Abstract
Calcium flux through L-type voltage-activated calcium (Cav1) channels is crucial for regulating brain functions including memory formation and behavior. Alterations in Ca2+ homeostasis have been linked to many cognitive disorders and understanding into the regulation of this process is crucial for their remedy. Therefore, here, we have evaluated the effect of a multifunctional protein known to be involved in memory functions called regulator of G protein signaling-14 (RGS-14) on Cav1 channel activity in neuronal cell lines NG108-15 and SH-SY5Y. RGS-14 protein produced significant reduction in Ca2+ influx in both cell lines and this effect was dependent on nifedipine-sensitive Cav1 channels. Thus, our results provide evidence supporting the idea that RGS-14 may facilitate the cognitive processing by modulating Cav1 channel-mediated intracellular Ca2+ transients.
Keywords: Ca2+ influx, L-type voltage-activated calcium channels, Cav1 channels, RGS-14 protein, Neuronal cell lines
Introduction
Plasma membrane voltage-activated calcium channels (Cav channels) regulate intracellular Ca2+ concentration. Ca2+ influx through these channels leads to a wide range of intracellular processes such as, activation of calcium-dependent enzymes, gene transcription, and neurotransmitter secretion [1]. Cav channels are classified into three families: Cav1 (L-type), Cav2 (P/Q-, N-, and R-type), and Cav3 (T-type) [2]. Cav1 channels comprise a family of 4 members, namely Cav1.1 to Cav1.4 and of which, Cav 1.2 and Cav1.3 are main isoforms present in the brain [3]. Cav1 channels play a critical role in somatodendritic Ca2+ influx and are involved in action potentials, synaptic plasticity, and learning and memory [1,4–7].
In addition to influx through Cav channels, Ca2+ can also be released from internal stores through inositol 1,4,5-trisphosphate (IP3) receptors or ryanodine receptors (RyRs) [8]. RyR3-deficient mice showed better learning and memory in the spatial version of the Morris water maze, which was thought to be due to altered Ca2+ recruitment dynamics between intracellular and extracellular Ca2+ resources involved in long-term potentiation (LTP) [9].
All known regulator of G protein signaling (RGS) proteins act as GTPase-activating proteins (GAP) targeting Gα subunit to terminate the G protein-mediated signal transmission in mammalian cells [10]. Within the family of RGS proteins, RGS-14 is a multidomain protein and belongs to a subfamily that in addition to a conserved 120 amino acids RGS domain for binding with active Gi/o alpha-GTP to confer GAP activity, contain a tandem Rap1/2 binding domain (RBD), a GoLoco/GPR motif that bind to inactive Gi alpha-GDP and Gi alpha1/3, and other regions with unknown functions [10,11]. RGS-14 interacts selectively with members of the Giα and Goα subfamily of G proteins to regulate their guanine nucleotide binding and hydrolysis activity. Recently, our laboratory has shown that this protein is involved in the formation of long-term memory [12]. Therefore, in a quest to underpin the mechanism that underlie RGS-14 protein-mediated memory formation and with the consideration of the expression of RGS-14 proteins observed into brain [13] and of the role of Cav channels in memory [4,7], we have evaluated the participation of RGS-14 protein in regulation of intracellular Ca2+ levels. With the use of two different cell lines of neuronal origin and two different Ca2+ measurement methods, we found that the expression of RGS-14 protein into these cell lines substantially reduced Ca2+ influx and that this reduction in Ca2+ was mediated through Cav1 channels.
Methods
Preparation of the RGS-14 recombinant vector
Human RGS-14 gene was cloned in our laboratory (GenBank accession number AY987041). RGS-14 gene was inserted into pcDNA 6.2/C-EmGFP vector by Gateway recombination using Vivid Colors pcDNA 6.2/C-EmGFP Gateway vector system (Invitrogen, Barcelona, Spain). The co-expression of this tagged-EmGFP will allow easy identification of the cells expressing RGS-14 proteins. pcDNA 6.2/C-EmGFP/GW/CAT vector without gene insert was used as control. The recombinant vector of RGS-14 gene was transformed into OmniMAX 2 T1 Phage-Resistant E. coli (Invitrogen) for the expansion of plasmids and DNA was purified with Wizard Plus Maxipreps DNA Purification System Kit (Promega Biotech, Madrid, Spain). A260/280 of isolated DNA was above 1.7.
