Abstract
Background
Aprotinin was a commonly utilized pharmacological agent for homeostasis in cardiac surgery but was discontinued resulting in the extensive use of lysine analogues. This study tested the hypothesis that early post-operative adverse events and blood product utilization would affected in this post-aprotinin era.
Methods/Results
Adult patients (n=781) undergoing coronary artery bypass (CABG), valve replacement, or both from November 1, 2005-October 31, 2008 at a single institution were included. Multiple logistic regression modeling and propensity scoring were performed on 29 pre-operative and intra-operative variables in patients receiving aprotinin (n=325) or lysine analogues (n=456). The propensity adjusted relative risk (RR;95% confidence interval;CI) for the intra-operative use of packed red blood cells (RR:0.75;CI:0.57–0.99), fresh frozen plasma (RR:0.37;0.21–0.64), and cryoprecipitate (RR:0.06;CI:0.02–0.22) were lower in the aprotinin versus lysine analogue group (all p<0.05). The risk for mortality (RR:0.53;CI:0.16–1.79) and neurological events (RR:0.87;CI:0.35–2.18) remained similar between groups, whereas a trend for reduced risk for renal dysfunction was observed in the aprotinin group.
Conclusions
In the post-aprotinin era with the exclusive use of lysine analogues, the relative risk of early post-operative outcomes such as mortality and renal dysfunction have not improved, but the risk for the intra-operative use of blood products has increased. Thus, improvements in early post-operative outcomes have not been realized with the discontinued use of aprotinin, but rather increased blood product utilization has occurred with the attendant costs and risks inherent with this strategy.
Introduction
Cardiac surgery and cardiopulmonary bypass (CPB) can cause excessive bleeding and potential post-operative complications. The serine protease inhibitor aprotinin was commonly used in this clinical context as the only FDA approved pharmacological agent to minimize blood loss.1–5 However, retrospective studies suggested that aprotinin may have post-operative consequences with respect to renal dysfunction and mortality, and that these adverse events may be dose dependent.3,4,6–10 One prospective study, the Blood Conservation using Antifibrinolytics Trial (BART), which utilized a high dose aprotinin protocol in patients undergoing cardiac surgery, was terminated early because of concerns regarding increased mortality with aprotinin.11 However, there were a number of potential confounding issues in these past studies which included but were not limited to patient selection bias, use of off-pump procedures versus CPB, variability in the empirical dosing strategy of aprotinin, and pre-operative medication profiles which can make the interpretation on outcomes difficult. While a continued subject of debate,12–19 the concerns regarding potential adverse events led to the voluntary withdrawal and discontinuation of aprotinin in November, 2007, with the exception of its availability for compassionate use. As a result, tranexamic acid (TXA) and epsilon aminocaproic acid (EACA), which are generically termed lysine analogues, have now become the most common pharmacological treatment option for antifibrinolytic therapy following cardiac surgery, particularly in patients requiring CPB.3,11–19 Unlike aprotinin, lysine analogues have not been approved as a pharmacological approach to address post-cardiac surgery related bleeding. Furthermore, it remains unknown whether and to what degree adverse events and blood product utilization have been significantly affected with the exclusive use of these lysine analogues when compared to the “aprotinin era” when potential confounding variables are taken into consideration. The present study tested the central hypothesis that following adjustment for pre-operative covariates and/or through propensity scoring, improved blood product utilization and early post-operative outcomes have not been realized in this post-aprotinin era.
Methods
The overall objective of this study was to perform a retrospective single center analysis with respect to early post-operative outcomes and blood product utilization in the aprotinin era (November 2005-October 2007) where there was uniformity in the aprotinin dosing protocol at this single center, to the post-aprotinin era (November 2007-October 2008) in which only lysine analogues, using consistent dosing protocols were utilized. Moreover, the present study addressed potential confounding factors such as pre-operative medication profiles, patient status and intraoperative variables- all of which have been shown previously to independently influence early post-operative outcomes, particularly in the context of evaluating the effects of antifibrinolytics.5,10,12–19
Patients
A retrospective chart review of all patients undergoing cardiac surgery was conducted from October 2005 - October 2008 at the Medical University of South Carolina (MUSC). All patients greater than 21 years of age that underwent the following cardiac surgical procedures requiring cardiopulmonary bypass (CPB) were included in the construction of the database: coronary revascularization with or without concomitant valve replacement/repair, isolated valve replacement/repair, and heart transplantation. Less frequent procedures that required CPB, such as congenital heart surgery and left ventricular assist devices were not included in this analysis. This data collection process and analysis was reviewed and approved by the MUSC Institutional Review Board (HR 18816). Standardized doses of the antifibrinolytics were used: Aprotinin (Trasylol, Bayer, Westhaven, CT)-2 million KIU loading dose followed by 0.5 million KIU/hr infusion during CPB; Tranexamic acid (Cyklokapron, Pfizer, NY, NY) - 15–30 mg/kg loading dose followed by 4–16mg/kg/hr infusion during CPB; Epsilon aminocaproic acid (Amicar, Hospira, Lake Forest, Il) - 150 mg/kg loading dose followed by 25 mg/kg/hr infusion during CPB. Five cardiothoracic surgeons participated in these cases and remained constant throughout the data collection interval.
