Abstract
Neuroimaging and neuropathological studies of major depressive disorder (MDD) and bipolar disorder (BD) have identified abnormalities of brain structure in areas of the prefrontal cortex, amygdala, striatum, hippocampus, parahippocampal gyrus, and raphe nucleus. These structural imaging abnormalities persist across illness episodes, and preliminary evidence suggests they may in some cases arise prior to the onset of depressive episodes in subjects at high familial risk for MDD. In other cases, the magnitude of abnormality is reportedly correlated with time spent depressed. Postmortem histopathological studies of these regions have shown abnormal reductions of synaptic markers and glial cells, and, in rare cases, reductions in neurons in MDD and BD. Many of the regions affected by these structural abnormalities show increased glucose metabolism during depressive episodes. Because the glucose metabolic signal is dominated by glutamatergic transmission, these data support other evidence that excitatory amino acid transmission is elevated in limbic-cortical-striatal-pallidal-thalamic circuits during depression. Some of the subject samples in which these metabolic abnormalities have been demonstrated were also shown to manifest abnormally elevated stressed plasma cortisol levels. The co-occurrence of increased glutamatergic transmission and Cortisol hypersecretion raises the possibility that the gray matter volumetric reductions in these depressed subjects are partly accounted for by processes homologous to the dendritic atrophy induced by chronic stress in adult rodents, which depends upon interactions between elevated glucocorticoid secretion and N-meihyl-D-aspartate (NMDA)-glutamate receptor stimulation. Some mood-stabilizing and antidepressant drugs that exert neurotrophic effects in rodents appear to reverse or attenuate the gray matter volume abnormalities in humans with mood disorders. These neurotrophic effects may be integrally related to the therapeutic effects of such agents, because the regions affected by structural abnormalities in mood disorders are known to play major roles in modulating the endocrine, autonomic, behavioral, and emotional experiential responses to stressors.
Keywords: major depressive disorder, bipolar disorder, neuroplasticity, neuro-imaging abnormalities, postmortem studies
Abstract
Los estudios neuropatológicos y de neuroimágenes en la depresión mayor (DM) y en el trastorno bipolar (TB) han identificado anormalidades de la estructura cerebral en áreas de la corteza prefrontal, la amígdala, el cuerpo estriado, el hipocampo, el giro parahipocámpico y el núcleo del rafe. Estas anormalidades estructurales en las neuroimágenes se mantienen más allá de los episodios de la enfermedad y las evidencias preliminares sugieren que en algunos casos ellas pueden aparecer antes del inicio de los episodios depresivos en sujetos con alto riesgo familiar de DM. En otros casos, la magnitud de la anormalidad se correlaciona con el tiempo que lleva la depresión. Estudios histopatológicos postmortem de estas regiones han mostrado disminuciones anormales de marcadores sinápticos y de células gliales y, en raros casos, disminución de neuronas en la DM y el TB. Muchas de las regiones afectadas por estas alteraciones estructurales muestran un aumento del metabolismo de la glucosa durante los episodios depresivos. Dado que la señal metabólica de glucosa está comandada por la transmisión glutamatérgica, estos datos sustentan el argumento a favor del incremento de la transmisión del aminoácido excitatorio en los circuitos límbico-córtico-estriato-pálido-talámicos durante la depresión. Algunos de los sujetos de las muestras en que se encontraron estas anormalidades metabólicas también tuvieron cifras elevadas de cortisol plasmático en respuesta al estrés. La aparición concomitante del aumento de la transmisión glutamatérgica y de la hipersecreción de cortisol incrementa la probabilidad que las disminuciones del volumen de sustancia gris en los sujetos con depresión se deba en parte a los procesos equivalentes a los de la atrofia dendrítica inducida por el estrés crónico en roedores adultos, lo que depende de las interacciones entre el aumento de la secreción de glucocorticoides y la estimulación del receptor de glutamato N-metil-D-aspártico (NMDA). Algunos antidepresivos y estabilizadores del ánimo que ejercen efectos neurotróficos en roedores parece que revierten o disminuyen las anormalidades del volumen de sustancia gris en humanos con trastornos afectivos. Estos efectos neurotróficos pueden estar relacionados integramente con los efectos terapéuticos de dichos fármacos, ya que se sabe que las regiones afectadas por alteraciones estructurales en los trastornos afectivos tienen un papel importante en la modulación de las respuestas endocrina, autonómica, conductual y emocional que se experimenta frente al estrés.
Abstract
Les études de neuropathologie et de neuro-imagerie des troubles dépressifs majeurs (TDM) et des troubles bipolaires (TB) ont identifié des anomalies de la structure cérébrale dans les aires du cortex prefrontal, de l'amygdale, du striatum, de l'hippocampe, du gyrus parahippocampique et du noyau du raphé. Ces anomalies structurelles à l'image persistent à travers les épisodes de la maladie et des arguments antérieurs suggèrent qu'elles peuvent se produire avant l'apparition des épisodes dépressifs chez les sujets à haut risque familial de TDM. Dans d'autres cas, l'importance des anomalies serait liée à la durée de la dépression. Des études histopathologiques post mortem de ces régions ont montré des réductions anormales des marqueurs synaptiques et des cellules gliales et, dans quelques rares cas, des diminutions du nombre des neurones dans les TDM et les TB. De nombreuses régions atteintes par ces anomalies structurelles présentent un métabolisme du glucose augmenté pendant ces épisodes dépressifs, La transmission glutamatergique dominant le signal métabolique du glucose, ces données confortent un autre argument à savoir que la transmission de l'acide aminé excitateur est élevée dans les circuits limbiques-corticaux-striataux-pallidaux-thalamiques pendant la dépression. Certains échantillons, chez des sujets chez qui on a trouvé des anomalies métaboliques, ont également montré des concentrations anormalement élevées de Cortisol plasmatique en réponse au stress. L'apparition concomitante de l'augmentation de la transmission glutamatergique et de l'hypersécrétion de Cortisol accroît la possibilité que les réductions de volume de la substance grise chez ces personnes dépressives soient en partie justifiées par des processus identiques à ceux de l'atrophie dendritique induite par le stress chronique chez les rongeurs adultes, qui dépend des interactions entre la sécrétion élevée des glu cocorticoïdes et la stimulation du récepteur glutamatergique N-méthyl-D-aspartate (NMDA). Certains médicaments antidépresseurs et thymorégulateurs exerçant des effets neurotrophiques chez les rongeurs semblent inverser ou atténuer les anomalies du volume de la substance grise chez les humains atteints de troubles de l'humeur. Ces effets neurotrophiques peuvent être intégralement liés aux effets thérapeutiques de tels médicaments, parce qu'il est reconnu que les régions affectées par des anomalies structurelles au cours des troubles de l'humeur jouent un rôle majeur dans la modulation des réponses aux agents stressants au niveau de l'expérience endocrine, autonome, comportementale et émotionnelle.
The recent development of neuroimaging technologies that permit in vivo characterization of the anatomical, physiological, and receptor pharmacological correlates of mood disorders have enabled significant advances toward delineating the neurobiological correlates of mood disorders. Because these conditions were not associated with gross brain pathology or with clear animal models for spontaneous, recurrent, mood episodes, the availability of tools allowing noninvasive assessment of the human brain proved critical to illuminating the pathophysiology of major depressive disorder (MDD) and bipolar disorder (BD). The results of studies applying imaging technologies and postmortem studies have guided clinical neuroscience toward models in which both functional and structural brain pathology play roles in the pathogenesis of mood disorders.
Longitudinal positron emission tomography (PET) imaging studies of MDD and BD identified abnormalities of regional cerebral glucose metabolism and cerebral blood flow (CBF), which, in some cases, persisted beyond symptom remission, and in other cases appeared mood state-dependent (reviewed in reference 1; Figure 1). These reversible abnormalities presumably reflect areas where metabolic activity increases or decreases to mediate or respond to emotional and cognitive manifestations of the depressive syndrome, because local glucose metabolism and CBF (which is tightly coupled to glucose metabolism) reflect summations of the energy utilization associated with terminal field synaptic transmission during neural activity.2-4 In contrast, abnormalities that persist independently of the mood state may instead reflect neuropathological sequelae of recurrent illness or neurodevelopmental abnormalities that may confer vulnerability to MDD (eg, in cases where they are evident in otherwise healthy individuals at high familial risk for developing mood disorders). Such abnormalities in CBF and metabolism may reflect pathological changes in synaptic transmission associated with altered neurotransmitter receptor function, cerebrovascular disease, changes in neuronal arborization or synapse formation, or abnormalities in cellular viability or proliferation.5 For example, areas where CBF and metabolism appeared irreversibly decreased in depressives relative to controls in PET studies of MDD and BD were subsequently associated with focal tissue reductions in magnetic resonance imaging (MRI)-based morphometric and postmortem histopathological studies of MDD and BD.6-10
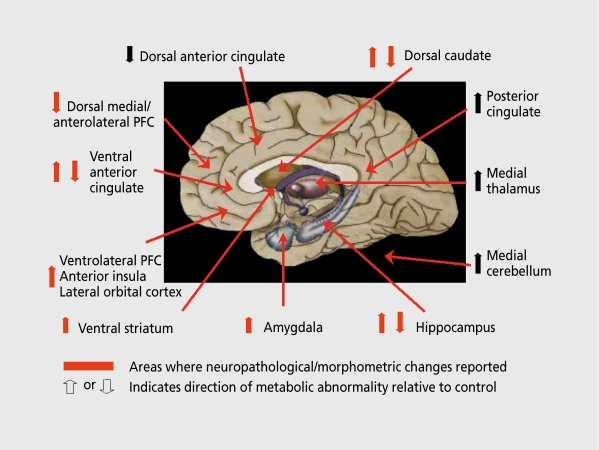
Figure 1. Summary of neuroimaging abnormalities in early-onset, primary, major depressive disorder (MDD). The regions where neurophysiological imaging abnormalities have been consistently reported in unmedicated MDD samples are listed and approximately shown on this midsagittal brain diagram in which subcortical structures are highlighted onto the medial surface. Because only the medial wall of the cortex is shown, the location of the lateral orbital/ventrolateral prefrontal cortex (PFC)/anterior insular region is better illustrated in Figure 2B. The “ventral anterior cingulate” region refers to both pregenual and subgenual portions (see text and Figure 2). The arrows in front of each region name indicate the direction of resting state abnormalities in glucose metabolism in unmedicated, depressed MDD samples relative to healthy control samples. In some cases, abnormalities in both directions have been reported which may depend either on the specific region involved or on the clinical state (eg, treatment responsive vs nonresponsive; see text). The red arrows have indicate histopathological and/or gray matter volumetric abnormalities in postmortem studies of primary mood disorders.