Cell culture
Two cell lines SH-SY5Y and NG108-15 of neuronal origin (LGC Standards, Barcelona, Spain) were used in this study. They were selected because of their functional expression of Cav channels and the capacity for KCl-stimulated Ca2+ turnover [14,15]. NG108-15 (neuroblastoma-glioma cell line) was cultured in high glucose D-MEM supplemented with 2% HAT complement, 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (P/S). The SH-SY5Y (neuroblastoma cell line) was cultured in 1:1 E-MEM and F12 Medium supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, and 1500 mg/l NaHCO3, 10% FBS, 1% P/S. A humidified incubator of 5% CO2 at 37°C was used.
Transfection with Lipofectamine 2000
Cells were plated in 2 ml of complete growth medium (1–3 × 105 cells in 35 mm culture dishes). After 48 hours, a period when cells with 90–95% confluence have been observed, cells were processed for the transfection with Lipofectamine 2000 (Invitrogen). On the day of transfection, medium was replaced with fresh one but without antibiotics. For the transfection, complex A (4 μg DNA into 250 μl of Opti-MEM I) and complex B (10 μl Lipofectamine 2000 in 250 μl of Opti-MEM I) were prepared. Complexes A and B were combined and incubated for 20 min at room temperature. 500 μl of this combined solution was added to culture plate with cells. Plate was incubated for 4 h in CO2-incubator. The medium was then replaced with growth medium without antibiotics. The incubation continued 48 h to allow the maximal protein expression. The expression of RGS-14 protein was confirmed by immunocytochemistry using specific antibody [13].
Fluorescence immunocytochemistry
After transfection and protein expression as described above, cells grown on Flask-style glass slides (Nunc, Barcelona, Spain) were fixed with 4% paraformaldehyde and 0.2% glutaraldehyde for 10 min and permeabilized with 0.3% Triton X-100. Immunofluorescence staining of cells was performed as described. Briefly, after incubation with RGS-14 antibody (1:200), cells were incubated with Alexa 568 (red) coupled to anti-rabbit IgG (1:1000; Invitrogen). Images of EmGFP (green) and RGS-14 antibody (red) were taken with a Leica fluorescence microscope.
Dye loading
Culture medium was replaced by a Na-HEPES buffer containing (in mM): 140 NaCl, 4.7 KCl, 1.3 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 0.2 % bovine serum albumin (pH 7.4). SH-SY5Y cells were incubated with Fura-Red AM (10 μM) for 30 min in a CO2-incubator at 37°C (Invitrogen). However, NG108-15 cells were incubated with Rhod-2 AM (8 μM) for 30 min at 23–25 °C (Invitrogen). Alternatively, NG108-15 cells were co-incubated with Rhod-2 AM and Mito-Tracker Green FM (Invitrogen).
Ca2+ measurement using Fura-Red AM
After dye loading, the coverslips with cells were mounted in a perfusion chamber placed on the stage of a confocal laser scanning microscope (LSM 410, Zeiss, Jena, Germany) equipped with an image acquisition and analysis system. The fluorescence change after application of 50 mM KCl was measured. Changes in fluorescence intensity were calculated by dividing the fluorescence level after KCl application (F) with average baseline fluorescence (F0). Non-stimulus related spontaneous changes in fluorescence were less than 3%.
Ca2+ measurement using Rhod-2 AM
Similar to above, coverslips with dye-loaded cells were mounted into a perfusion chamber located on the stage of an inverted microscope (Nikon Eclipse TE300, Nikon, Tokio, Japan). Fluorescence changes were determined by a MRC-1024 confocal laser scanning system (Bio-Rad, Madrid, Spain). After application of 50 mM KCl, images were recorded and fluoresecence changes were plotted as F/F0.
Cells double-loaded with Rhod-2 and Mito-Tracker Green, were simultaneously excited and emitted fluorescence was recorded. Neutral density filters were employed to reduce laser intensity to 0.3–1% to diminish photo-bleaching.
