Abstract
More than 25 million people in the world today are affected by dementia, most suffering from Alzheimer's disease. In both developed and developing nations, Alzheimer's disease has had tremendous impact on the affected individuals, caregivers, and society. The etiological factors, other than older age and genetic susceptibility, remain to be determined. Nevertheless, increasing evidence strongly points to the potential risk roles of vascular risk factors and disorders (eg, cigarette smoking, midlife high blood pressure and obesity, diabetes, and cerebrovascular lesions) and the possible beneficial roles of psychosocial factors (eg, high education, active social engagement, physical exercise, and mentally stimulating activity) in the pathogenetic process and clinical manifestation of the dementing disorders. The long-term multidomain interventions toward the optimal control of multiple vascular risk factors and the maintenance of socially integrated lifestyles and mentally stimulating activities are expected to reduce the risk or postpone the clinical onset of dementia, including Alzheimer's disease.
Keywords: aging, Alzheimer's disease, epidemiology, incidence, prevalence, vascular risk factor, psychosocial factor, intervention
Abstract
Hoy en día más de 25 millones de personas en el mundo están afectadas por demencia, la mayor parte con Enfermedad de Alzheimer. Tanto en los países desarrollados como en aquellos en vías de desarrollo, la Enfermedad de Alzheimer ha tenido un tremendo impacto en los individuos que la padecen, en los cuidadores y en la sociedad. Los factures etiológicos, más allá de la edad avanzada y la susceptibilidad genética, tienen que ser determinados. Sin embargo, hay una creciente evidencia que apunta fuertemente al papel potencialmente dañino de los factores de riesgo vascular y otros trastornos (fumar cigarrillos, presión alta y obesidad en la edad media de la vida, diabetes y lesiones cerebrovasculares) y al posible papel benéfico de los factores psicosociales (mayor escolaridad, participación social activa, ejercicio físico y actividades de estimulación mental) en el proceso patogénico y en las manifestaciones clínicas de las demencias.
Se espéra que las intervenciones a largo plazo, desde múltiples áreas, orientadas al control óptimo de los múltiples factores de riesgo vascular y el mantenimiento de estilos de vida con buena integración social y actividades de estimulación mental reduzcan el riesgo o retarden la instalación de las demencias, incluyendo la Enfermedad de Alzheimer.
Abstract
Plus de 25 millions de personnes dans le monde sont aujourd'hui touchées par la démence, la plupart par la maladie d'Alzheimer. Cette maladie affecte de façon très importante les patients atteints, leurs soignants et la société qu'il s'agisse des pays développés ou en voie de développement. Les facteurs étiologiques autres qu'un âge avancé et une susceptibilité génétique restent à déterminer. Le rôle potentiel des facteurs de risque et des maladies vasculaires (tabagisme, hypertension artérielle et obésité de la quarantaine, diabète, lésions cérébrovasculaires) et l'éventuel rôle bénéfique des facteurs psychosociaux (niveau élevé d'éducation, engagement social actif, exercice physique, activité mentalement stimulante) sont cependant de plus en plus fortement mis en évidence dans le processus de la pathogenèse et les manifestations cliniques des troubles démentiels. Les interventions dans plusieurs domaines à long terme pour un contrôle optimal des multiples facteurs de risque vasculaire et le maintien d'un style de vie intégré socialement et d'activités mentalement stimulantes font espérer une réduction du risque ou un report du début clinique de la démence, y compris de la maladie d'Alzheimer.
Dementia can be defined as a clinical syndrome characterized by a cluster of symptoms and signs manifested by difficulties in memory, disturbances in language and other cognitive functions, changes in behaviors, and impairments in activities of daily living. Alzheimer's disease (AD), which is named after the German psychiatrist Alois Alzheimer, who first described this disorder more than one century ago, is the most common cause of dementia, accounting for up to 75 % of all dementia cases. Alzheimer's disease is a progressive neurodegenerative disorder. During the last a few decades, research in epidemiology of dementia and AD has made tremendous progress. In this review, we briefly summarize the major findings from the recent epidemiologic studies of AD concerning occurrence (global prevalence, incidence, and impact), determinants (risk and protective factors), and possible strategies toward intervention.