Abnormalities of gray matter volume and histology have now been identified in several brain structures using volumetric MRI and postmortem neuropathological assessments, which in many cases were guided by initial application of functional imaging approaches. The regions affected by these abnormalities have been shown to play major roles in modulating emotional behavior by electrophysiological, lesion analysis, and functional neuroimaging studies in experimental animals and healthy humans. Thus, the structural abnormalities in these regions may prove relevant to the emotional dysregulation that is clinically manifest in mood disorders.
Sensitivity for detecting neuroimaging abnormalities in depression
The neuroimaging abnormalities discovered to date have not had effect sizes sufficient to permit sensitive or specific classification of individual cases. Moreover, the psychiatric imaging literature is in disagreement regarding the specific location and direction of some abnormalities. Many limitations in the sensitivity in reproducing findings across studies appear to be accounted for simply by technical issues of image acquisition and/or analysis.1 In other cases, however, disagreements within the literature appear to reflect differences in subject selection criteria applied across studies, because the conditions encompassed by the diagnostic criteria for MDD appear to be heterogeneous with respect to pathophysiology and etiology.
It is noteworthy that neuroimaging laboratories selecting depressed subjects according to MDD criteria alone have rarely been able to replicate their own previous findings in independent subject samples. Instead, neuroimaging abnormalities appear to be specific to subsets of MDD subjects.1 For example, requiring that subjects have familial aggregation of illness and an early age at illness onset improved sensitivity for identifying subject samples with reproducible neuroimaging abnormalities. Clinical differences related to the capacity for developing mania or psychosis or having a late age at illness onset have also been shown to influence neuroimaging data. For example, elderly MDD subjects with a late age at depression onset have an elevated prevalence of MRI signal hyperintensities (in T2-weighted MRI scans, as putative correlates of cerebrovascular disease) in the deep and periventricular white matter, which is not the case for elderly depressives with an early age at depression onset. Similarly, elderly MDD cases with a late-life onset, and delusional MDD cases have been shown to have lateral ventricular enlargement - a feature which is generally not present in MDD cases who are elderly but have an early age of MDD onset, or in midlife depressives who are not delusional. In addition, enlargement of the third ventricle has been consistently reported in BD, but not in MDD.
A major technical issue that influences the sensitivity for detecting neuroimaging abnormalities across studies is the low spatial resolution of imaging technology relative to the size of brain structures of primary interest. With respect to morphometric assessments of gray matter volume, the volumetric resolution of state-of-the-art image data has recently been about 1 mm3, compared with the cortex thickness of only 3 to 4 mm. MRI studies involving images of this resolution have been able to repro ducibly show regionally specific reductions in mean gray matter volume across groups of clinically similar depressives versus controls. However, they have lacked sensitivity to detect the relatively subtle tissue reductions extant in mood disorders in individual subjects. Moreover, studies attempting to replicate such findings using data acquired at lower spatial resolutions (ie, voxel sizes ≥1.5 mm3) have commonly been negative because of the substantial partial volume effects that arise when attempting to segment, regions of only 3- to 4-mm cortex thickness in such low-resolution MRI images.
Volumetric MRI imaging abnormalities in mood disorders
Frontal lobe structures
Volumes of the whole brain and entire frontal lobe generally have not differed between depressed and healthy control samples. In contrast, volumetric abnormalities have been identified in specific prefrontal cortical (PFC), mesiotemporal, and basal ganglia structures in mood disorders. The most prominent reductions in the cortex have been identified in the anterior cingulate gyrus ventral to the genu of the corpus callosum, where gray matter volume has been abnormally decreased 20% to 40% in depressed subjects with familial pure depressive disease (FPDD), familial BD, and psychotic depression6,11-13 relative to healthy controls or mood-disordered subjects with no first-degree relatives with mood disorders. These findings were confirmed by postmortem studies of clinically similar samples (see below). Effective treatment with selective serotonin (5-hydroxytryptamine [5-HT]) reuptake inhibitors did not alter the subgenual PFC volume in MDD,6 although the PFC appeared significantly larger in BD subjects chronically medicated with lithium or divalproex than BD subjects who were cither unmedicated or medicated with other agents,1 compatible with evidence that chronic administration of these mood stabilizers increases expression of the neurotrophic factors in rodents.14
In the posterior orbital, cortex, and ventrolateral PFC, volume has also been shown to be reduced in in vivo volumetric MRI studies15,16 and in postmortem neuropathological studies of MDD.17,18 Reductions in gray matter volume were also found in the dorsomedial/dorsal anterolateral PFC in M'DD subjects versus controls,19 and postmortem studies of MDD and BD reported abnormal reductions in the size of neurons and/or the density of glia.18,20,21
Temporal lobe structures
Morphometric MRI studies of specific temporal lobe structures reported significant, reductions in the hippocampal volume in MDD, with magnitudes of difference ranging from 8% to 19% with respect to healthy controls.22,28 Sheline et al23 and MacOueen et al28 reported that the hippocampal volume was negatively correlated with the total time spent, depressed or with the number of depressive episodes in MDD. Other groups found no significant differences between MDD and control samples.29-35 The inconsistency in the results of MDD studies may reflect pathophysiological heterogeneity within the MDD samples studied. For example, Vythilingam et al36 reported that the hippocampal volume was abnormally decreased in depressed women who also had suffered early-life trauma, but not in women who had depression without early-life trauma.
In BD, reductions in hippocampal volume were identified by Noga et al37 and Swayze et al38 relative to healthy controls, although Pearlson et al39 and Nugent et al27 found no differences between BD and control samples. In postmortem studies of BD, abnormal reductions in the mRNA concentrations of synaptic proteins40 and in apical dendritic spines of pyramidal cells41 were specifically observed in the subicular and ventral CA1 subregions of the hippocampus. A recent study using high-resolution MRI scans found that the volume of the subiculum, but not the remainder of the hippocampus, was decreased in BD relative to control samples.27
Two studies reported abnormalities of the hippocampal T1 MRI signal in MDD. Krishnan et al42 observed that the T1 relaxation time was reduced in the hippocampus, but not in the entire temporal lobe, in unipolar depressives relative to healthy controls, and Sheline et al23 observed that elderly subjects with MDD have a higher number of areas with a low MRI signal than age-matched controls in T1-weighted images. The significance of such abnormalities remains unclear.
In the amygdala, the literature is in disagreement. Studies of MDD have reported that amygdala volume is decreased,43,44 increased,45 or not different26 in depressives relative to healthy controls. Similarly, in BD, amygdala volume was reported to be increased,46-48 decreased,39,49,50 or not different38 relative to healthy controls. Although the extent to which disagreements in the results across studies are accounted for by confounding factors (such as medication effects) remains unclear, it appears more likely that MRI images acquired at ≤1.5 tesla lack the spatial and tissue contrast resolution needed to measure amygdala volumes with sufficient validity and reliability. The amygdala's small size and proximity to other gray matter structures seriously limits the specificity (accuracy) for delimiting amygdala, boundaries in images acquired using MRI scanners of ≤1.5 tesla field strength. High-resolution MRI images acquired at 3-tesla magnetic field strengthen contrast, permit valid and reliable volumetric measures of the human amygdala. A recent study employing this technique established that mean amygdala volumes are decreased bilaterally (P<0.001) in MDD relative to healthy control samples.51 Amygdala volumes were decreased both in currently depressed and currently remitted MDD subsamples. Although mean amygdala volumes did not differ between BD and control samples, they were smaller in BD subjects who had not been recently medicated with mood stabilizers than in BD subjects who had been taking such agents, consistent with evidence that some mood stabilizers exert neurotrophic effects.14
Basal ganglia
Volumes of some basal ganglia structures have also been reported to be abnormally decreased in mood disorders. Husain et al52 reported that the putamen was smaller in depressives (mean age 55) than controls, and Krishnan et al53 found a smaller caudate nucleus volume in depressives (mean age 48) than controls. In a sample limited to elderly depressives, Krishnan et al54 also reported smaller putamen and caudate volumes relative to controls. These findings were consistent with the postmortem study of Baumann et al,55 which found that caudate and accumbens area volumes were markedly decreased in both MDD and BD samples relative to control samples. Nevertheless, Dupont et al56 and Lenze et al57 failed to find significant differences in caudate or lentiform nucleus (putamen plus globus pallidus) volumes between younger MDD subjects and controls. The factors accounting for the discrepant results across studies remain unclear.
Abnormalities of corpus callosal volume in mood disorders
The genual subsection of the corpus callosum was reduced in volume in both depressed women with MDD and their high-risk, female offspring (insufficient numbers of males were studied to determine whether the abnormality extends to males).58,59 These white matter regions contain the transcallosal fibers connecting the orbital cortex, anterior cingulate cortex (ACQ, and medial PFC with their homologous cortices in the contralateral hemisphere. The volumes of the splenial subregion of the corpus callosum was also decreased in mood-disordered versus control samples, which contains transcallosal fibers from the posterior cingulate cortex.