The results are reported as mean ± SEM of 3–6 independent experiments of at least 6–10 cells in each. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey post-hoc test, and p values <0.05 were considered significant. For individual comparisons and statistics Student’s t-test was used.
Results
To understand the role of RGS-14 protein in the mediation of Ca2+ influx through plasma membrane Cav channels, SH-SY5Y and NG108-15 cells containing RGS-14 protein were exposed to 50 mM KCl to induce cellular depolarization. This process of chemical depolarization drives Cav channel pore opening and subsequently Ca2+ entry into cells [15]. The determination of Ca2+ increase into cytoplasm was used as a tool to evaluate the effect of RGS-14 protein into this process. Here, Fig. 1 represents the study performed in SH-SY5Y cells with Fura-Red and Fig. 2 shows the results from NG108-15 cells with Rhod-2. The difference between both dyes is that the binding of free Ca2+ with Fura-Red proportionally decreases the fluorescence intensity and in contrary, binding with Rhod-2 increases the intensity of fluorescence.
Fig. 1.
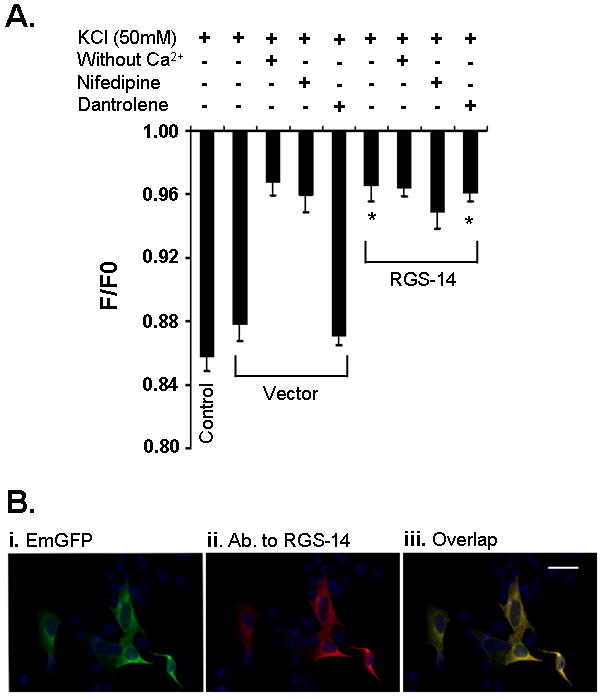
Expression of RGS-14 protein reduces cytoplasmic Ca2+ concentration in SH-SY5Y cells loaded with Fura-Red dye through Cav1 channels. A, Exposure of non-transfected (control) and control vector (vector) to 50mM KCl produced increase in Ca2+ concentration. Absence of Ca2+ in medium and the presence of Cav1 channels blocker, nifedipine, completely abolished this activity in control vector cells. Addition of dantrolene, an inhibitor of intracellular Ca2+ storage, did not produce any effect on the increase in Ca2+ level. Expression of RGS-14 protein in these cells resulted in reduction of KCl-mediated Ca2+ increase. B, is to demonstrate the expression of tagged EmGFP (i, green) as well as RGS-14 protein (ii, red) in cells. RGS-14 protein was detected by a specific antibody (Ab.) developed against this protein. As expected, both proteins were found to be co-localized (iii). Scale bar is 75 μm. *, indicates significantly different from their counterpart control vectors.
Fig. 2.