Global population aging, occurrence, and impact of Alzheimer's disease
Worldwide population aging
Population aging has become a worldwide universal phenomenon. Hie reports from the UN Aging Program and the US Centers for Disease Control and Prevention have projected that the number of older people (65+ years) in the world is expected to increase from 420 million in 2000 to nearly 1 billion by 2030, with the proportion of older people, being increased from 7 % to 12 %. 1,2 Developing countries will see the largest increase in absolute numbers of older persons. Thus, the developing nations' share of the worldwide aging population will increase from 59 % to 71 %. Because occurrence of AD is strongly associated with increasing age, it is anticipated that this dementing disorder will pose huge challenges to public health and elderly care systems in all countries across the world.
Prevalence of Alzheimer's disease
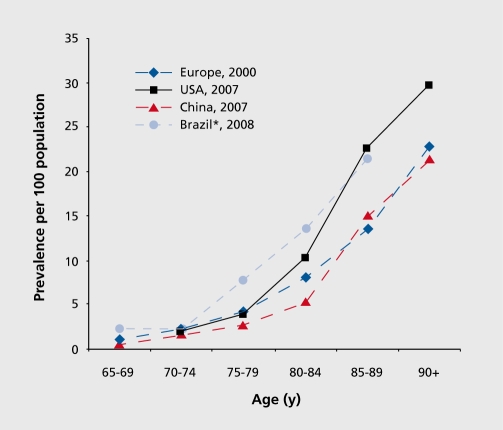
The pooled data of population-based studies in Europe suggests that the age-standardized prevalence in people 65+ years old is 6.4 % for dementia and 4.4 % for AD.3 In the US, the study of a national representative sample of people aged >70 years yielded a prevalence for AD of 9.7 %. 4 Worldwide, the global prevalence of dementia was estimated to be 3.9 % in people aged 60+ years, with the regional prevalence being 1.6 % in Africa, 4.0 % in China and Western Pacific regions, 4.6 % in Latin America, 5.4 % in Western Europe, and 6.4 % in North America.5 More than 25 million people in the world are currently affected by dementia, most suffering from AD, with around 5 million new cases occurring every year.5-7 The number of people with dementia is anticipated to double every 20 years. Despite different inclusion criteria, several meta-analyses and nationwide surveys have yielded roughly similar age-specific prevalence of AD across regions (Figure 1).3,4,8,9 The age-specific prevalence of AD almost doubles every 5 years after aged 65. Among developed nations, approximately 1 in 10 older people (65+ years) is affected by some degree of dementia, whereas more than one third of very old people (85+ years) may have dementia-related symptoms and signs.10,11 There is a similar pattern of dementia subtypes across the world, with AD and vascular dementia, the two most common forms of dementia, accounting for 50 % to 70 % and 15 % to 25 %, respectively, of all dementia cases.
Figure 1. Age-specific prevalence of Alzheimer's disease (per 100 population) across continents and countries. *, prevalence of all types of dementia .
Epidemiologic research of dementia and AD in low- and middle-income countries has drawn much attention in recent years. A systematic review estimated that the overall prevalence of AD in developing countries was 3.4 % (95 % CI,1.6 % - 5.0 %).12 The 10/66 Dementia Research Group found that the prevalence of dementia (DSM-IV criteria) in people aged 65+ years in seven developing nations varied widely from less than 0.5 % to more than 6 %, which is substantially lower than in developed countries.13 Indeed, the prevalence rates of dementia in India and rural Latin America were approximately a quarter of the rates in European countries. However, the prevalence of AD in persons 65+ years in urban areas of China was 3.5 %, and even higher (4.8 %) after post-hoc correction for negative screening errors,14 which is generally comparable with those from Western nations. Similar prevalence rates of dementia were also reported from the urban populations of Latin American nations such as Havana in Cuba (6.4 %) and São Paulo in Brazil (5.1 %).9-15
Incidence of Alzheimer's disease
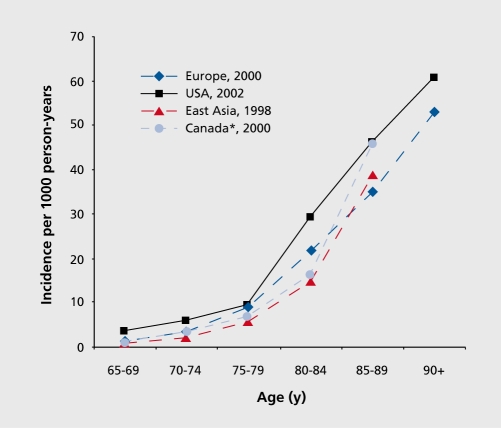
The pooled incidence rate of AD among people 65+ years of age in Europe was 19.4 per 1000 person-years.16 The pooled data from two large-scale community-based studies of people aged 65+ years in the US Seattle and Baltimore, areas yielded an incidence rate for AD of 15.0 (male, 13.0; female, 16.9) per 1000 person-years.17,18 The incidence rate of AD increases almost exponentially with increasing age until 85 years of age (Figure 2).16,18-20
Figure 2. Age-specific incidence of Alzheimer's disease (per 1 000 person years) across continents and countries. *, incidence of all types of dementia.