Other cerebral structures
Morphometric studies of other brain structures in depression have produced less consistent results. Of MRI studies of the thalamus, Dupont et al56 reported that the thalamic volume was decreased in unipolar depressives relative to controls, but Krishnan et al42,54 found no differences between depressives and controls. Two studies of thalamic volume in BD also have reported conflicting results. Of MRI studies of the cerebellum, two reported that the vermal volume is reduced in depressives relative to controls,60,61 while a third did not.62
Consistent with evidence that the hypothalamic-pituitary-adrenal (HPA) axis function is elevated in some mood-disordered subgroups, enlargement of the pituitary and adrenal glands has been reported in MDD. Krishnan et al63 showed that MRI-based measures of cross-sectional area and volume of the pituitary were increased (by 34% and 41%, respectively) in depressives (n=19) versus controls (n=19). This observation is consistent with evidence that the adrenal gland is also abnormally enlarged in MDD,1 which would putatively result, from chronically elevated stimulation of the adrenal cortex by adrenocorticotropic hormone (ACTH).
Postmortem neuropathological assessments of mood disorders
Most of the regions where MRI studies demonstrated volumetric abnormalities in mood disorders were also shown to contain histopathological changes or gray matter volumetric reductions in postmortem studies of MDD and BD. Reductions in gray matter volume, thickness, or wet weight have been reported in the subgenual ACC, posterolateral orbital cortex, and ventral striatum in MDD and/or BD subjects relative to controls.7,9,18,55 The histopathological correlates of these abnormalities included reductions in glial cells with no equivalent loss of neurons, reductions in synapses or synaptic proteins, elevations in neuronal density, and reductions in neuronal size.9,17,18,20,40,64,65 Abnormal reductions in glial cell counts and density, and/or glia-to-neuron ratios have also been found in MDD in Brodmann area (BA) 24 cortex of the pregenual ACC,20 the dorsal anterolateral PFC (BA9),21,66 and the amygdala.1,67 Finally, the mean size of neurons was reduced in the dorsal anterolateral PFC (BA9) in M'DD subjects relative to controls,18 and the density of neurons was decreased in the ACC in BD.68 In several of these studies, the decreases were largely accounted for by differences in the left, hemisphere.1,7,9,17,67 In the amygdala and the dorsal anterolateral PFC (BA9), the glial type that specifically differed between MDD and control samples was the oligodendrocytes. In contrast, astrocyte and microglial cell counts did not differ significantly between MDD or BD samples and healthy control samples in the amygdala.1 Oligodendroglia are best characterized for their role in myelination, and the reduction in oligodendrocytes may conceivably arise secondary to an effect on myelin, either through demyelination, abnormal development, or atrophy in the number of myelinated axons. Notably, the myelin basic protein concentration was found to be decreased in the frontal polar cortex (BA10) in MDD subjects.69 Compatible with these data, the concentration of white matter within the vicinity of the amygdala27 and the white matter volume of the genual and splenial portions of the corpus callosum are abnormally reduced in MDD and BD.58,59 These regions of the corpus callosum were also smaller in child and adolescent offspring of women with MDD who had not yet experienced a major depressive episode, in comparison to age-matched controls, suggesting that the reduction in white matter in MDD reflects a developmental defect that exists prior to the onset of depressive episodes.58 All of these observations support, the hypothesis that, the glial cell loss in mood disorders is accounted for by a reduction in myelinating oligodendrocytes.
Further evidence supporting this hypothesis comes from several reports that, deficits in glia in the cerebral cortex depend upon laminar analysis, with the greatest effects in layers III, V, and VI.18,20,70,71 The intracortical plexuses of myelinated fibers known as “bands of Baillarger” are generally concentrated in layers III and V. The size of these plexuses varies across cortical areas, so if the oligodendrocytes related to these plexuses were affected, different areas would be expected to show greater or lesser deficits. Layer VI in particular has a relatively large component of myelinated fibers running between the gray and white matter.
Finally, a population of satellite oligodendrocytes exists next to neuronal cell bodies that have largely unknown functions, but do not appear to have a role in myelination under normal conditions.72 An electron microscopic study of the PFC in BD revealed decreased nuclear size, clumping of chromatin, and other types of damage to satellite oligodendrocytes, including indications of both apoptotic and necrotic degeneration.73 Fewer signs of degeneration were seen in myelin-related oligodendrocytes in white matter. Satellite oligodendrocytes may play a role in maintaining the extracellular environment, for the surrounding neurons, which resembles the functions mediated by astrocytes. These oligodendrocytes are immunohistochemically reactive for glutamine synthetase, suggesting that they function like astrocytes and take up synaptically released glutamate for conversion to glutamine and cycling back into neurons.74 Many studies of glial function have not distinguished astrocytes from oligodendrocytes, and the two glial types may share several functions.
In other brain regions, reductions in astroglia have been reported by postmortem studies of mood disorders. In the frontal cortex, Johnston-Wilson et al75 found that, four forms of the astrocytic product, glial fibrillary acidic protein (GFAP) were decreased in mood-disordered subjects relative to controls, although it remained unclear whether this decrement reflected a reduction in the astrocyte density or in GFAP expression. Using immunohistochemical staining for GFAP, Webster et al76 did not find significant differences in cortical astrocytes between controls, and MDD or BD cases. Other studies also did not find differences in GFAP between mood disorder cases and controls.66
Factors that may conceivably contribute to a loss of oligodendroglia in mood disorders include the elevated glucocorticoid secretion and glutamatergic transmission evident during depression and mania. Glucocorticoids affect glia as well as neurons,77 and elevated glucocorticoid levels decrease the proliferation of oligodendrocyte precursors.78 Moreover, oligodendrocytes express α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and kainatetype glutamate receptors, and are sensitive to excitotoxic damage from excess glutamate as well as to oxidative stress.1 These vulnerabilities putatively contribute to oligodendrocyte degeneration in ischemic brain injury and demyelinating diseases,79,80 although no data exist to establish a similar role in mood disorders. The targeted nature of the reductions in gray matter volume and glial cells to specific areas of the limbic-cortical circuits that show increased glucose metabolism during depressive episodes is noteworthy given the evidence reviewed below that the glucose metabolic signal is dominated by glutamatergic transmission. The hypothesis that glutamate transmission is elevated in these areas in depression was also supported by a postmortem study in depressed suicide victims.81
Elevations of glutamate transmission and Cortisol secretion in mood disorders may also contribute to reductions in gray matter volume and synaptic markers by inducing dendritic atrophy in some brain structures. In the medial PFC and parts of the hippocampus and amygdala of adult rodents, the dendritic arbors undergo atrophy or debranching in response to specific types of repeated or chronic stress.82 The effects of stress on dendritic arborization depend both upon the type of stress applied and anatomical location. For example, chronic unpredictable stress produces dendritic atrophy in the basolateral amygdala, whereas chronic immobilization stress increased dendritic branching in pyramidal and stellate neurons within the basolateral amygdala, but did not affect dendritic arborization in the central nucleus of the amygdala.83,84 These dendritic reshaping processes depend upon interactions between N-methyl-D-aspartate (NM'DA) glutamatergic receptor stimulation and glucocorticoid secretion associated with repeated stress.82
The depressives with BD and FPDD who show regional reductions in gray matter volume also show evidence of having increased Cortisol secretion and glutamate transmission. Specifically, depressives with FPDD or BD are more likely to show abnormal suppression of Cortisol secretion by dexamethasone and blunted hypoglycemic response to insulin8 and to release excessive amounts of Cortisol during stress.8-85 Subjects with FPDD or familial BD also show elevations of glucose metabolism, which largely reflects glutamate transmission, in the medial and orbital PFC, amygdala, ventral striatum, and cingulate cortex regions that show reductions in gray matter volume and cellular elements.
Association between structural and metabolic abnormalities
The glucose metabolic signal is dominated by changes in glutamate transmission, and so the findings that gray matter reductions appear to occur specifically in regions that show hypermetabolism during depression raise the possibility that excitatory amino acid transmission plays a role in the neuropathology of mood disorders. At least 85% to 90% of the glucose metabolic measure is accounted for by glutamate transmission from afferent projections originating within the same structure or from distal structures.4,86-89 In the depressed phase of familial MDD and BD, regional cerebral metabolism and CBF arc abnormally increased in the amygdala, lateral orbital/ventrolateral PFC, ACC anterior to the genu of the corpus callosum (“pregenual” ACC), posterior cingulate cortex, ventral striatum, medial thalamus, and medial cerebellum.1 During effective antidepressant, drug or electroconvulsive therapy, metabolic activity decreases in all of these regions,1,8 compatible with evidence that these treatments result in desensitization of NMDA glutamatergic receptors in the frontal cortex.90 In addition to these areas of increased metabolic activity, areas of reduced CBF and metabolism in depressives relative to controls were found in the ACC ventral to the genu of the corpus callosum (ie, “subgenual” ACC7) and the dorsomedial/ dorsal anterolateral PFC.19,91,92 Yet even in these regions, metabolic activity increases during the depressive relapse induced by tryptophan depletion (a dietary challenge that depletes central 5-HT transmission),93 and metabolism is increased in the subgenual ACC in the unmedicated-depressed phase relative to the unmedicated-remitted phase. In all of these regions where glucose metabolism is increased in the depressed phase relative to the remitted phase, reductions in cortex volume and/or histopathological changes have been found in in vivo MRI studies and/or postmortem studies of MDD and/or BD.