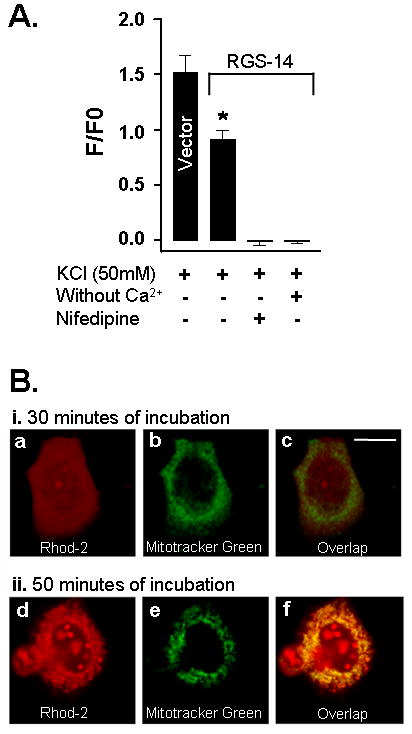
Presence of RGS-14 protein reduces cytoplasmic Ca2+ level in NG108-15 cells loaded with Rhod-2 dye via Cav1 channels. A, Similar to SH-SY5Y cells in Fig. 1, control vector (vector) transfected NG108-15 cells in combination with Rhod-2 dye produced an increase in cytoplasmic Ca2+ concentration when exposed to 50 mM KCl. Expression of RGS-14 protein led to a substantial reduction in the Ca2+ level. Both the absence of Ca2+ in medium and presence of nifedipine completely abolished the cytoplasmic Ca2+ level. B, Incubation for 30 min with Rhod-2 dye showed no dye presence in mitochondria of NG108-15 cells. i, shows the presence of fluorescence after co-incubation with Rhod-2 (a) and Mito-Tracker Green (b), a dye specific for mitochondria, for 30 min. Merged images in (c) demonstrates no co-localization of Mito-Tracker Green with Rhod-2 and no presence of Rhod-2 dye in mitochondria. ii, fluorescence level observed after co-incubation with Rhod-2 (d) and Mito-Tracker Green (e) for 50 min. Merged images of Mito-Tracker Green with Rhod-2 show co-localization of both dyes in mitochondria (f). Scale bar is 75 μm. *, indicates significantly different from control vector.
In non-transfected SH-SY5Y cells (control), KCl stimulation substantially increases the cytoplasmic free Ca2+ level (Fig. 1A). A similar level increase was also observed in cells transfected with control vector (vector). However, in contrast to control vector transfected and non-transfected cells, the expression of RGS-14 protein in SH-SY5Y cells reduced this increase in cytoplasmic Ca2+ level to KCl stimulation (Fig. 1A), indicating that this protein may be involved in regulating Ca2+ influx into the cell. The KCl-mediated Ca2+ increase in control vector transfected cells was dependent on the presence of Ca2+ extracellular (Fig. 1A) because the use of Ca2+ free medium instead of normal medium resulted in complete loss in Ca2+ transients. In addition, pretreatment with nifedipine (5μM), a specific Cav1 channel blocker, completely blocked the KCl-mediated Ca2+ influx in both control vector and RGS-14 transfected cells (Fig. 1A). These results suggest that KCl-mediated Ca2+ increase in the SH-SY5Y cells is through membrane bound Cav1 channels. The finding of no effect in the Ca2+ concentration after treatment with dantrolene (100 μM), an intracellular Ca2+ storage inhibitor, in both control vector and RGS-14 transfected cells, further ascertains the concept of the involvement of Cav1 channels into this signaling process and furthermore, excludes the possibility of participation of intracellular Ca2+ storage. As expected, in immunocytochemistry study, we found a reasonably good level of RGS-14 protein expression in these cells (Fig. 1Bii) and that these proteins were co-localized with tagged EmGFP (Fig. 1Bi and iii).
Similar to SH-SY5Y cells, transfection of the same recombinant RGS-14 plasmid into NG108-15 cells also resulted in the reduction of KCl-stimulated increase in the cytoplasmic Ca2+ level (Fig. 2A). Presence of RGS-14 protein produces a significant decrease in Ca2+ levels. This effect seems to be specific to RGS-14 protein because sGi2 protein, a member of G-protein family (data not shown), and EmGFP protein (control vector) did not produce any effect. As in SH-SY5Y cells, the reduction in Ca2+ influx in NG108-15 cells was dependent on the presence of Ca2+ extracellular (Fig. 2A). Further, pretreatment of these RGS-14-cells with nifedipine completely abolished this activity. Thus, results from NG108-15 cells further suggest for the participation of Cav1 channels into RGS-14 protein-mediated Ca2+ influx.