However, it remains uncertain whether the incidence continues to increase, even at more advanced ages, or reaches a plateau at a certain age; this is relevant for projecting the burden of the disease as well as for understanding its etiology. For instance, a consistently exponential increase, with advancing age in Alzheimer incidence suggests that AD is an inevitable consequence of aging, whereas a convergence to or a decline at certain age may suggest that very old people may have reduced vulnerability, owing perhaps to genetic or environmental factors.21 The Cache County Study further found that the incidence of AD increased with age, peaked, and then started to decline at extreme old ages for both men and women.21 However, some meta-analyses and large-scale studies in Europe provided no evidence for the potential decline, in the incidence of dementia and AD among the oldest-old age groups.16,22,23 The apparent decline suggested in some studies may be an artifact of poor response rate and survival effect in these very old age groups. Several studies from Europe observed a higher incidence rate of AD among women than men, especially among the oldest-old age groups,16 whereas studies in North America generally found no significant gender difference.17,18
There appears to have been some geographic variations in the incidence of AD. The pooled data of eight European studies suggested a geographical dissociation across Europe, with higher incidence rates being found among the oldest-old people of north-western countries than among southern countries.16 The incidence rates of AD were reported to be slightly lower in North America than in Europe. Differences in methodology (eg, differences in study design and procedure of case ascertainment), rather than real different regional distributions of the disease, may be partly responsible for the geographic variations. The study using identical methods in UK found no evidence of variation in dementia incidence among five areas in England and Wales.22 Studies have confirmed that AD incidence in developing countries is generally lower than in North America and Europe. For example, the incidence rate of AD among people aged 65+ years was 7.7 per 1 000 person-years in Brazil and 3.2 per 1 000 person-years in India.12,24
Impact of Alzheimer's disease at individual and societal levels
At the individual level, AD significantly shortens life expectancy and is one of the principal causes of physical disability, institutionalization, and decreased quality of life among the elderly. First, AD is strongly associated with functional disability and institutionalization. It is estimated that among individuals over 60 years of age dementia contributes 11.2 % of the years lived with disability, compared with 9.5 % for stroke, 8.9 % for musculoskeletal disorders, and 5.0 % for cardiovascular disease.25 The follow-up studies of people, aged 75+ years in Sweden have shown that approximately half of the elderly people who develop functional dependence over a 3-year period can be attributable to dementia and AD.26 In many industrialized countries, dementia is the most common disease among older adults living in nursing homes or in institutions. Second, epidemiologic studies have confirmed the malignant nature of AD that could confers an excess risk of death for older people, in a similar extent to that of malignant tumors.27 Several community-based follow-up studies of incident cases showed that AD was associated with a twoto fivefold increased risk of death.28,29 The long-term follow-up study also showed that AD was associated with relative risk of 2.6 for mortality, although the strength of association was diluted after controlling for multimorbidities.30 Overall, the median survival time for people with newly diagnosed AD ranges from 3 to 6 years.28 Older age, male sex, white race, low education, comorbidities (eg, hypertension, diabetes, and heart disease), poorer cognitive function, and physical disability are frequently reported to predict a shorter survival in persons with AD.28-32
The rapid increase in the number of patients with dementia and AD will result in tremendous consequence for our society and economy. The number of persons with AD in the US population in 2000 was estimated to be 4.5 million, and by 2050 this number was projected to increase by almost threefold, to 13.2 million.33 The more recent study indicated that in 2006 the worldwide total number of patients with AD was 26.6 million, and by 2050 the number will quadruple.7 It was estimated that about 43 % of AD patients require a high level of care such as nursing home and institutions. The long-term institutional care will be the main cost in many developed countries, whereas in developing countries informal home care provided byfamily members is usually the only source available for patients with dementia.12 Even in the US, almost 10 million Americans (eg, family members, friends, and neighbors) provided unpaid care for persons with AD or other dementia.34 Thus, enormous resources will be needed for adequate care of Alzheimer and dementia patients. The worldwide, overall societal costs of dementia were estimated to be more than US$315 billion in 