation. Journal of bacteriology. 2007;189:3462–3470. doi: 10.1128/JB.01911-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline KA, Sechman EV, Skaar EP, Seifert HS. Recombination, repair and replication in the pathogenic Neisseriae: the 3 R’s of molecular genetics of two human-specific bacterial pathogens. Molecular microbiology. 2003;50:3–13. doi: 10.1046/j.1365-2958.2003.03679.x. [DOI] [PubMed] [Google Scholar]
- Kline KA, Seifert HS. Mutation of the priA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. Journal of bacteriology. 2005a;187:5347–5355. doi: 10.1128/JB.187.15.5347-5355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline KA, Seifert HS. Role of the Rep helicase gene in homologous recombination in Neisseria gonorrhoeae. J Bacteriol. 2005b;187:2903–2907. doi: 10.1128/JB.187.8.2903-2907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey M, Gotschlich EC, Robbins K, Bergstrom S, Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987;117:391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer P, Albertson NH, Koomey M. Conservation of genes encoding components of a type IV pilus assembly/two-step protein export pathway in Neisseria gonorrhoeae. Molecular microbiology. 1993;8:357–368. doi: 10.1111/j.1365-2958.1993.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Lin T, Gao L, Edmondson DG, Jacobs MB, Philipp MT, Norris SJ. Central role of the Holliday junction helicase RuvAB in vlsE recombination and infectivity of Borrelia burgdorferi. PLoS Pathog. 2009;5:e1000679. doi: 10.1371/journal.ppat.1000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveris D, Mulay V, Sandigursky S, Schwartz I. Borrelia burgdorferi vlsE antigenic variation is not mediated by RecA. Infection and immunity. 2008;76:4009–4018. doi: 10.1128/IAI.00027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, van Putten JP, Meyer TF. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. The EMBO journal. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch R, Barry JD. A role for RAD51 and homologous recombination in Trypanosoma brucei antigenic variation. Genes Dev. 1999;13:2875–2888. doi: 10.1101/gad.13.21.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch R, Horn D. What has DNA sequencing revealed about the VSG expression sites of African trypanosomes? Trends Parasitol. 2009;25:359–363. doi: 10.1016/j.pt.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Mehr IJ, Long CD, Serkin CD, Seifert HS. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics. 2000;154:523–532. doi: 10.1093/genetics/154.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehr IJ, Seifert HS. Random shuttle mutagenesis: gonococcal mutants deficient in pilin antigenic variation. Molecular microbiology. 1997;23:1121–1131. doi: 10.1046/j.1365-2958.1997.2971660.x. [DOI] [PubMed] [Google Scholar]
- Mehr IJ, Seifert HS. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair. Molecular microbiology. 1998;30:697–710. doi: 10.1046/j.1365-2958.1998.01089.x. [DOI] [PubMed] [Google Scholar]
- Morrison LJ, Majiwa P, Read AF, Barry JD. Probabilistic order in antigenic variation of Trypanosoma brucei. Int J Parasitol. 2005;35:961–972. doi: 10.1016/j.ijpara.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Ohnishi J, Schneider B, Messer WB, Piesman J, de Silva AM. Genetic variation at the vlsE locus of Borrelia burgdorferi within ticks and mice over the course of a single transmission cycle. J Bacteriol. 2003;185:4432–4441. doi: 10.1128/JB.185.15.4432-4441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Wolfgang M, van Putten JP, Dorward D, Hayes SF, Koomey M. Structural alterations in a type IV pilus subunit protein result in concurrent defects in multicellular behaviour and adherence to host tissue. Molecular microbiology. 2001;42:293–307. doi: 10.1046/j.1365-2958.2001.02629.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot C, McCulloch R. Distinct roles for two RAD51-related genes in Trypanosoma brucei antigenic variation. Nucleic acids research. 2005;33:6906–6919. doi: 10.1093/nar/gki996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot C, McCulloch R. Trypanosoma brucei DMC1 does not act in DNA recombination, repair or antigenic variation in bloodstream stage cells. Mol Biochem Parasitol. 2006;145:245–253. doi: 10.1016/j.molbiopara.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Ren J, Chaires JB. Sequence and structural selectivity of nucleic acid binding ligands. Biochemistry. 1999;38:16067–16075. doi: 10.1021/bi992070s. [DOI] [PubMed] [Google Scholar]