During dye loading with Rhod-2 AM in different time frames, it has been shown that dye was not only present in cytoplasm but was also observed in mitochondria [16]. Therefore, to determine the time point for labeling only into cytoplasm in our experimental conditions and to discard the possible interference of mitochondrial fluorescence during the analysis of Ca2+ levels, we have evaluated Rhod-2 labeling in mitochondria using Mito-Tracker Green, a dye specific for mitochondria. The co-incubation of NG108-15 cells with Rhod-2 AM and Mito-Tracker Green FM for 30 min showed no indication of Rhod-2 presence in mitochondria because no overlap of Mito-Tracker Green with Rhod-2 was observed (Fig. 2Bi). However, incubation performed for 50 min during dye loading showed substantial labeling of Rhod-2 in mitochondria that was evident from the co-localization of both dyes (Fig. 2Bii). These results demonstrate that mitochondrial fluorescence did not participate in the Ca2+ level determination in our experiments of NG108-15 cells with Rhod-2.
Discussion
Cav channels play a critical role in many aspects of neuronal function [7]. However, the interplay between Ca2+ and memory processing has recently revealed unexpected complexities. Much excitement now centers on work showing that Ca2+ regulates both forms of synaptic plasticity named LTP and long-term depression (LTD), which means that it may determine whether memory is strengthened or weakened [17]. Ca2+ transients-mediated through Cav channels initiates downstream signaling not only required for the acquisition and consolidation of memory but also is considered important for synaptic plasticity [18]. It has also been shown that Ca2+ transients of different amplitude induced by varying levels of Ca2+ influx can lead to a switch between LTP and LTD depending on the status of cytosolic Ca2+ concentration [17]. These results suggest that different levels of Ca2+ may lead to differential effects on memory processing and consolidation. There are ample evidence that indicate the association of Cav1 channels in the mediation of learning and memory and correlated adaptations in synaptic plasticity [4–6]. Mutants of Cav1 channel isoform Cav1.2 have deficits in synaptic plasticity in the central nervous system and in spatial learning [19]. Conditional forebrain deletion of the same isoform disrupts remote spatial memories while sparing recently acquired spatial memories [20]. In addition to Cav 1.2, deletion of another Cav1 channel isoform Cav1.3 results in deficit in consolidation of memory for fear conditioning but does not alter in the hidden platform version of the water maze [21]. These results indicate that malfunction in Ca2+ influx mediated through Cav1 channels produces defects in learning and memory.
Apart from the genetic manipulations of Cav channels, pharmacological activation has been shown to promote Ca2+ influx through Cav1 channels and triggers processes that underlie long-term retention or amygdala-dependent conditioned fear memory [4]. However, behavioral experiments using Cav1 channel blockers either administered systemically or as intracranial infusions have yielded contradictory results. In fact, a number of investigations suggest that pharmacological blockage of Cav1 channels enhance and not impair learning and memory. Chronic treatment with Cav1 channels antagonist nimodipine ameliorated age-related working memory deficits and accelerated the rate of learning in mice at different behavioral tasks [5,6]. Similarly, mice in which the β3 subunit of the voltage-dependent Ca2+ channels (Cavβ3) was knocked out also showed enhanced LTP and memory [22]. It has been suggested that excessive Ca2+ influx through Cav1 channels may in fact be detrimental to memory formation. For instance, increases in Cav1 channels currents in area CA1 of the aged hippocampus correlated with the degree of learning impairment on a hippocampal-dependent task [23]. In addition, continuously maintained inward Ca2+ currents due to Cav1.2 missense mutation produce cognitive deficits [24]. Therefore, we argue that our results of RGS-14 protein-mediated reduction of intracellular Ca2+ level through modulation of Cav1 channels may serve as a mechanism in the facilitation of memory [12]. It is suggested that RGS-14 proteins may interact with Cav1 channels to promote reduction in Ca2+ influx into the cell similar to as observed in case of RGS-12 protein, another protein that belongs to the same family, where modulation was observed through N-type Ca2+ channels [25]. Thus, RGS-14 protein may in fact prevent higher Ca2+ levels into cytoplasm by reducing Ca2+ influx through Cav1 channels.