2005, including one third for informal care;35 approximately three fourths of the global costs for dementia occurred in middleincome countries where about 46% of dementia patients reside.12 The 2009 reports from Alzheimer's Association showed that in US the annual costs for patients with AD and other dementia were estimated to be US$148 billion plus US94 billion unpaid care service, and that AD tripled health care costs for Americans aged 65+ years.34 It has reported that the costs for dementia are higher than those related to diabetes and smoking.36 Thus, AD will place heavy economic burden on the family and society due to the needs of persistent care and therapy. It was anticipated that modest advances in therapeutic and preventive strategies that lead to even a 1-year delay in the onset and progression of clinical AD, would significantly reduce the global burden of this disease.7,37
Determinants of Alzheimer's disease
Alzheimer's dementia is a multifactorial disease, in which older age is the strongest risk factor, suggesting that the aging-related biological processes may be implicated in the pathogenesis of the disease. Furthermore, the strong association of AD with increasing age may partially reflect the cumulative effect of different risk and protective factors over the lifespan, including the effect of complex interactions of genetic susceptibility, psychosocial factors, biological factors, and environmental exposures experienced over the lifespan. Following various etiologic hypotheses, Table I summarizes the major risk and protective factors for AD.38 Moderate to strong evidence, most from epidemiologic, neuroimaging, and neuropathological research, supports the role of genetic, vascular, and psychosocial factors in the development of AD, whereas evidence for the etiologic role of other factors (eg, dietary or nutritional factors, occupational exposures, and inflammation) is mixed or insufficient.
Table I. Summary of risk and protective factors for Alzheimer's disease by various etiologic hypotheses.
| Etiologic hupothesis | Contents: risk ans protective factors | Epidemiologic evidence |
| Genetic susceptibility | Risk factors: APOE ε4 allele and familial aggregation | Strong |
| Vascular pathway hypothesis | Risk factors: midlife high blood pressure and high BMI, diabet, cerebrovascular disease, and smoking; Protective factors: light-to-moderate alchohol consumption, and antihypertensive therapy | Moderate or sufficient |
| Psychosocial hypothesis | Protecive factors: high educational attainment, mentaly stimulating activities, social activity and enriched social network, and physical activity | Moderate or sufficient |
| Nutritional and dietary hypothesis | Risk factor deficiency in folate, vitamin B12, and antioxidants (vitamins A, E, and C); Protective factors: Fish (omege-3 fatty acids) and vegetable consumption. | Insufficient or limited/mixed |
| Others (eg, toxic or inflammatory factors) | Risk factors: traumatic head injuries, occupational exposure to toxins and electromagnetic fields, depression, and hormone replacement therapy; Protective factors: nosteroidal anti-inflammatory drugs | Insufficient or limited/mixed |
Genetic hypothesis
Early-onset familial AD is often caused by autosomal dominant mutations (eg, mutations in amyloid precursor protein, presenilin-1, and presenilin-2 genes), which accounts for only about 2 % to 5 % of all Alzheimer patients.39 The majority of AD cases are sporadic and present considerable heterogeneity in terms of risk factor profiles and neuropathological features. First-degree relatives of Alzheimer patients have a higher lifetime risk of developing AD than the general population or relatives of nondemented individuals40; both genetic and shared environmental factors contribute to the phenomenon of familial aggregation. In addition, some studies suggest that the familial aggregation of AD can only be partially explained by known genetic components such as the apolipoprotein E (APOE) ε4 allele, indicating that other susceptibility genes may be involved.41 The APOE ε4 allele is the only established genetic factor for both early- and late-onset AD; it is a susceptibility gene for AD, being neither necessary nor sufficient for AD development. With increasing number of the APOE ε4 alleles, the risk of AD increases and the age of AD onset deceases, in a dose-dependent manner.42 The risk effect of APOE ε4 allele on AD decreases with increasing age, and overall approximately 15 % to 20 % of Alzheimer cases are attributable to the ε4 allele.39,42 Several other candidate genes, many of them vascular related such as angiotensin-I converting enzyme, cholesterol 24-hydroxylase, and insulin-degrading enzyme genes, have been studied, but with inconsistent findings.44,45
Vascular pathway hypothesis
Moderate to strong evidence from multidisciplinary research (epidemiologic, neuroimaging, and neuropathological studies) has emerged supporting the hypothesis that vascular risk factors (eg, smoking, obesity, and high total cholesterol) and vascular morbidity (eg, high blood pressure, diabetes, and silent brain infarcts and white matter lesions) are associated with an increased risk of dementia, including AD.