- Robertson JN, Vincent P, Ward ME. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977;102:169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Robinson NP, McCulloch R, Conway C, Browitt A, Barry JD. Inactivation of Mre11 does not affect VSG gene duplication mediated by homologous recombination in Trypanosoma brucei. J Biol Chem. 2002;277:26185–26193. doi: 10.1074/jbc.M203205200. [DOI] [PubMed] [Google Scholar]
- Rudel T, van Putten JP, Gibbs CP, Haas R, Meyer TF. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Molecular microbiology. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Rytkonen A, Albiger B, Hansson-Palo P, Kallstrom H, Olcen P, Fredlund H, Jonsson AB. Neisseria meningitidis undergoes PilC phase variation and PilE sequence variation during invasive disease. J Infect Dis. 2004;189:402–409. doi: 10.1086/381271. [DOI] [PubMed] [Google Scholar]
- Scherp HW, Fitting C. The Growth of Neisseria Meningitidis in Simple Chemically Defined Media. Journal of bacteriology. 1949;58:1–9. doi: 10.1128/jb.58.1.1-9.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechman EV, Kline KA, Seifert HS. Loss of both Holliday junction processing pathways is synthetically lethal in the presence of gonococcal pilin antigenic variation. Molecular microbiology. 2006;61:185–193. doi: 10.1111/j.1365-2958.2006.05213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechman EV, Rohrer MS, Seifert HS. A genetic screen identifies genes and sites involved in pilin antigenic variation in Neisseria gonorrhoeae. Molecular microbiology. 2005;57:468–483. doi: 10.1111/j.1365-2958.2005.04657.x. [DOI] [PubMed] [Google Scholar]
- Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. The Journal of clinical investigation. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serkin CD, Seifert HS. Iron availability regulates DNA recombination in Neisseria gonorrhoeae. Molecular microbiology. 2000;37:1075–1086. doi: 10.1046/j.1365-2958.2000.02058.x. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Lazio MP, Seifert HS. Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae. J Bacteriol. 2002;184:919–927. doi: 10.1128/jb.184.4.919-927.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling PF. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. Journal of bacteriology. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A, Brown M, Nickel P, Meyer TF. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Stohl EA, Brockman JP, Burkle KL, Morimatsu K, Kowalczykowski SC, Seifert HS. Escherichia coli RecX inhibits RecA recombinase and coprotease activities in vitro and in vivo. The Journal of biological chemistry. 2003;278:2278–2285. doi: 10.1074/jbc.M210496200. [DOI] [PubMed] [Google Scholar]
- Stohl EA, Seifert HS. The recX gene potentiates homologous recombination in Neisseria gonorrhoeae. Molecular microbiology. 2001;40:1301–1310. doi: 10.1046/j.1365-2958.2001.02463.x. [DOI] [PubMed] [Google Scholar]
- Tan KS, Leal ST, Cross GA. Trypanosoma brucei MRE11 is non-essential but influences growth, homologous recombination and DNA double-strand break repair. Mol Biochem Parasitol. 2002;125:11–21. doi: 10.1016/s0166-6851(02)00165-2. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, Gwinn ML, DeBoy R, Peterson JD, Hickey EK, Haft DH, Salzberg SL, White O, Fleischmann RD, Dougherty BA, Mason T, Ciecko A, Parksey DS, Blair E, Cittone H, Clark EB, Cotton MD, Utterback TR, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith HO, Fraser CM, Moxon ER, Rappuoli R, Venter JC. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- Tobiason DM, Seifert HS. The obligate human pathogen, Neisseria gonorrhoeae, is polyploid. PLoS biology. 2006;4:e185. doi: 10.1371/journal.pbio.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiason DM, Seifert HS. Genomic content of Neisseria species. Journal of bacteriology. 2010;192:2160–2168. doi: 10.1128/JB.01593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CM, Barry JD. High frequency of antigenic variation in Trypanosoma brucei rhodesiense infections. Parasitology. 1989;99(Pt 1):67–75. doi: 10.1017/s0031182000061035. [DOI] [PubMed] [Google Scholar]
- van Vliet SJ, Steeghs L, Bruijns SC, Vaezirad MM, Snijders Blok C, Arenas Busto JA, Deken M, van Putten JP, van Kooyk Y. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog. 2009;5:e1000625. doi: 10.1371/journal.ppat.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang M, Lauer P, Park HS, Brossay L, Hebert J, Koomey M. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Molecular microbiology. 1998;29:321–330. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Maizels N. Substrate-specific inhibition of RecQ helicase. Nucleic acids research. 2001;29:1765–1771. doi: 10.1093/nar/29.8.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]