Sources of Data Collection
The master list for all patients that could be included in this retrospective study was first identified utilizing the computerized database that exists within the Division of Cardiothoracic Surgery, MUSC which is maintained following the Society of Thoracic Surgeons format. For the purposes of capturing blood product utilization, a merged dataset was obtained by using the MUSC electronic pharmacy records system (Emeds). Following this merging, the database was stripped of all patient identification and combined to form the master database. We have utilized this approach previously in order to examine the effects of statin pretreatment in patients undergoing cardiac surgery.20
During the study interval, standardized set points had been established in terms of administration of packed red blood cells, fresh frozen plasma and cryoprecipitate in the perioperative period. First, clinically significant bleeding was defined as >300 mL of chest tube output within 1 hour, or >400 mL of chest tube output in any 2 hour interval, or >100 mL per hour of output on 2 occasions after the first 2 hours. Second, if the threshold for clinically significant bleeding was met, then a pre-specified age dependent setpoint for hematocrit was used to determine packed red blood cell administration (~<70 years old then a hematocrit of <23%, >70 years old then a hematocrit of <27%). Third, if the platelet count was <75,000, or recent history of platelet antagonist therapy, or an elevated prothrombin time, then platelet transfusion would be considered. Fourth, if the above criteria were met and the fibrinogen levels were < 150 mg/dL, then transfusion of fresh frozen plasma would be considered. The clinical decision for utilization of recombinant factor VIIa (rFVlla; NovoSeven, Novo Nordisk, Princeton, NJ) was based upon encountering significant, non-surgical intra-operative bleeding or postoperative bleeding refractory to conventional treatments.
Variables Measured
Demographic variables included: patient age, gender, body surface area, smoking status. Comorbidities considered included diabetes, hypertension, coagulation disorders, re-operation, chronic renal/liver disease, and pre-operative medication profiles (including aspirin). The composite risk score, EuroSCORE was computed for each patient.21 The pre-operative medication profiles were carefully scrutinized for angiotensin converting enzyme (ACE) inhibitors as it has been shown previously to be a significant covariate when considering post-operative renal function in the context of anti-fibrinolytic therapy.15 Operative variables included procedure type, cardiopulmonary bypass time, cross-clamp time, total operative time, intubation time, and length of hospital stay. Blood product utilization was broken out by units of packed red blood cells, units of fresh frozen plasma, units of platelets, cryoprecipitate, and rFVlla administration. Blood product utilization was dichotomized into blood products utilized in the first 24 hours and those utilized greater than 24 hours after the initial procedure and until discharge. The exception to this was rFVlla, where the number of patients receiving rFVlla over the entire admission-discharge period was recorded. Post-operative outcome variables included MACCE (major adverse cardiac and cerebrovascular events), repeat surgery due to hemorrhage or cardiac tamponade, renal failure defined as > 2.0 mg/dL increase in creatinine and/or the need for dialysis treatment, and respiratory failure as defined as ventilatory support for more than 48 hours.
Statistical Analysis
In this analysis, 325 patients were included in the aprotinin group and for the lysine analogues, 250 patients received EACA and 206 patients received TXA. For the purposes of this study, and in order to provide a robust means for adjusting for potential confounding variables, the lysine analogues were combined and simply identified as the lysine-analogue group (n=456 combined). Baseline demographic variables for patients assigned to the aprotinin versus lysine-analogue groups were compared using chi-square tests for categorical variables. Nonparametric Wilcoxon rank sum test were used to compare the groups on continuous variables.
A step-wise approach for analyzing the blood product and other post-operative outcomes was utilized. First, an unadjusted analysis with group as the only predictor was performed. Second, a covariate adjusted analysis in which group and the covariates found to be significant confounding variables of group, was performed. Third, a propensity matched, covariate adjusted analysis was performed.22 The reason that both covariate adjusted and propensity matched analyses were considered was to be able to use all of the data (i.e., in the covariate adjusted analysis), as well as to analyze a selected subset of aprotinin and lysine-analogue subjects that were able to be very closely matched on underlying confounders (i.e., in the propensity matched analysis). To determine important covariates for predicting group, all variables meeting alpha = 0.15 in univariate analysis were included in a logistic regression model with aprotinin or lysine-analogue group as the outcome, and insignificant predictors were removed using a backwards elimination algorithm setting alpha = 0.05. Propensity scores were then generated from the logistic regression model, and aprotinin and lysine-analogue subjects were matched 1:1 using a greedy matching algorithm that matches the nearest available pair with a specified maximum caliper of 0.123 A total of 434 subjects were able to be matched using this algorithm.
For unmatched analyses, the association between aprotinin and number of units of intra or post operative blood product utilized was assessed using Poisson regression, with the number of blood products as the outcome and group as the exposure of interest. Matched analyses of blood product utilization were performed using a generalized estimating equations (GEE) based Poisson regression approach to account for the correlation induced by matching.24 The resulting parameter estimates are interpreted as the ‘relative risk of one additional unit of blood product usage in aprotinin versus lysine-analogue group.’ All Poisson regression results are presented in terms of a relative risk (RR) and 95% confidence interval (CI).
For all unmatched analyses, binary post operative outcomes were analyzed using logistic regression models with a binary indicator of the event as the outcome and aprotinin as the exposure of interest. Matched analyses of binary outcomes were performed using a GEE based logistic regression approach to account for the correlation induced by matching. For rare outcomes (which included all binary outcomes except for prolonged ventilator usage), odds ratios can be interpreted as approximate relative risks. Therefore, results are presented as relative risks and 95% confidence intervals.