Tobacco use
Earlier cross-sectional studies often reported lower prevalence rates of AD among smokers than nonsmokers.46 However, this protective effect was probably due to selective survival bias related to smoking because smokers are proportionally less numerous among prevalent cases; when incident AD cases were examined, such an effect was no longer present.47-49 Contrary to the cross-sectional studies, many follow-up studies found a significantly increased risk of AD associated with cigarette smoking, especially among noncarriers of the APOE ε4 allele.50-52
Meta-analyses of follow-up studies concluded that current smoking was associated with an increased risk of AD (pooled relative risk, 1.79; 95 % CI, 1.43-2.23) ,53,54 Thus, in contrast to the initial hypothesis of a possible protective effect, cigarette smoking actually increases the risk of AD.
Alcohol consumption
It is known that alcohol abuse can cause “alcoholic dementia.” The deleterious effect of heavy alcohol intake emerges from a study suggesting that heavier drinkers at middle age had a more than threefold increased risk of dementia and AD in late life, especially among carriers of the APOE ε4 allele.55 By contrast, light-to-moderate alcohol consumption was frequently reported to be associated with a reduced incidence of dementia and AD,56,57 leading to the hypothesis that light-to-moderate alcohol intake may protect against the development of dementia. However, the role of moderate alcohol consumption in dementia remains controversial because the inverse association may be due to information bias, the confounding of healthy lifestyles and high socioeconomic status, different approaches in assessments of alcohol consumption, or outcome misclassification.
Indeed, alcohol may have beneficial influences on several cardiovascular factors, including lipid and lipoprotein levels, and inflammatory, and hemostatic factors. Moderate alcohol drinking has been related to a reduced risk of cardiovascular diseases, and may be associated with fewer brain infarcts. Furthermore, a U-shaped relationship of alcohol consumption with the burden of white matter lesions has been described.58 On the other hand, excessive alcohol drinking has clear detrimental effects on the brain, and even light-to-moderate alcohol drinking has been related to increased brain atrophy and to smaller brain volumes.59,60
Overweight and obesity
The lifespan-dependent relationship between body mass index (BMI) and risk of dementia has emerged in a systematic review such that a higher BMI in midlife is a risk factor for AD and dementia, whereas an accelerated decline, in BMI during late life may anticipate the occurrence of the dementing disorders.61 A higher BMI or obesity (especially central obesity) at the age of around 50 years was related to an increased risk of dementia occurring 20 to 25 years later.62-65 The long-term follow-up study of Japanese-American men revealed a greater decline in BMI approximately 10 years prior to dementia onset.66 The Cardiovascular Health Study also provided insight into the age-dependent BMI-dementia relationship, in which obesity at midlife (around 50 years of age) was related to a higher risk of late-life development of dementia, whereas BMI measured after age 65 years was inversely related to dementia risk.65 In line with these findings, several follow-up studies of older people suggested that accelerated decline in BMI was associated with future development of AD.67-69 Low BMI in late life was related to a higher risk for AD over a subsequent 5-to 6-year period.70,71 Thus, late-life low BMI and weight loss can be interpreted as markers for preclinical AD, particularly when measured just a few years prior to clinical diagnosis of the disease.