The hypothesis that the elevations in glucose metabolism seen in these circuits reflect, elevations in glutamatergic transmission is supported by evidence that the anatomical projections between affected areas are excitatory in nature. The abnormally increased CBF and metabolism in the ventrolateral and orbital PFC, ventral ACC, amygdala, ventral striatum, and medial thalamus evident in depression (Figure 2) implicate a limbic-thalamo-cortical circuit involving the amygdala, the mediodorsal nucleus of the thalamus and the orbital and medial PFC, and a limbic-striatal-pallidal-thalamic circuit involving related parts of the striatum and the ventral pallidum along with the components of the other circuit.95 The first of these circuits can be conceptualized as an excitatory triangular circuit, whereby the basolateral nucleus of the amygdala and the orbital and medial prefrontal regions are interconnected by excitatory (especially glutamatergic) projections with each other and with the mediodorsal nucleus.96-100 This means that increased metabolic activity in these structures would presumably reflect increased synaptic transmission through the limbic-thalamo-cortical circuit. The limbic-striatal-pallidal-thalamic circuit constitutes a disinhibitory side loop between the amygdala or PFC and the mediodorsal nucleus. The amygdala and the PFC send excitatory projections to overlapping parts of the ventromedial striatum.101 This part of the striatum sends an inhibitory projection to the ventral pallidum,102 which in turn sends GABAergic (GABA, γ-aminobutyric acid), inhibitory fibers to the mediodorsal nucleus.99
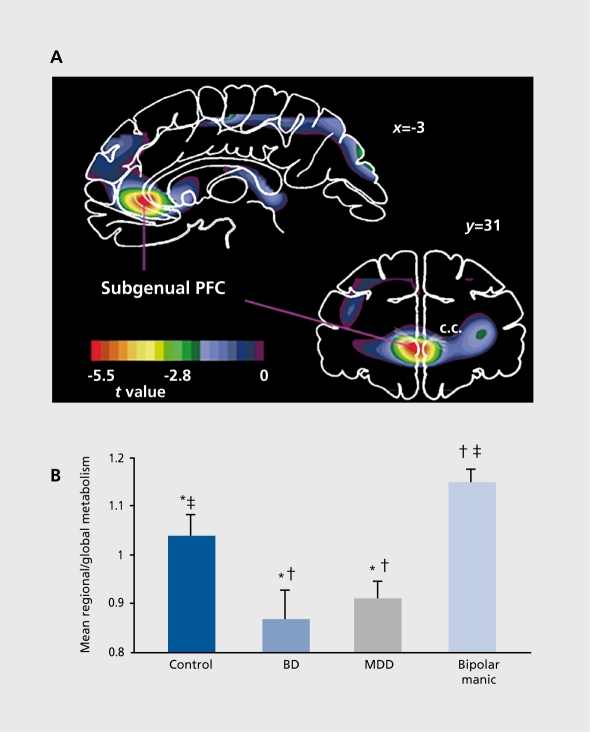
Figure 2. Altered metabolism in the prefrontal cortex (PFC) ventral to the genu of the corpus callosum (c.c.) (ie, subgenual PFC) in mood disorders. A. Negative voxel t values where glucose metabolism is decreased in dépressives relative to controls in coronal (31 mm anterior to the anterior commissure, or y=31)and sagittal (3 mm left of midline, or x=-3) planes of a statistical parametric image comparing depressives relative to controls.7 This image localized an abnormality in the subgenual portion of the anterior cingulate cortex (subgenual ACC7), which was subsequently shown to be accounted for by a corresponding reduction in cortex volume on the left side (see text). Anterior (or left) is to the left of the image. B. Mean, normalized, glucose metabolic values for the left subgenual ACC measured using magnetic resonance imaging (MRI)-based region-of-interest analysis. Metabolism is decreased in depressed subjects with either bipolar disorder (BD) or major depressive disorder (MDD) relative to healthy controls. In contrast, subjects scanned in the manic phase of BD (“bipolar manic”) have higher metabolism than either depressed or control subjects in this region. *P<0.025 controls versus depressed; tP<0.01 depressed versus manic; P<0.05 controls versus manic. Although none of these subjects were involved in the study that generated the images shown in Figure 3, the mean glucose metabolism in this independent sample of depressives and controls also confirmed the areas of abnormally increased activity in the depressives in the amygdala, lateral orbital cortex, ventrolateral PFC, and medial thalamus (not shown in A, which only illustrates negative f values corresponding to hypometabolic areas in the depressives). Figure 2A reproduced with permission from reference 6: Drevets WC, Price JL, Simpson JR, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824-827. Copyright © 1997, Nature Publishing Group. Figure 2B reproduced with permission from reference 94: Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive emotional manifestations of mood disorders. Curr Opin Neurobiol. 2001;11:240-249. Copyright © 2001 , Elsevier.
Implications for the pathogenesis of emotion dysregulation
The circuits described above have also been implicated in the depressive syndromes arising secondary to lesions or degenerative illnesses. Lesions involving the PFC (eg, tumors or infarctions) and the diseases of the basal ganglia, (eg, Parkinson's disease or Huntington's disease) are associated with higher rates of depression than other similarly debilitating conditions and result in dysfunction at distinct points within these circuits and affect synaptic transmission in diverse ways.103 Consistent with this hypothesis, imaging studies of depressive syndromes arising secondary to neurological disorders have generally shown results that differ from those reported for primary mood disorders. For example, in contrast to the findings of increased CBF or metabolism in parts of the orbital cortex in primary depressives, orbital cortex flow is reportedly decreased or not significantly different in subjects with depressive syndromes arising secondary to Parkinson's disease, Huntington's disease, or basal ganglia infarction relative to nondepressed subjects with the same illnesses.104-107 Primary and secondary depressive syndromes may thus involve the same neural network, although the direction of the physiological abnormalities within individual structures may differ across conditions. A common substrate in these cases may be dysfunction of the PFC-striatal modulation of limbic and visceral functions, because the idiopathic neuropathological changes evident in the orbital and medial PFC and ventral striatum in primary mood disorders (see above) and those found in neurodegenerative conditions all appear to be capable of inducing depressive syndromes (eg, Parkinson's disease, Huntington's disease, and cerebrovascular disease).
PFC-amygdalar projections may also play a role in the pathogenesis of depressive and anxiety symptoms in mood disorders. Although the reciprocal PFC-amygdalar projections are excitatory in nature, these connections ultimately appear to activate inhibitory interneurons, which, in turn, lead to functional inhibition in the projected field of the amygdala (for PFC-amygdalar projections) or the medial PFC and ventrolateral PFC.96,108-110 The function of the PFC in modulating the amygdala appears to be impaired in mood disorders, according to functional MRI data showing that abnormally sustained amygdala activity in response to aversive words or sad faces in MDD is associated with blunted activation of PFC areas.108,111 Thus, the volumetric and/or histopathological changes evident in the subgenual and pregenual ACC, lateral orbital cortex, dorsomedial/dorsal anterolateral PFC, hippocampal subiculum, amygdala, and ventral striatum may interfere with the modulation of emotional behavior, as discussed below.
Ventral ACC
The ACC ventral and anterior to the genu of the corpus callosum (“subgenual” and “pregenual,” respectively; Figure 2) shows complex relationships between CBF, metabolism, and illness state, which appear to be accounted for by a left-lateralized reduction in the corresponding cortex, initially demonstrated by MRI-based morphometric measures6,12-16,112 and later by postmortem neuropathological studies of familial BD and MDD.9 Thus, computer simulations that correct the PET data acquired from this region for the partial volume effect of the reduction in gray matter volume measured in MRI scans of the same subject conclude the “actual” metabolic activity in the remaining subgenual PFC tissue is increased in depressives relative to controls, and decreases to normative levels during effective treatment.113 This hypothesis appears to be compatible with the observations that effective antidepressant pharmacotherapy results in a decrease in metabolic activity in this region in M'DD,8,10,114 that during depressive episodes metabolism shows a positive relationship with depression severity,8,115,116 and that flow increases in this region in healthy, nondepressed humans during sadness induced via. contemplation of sad thoughts or memories.114,117,118
The reduction in volume in this region exists early in the illness in familial MDD11 and BD.12The gray matter deficit may nevertheless worsen or initially become apparent, following illness onset based upon preliminary evidence in twins discordant for MDD that the affected twin has a smaller volume than their unaffected cotwin.119 Kimbrell et al120 reported that the subgenual ACC metabolism correlated inversely with the number of lifetime depressive episodes, compatible with the possibility that the reduction in metabolism in this region measured via PET reflects a partial volume effect of a gray matter reduction that worsens with repeated illness.
In the pregenual ACC, Drevets et al95 initially found increased CBF in MDD, and subsequent studies extended this observation by demonstrating complex relationships between pregenual ACC activity and subsequent antidepressant treatment outcome. Wu et al121 reported that depressed subjects whose mood improved during sleep deprivation showed elevated metabolism in the pregenual ACC and amygdala in their pretreatment scans. Mayberg ct al122 reported that, while metabolism in the pregenual ACC was abnormally increased in depressives who subsequently responded to antidepressant drugs, metabolism was decreased in depressives who later had poor treatment response. Finally, in a tomographic electroencephalographic (EEG) analysis, Pizzagalli et al123 reported that depressives who ultimately showed the best response to nortriptyline showed hyperactivity (higher theta activity) in the pregenual ACC at baseline, compared with subjects showing the poorer response. During effective antidepressant, treatment, most PET studies have shown that pregenual ACC flow and metabolism decrease in posttreatment scans relative to pretreatment scans.1 The finding that this region contains histopathological changes in MDD and BD20,64,68 suggests the hypothesis that, the abnormal reduction in metabolism in treatment-nonresponsive cases reflects more severe reductions in cortex.