Conclusion
In conclusion, we have shown that RGS-14 protein promotes reduction in cytoplasmic Ca2+ concentration in two different neuronal cell lines and that this effect was dependent on nifedipine-sensitive Cav1 channels. It is proposed that this RGS-mediated reduction in Ca2+ signaling may facilitate the cognitive processing and memory formation. This theory is currently under investigation in our laboratory.
Acknowledgments
This work was supported financially by Ministerio de Ciencia e Innovacion grant BFU 09-07641 and Junta de Andalucia grant CTS 586/09 to Z.U.K. and in part by grants EY014227 from NIH/NEI, RR022570 from NIH/NCRR and AG010485, AG022550, and AG027956 from NIH/NIA to P.K.
References
- 1.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann F, Lacinova L, Klugbauer N. Voltage-dependent calcium channels: from structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 3.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1. 3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res. 2003;110:193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- 6.Quartermain D. Chronic administration of the Ca(2+) channel blocker amlodipine facilitates learning and memory in mice. Eur J Pharmacol. 2000;399:57–63. doi: 10.1016/s0014-2999(00)00368-x. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy MB. Regulation of neuronal function by calcium. Trends Neurosci. 1989;12:417–420. doi: 10.1016/0166-2236(89)90089-1. [DOI] [PubMed] [Google Scholar]
- 8.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 9.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 10.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 11.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Aranda MF, Lopez-Tellez JF, Navarro-Lobato I, Masmudi-Martin M, Gutierrez A, Khan ZU. Role of layer 6 of V2 visual cortex in object-recognition memory. Science. 2009;325:87–89. doi: 10.1126/science.1170869. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Aranda MF, Acevedo MJ, Carballo FJ, Gutierrez A, Khan ZU. Localization of the GoLoco motif carrier regulator of G-protein signalling 12 and 14 proteins in monkey and rat brain. Eur J Neurosci. 2006;23:2971–2982. doi: 10.1111/j.1460-9568.2006.04838.x. [DOI] [PubMed] [Google Scholar]
- 14.Kukkonen JP, Shariatmadari R, Courtney MJ, Akerman KE. Localization of voltage-sensitive Ca2+ fluxes and neuropeptide Y immunoreactivity to varicosities in SH-SY5Y human neuroblastoma cells differentiated by treatment with the protein kinase inhibitor staurosporine. Eur J Neurosci. 1997;9:140–150. doi: 10.1111/j.1460-9568.1997.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 15.Imanishi T, Matsushima K, Kawaguchi A, Wada T, Yoshida S, Ichida S. Increased response to high KCl-induced elevation in the intracellular-Ca(2+) concentration in differentiated NG108-15 cell and the inhibitory effect of the L-type Ca(2+) channel blocker, calciseptine. Neurochem Res. 2006;31:33–40. doi: 10.1007/s11064-005-9003-9. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez A, Granados MP, Salido GM, Pariente JA. Changes in mitochondrial activity evoked by cholecystokinin in isolated mouse pancreatic acinar cells. Cell Signal. 2003;15:1039–1048. doi: 10.1016/s0898-6568(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 17.Cho K, Aggleton JP, Brown MW, Bashir ZI. An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of rat. J Physiol. 2001;532:459–466. doi: 10.1111/j.1469-7793.2001.0459f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 19.Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Müller J, et al. Role of hippocampal Cav1. 2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Ca V 1. 2 disrupts remote spatial memories in mice. Learn Mem. 2008;15:1–5. doi: 10.1101/lm.773208. [DOI] [PubMed] [Google Scholar]
- 21.McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1. 3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon D, Song I, Guido W, Kim K, Kim E, Oh U, et al. Ablation of Ca2+ channel beta3 subunit leads to enhanced N-methyl-D-aspartate receptor-dependent long term potentiation and improved long term memory. J Biol Chem. 2008;283:12093–12101. doi: 10.1074/jbc.M800816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 24.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Schiff ML, Siderovski DP, Jordan JD, Brothers G, Snow B, De Vries L, et al. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 2000;408:723–727. doi: 10.1038/35047093. [DOI] [PubMed] [Google Scholar]