Blood pressure and blood pressure-lowering therapy
Elevated blood pressure in middle age, especially uncontrolled midlife high blood pressure, was linked to an increased risk of late-life AD in several observational studies.72,73 Findings from follow-up studies of late-life blood pressure in relation to the risk of dementia have been inconsistent; several follow-up studies, especially those with a relatively short period of follow-up (eg, <3 years), found no association or even an inverse association between level of blood pressure and risk of dementia and AD.74 As dementia has a long latent period and blood pressure may be lowered at its preclinical phase, the lack or an inverse association can be interpreted as a consequence of the disease process.75,76 However, studies with a longer follow-up period follow-up (eg, >6 years) have also reported an inverse association,77-79 suggesting that low blood pressure in late life may contribute to the development or clinical expression of dementia and AD.74,80
Longitudinal studies repeatedly show a protective effect of use of antihypertensive, drugs against dementia and AD.74,81,82 Recent follow-up studies have suggested that the protective effect of antihypertensive therapy on dementia and AD may depend on the duration of treatment and the age when people take the medications; the more evident efficacy was seen among younger-old people (eg, <75 years) and those with long-term treatment.83,84 Evidence from clinical trials of antihypertensive therapy and dementia is summarized in the section on intervention trials towards primary prevention. Antihypertensive treatment may protect against dementia and AD by postponing atherosclerotic process, reducing the number of cerebrovascular lesions, and improving cerebral perfusion.74 It has also been suggested that some antihypertensive agents (eg, calcium-channel antagonists) may have neuroprotective effects. The recent neuropathological study found substantially less Alzheimer neuropathological changes (ie, neuritic plaque and neurofibrillary tangle densities) in the medicated hypertension group than nonhypertensive group, which may reflect a salutaryeffect of antihypertensive therapy against AD-associated neuropathology.85
High serum cholesterol and use of cholesterol-lowering drugs (statins)
High serum total cholesterol at midlife was linked to an increased risk of late-life AD.86,87 Hie late-life high cholesterol in relation to dementia and AD is less clear, with studies indicating either no association or an inverse association of hypercholesterolemia with subsequent development of AD.88-90 A bidirectional influential relationship between serum total cholesterol and dementia has been suggested; high total cholesterol at middle age is a risk factor for the development of AD and dementia 20 years later, but decreasing serum cholesterol after midlife mayreflect ongoing disease processes and may represent a marker for late-life AD and dementia.91 A pattern of decrease in blood pressure and BMI from midlife to older adults has also been described, but decline in total cholesterol shows somewhat different patterns. The dementiaassociated additional decline in blood pressure and BMI generally becomes detectable about 3 to 6 years before the clinical expression of the disease, while the decline in total cholesterol seems to start much earlier, and with less evident acceleration prior to dementia onset.92 These changes may explain, at least partly, the inconsistent results from the cross-sectional and short-term follow-up studies as well as studies having the measurement of serum cholesterol later in life.
Little information is currently available regarding the roles of subtype cholesterols (low-density lipoprotein, high-density lipoprotein, and triglycerides) in AD. It is important to note that serum and brain cholesterol are two separate pools, and links between them are not totally understood. One possible pathway is represented by oxysterols, mono-oxygenated metabolites of cholesterol, with the unique ability to cross the blood-brain barrier.93 The currently available epidemiological and clinical data on use of lipids lowering drugs (statins) and risk of AD give a rather mixed picture. Several cross-sectional and case-control studies have reported that statin users have a considerable lower prevalence of AD.94,95 While the follow-up data from the Rotterdam Study showed that use of statins was associated with a lower risk of AD independent of lipophilicity of statins,96 other prospective studies have indicated that there is no beneficial effect or only modestly decreased risk of AD related to statin use.97,98 Neuropathological studies also showed inconsistent findings as to whether the use of statins was associated with the burden of Alzheimer pathological changes and infarcts in the brain.99,100 Experimental studies suggest that statins may reduce β-amyloid production in vitro and in vivo. Statins also have a variety of actions that may benefit the central nervous system and reduce the risk of AD, including endothelial protection via actions on the nitric oxide synthase system, antioxidant, anti-inflammatory, and antiplatelet effects.