In rodents and nonhuman primates, the regions that appear homologous to human subgenual and pregenual ACC, namely the infralimbic, prelimbic, and ventral ACCs, have extensive reciprocal connections with areas implicated in the expression of behavioral, autonomic, and endocrine responses to threat, stress, or reward/nonreward, such as the orbital cortex, lateral, hypothalamus, amygdala, accumbens, subiculum, ventral tegmental area (VTA), raphe, locus ceruleus, periaqueductal grey (PAG), and nucleus tractus solitarius.7,124 Humans with lesions that include these ventromedial PFC structures show abnormal autonomic responses to emotionally provocative stimuli and an inability to experience emotion related to concepts that ordinarily evoke emotion.125 Electrical stimulation of the ACC elicits fear, panic, or a sense of foreboding in humans, and vocalization in experimental animals.126 Similarly, rats with experimental lesions of prelimbic cortex demonstrate altered autonomic, behavioral, and neuroendocrine responses to stress and fear-conditioned stimuli. The prelimbic and infralimbic cortices contain abundant concentrations of glucocorticoid receptors, which, when stimulated by corticosterone (CORT), reduce stress-related HPA activity.127 Lesions of these cortices consequently result in exaggerated plasma ACTH and CORT responses to restraint stress.127 In rats, bilateral or right-lateralized lesions of the ACC and prelimbic and infralimbic cortex attenuate sympathetic autonomic responses, stressinduced CORT secretion, and gastric stress pathology during restraint stress or exposure to fear-conditioned stimuli.128-130 In contrast, left-sided lesions of this area increase sympathetic autonomic arousal and CORT responses to restraint stress.130 These data suggest that the right subgenual PFC facilitates expression of visceral responses during emotional processing, while the left subgenual PFC inhibits or modulates such responses.130 It is thus noteworthy that the gray matter reduction in this region in MDD and BD was lateralized to the left side, suggesting that it may contribute to disinhibition of neuroendocrine and autonomic function in depression.127,131,132
The ventral ACC also appears to participate in processing of behavioral incentive and motivated behavior. These areas send efferent projections to the VTA and substantia nigra, and receive dense dopaminergic innervation from VTA.124 In rats, electrical or glutamatergic stimulation of medial PFC areas that include prelimbic cortex elicits burst firing patterns from dopamine (DA) cells in the VTA and increases DA release in the accumbens.113 These phasic, burst firing patterns of DA neurons appear to encode information regarding stimuli that predict, reward and deviations between such predictions and actual occurrence of reward.133 Ventral ACC dysfunction may thus conceivably contribute to disturbances of motivated behavior and hedonic perception in mood disorders.
Dorsomedial/dorsal anterolateral PFC
Metabolism and CBF arc abnormally decreased in the dorsolateral and dorsomedial PFC in MDD.1 The dorsomedial PFC region includes the dorsal ACC92 and an area rostral to the dorsal ACC involving cortex on the medial and lateral surface of the superior frontal gyrus (approximately corresponding to BA9 and BA32).8,19,91 Postmortem studies of MDD and BD found abnormal reductions in the size of neurons and/or the density of glia in this portion of BA9,18,20,134 which may account for the reduction in metabolism in this region in MDD, and for the failure of antidepressant drug treatment, to correct metabolism in these areas.8,19 Nevertheless, currently remitted MDD subjects who experience depressive relapse during tryptophan depletion show increased metabolic activity within these areas in the depressed versus the remitted conditions,93 similar to other structures where histopathological and gray matter volume changes exist, in MDD.
Flow normally increases in the vicinity of this dorsomedial/dorsal anterolateral PFC in healthy humans as they perform tasks that elicit emotional responses or require emotional evaluations.1 In healthy humans, CBF increases in this region during anxious anticipation of an electrical shock to an extent that correlates inversely with changes in anxiety ratings and heart rate, suggesting that this region functions to attenuate emotional expression. In rats, lesions of the dorsomedial PFC result in exaggerated heart rate responses to fear-conditioned stimuli, and stimulation of these sites attenuate defensive behavior and cardiovascular responses evoked by amygdala stimulation,128 although the homologue to these areas in primates has not been clearly established. In primates, the BA9 cortex sends efferent projections to the lateral PAG and the dorsal hypothalamus through which it may modulate cardiovascular responses associated with emotional behavior.124 It is thus conceivable that dysfunction of the dorsomedial/dorsal anterolateral PFC may contribute to impairments in the ability to modulate emotional responses in mood disorders.
Lateral orbital/ventrolateral PFC.
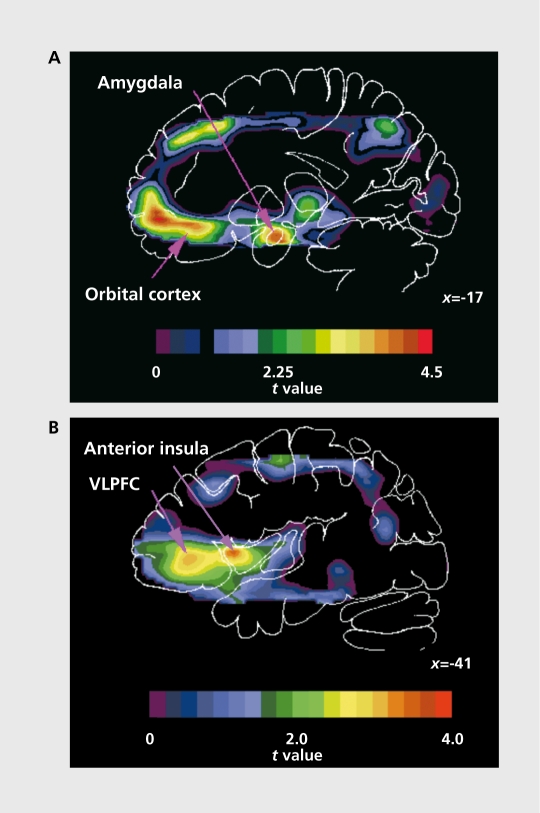
In the lateral orbital cortex, ventrolateral PFC, and anterior insula, the resting CBF and metabolism have been abnormally increased in unmedicated subjects with primary MDD (Figure 3) 1 The elevated activity in these
areas in MDD appears to be mood-state dependent,95 and, during treatment with somatic antidepressant therapies, flow and metabolism decreases in these regions.1 The relationship between depression severity and physiological activity in the lateral orbital cortex/ventrolateral PFC is complex. While CBF and metabolism increase in these areas in the depressed phase relative to the remitted phase of MDD, the magnitude of these measures is inversely correlated with ratings of depressive ideation and severity.95-116,135 Moreover, while metabolic activity is abnormally increased in these areas in treatment-responsive unipolar and bipolar depressives, more severely ill or treatment-refractory samples show CBF and metabolic values lower than or not different from those of controls.81,139 These inverse relationship between orbital cortex/ventrolateral PFC activity and ratings of depression severity extends to some other emotional states as well. Posterior orbital cortex flow also increases in subjects with obsessive-compulsive disorder or simple animal phobias during exposure to phobic stimuli and in healthy subjects during induced sadness,140-142 with the change in posterior orbital CBF correlating inversely with changes in obsessive thinking, anxiety, and sadness, respectively.
These data appear to be consistent with electrophysiological and lesion analysis data, showing that parts of the orbital cortex participate in modulating behavioral and visceral responses associated with defensive, emotional, and reward-directed behavior as reinforcement contingencies change.124,143,144 The orbital cortex and amygdala send overlapping projections to each of these structures and to each other through which they appear to modulate each other's neural transmission.124,143,145
Activation of the orbital cortex during depression may thus reflect compensatory attempts to attenuate emotional expression or interrupt unreinforced aversive thought and emotion. Consistent, with this hypothesis, cerebrovascular lesions of the orbital cortex are associated with an increased risk for depression.146 These observations also suggest, that, the reduction of CBF and metabolism in the orbital cortex and ventrolateral PFC during antidepressant drug treatment may not be a primary mechanism through which such agents ameliorate depressive symptoms. Instead, direct inhibition of pathological limbic activity in areas such as the amygdala and ventral ACC may attenuate the mediation of depressive symptoms.8 The orbital cortex neurons may thus “relax,” as reflected by the return of metabolism to normal levels, as antidepressant drug therapy attenuates the pathological limbic activity to which these neurons putatively respond.145
The amygdala
In the amygdala, neurophysiological activity is altered both at rest and during exposure to emotionally valenced stimuli in some depressive subgroups. The basal CBF and metabolism are elevated in mood-disordered subgroups who meet criteria for FPDD (Figure 3),8,95,135,136 for MDD melancholic subtype,148 type II or nonpsychotic type I BD,136,149 or for those who are responsive to sleep deprivation.121 In contrast, metabolism has not been abnormal in unipolar depressives meeting criteria for depression spectrum disease,136,137 or in MDD samples meeting Diagnostic and Statistical Manual, of Mental. Health Disorders (DSM) criteria,150-152 although the interpretation of the latter results was confounded by technical problems that reduced sensitivity for measuring amygdala. activity.136 During antidepressant treatment, that both attenuates depressive symptoms and prevents relapse, amygdala metabolism decreases toward normative levels.8
Figure 3. Areas of abnormally increased blood flow in subjects with major depressive disorder (MDD). The image sections shown are from an image of t values, produced by a voxel-by-voxel computation of the unpaired t statistic to compare regional CBF between a depressed sample selected according to criteria for familial pure depressive disease (FPDD) (n=13) and a healthy control sample (n=33).95 The positive t values shown correspond to areas where flow is increased in the depressives relative to the controls. The abnormal activity in these regions was replicated using glucose metabolism imaging in independent subject samples.8,135,136 A. Sagittal section at 17 mm left of midline illustrating areas of increased CBF in depression in the amygdala and orbital cortex. B. Area of increased flow extended through the lateral orbital cortex to the ventrolateral prefrontal cortex (VLPFC) and anterior insula.8,95 The x coordinate locates sagittal sections in millimeters to the left of midline. The PET images in A and B from which the t image was generated have been stereotaxically transformed to the coordinate system of Talairach and Tournoux,137 from which the corresponding atlas outline is shown. Anterior is left. Figure 3A reproduced with permission from reference 126: Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex: a substrate for emotional behavior? Prog Brain Res. 1996;107:523-536. Copyright © 1996, Elsevier. Figure 3B reproduced with permission from reference 138: Drevets WC, Videen TO, Snyder AZ, MacLeod AK, Raichle ME. Regional cerebral blood flow changes during anticipatory anxiety. Soc Neurosci Abstr. 1994;20:368. Copyright © 1994, Society for Neuroscience.