Finally, length of stay outcomes were considered as time to event data. Correspondingly, these were analyzed using Cox proportional hazards models for unmatched data, and the Wei, Lin, and Weissfeld pseudolikelihood approach for matched data.25 This approach is similar to GEE estimation and uses a robust sandwich estimate of the covariance matrix. Results are presented as hazard ratios (HR, which may be interpreted as relative risks) and 95% confidence intervals. A 2-sided alpha level of 0.05 indicated significance. Similarly, in adjusted analyses, 95% confidence intervals excluding 1 indicate significance at the 0.05 alpha level. All analyses were performed in SAS Version 9.2 (SAS Institute, Cary, NC).26
Results
Patient Demographics, Pre-operative and Intra-operative Variables
Baseline and intraoperative characteristics of the patients in the aprotinin and lysine-analogue groups are presented in Table 1. Patients were slightly older (63 vs 61 years, p=0.04) with a greater incidence of hypercholesterolemia (61.8% vs 51.3%, p=0.003) and diabetes (44.7% vs 24.9%, p<0.0001) in the lysine-analogue versus aprotinin group. There were a higher percentage of aprotinin versus lysine-analogue patients with valve replacement/repair (41.9% vs 27.2%, p<0.0001. LV ejection fraction was lower (47.5% vs 52.1%, p<0.0001) and the percentage of patients with a positive smoking history was higher (29.4% vs 23.3%, p=0.0004) in the aprotinin versus lysine-analogue group. Pre-operative use of ACE inhibitors, diuretics, positive inotropic agents and aspirin were also all significantly higher in the aprotinin group, consistent with LV dysfunction and symptomatic heart failure. The aprotinin group had significantly higher EuroSCORE than lysine-analogue group (7.0 vs 5.3, p<0.0001). With respect to inter-operative variables, both cross-clamp time (92.1 vs 79.7 minutes, p<0.0001) and cardiopulmonary bypass time (144.6 vs 111.4 minutes, p<0.0001) were higher in the aprotinin versus lysine-analogue group. Thus, in the aprotinin group, the incidence of pre-existing LV dysfunction was higher, and the duration and complexity of the surgical procedures greater. These pre-operative and intra-operative differences between the aprotinin and lysine analogue groups underscores the rationale and purpose for utilizing covariate adjusted and propensity score analysis.
Table 1.
Pre-operative Demographics and Intra-operative Variables in Patients Receiving Aprotinin or Lysine Analogues
| Aprotinin(N=325) | Lysine Analogues*(N=456) | p-value | |
|---|---|---|---|
| Patient History Profile | |||
| LV Ejection Fraction (%) | 47.5 | 52.1 | 0.0001 |
| BSA (m2) | 2.0±0.26 | 2.0±0.24 | 0.92 |
| Age | 61.2±15.2 | 63.3±13.2 | 0.04 |
| Gender (M) % | 63.2 | 64.4 | 0.74 |
| Current Smoker (Y) % | 29.4 | 23.3 | 0.0004 |
| Diabetes (Y) % | 24.9 | 44.7 | <0.0001 |
| Hypercholesterolemia(Y) % | 51.3 | 61.8 | 0.003 |
| History of Renal Failure (Y)% | 7.7 | 5.2 | 0.14 |
| Chronic Obstructive Pulmonary | 12.2 | 15.1 | 0.23 |
| Disease (Y)% | |||
| Peripheral Vascular Disease ( Y)% | 12.5 | 12.1 | 0.88 |
| Cerebrovascular Disease (Y)% | 12.2 | 13.0 | 0.74 |
| Previous Myocardial Infarction (Y)% | 20.8 | 23.1 | 0.43 |
| EuroScore Mean (SD) | 7.0(3.3) | 5.3(3.1) | <0.0001 |
| Pre-operative cardiac medications | |||
| Beta Blockers (Y) % | 61.4 | 66.1 | 0.17 |
| Ace Inhibitors (Y) % | 40.7 | 16.2 | <0.0001 |
| Nitrates (Y) % | 7.4 | 9.9 | 0.22 |
| AntiArrythmics (Y) % | 11.9 | 8.6 | 0.13 |
| Antiplatelet (Y) % | 3.6 | 5.4 | 0.22 |
| Diuretic (Y) % | 19.0 | 6.5 | <0.0001 |
| Inotropes (Y )% | 7.1 | 2.2 | 0.0006 |
| Steroids (Y )% | 5.3 | 5.2 | 0.92 |
| Aspirin (Y) % | 47.5 | 27.4 | <0.0001 |
| Anticoagulant (Y) % | 42.1 | 57.9 | 0.73 |
| Procedure Type | <0.0001 | ||
| CABG (%) | 41.2 | 65.8 | |
| Valve (%) | 41.9 | 27.2 | |
| Transplant/Other (%) | 16.9 | 7.0 | |
| Intra/Post Operative Profile | |||
| Cardiopulmonary Bypass Time (min) | 144.6±63.0 | 111.4±53.8 | <0.0001 |
| Cross Clamp Time (min) | 92.1±34.9* | 79.7±39.0 | <0.0001 |
| Total Length of Stay (days) | 9 [6, 12] | 8 [5, 12] | 0.002 |
| Post operative Length of Stay (days) | 7 [5, 10] | 6 [5. 9] | 0.005 |
-Lysine Analogues: tranexamic acid or aminocaproic acid. Values presented as mean+/− standard deviation or percent. Length of stay presented as median value [interquartile range]. P-values computed from t-tests (with Satterthwaite approximation) for mean values, chi-Square for frequencies, and Wilcoxon Rank sum for length of stay.
Unadjusted Comparisons of Blood Product Utilization and Post-operative Outcomes
Table 2 presents early post-operative outcomes which were defined as those which occurred during the initial admission and discharge period. These values reflect the unadjusted, unmatched results for the aprotinin and lysine analogue groups. In the early intra-operative/post-operative time point, the average number of red blood cell units was higher in the aprotinin group. In the late postoperative period, the average number of units of fresh frozen plasma and cryoprecipitate were higher in the aprotinin group. With respect to unadjusted post-operative outcomes, the percent of patients requiring re-operation for bleeding, and the incidence of transient neurological events and the requirement for renal dialysis appeared higher in the aprotinin group.
Table 2.
Unadjusted Values for Blood Product Utilization and Early Post-operative Outcomes in Patients Receiving Aprotinin or Lysine Analogues
| Aprotinin(N=325) | Lysine Analogues*(N=456) | p-value | |
|---|---|---|---|
| Blood Product Utilization | |||
| Intra-operative/Early-post Operative (<24 hours) | |||
| Packed Red Blood Cells (units) | 1.37 ± 1.79 | 1.14 ± 1.88 | 0.01 |
| Fresh Frozen Plasma (units) | 0.68 ± 1.80 | 0.63 ± 1.78 | 0.65 |
| Cryoprecipitate (units) | 0.13 ± 0.98 | 0.18 ± 1.02 | 0.09 |
| Platelets (units) | 1.77 ± 4.58 | 1.54 ± 4.65 | 0.98 |
| Late Post-operative Period (>24 hours) | |||
| Packed Red Blood Cells (units) | 2.21 ± 5.42 | 1.64 ± 3.22 | 0.99 |
| Fresh Frozen Plasma (units) | 1.22 ± 3.25 | 0.61 ± 2.22 | 0.002 |
| Cryoprecipitate (units) | 0.13 ± 0.46 | 0.06 ± 0.25 | 0.04 |
| Platelets (units) | 0.65 ± 2.74 | 0.39 ± 1.29 | 0.18 |
| Factor VIIa % | 7.7 | 9.9 | 0.52 |
| Post-operative Outcome | |||
| MACCE | |||
| Operative Death (Y) % | 5.5 | 3.3 | 0.12 |
| Perioperative MI (Y) % | 0.0 | 0.2 | 1.00 |
| Reoperation for Bleeding (Y) % | 4.9 | 1.8 | 0.01 |
| Neurological Event Permanent (Y) % | 2.2 | 1.8 | 0.69 |
| Neurological Event Transient (Y) % | 3.4 | 1.3 | 0.05 |
| Intra-aortic Balloon Pump (Y)% | 1.9 | 2.9 | 0.37 |
| Prolonged Ventilatory/Respiratory Failure(Y) % | 22.5 | 20.2 | 0.44 |
| Renal | |||
| Renal Failure (Y) % | 9.2 | 9.0 | 0.91 |
| Renal Dialysis Required (Y) % | 4.9 | 2.4 | 0.06 |
-Lysine Analogues: tranexamic acid or aminocaproic acid. Values presented as mean+/− standard deviation or percent. P-values computed from Wilcoxon rank sum tests, or chi-Square test (Fisher’s exact test for vell counts less than 5). MACCE- Major adverse cardiovascular or cerebrovascular events.
Unadjusted Comparisons of Blood Product Utilization and Post-operative Outcomes
In light of the significant differences in pre-operative cardiac disease states and comorbidities, aprotinin and lysine analogue groups were not well-matched as multivariable logistic regression modeling allowed for the identification of group designation (ie aprotinin vs lysine analogue). Variables that were predictive for group designation included EuroSCORE, CBP time, surgery type (i.e. valve replacement/repair vs CABG alone), presence or absence of diabetes, and the pre-operative use of ACE inhibition, diuretics or aspirin (goodness of fit statistic; C-statistic=0.77). As a result, these variables were utilized in the covariate adjusted analyses presented in Table 3.
Table 3.
Unadjusted and Covariate Adjusted Relative Risk for Blood Product Utilization and Post-operative Events in Patients Receiving Aprotinin when Compared to Patients Receiving Lysine Analoguesa
| Unadjusted Relative Risk | Covariate Adjusted Relative Risk | |
|---|---|---|
| Blood Product Utilization | ||
| Intra-operative/Early-post Operative (<24 hours) | ||
| Packed Red Blood Cells | 1.20 [1.05, 1.36]* | 0.94 [0.81, 1.09] |
| Fresh Frozen Plasma | 1.07 [0.90, 1.28] | 0.69 [0.55, 0.86]* |
| Cryoprecipitate | 0.70 [0.46, 1.01] | 0.30 [0.18, 0.52]* |
| Platelets | 1.16 [1.04, 1.29]* | 0.95 [0.83, 1.09] |
| Late Post-operative Period (>24 hours) | ||
| Packed Red Blood Cells | 1.35 [1.22, 1.49]* | 0.91 [0.80, 1.04] |
| Fresh Frozen Plasma | 1.99 [1.70, 2.32]* | 1.38 [1.15, 1.66]* |
| Cryoprecipitate | 2.23 [1.38, 3.62]* | 1.66 [0.93, 2.97] |
| Platelets | 1.68 [1.38, 2.05]* | 0.97 [0.76, 1.25] |
| Recombinant Factor VIIa | 0.84 [0.50, 1.42] | 0.22 [0.10, 0.49]* |
| Post-operative Outcome | ||
| MACCE | ||
| Operative Death | 1.72 [0.85, 3.47] | 0.67 [0.25, 1.80] |
| Perioperative MI | --- | |
| Reoperation for Bleeding | 2.90 [1.23, 6.86]* | 2.39 [0.88, 6.48] |
| Neurological Event Permanent | 1.23 [0.44,3.43] | 0.87 [0.25, 3.10] |
| Neurological Event Transient | 2.63 [0.96, 7.18]* | 1.61 [0.50, 5.20] |
| Intra-aortic Balloon Pump | 0.64 [0.24,1.70] | 0.83 [0.25, 2.78] |
| Prolonged Ventilatory/Respiratory Failure | 1.15 [0.81, 1.62] | 0.63 [0.40, 1.00] |
| Renal | ||
| Renal Failure | 1.03 [0.63, 1.69] | 0.59 [0.31, 1.13] |
| Renal Dialysis Required | 2.10 [0.96, 4.58]* | 0.77 [0.27, 2.20] |
| Composite Events | ||
| Renal | 1.03 [0.63, 1.69] | 0.59 [0.31, 1.13] |
| Neurological | 1.85 [0.91, 3.78]* | 1.18 [0.50, 2.78] |
| Length of Stay | ||
| Total Length of Stay | 0.85 [0.73, 0.98]* | 0.98 [0.79, 1.10] |
| Post operative Length of Stay | 0.85 [0.74, 0.98]* | 1.01 [0.83, 1.16] |
-Lysine Analogues: tranexamic acid or aminocaproic acid. Values presented as relative risk (RR) and 95% confidence interval as estimated using Poisson or logistic regression for unmatched data or generalized estimating equations (GEE) for matched data. Length of stay outcomes were analyzed using proportional hazards models. MACCE- Major adverse cardiovascular or cerebrovascular event.