Functional imaging data, acquired as subjects view emotionally valenced stimuli that normally activate the amygdala also demonstrate altered physiological responses in MDD. In the left, amygdala, the hemodynamic response to viewing fearful faces was blunted in depressed children153 and depressed adults,94 consistent with the elevation of basal CBF and metabolism in the left amygdala in such cases (physiologically activated tissue is expected to show an attenuation of further rises in the hemodynamic/metabolic signal in response to tasks that normally engage the same tissue). The duration of the amygdala response to emotionally valenced stimuli is also abnormally prolonged in response to sad stimuli in depression. Drevets et al94 observed that, although the initial amygdala CBF response to sad faces was similar in depressives and controls, this response habituated during repeated exposure to the same stimuli in the controls, but not in the depressives over the imaging period. Similarly, Siegle ct al44 reported that hemodynamic activity increased in the amygdala during exposure to negatively valenced words to a similar extent in depressives and controls, but, while the hemodynamic response rapidly fell to baseline in the controls, it remained elevated in the depressives. The amygdala plays major roles in organizing other behavioral, neuroendocrine, and autonomic aspects of emotional and stress responses to experiential stimuli. For example, the amygdala facilitates stress-related corticotropin-releasing hormone (CRH) release154 and electrical stimulation of the amygdala, in humans increases Cortisol secretion,155 suggesting a mechanism via which excessive amygdala activity may participate in inducing the CRH and Cortisol hypersecretion that, is evident in MDD. In PET studies of MDD and BD, CBF and metabolism in the left amygdala correlates positively with stressed plasma Cortisol secretion, which may reflect the effect of either amygdala activity on CRH secretion or Cortisol or CRH on amygdala, function.136
If the reduction in amygdala volume is associated with reductions in synaptic contacts formed by afferent projections from regions known to modulate amygdala function, then amygdala neuronal activity may become disinhibited. The above reports that amygdala blood flow and metabolism arc abnormally elevated and hemodynamic responses to emotional stimuli are abnormally persistent, in MDD support this hypothesis. Notably, Siegle et al44 reported that the abnormally prolonged hemodynamic responses of the amygdala to sad words occurred particularly in the MDD subjects who had reduced amygdala volumes. If the neurotrophic effects of mood-stabilizing drugs restore and protect modulatory connections formed between the amygdala and cortex,1 then the volumetric changes observed during treatment may contribute to their therapeutic effects in mood disorders.
Abnormalities in anatomically related limbic and subcortical structures
In the medial thalamus and ventral striatum, CBF and metabolism are abnormally elevated in the depressed phase of MDD and BD, and decrease during antidepressant pharmacotherapy.8,95,134,136,154,156,157 Several groups also reported abnormally increased CBF in the posterior cingulate cortex in the unmedicated, depressed phase of MDD.8,112,158 Bench et al158 specifically reported that the elevation of posterior cingulate flow in depressives relative to controls correlated positively with anxiety ratings. Exposure to aversive stimuli of various types results in increased physiological activity in the posterior cingulate cortex.159 The posterior cingulate cortex sends major anatomical projections to the pregenual ACC.160
Neuroreceptor imaging abnormalities in mood disorders
Neuroreceptor imaging studies of mood disorders have demonstrated reductions in 5-HT1a receptor binding in mood disorders, which would appear to hold major implications for alterations in neuroplasticity in these conditions. Both presynaptic (in the raphe) and postsynaptic (insula, anterior, and posterior cingulate cortices, parietooccipital cortex, orbital/ventrolateral PFC) 5-HT1a binding is abnormally decreased in MDD and panic disorder (irrespective of the current presence of comorbid depression), and postsynaptic 5-HT1a receptor binding is also decreased in RD.85,116,161-165 The magnitudes of these differences have been similar to those found by postmortem studies of primary mood-disordered samples17,165 and depressed suicide victims.166 These data are also compatible with results of studies showing that M'DD and panic disorder subjects show blunted thermic and adrenocorticotropin/cortisol responses to 5-HT1a receptor agonist challenge.85,162
The 5-HT1a receptor plays major roles in the neuroplasticity involving serotonergic and other neurons.167,168 In addition, during fetal development and subsequently during 5-HT1a neuronal injury, stimulation of astrocyte and radial glial cell-based 5-HT1a receptors results in release of the trophic factor S100β which promotes 5-HT neuronal arborization.168,169 If glial function is reduced during 5-HT system development in BD and MDD, it is conceivable that arborization of the 5-HT neurons may be attenuated, potentially reflected by the widespread reductions of 5-HT transporter and postsynaptic 5-HT1a receptor expression seen in MDD.17,85,116,163,166,170 Such a hypoplastic process may also underlie the finding that the area, expressing 5-HT1a receptors in the dorsal raphe nucleus is abnormally decreased in depressed suicides.166 It is conceivable that the persistently increased anxiety behaviors and the exaggerated fear and behavioral despair responses shown by 5-HT1a receptor knockout mice at least partly reflect effects of deficient 5-HT1a receptor function on neuroplasticity during neurodevelopment.162 It remains unclear, however, whether the reduction in 5-HT1a receptor function and expression constitutes a neurodevelopmental or an acquired abnormality in mood disorders.165
Concluding remarks
The convergent, results from studies of mood disorders conducted using neuroimaging, lesion analysis, and post-mortem techniques support, models in which the signs and symptoms of major depression can emanate from dysfunction within PFC, striatal, and brain stem systems that modulate emotional behavior. Antidepressant therapies may compensate for this dysfunction by attenuating the pathological limbic activity that mediates such symptoms,9 and by increasing genetic transmission of neurotrophic factors that exert neuroplastic effects within the pathways modulating emotional expression.14
Selected abbreviations and acronyms
- ACC
anterior cingulate cortex
- BD
bipolar disorder
- FPDD
familial pure depressive disease
- 5-HT
5-hydroxytryptamine (serotonin)
- MDD
major depressive disorder
- NMDA
N-methyl-D-aspartate
- PFC
prefrontal cortex
- VTA
ventral tegmental area
REFERENCES
- 1.Drevets WC., Gadde K., Krishnan R. Neuroimaging studies of depression. In: Charney DS, Nestler EJ, Bunney BJ, eds. The Neurobiological Foundation of Mental Illness. 2nd ed. New York, NY: Oxford University Press. 2004:461–490. [Google Scholar]
- 2.Raichle ME. Circulatory and metabolic correlates of brain function in normal humans. In: Brookhart JM, Mountcastle VB, eds. Handbook of Physiology The Nervous System. Baltimore, Md: American Physiology Society. 1987;5:643–674. [Google Scholar]
- 3.DiRocco RJ., Kageyama GH., Wong-Riley MT. The relationship between CNS metabolism and cytoarchitecture: a review of 14C-deoxyglucose studies with correlation to cytochrome oxidase histochemistry. Comput Med imaging Graph. 1989;13:81–92. doi: 10.1016/0895-6111(89)90080-3. [DOI] [PubMed] [Google Scholar]
- 4.Magistretti PJ., Pellerin L. Cellular mechanisms of brain imaging metabolism and their relevance to functional brain imaging. Phil Trans Roy Soc London B. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wooten GF., Collins RC. Metabolic effects of unilateral lesion of the substantia nigra. J Neurosci. 1981;1:285–291. doi: 10.1523/JNEUROSCI.01-03-00285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drevets WC., Price JL., Simpson JR., et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 7.Drevets WC., Öngür D., Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 8.Drevets WC., Sogers W., Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur J Neuropharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 9.Öngür D., Drevets WC., Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazziotta JC., Phelps ME., Plummer D., Kuhl DE. Quantitation in positron emission computed tomography. 5. Physical-anatomical effects. J Comput Assist Tomogr. 1981;5:734–743. doi: 10.1097/00004728-198110000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Botteron KN., Raichle ME., Drevets WC., Heath AC., Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 12.Hirayasu Y., Shenton ME., Salisbury DE., et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coryell W., Nopoulos P., Drevets WC., Andreasen NC. Subgenual PFC volumes in MDD and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. In press. 2004 doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 14.Du J., Quiroz JA., Gray NA., Szabo ST., Zarate CA Jr., Manji HK. Regulation of cellular plasticity and resilience by mood stabilizers: the role of AMPA receptor trafficking. Dialogues Clin Neurosci. 2004;6:143–155. doi: 10.31887/DCNS.2004.6.2/jdu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai T., Payne ME., Byrum CE., Steffens DC., Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 16.Bremner JD., Vythilingham M., Vermetten E., et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 17.Bowen DM., Najlerahim A., Procter AW., Francis PT., Murphy E. Circumscribed changes of the cerebral cortex in neuropsychiatrie disorders of later life. Proc Natl Acad Sci U S A. 1989;86:9504–9508. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkowska G., Miguel-Hidalgo JJ., Wei J., et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 19.Bell KA., Kupfer DJ., Drevets WC. Decreased glucose metabolism in the dorsomedial prefrontal cortex in depression. Biol Psychiatry. 1999;45:118S. [Google Scholar]
- 20.Cotter DR., Mackay D., Landau S., Kerwin R., Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 21.Uranova NA., Vostrikov VM., Orlovskaya DD., Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 22.Sheline Yl., Sanghavi M., Mintun MA., Gado M. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheline Yl., Wang PW., Gado MH., Csernansky JG., Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremner JD., Narayan M., Anderson ER., Staib LH., Miller HL., Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 25.Steffens DC., Byrum CE., McQuoid DR., et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 26.Mervaala E., Fohr J., Kononen M., et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 27.Nugent AC., Wood S., Bain EE., et al. High resolution MRI neuromorphometric assesment of the hippocampal subiculum in mood disorders. Presented at the International Society for Magnetic Resonance in Medicine, 12th Annual Meeting, Kyoto, Japan; 2004 [Google Scholar]