Indicates a significant finding (p<0.05).
The unadjusted and covariate adjusted relative risks and 95% confidence intervals for blood product utilization resulting from Poisson regression are presented in Table 3. The unadjusted risk for intra-operative administration of packed red blood cells and intra-operative platelets was higher in the aprotinin group, and the unadjusted risk for the use of all blood products (with the exception of rFVlla) in the late post-operative period was higher. However, in covariate adjusted analysis, the risk for utilization of specific blood products in the intra-operative period was significantly lower in the aprotinin group. Specifically, as shown in Table 3, the covariate adjusted risk for the use of fresh frozen plasma and cryoprecipitate was significantly lower in the intra-operative period. However, the covariate adjusted risk for the use of fresh frozen plasma in the later post-operative period remained higher in the aprotinin group. In contrast, the covariate adjusted risk for the use of rFVlla in the entire intra-operative and postoperative period was substantially lower in the aprotinin group.
The unadjusted and covariate adjusted relative risks and 95% confidence intervals for early post-operative outcomes are also presented in Table 3. The unadjusted risk for re-operation due to bleeding, transient neurological events, and the need for dialysis, was all higher in the aprotinin group. The unadjusted risk for any neurological event (whether transient or permanent) was higher in the aprotinin group. The unadjusted risk for a prolonged length of hospital stay was also higher in the aprotinin group. However, when adjusted for the aforementioned pre-operative and intra-operative covariates, the risk for all early post-operative events in the aprotinin group was similar to that of the lysine analogue group (i.e., the relative risk was not significantly different from 1).
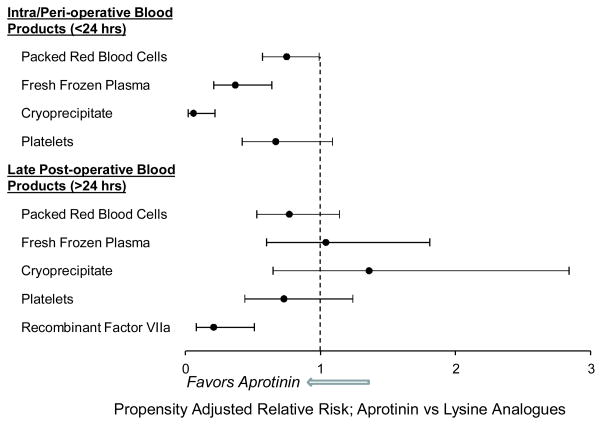
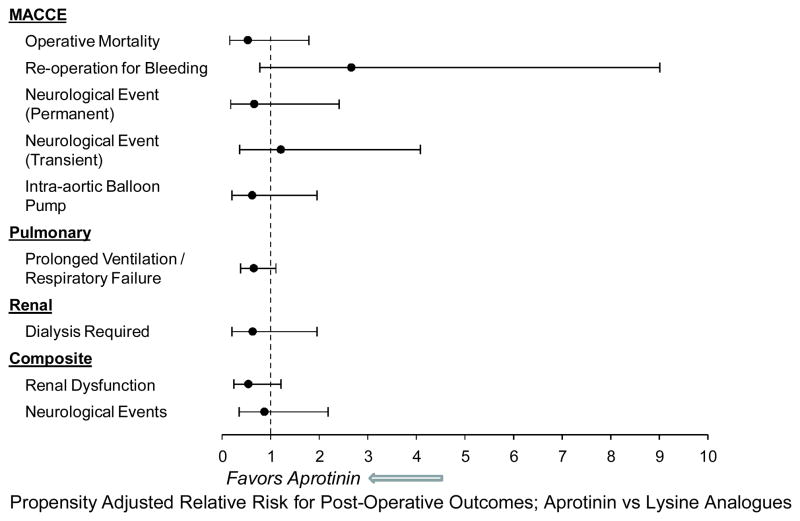
Past studies have provided evidence that covariate adjustment does not sufficiently match patients on underlying prognostic indicators.6,10 Accordingly, a propensity adjusted analysis to determine a more unbiased relative risk of blood product utilization and post-operative adverse events was performed. In this approach, the patients were matched on the propensity score generated by the above-mentioned covariates that significantly predicted group membership, which yielded a final matched data set of 434 patients. The results from this approach for blood product utilization are shown in Figure 1. The propensity adjustsed risk for early (<24 hours) utilization of packed red blood cells (RR:0.75;CI:0.57–0.99), fresh frozen plasma (RR:0.37;0.21–0.64), and cryoprecipitate (RR:0.06;CI:0.02–0.22) was significantly lower in the aprotinin versus lysine analogue group. In the late (>24 hours) post-operative period, the relative risk of blood product utilization was not significantly different from 1, with one notable exception; the propensity adjusted risk for the use of rFVlla was significantly lower in the aprotinin group (RR:0.21;0.08–0.51). The propensity adjusted relative risks for postoperative adverse events following propensity adjusted analyses, are shown in Figure 2. Most of these relative risks were less than 1 and none were significantly different from 1 indicating a similar risk of these outcomes in the aprotinin and lysine-analogue groups. Finally, the propensity adjusted total length of stay (HR:1.06;CI:0.88,1.30) and post operative length of stay (HR:1.08;CI:0.88,1.32) were not significantly different between the two groups.