- 28.MacQueen GM., Campbell S., McEwen BS., et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashtari M., Greenwald BS., Kramer-Ginsberg E., et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 30.Axelson D., Doraiswamy PM., McDonald WM., et al. Hypercortisolemia and amygdala hippocampal changes in depression. Psychiatry Res. 1993; 47:167–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- 31.Hauser P., Altschuler LL., Berrettini W., et al. Temporal lobe measurement in primary affective disorder by magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1989;1:128–134. doi: 10.1176/jnp.1.2.128. [DOI] [PubMed] [Google Scholar]
- 32.Pantel J., Schroder J., Essig M., et al. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord. 1997;42:69–83. doi: 10.1016/s0165-0327(96)00105-x. [DOI] [PubMed] [Google Scholar]
- 33.Shah PJ., Ebmeier KP., Glabus MF., Goodwin GM. Cortical gray matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 34.Vakili K., Pillay SS., Lafer B., Fava M., Renshaw PF., Bonello-Cintron CM. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 35.Von Gunten A., Fox NC., Cipolotti L., Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci. 2000;12:493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- 36.Vythilingam M., Heim C., Newport J., et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noga JT., Vladar K., Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res. 2001;106:25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 38.Swayze VW 2nd., Andreasen NC., Alliger RJ., Yuh WT., Ehrhartdt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 39.Pearlson GD., Barta PE., Powers RE., et al. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 40.Eastwood SL., Harrison PJ. Hippocampal synaptic pathology in schizophrenia, bipolar disorder, and major depression: a study of complexin mRNAs. Mol Psychiatry. 2000;5:425–432. doi: 10.1038/sj.mp.4000741. [DOI] [PubMed] [Google Scholar]
- 41.Rosoklija G., Toomayan G., Ellis SP., et al. Structural abnormalities in subicular dendrites in subjects with schizophrenia and mood disorders. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan KRR., Doraiswamy PM., Figiel GS., et al. Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci. 1991;3:387–391. doi: 10.1176/jnp.3.4.387. [DOI] [PubMed] [Google Scholar]
- 43.Sheline Yl., Gado MH., Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 44.Siegle GJ., Konecky RO., Thase ME., Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acac Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 45.Frodl T., Meisenzahl E., Zetzsche T., et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 46.Altshuler LL., Bartzokis G., Thomas G., Curran J., Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 47.Strakowski SM., DelBello MP., Sax KW., et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 48.Brambilla P., Harenski K., Nicoletti M., et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 49.Blumberg HP., Kaufman J., Martin A., et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 50.DelBello MP., Zimmerman ME., Mills NP., Getz GE., Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC., Sills R., Nugent A., et al. Volumetric assessment of the amygdala in mood disorders using high resolution, 3T MRI. Biol Psychiatry. 2004;SS:182S. [Google Scholar]
- 52.Husain MM., McDonald WM., Doraiswamy PM., et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 53.Krishnan KRR., McDonald WM., Escalona PR., et al. Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan KRR., McDonald WM., Doraiswamy PM., et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Neurosci. 1993;243:41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 55.Baumann B., Danos P., Krell D., et al. Reduced volume of limbic systemaffiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychoi Clin Neurosci. 1999;11:71–78. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- 56.Dupont RM., Jernigan TL., Heindel W., et al. Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry. 1995;52:747–755. doi: 10.1001/archpsyc.1995.03950210041009. [DOI] [PubMed] [Google Scholar]
- 57.Lenze EJ., Sheline Yl. Absence of striatal volume differences between depressed subjects with no comorbid medical illness and matched comparison subjects. Ami J Psychiatry. 1999;156:1989–1991. doi: 10.1176/ajp.156.12.1989. [DOI] [PubMed] [Google Scholar]
- 58.Martinez P., Ronsaville D., Gold PW., Hauser P., Drevets WC. Morphometric abnormalities in adolescent offspring of depressed mothers. Soc Neurosci Abstr. 2002;32 [Google Scholar]
- 59.Brambilla P., Nicoletti M., Sassi RB., et al. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75:221–225. [PMC free article] [PubMed] [Google Scholar]
- 60.Shah SA., Doraiswamy PM., Husain MM., et al. Posterior fossa abnormalities in major depression: a controlled magnetic resonance imaging study. Acta Psychiatr Scand. 1992;85:474–479. doi: 10.1111/j.1600-0447.1992.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 61.Escalona PR., McDonald WM., Doraiswamy PM., et al. Reduction of cerebellar volume in major depression. A controlled MRI study. Depression. 1993;1:156–158. [Google Scholar]
- 62.Parashos IA., Tupler LA., Blitchington T., Krishnan KR. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res. 1998;84:715. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan KRR., Doraiswamy PM., Lurie SN., et al. Pituitary size in depression. J Clin Endocrinol Metab. 1991;72:256–259. doi: 10.1210/jcem-72-2-256. [DOI] [PubMed] [Google Scholar]
- 64.Cotter D., Mackay D., Beasley C., Kerwin R., Everall I. Reduced glial density and neuronal volume in major depressive disorder and schizophrenia in the anterior cingulate cortex. Schizophr Res. 2000;41:106. [Google Scholar]
- 65.Eastwood SL., Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP 43, and the complexins. Brain Res Bull. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 66.Cotter DR., Pariante CM., Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 67.Bowley MP., Drevets WC., Öngür D., Price JL. Low glial numbers in the amygdala in mood disorders. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 68.Benes FM., Vincent SL., Todtenkopf M. The density of pyramidal and nonpyramidal neurons in ACC of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 69.Honer WG., Falkai P., Chen C., Arango V., Mann JJ., Dworks AJ. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience. 1999;91:1247–1255. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]
- 70.Rajkowska G., Halaris A., Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 71.Cotter D., Mackay D., Chana G., Beasley C., Landau S., Everall I. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 72.Ludwin SK. The function of perineuronal satellite oligodendrocytes: an immunohistochemical study. Neuropathol Appl Neurobiol. 1984;10:143–149. doi: 10.1111/j.1365-2990.1984.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 73.Uranova N., Orlovskaya D., Vikhreva O., et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 74.D'Amelio F., Eng LF., Gibbs MA. Glutamine synthetase immunoreactivity present in oligodendroglia of various regions of the central nervous system. Glia. 1990;3:335–341. doi: 10.1002/glia.440030504. [DOI] [PubMed] [Google Scholar]
- 75.Johnston-Wilson NL., Sims CD., Hofmann JP., et al. Disease-specif ic alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry. 2000;5:142–149. doi: 10.1038/sj.mp.4000696. [DOI] [PubMed] [Google Scholar]
- 76.Webster MJ., Knable MB., Johnston-Wilson N., Nagata K., Inagaki M., Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immunol. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- 77.Cheng JD., de Vellis J. Oligodendrocytes as glucocorticoids target cells: functional analysis of the glycerol phosphate dehydrogenase gene. J Neurosci Res. 2000;59:436–445. doi: 10.1002/(SICI)1097-4547(20000201)59:3<436::AID-JNR19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 78.Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 79.Dewar D., Underhill SM., Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 80.Matute C., Sanchez-Gomez MV., Martinez-Millan L., Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nowak G., Ordway GA., Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 82.McEwen BS. Structural plasticity of the adult brain: how animal models help us understand brain changes in depression and systemic disorders related to depression. Dialogues Clin Neurosci. 2004;6:119–133. doi: 10.31887/DCNS.2004.6.2/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vyas A., Mitra R., Shankaranarayana Rao BS., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vyas A., Bernai S., Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- 85.Drevets WC., Frank E., Price JC., et al. PET imaging of serotonin 1 A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 86.Rothman DL., Sibson NR., Hyder F., Shen J., Behar KL., Shulman RG. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Phil Trans Roy Soc London B. 1999;354:1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sibson NR., Dhankhar A., Mason GF., Rothman DL., Behar KL., Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen J., Petersen KF., Behar KL., et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shulman RG., Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci USA. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paul IA., Nowak G., Layer RT., Popik P., Skolnick P. Adaption of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269:95–102. [PubMed] [Google Scholar]
- 91.Baxter LR., Schwartz JM., Phelps ME., et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 92.Bench CJ., Friston KJ., Brown RG., Scott LC., Frackowiak SJ., Dolan RJ. The anatomy of melancholia - focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 93.Neumeister A., Nugent AC., Waldeck T., et al. Behavioral and neural responses to tryptophan depletion in unmedicated remitted patients with major depressive disorder and controls. Arch Gen Psychiatry. 2004. In press. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 94.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive emotional manifestations of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 95.Drevets WC., Videen TO., Price JL., Preskorn SH., Carmichael ST., Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amaral DG., Insausti R. Retrograde transport of D-[3H]aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- 97.Bacon SJ., Headlam AJ., Gabbot PL., Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- 98.Jackson ME., Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuroda M., Price JL. Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol. 1991;303:513–533. doi: 10.1002/cne.903030402. [DOI] [PubMed] [Google Scholar]
- 100.Amaral DG., Price JL. Amygdalo-cortical projections in the monkey. (Macaca fascicularis). J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 101.Russchen FT., Price JL. Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-Las an anterograde tracer. Neurosci Lett. 1984;47:15–22. doi: 10.1016/0304-3940(84)90379-3. [DOI] [PubMed] [Google Scholar]