Figure 1.
The propensity adjusted relative risk blood product utilization in the early intra-operative/post-operative period (<24 hours) and the late post-operative period (>24 hours) in the aprotinin group versus the lysine analogue group. The relative risk for the use of rVlla was computed for the entire intra-operative/post-operative course. The risk for early utilization of red blood cells, fresh frozen plasma and cryoprecipitate was significantly lower in the aprotinin group (p<0.05). In the late post-operative period, the risk of blood product utilization was equivalent between the aprotinin group and the lysine analogue group, with one notable exception. The propensity adjusted risk for the use of rVlla was substantially lower in the aprotinin group (p<0.05).
Figure 2.
The propensity adjusted relative risk for early post-operative adverse events in the aprotinin group versus the lysine analogue group. Overall, the propensity adjusted analysis failed to identify any risk reduction in significant peri-operative adverse events in the post-aprotinin era. Indeed, there was a trend for a reduction in the risk of respiratory in the aprotinin group (p=0.06). Although the propensity adjusted risk of renal failure was reduced in the aprotinin group, this was not significant (p=0.13).
Discussion
Management of early peri-operative bleeding is a basic objective in cardiac surgery as it is a fundamental determinant of post-operative outcomes. While past studies have provided evidence that aprotinin reduced peri-operative blood loss, concerns regarding potential adverse events resulted in the removal of aprotinin from routine clinical use. This resulted in the lysine analogues becoming the primary means of anti-fibrinolytic therapy in the context of cardiac surgery. However, whether and to what degree this “post aprotinin era” has yielded improvements in early post-operative outcomes and whether blood product utilization has been affected, remain to be fully examined. The unique findings from the present study were 3-fold. First, pre-operative co-morbidities such as reduced LV systolic function and use of cardiovascular medications were more likely in the aprotinin group, as was the complexity of the cardiac procedure. These findings suggest that in the post-aprotinin era, lysine analogues are not being utilized to the same degree in high risk patients. Second, using propensity scoring, the risk for intra-operative/peri-operative use of blood products such as fresh frozen plasma and cryoprecipitate was lower in the aprotinin era patients, as was the overall risk for the use of rFVlla. Third, the adjusted risk for peri-operative renal dysfunction, has not improved with the exclusive availability of lysine analogues. These new findings suggest that this post-aprotinin era has resulted in a higher risk for the use of refined and recombinant blood products with the potential attendant costs and risks inherent with this strategy and improvements in post-operative adverse events have not been realized.
The majority of past studies that compared the relative effects of aprotinin or lysine analogue administration were performed when both agents were available. These past retrospective studies underscored the importance of considering confounding variables in the analysis which may prevent interpretation of the effects of these anti-fibrinolytic agents with respect to early post-operative outcomes.1,3–12 Previous studies identified that pre-operative variables such as cardiac functional status, diabetes, pre-existing renal dysfunction and the complexity of the procedure (combined CABG/valve replacement), may directly influence the outcome response variables.1,5,7,8,10,15–19 In addition, other studies demonstrated that pre-operative use of ACE inhibitors, the use of CPB, and the duration of the CPB time could independently influence post-operative outcomes such as renal dysfunction, neurological events and mortality.15–17 Finally, the effects of aprotinin may be dependent on the dosing strategy used, as several studies reporting detrimental findings with respect to post operative outcomes did not use a standardized dosing procedure7–10 The present study built upon past observations by considering all of these potentially confounding variables in the analysis of patients requiring CPB who received a uniform anti-fibrinolytic dosing strategy at a single institution. Moreover, the present study examined blood product utilization and postoperative events using established set points for transfusions, and over an interval of time in which the cardiac surgeons performing the cardiac procedures were consistent. Finally, this study is the first to perform a quantitative analysis of blood product utilization and early adverse events in the “post aprotinin-era” whereby only lysine analogues were available for anti-fibrinolytic therapy.
There were several important differences in the patient demographics between the aprotinin and lysine analogue groups. Specifically, there was a higher incidence of LV dysfunction requiring cardiovascular medications than in the lysine analogue group. There are several possible explanations for this observation. First, aprotinin administration was routinely considered for relatively high risk patients, such as those with pre-existing LV dysfunction, whereas the off-label use of lysine analogues is not. Thus, in the post-aprotinin era, it is possible that patients with pre-existing LV dysfunction are not routinely considered for administration of lysine analogues.