- 102.Graybiel AM. The basal ganglia and the initiation of movement. Rev Neurol (Paris). 1990;146:570–574. [PubMed] [Google Scholar]
- 103.Drevets WC., Todd RD. Depression, mania and related disorders. In: Guze SB, ed. Adult Psychiatry. St Louis, Mo: Mosby Press; 2004:99–141. [Google Scholar]
- 104.Mayberg HS., Starkstein SE., Sadzot B., et al. Selective hypometabolisrn in the inferior frontal lobe in depressed patients with Parkinson's disease. Ann Neurol. 1990;28:57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- 105.Mayberg HS., Starkstein SE., Morris PL., et al. Remote cortical hypometabolisrn following focal basal ganglia injury: relationship to secondary changes in mood. Neurology. 1991;41(suppl):266. [Google Scholar]
- 106.Mayberg HS., Starkstein SE., Peyser CE., Brandt J., Dannals RF., Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with Huntington's disease. Neurology. 1992;42:1791–1797. doi: 10.1212/wnl.42.9.1791. [DOI] [PubMed] [Google Scholar]
- 107.Ring HA., Bench CJ., Trimble MR., Brooks DJ., Frackowiak RSJ., Dolan RJ. Depression in Parkinson's disease: a positron emission study. Br J Psychiatry. 1994;165:333–339. doi: 10.1192/bjp.165.3.333. [DOI] [PubMed] [Google Scholar]
- 108.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 109.Garcia R., Vouimba RM., Baudry M., Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 110.Rosenkranz JA., Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siegle GJ., Steinhauer SR., Thase ME., Stenger VA., Carter CC. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 112.Buchsbaum MS., Wu J., Siegel BV., et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 113.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 114.Mayberg HS., Liotti M., Brannan SK., et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 115.Osuch EA., Ketter TA., Kimbrell TA., et al. Regional cerebral metabolism associated with anxiety symptoms in affective disorder patients. Biol Psychiatry. 2000;48:1020–1023. doi: 10.1016/s0006-3223(00)00920-3. [DOI] [PubMed] [Google Scholar]
- 116.Drevets WC., Thase M., Sogers W., Greer P., Kupfer DJ. Glucose metabolic correlates of depression severity and antidepressant treatment response. Biol Psychiatry. 2002;51:176S. [Google Scholar]
- 117.George MS., Ketter TA., Parekh PI., Horwitz B., Herscovitch P., Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 118.Damasio A., Grabowski TJ., Bechara A., et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 119.Botteron KN., Raichle ME., Heath AC., et al. An epidemiological twin study of prefrontal neuromorphometry in early onset depression. Biol Psychiatry. 1999;45:59S. [Google Scholar]
- 120.Kimbrell TA., Ketter TA., George MS., et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 121.Wu JC., Gillin JC., Buchsbaum MS., Hershey T., Johnson JC., Bunney WE. Effect of sleep deprivation on brain metabolism of depressed patients. Am J Psychiatry. 1992;149:538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]
- 122.Mayberg HS., Brannan SK., Mahurin RK., et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 123.Pizzagalli D., Pascual Marqui RD., Nitschke JB., et al. Anterior cingulate activity predicts degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 124.Öngür D., Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 125.Damasio AR. Descarte's Error; Emotion, Reason, and the Human Brain. New York, NY: Grosset/Putnam; 1995 [Google Scholar]
- 126.Price JL., Carmichael ST., Drevets WC. Networks related to the orbital and medial prefrontal cortex: a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 127.Dioro D., Viau V., Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frysztak RJ., Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 129.Morgan MA., LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 130.Sullivan RM., Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Veith RC., Lewis N., Linares OA., et al. Sympathetic nervous system activity in major depression. Arch Gen Psychiatry. 1994;51:411–422. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- 132.Carney RM., Freedland KE., Rich MW., Smith LJ., Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. Am J Med. 1993;95:23–28. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- 133.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 134.Uranova NA., Vostrikov VM., Orlovskaya DD., Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 135.Drevets WC., Spitznagel E., Raichle ME. Functional anatomical differences between major depressive subtypes. J Cereb Blood Flow Metab. 1995;15:S93. [Google Scholar]
- 136.Drevets WC., Price JL., Bardgett ME., Reich T., Todd R., Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and stressed plasma Cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 137.Talairach J., Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988 [Google Scholar]
- 138.Drevets WC., Videen TO., Snyder AZ., MacLeod AK., Raichle ME. Regional cerebral blood flow changes during anticipatory anxiety. Soc Neurosci Abstr. 1994;20:368. [Google Scholar]
- 139.Mayberg HS., Lewis PJ., Reginald W., Wanger HN Jr. Paralimbic hypoperfusion in unipolar depression. J Nucl Med. 1994;35:929–934. [PubMed] [Google Scholar]
- 140.Rauch SL., Jenike MA., Alpert NM., et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 141.Drevets WC., Simpson JR., Raichle ME. Regional blood flow changes in response to phobic anxiety and habituation. J Cereb Blood Flow Metab. 1995;15:S856. [Google Scholar]
- 142.Schneider F., Gur RE., Alavi A., et al. Mood effects on limbic blood flow correlate with emotion self-rating: a PET study with oxygen-15 labeled water. Psychiatry Res Neuroimag. 1995;61:265–283. doi: 10.1016/0925-4927(95)02678-q. [DOI] [PubMed] [Google Scholar]
- 143.Mogenson GJ., Brudzynski SM., Wu M., Yang CR., Yim CCY. From motivation to action: a review of dopaminergic regulation of limbic to nucleus to accumbens to ventral pallidum to pedunculopontine nucleus circitries involved in limbic-motor integration. In: Kalivas PW, Barnes CD, eds. Limbic Motor Circuits and Neuropsychiatry. London, UK: CRC Press; 1993:193–236. [Google Scholar]
- 144.Rolls ET. A theory of emotion and consciousness, and its application to understanding the neural basis of emotion. In: Gazzaniga M, ed. The Cognitive Neurosciences. Cambridge, Mass: MIT Press; 1995:1091–1106. [Google Scholar]
- 145.Timms RJ. Cortical inhibition and facilitation of the defence reaction. J Physiol Lond. 1977;266:98P–99P. [PubMed] [Google Scholar]
- 146.MacFall JR., Payne ME., Provenzale JE., Krishnan KRR. Medial orbital frontal lesions in late onset depression. Biol Psychiatry. 2001;49:803–806. doi: 10.1016/s0006-3223(00)01113-6. [DOI] [PubMed] [Google Scholar]
- 147.Oya H., Howard M., Kawasaki H., Adolphs R. Intracranial field potentials recorded from human amygdala and frontal cortex: amplitude and phase responses to emotional stimuli. Soc Neurosci Abstr. 2001;645:6. [Google Scholar]
- 148.Nofzinger EA., Nichols TE., Meltzer CC., et al. Changes in forebrain function from waking to REM-sleep in depression: preliminary analyses of [18F]FDG PET studies. Psychiatry Res. 1999;91:59–78. doi: 10.1016/s0925-4927(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 149.Ketter TA., Kimbrell TA., George MS., et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 150.Abercrombie HC., Schaefer SM., Larson CL., et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- 151.Brody AL., Saxena S., Stoessel P., et al. Regional brain metabolic changes in patients major depressive disorder from pre- to post-treatment with paroxetine. Arch Gen Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 152.Saxena S., Brody AL., Ho ML., et al. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 2002;59:250–261. doi: 10.1001/archpsyc.59.3.250. [DOI] [PubMed] [Google Scholar]
- 153.Thomas KM., Drevets WC., Dahl RE., et al. Abnormal amygdala response to faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 154.Herman JP., Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 155.Rubin RT., Mandell AJ., Crandall PH. Corticosteroid responses to limbic stimulation in man: localization of stimulus sites. Science. 1966;1 53:767–768. doi: 10.1126/science.153.3737.767. [DOI] [PubMed] [Google Scholar]
- 156.Videbech P., Ravnkilde B., Pedersen AR., et al. The Danish PET/depression project: PET findings in patients with major depression. Psychol Med. 2001;31:1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- 157.Wilson J., Kupfer DJ., Thase M., Sogers W., Greer P., Drevets WC. Ventral striatal metabolism is increased in depression, and decreases with treatment. Biol Psychiatry. 2002;51:122S. [Google Scholar]
- 158.Bench CJ., Friston KJ., Brown RG., Frackowiak RS., Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- 159.Charney DS., Drevets WC. The neurobiological basis of anxiety disorders. In: Davis K, Charney DS, Coyle J, Nemeroff CB, eds. Psychopharmacology. The Fifth Generation of Progress. New York, NY: Lippencott, Williams, and Wilkins; . 2002:901–930. [Google Scholar]
- 160.Vogt B. Structural organization of cingulate cortex. In: Vogt BA, Gabriel M, eds. Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston, Mass: Birkhauser; 1993 [Google Scholar]
- 161.Bain EE., Nugent AC., Carson RE., et al. Decreased 5-HT1A receptor binding in bipolar depression. Biol Psychiatry. 2004;55:178S. doi: 10.1016/j.biopsych.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 162.Neumeister A., Bain E., Nugent A., et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Parsey RV., Oquendo MA., Simpson NR., et al. Altered serotonin 1A binding in major depression: a [11C]QWAY100635 PET study. Biol Psychiatry. Abstract 300. 2002;51(8S):106S. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 164.Sargent PA., Kjaer KH., Bench CJ., et al. Brain serotonin 1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 165.Lopez JF., Chalmers DT., Little KY., Watson SJ. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. A. E. Bennett Research Award. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 166.Arango V., Underwood MD., Boldrini M., et al. Serotonin 1 A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacoiogy. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 167.Brewton LS., Haddad L., Azmitia EC. Colchicine-induced cytoskeletal collapse and apoptosis in N-18 neuroblastoma cultures is rapidly reversed by applied S-1003. Brain Res. 2001;912:9–16. doi: 10.1016/s0006-8993(01)02519-7. [DOI] [PubMed] [Google Scholar]
- 168.Azmitia EC., Whitaker-Azmitia PM. Awakening the sleeping giant: anatomy and plasticity of the brain serotonergic system. J Clin Psychiatry. 1991;52(12, suppl):4–16. [PubMed] [Google Scholar]
- 169.Azmitia EC., Gannon PJ., Kheck NM., Whitaker-Azmitia PM. Cellular localization of the 5-HT1a receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 170.Mann JJ., Huang YY., Underwood MD., et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]