One of the notable differences between the present study and past retrospective studies regarding aprotinin was the relative risk for post-operative renal failure. In a study by Furnary et al,17 an analysis of a cardiac surgery database revealed that the unadjusted risk for renal failure was higher with aprotinin, but when adjusted for the number of red blood cell transfusions, the independent effects of aprotinin in the risk model for renal failure was attenuated. This underscores the importance of inclusion of independent covariates when assessing the relative risk.. However, this past registry analysis was performed when selection bias between aprotinin and lysine analogues may have been operative, did not directly compare the adjusted risk of renal failure between aprotinin and lysine analogues, and did not utilize propensity matching. The present study evaluated the relative risk for renal failure as that of a clinically significant rise in post-operative creatinine levels, a need for post-operative dialysis, or a combination of these events. The present study as well as past reports,15 have identified that pre-operative use of ACE inhibitors is an independent contributory variable for the risk of post-operative renal dysfunction. Propensity adjusted analysis suggested that the risk for renal dysfunction was actually increased by 85% in the post-aprotinin era with the use of lysine analogues. These new findings build upon the results from past registry findings.17
This study examined an interval of time when aprotinin was the pharmacological agent of choice for anti-fibrinolytic therapy to an interval of time following when this serine protease had been withdrawn. One of the deciding factors for the withdrawal of aprotinin was the initial adverse events reported in the prospective study entitled the Blood Conservation Using Antifibrinolytic in a Randomized Trial (BART).11 The BART study was terminated early due to the fact that an interim analysis demonstrated a higher relative risk for 30 day mortality in the aprotinin group when compared to the lysine analogue groups.11 However, the BART study used a pre-specified definition for massive bleeding, and in the interim analysis, the risk for massive bleeding appeared to be reduced with aprotinin when compared to lysine analogues. But the BART study was not designed to evaluate the relative risk of blood product utilization in the early and late post-operative periods. In the present study, and consistent with the BART study, the unadjusted risk for re-operation due to excessive bleeding was higher in the aprotinin group. This risk was reduced with the introduction of confounding variables such as pre-operative functional status and CPB times- indicating that aprotinin was utilized in more complex cases where an inherent risk for post-operative bleeding was higher. However, one unique finding from the present study was that despite the higher incidence of comorbidities, surgical complexity, and as a consequence increased re-operation for bleeding, the propensity adjusted risk for intra-operative blood product utilization in the aprotinin group was substantially reduced when compared to lysine analogues. These findings would suggest that aprotinin reduced the risk for receiving intra-operative blood products such as packed red blood cells, fresh frozen plasma, and cryoprecipitate, independent of the incidence of significant post-operative bleeding.
Limitations and Summary
There are a number of limitations to the present study which must be recognized. First, this is a retrospective study and the inherent limitations of this approach have been discussed in the preceding sections. Second, this was a single center study and in relative terms, the sample size was small. However, the single center approach limited the variability that can occur with multi-center studies where the anti-fibrinolytic formulations and dosing regimens can differ as well as increase the procedure variability by increasing the number of cardiac surgeons. Third, the present study combined the lysine analogues together in order to provide a more robust comparison to the aprotinin era patients. While this approach has been commonly employed previously,2,11 it prevents comparison between the lysine analogues.
There are also limitations to covariate adjusted and propensity adjusted analysis approaches. Propensity matching inevitably reduces sample size as patients are closely matched on confounding variables, however, uniform results were obtained whether covariate adjustment or propensity matching were used. The analysis was predicated upon comparisons between aprotinin and lysine analogues in terms of the primary outcomes of blood product utilization and renal failure, and a robust statistical power was achieved for this purpose. However, the present study was likely underpowered in terms of identifying significant differences in post-operative outcomes such as mortality and neurological events. Utilizing the observed incidence in the propensity matched samples for the outcomes of mortality (aprotinin: 2.3% vs lysine analogues: 4.7%) and neurological events (aprotinin: 4.7% vs lysine analogues: 5.1%), a conditional power calculation was computed for these two outcomes.27,28 An observed incidence within the aprotinin group of at least 7.6% for mortality (assuming a continual rate of 4.7% in the lysine analogue group), and at least a 6.4% incidence in neurological outcomes (assuming a continual rate of 5.1% in the lysine analogue group) would have been required. Given the observed incidence of these outcomes in the present study as well as in past reports,4–13 then it follows that an extremely large sample size would be required, and ultimately yield similar conclusions to those of the present study.
Nevertheless, the unique findings from the present study suggest that the withdrawal of aprotinin has not yielded an improvement in post-operative outcomes such as a reduced incidence of renal failure, which would have been anticipated based upon past retrospective studies.5–8,13 Moreover, the relative risk for the use of refined blood products such as cryoprecipitate and rFVIIa has increased significantly in this post-aprotinin era. Indeed, a recent study has identified that increased use of rFVIIa can increase the risk of arterial thrombotic events.29 Thus, while remaining speculative, the risk of higher utilization of rFVIIa in this post-aprotinin era may in turn contribute to adverse post-operative outcomes such as neurological events. Excessive postoperative bleeding and the management of fibrinolysis remain contemporary problems confronting the cardiac surgeon.19,30 In light of the fact that increased blood product utilization holds inherent risks and costs, the findings from the present study underscore the need for improvements in pharmacological strategies which modulate the fibrinolytic cascade following cardiac surgery.
Acknowledgments
Funding Sources
This study was supported by NIH grant HL59165 and a Merit Award from the Veterans’ Affairs Health Administration.
Footnotes
Disclosures
None.
References
- 1.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–8. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briët E, Büller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940–7. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007;115:2801–13. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 4.Kristeller JL, Roslund BP, Stahl RF. Benefits and risks of aprotinin use during cardiac surgery. Pharmacotherapy. 2008;28:112–24. doi: 10.1592/phco.28.1.112. [DOI] [PubMed] [Google Scholar]
- 5.Ngaage DL, Cale AR, Cowen ME, Griffin S, Guvendik L. Aprotinin in primary cardiac surgery: operative outcome of propensity score-matched study. Ann Thorac Surg. 2008;86:1195–202. doi: 10.1016/j.athoracsur.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Mangano DT, Tudor IC, Dietzel C Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 7.Grant MC, Kon Z, Joshi A, Christenson E, Kallam S, Burris N, Gu J, Poston RS. Is aprotinin safe to use in a cohort at increased risk for thrombotic events: results from a randomized, prospective trial in off-pump coronary artery bypass. Ann Thorac Surg. 2008;86:815–22. doi: 10.1016/j.athoracsur.2008.04.047. discussion 815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olenchock SA, Jr, Lee PH, Yehoshua T, Murphy SA, Symes J, Tolis G., Jr Impact of aprotinin on adverse clinical outcomes and mortality up to 12 years in a registry of 3,337 patients. Ann Thorac Surg. 2008;86:560–6. doi: 10.1016/j.athoracsur.2008.04.048. discussion 566–7. [DOI] [PubMed] [Google Scholar]
- 9.Kluth M, Lueth JU, Zittermann A, Lanzenstiel M, Koerfer R, Inoue K. Safety of low-dose aprotinin in coronary artery bypass graft surgery: a single-centre investigation in 2,436 patients in Germany. Drug Saf. 2008;31:617–26. doi: 10.2165/00002018-200831070-00007. [DOI] [PubMed] [Google Scholar]
- 10.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–83. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J. Pretorius R; BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AD, Stafford-Smith M, White WD, Phillips-Bute B, Swaminathan M, Milano C, Welsby IJ, Aronson S, Mathew JP, Peterson ED, Newman MF. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358:784–93. doi: 10.1056/NEJMoa0707768. [DOI] [PubMed] [Google Scholar]
- 13.Lindvall G, Sartipy U, Ivert T, van der Linden J. Aprotinin is not associated with postoperative renal impairment after primary coronary surgery. Ann Thorac Surg. 2008;86:13–9. doi: 10.1016/j.athoracsur.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Umscheid CA, Kohl BA, Williams K. Antifibrinolytic use in adult cardiac surgery. Curr Opin Hematol. 2007;14:455–67. doi: 10.1097/MOH.0b013e328270b89e. [DOI] [PubMed] [Google Scholar]
- 15.Mouton R, Finch D, Davies I, Binks A, Zacharowski K. Effect of aprotinin on renal dysfunction in patients undergoing on-pump and off-pump cardiac surgery: a retrospective observational study. Lancet. 2008;371:475–82. doi: 10.1016/S0140-6736(08)60237-8. [DOI] [PubMed] [Google Scholar]
- 16.Van der Linden PJ, Hardy JF, Daper A, Trenchant A, De Hert SG. Cardiac surgery with cardiopulmonary bypass: does aprotinin affect outcome? Br J Anaesth. 2007;99:646–52. doi: 10.1093/bja/aem252. [DOI] [PubMed] [Google Scholar]
- 17.Furnary AP, Wu Y, Hiratzka LF, Grunkemeier GL, Page US., 3rd Aprotinin does not increase the risk of renal failure in cardiac surgery patients. Circulation. 2007 Sep 11;116(11 Suppl):I127–33. doi: 10.1161/CIRCULATIONAHA.106.681395. [DOI] [PubMed] [Google Scholar]
- 18.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Tait G, Beattie WS. The risk-benefit profile of aprotinin versus tranexamic acid in cardiac surgery. Anesth Analg. 2010 Jan 1;110(1):21–9. doi: 10.1213/ANE.0b013e3181c0ea6d. [DOI] [PubMed] [Google Scholar]
- 19.Koster A, Schirmer U. Re-evaluation of the role of antifibrinolytic therapy with lysine analogs during cardiac surgery in the post aprotinin era. Curr Opin Anaesthesiol. 2011 Feb;24(1):92–7. doi: 10.1097/ACO.0b013e32833ff3eb. [DOI] [PubMed] [Google Scholar]
- 20.Clark LL, Ikonomidis JS, Crawford FA, Jr, Crumbley A, 3rd, Kratz JM, Stroud MR, Woolson RF, Bruce JJ, Nicholas JS, Lackland DT, Zile MR, Spinale FG. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg. 2006;131:679–85. doi: 10.1016/j.jtcvs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Gogbashian A, Sedrakyan A, Treasure T. EuroSCORE: a systematic review of international performance. Eur J Cardiothorac Surg. 2004 May;25(5):695–700. doi: 10.1016/j.ejcts.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum PR. Optimal matching for observational studies. J Amer Stat Assoc. 1989;408:1024–1032. [Google Scholar]
- 24.Liang K-Y. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 25.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84:1065–1073. [Google Scholar]
- 26.SAS Version 9.2, Copyright © 2002–2008 by SAS Institute Inc., Cary, NC. All Rights Reserved.
- 27.Betensky RA, Tierney C. An examination of methods for sample size recalculation during an experiment. Stat Med. 1997 Nov 30;16(22):2587–98. doi: 10.1002/(sici)1097-0258(19971130)16:22<2587::aid-sim687>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Betensky RA. Conditional power calculations for early acceptance of H0 embedded in sequential tests. Stat Med. 1997 Feb 28;16(4):465–77. doi: 10.1002/(sici)1097-0258(19970228)16:4<465::aid-sim384>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010 Nov 4;363(19):1791–800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 30.Edmunds LH., Jr Managing fibrinolysis without aprotinin. Ann Thorac Surg. 2010;89:324–331. doi: 10.1016/j.athoracsur.2009.10.043. [DOI] [PubMed] [Google Scholar]