Nutritional and dietary factors
Several follow-up studies have reported a decreased risk of AD associated with increasing dietary or supplementary intake, of antioxidants (eg, vitamins E and C),101,102 although some negative findings were also reported.103 Furthermore, studies found that higher adherence to “Mediterranean diet” (ie, a dietary pattern with higher intake of fish, fruits, and vegetables rich in antioxidants) was associated with a reduced risk of AD independent of vascular pathways.104,105
In addition, mixed results have been reported on the association of serum vitamin B12, folate, and homocysteine with the risk of dementia and AD.106 Hie Cochrane systematic review concluded that folic acid and vitamin B12 supplementations have no benefits on cognition, although folate plus vitamin B12 are effective in reducing serum homocysteine.107 Finally, it has been reported that a diet rich in saturated fats and cholesterol increases the risk of AD,108 whereas polyunsaturated fatty acids and fish may be protective against dementia.109,110
Unsaturated fatty acids may confer protection through anti-inflammatory properties. Fatty acids may also play a part in the synthesis and fluidity of nerve cell membranes and for synaptic plasticity and neuronal degeneration. In addition, oxidative stress is one of the central features in the Alzheimer brain. Hius, it may be plausible that supplementation or diet rich in antioxidants such as fruits, vegetables, and vitamins E and C might protect against AD.
Diabetes
An increased risk of not only vascular dementia but also neurodegenerative type dementia among persons with diabetes has been reported in several longitudinal studies,111-113 and the risk effect was confirmed by a systematic review.114 Midlife diabetes or a longer duration of diabetes may play a crucial role in dementia and AD.115,116 In addition, borderline or prediabetes or impaired glucose tolerance, is also linked to an increased risk of dementia and AD in very old people.117 Such an association may reflect a long-term direct effect of uncontrolled hyperglycemia on neurodegenerative changes in the brain or an effect of hyperinsulinemia or impaired insulin response, or due to diabetes-related comorbidities such as hypertension and dyslipidemia.118-120
The metabolic syndrome, which is a cluster of multiple vascular risk factors characterized by abnormalities in insulin, blood glucose, lipoprotein metabolism, hypertension, and obesity, was found to be associated with an increased prevalence of AD in an elderly Finnish population.121 However, the follow-up study of multiethnic elderly cohort in the US found no association of the metabolic syndrome with either prevalent or incident AD, but two components of the syndrome, diabetes and hyperinsulinemia, were associated with an increased risk of incident AD122; the authors concluded that examining diabetes and hyperinsulinemia separately might be preferable to using the metabolic syndrome as a single factor to define the risk of AD.
Cerebral and cardiovascular disease
Stroke, and even clinically silent brain infarcts and white-matter hyperintensities seen on magnetic resonance imaging (MRI) scans, significantly increased the risk of dementia and AD,123,124 although the observed association with AD has been argued to actually reflect an association with mixed dementia. The follow-up data of the Cardiovascular Health Study showed that cardiovascular disease was associated with an increased incidence of dementia and AD, with the highest risk of dementia being seen in people with peripheral arterial disease, suggesting that extensive peripheral atherosclerosis is a risk factor for AD.125,126 Other cardiovascular disease, (eg, atrial fibrillation and heart failure) and more severe atherosclerosis measured with ankle-to-brachial index have been related to dementia and to AD as well.127-129 Neuropathological studies suggested that cerebrovascular lesions, atherosclerosis, and neurodegenerative changes in the brain often coexist, and may be coincident processes converging to cause additive damage to the aging brain and to promote clinical expression of the dementia syndrome.130,131
Psychosocial hypothesis
A systematic review found that psychosocial factors and actively integrated lifestyle over the lifespan may reduce the risk of AD and dementia.132 These factors include early-life high educational attainment, adult-life high work complexity, late-life rich social network and high levels of social engagement, and more frequently participating in physically and mentally stimulating activity.
High educational attainments and socioeconomic status
An association of low education with an increased risk of dementia and AD has been reported in numerous cross-sectional and longitudinal studies.133,134 Education and socioeconomic status are highly correlated; when both measures were examined simultaneously, the independent association was detected only with education.135 The reserve hypothesis has been proposed to interpret this association such that education could enhance neural and cognitive reserve that may provide compensatory mechanisms to cope with degenerative pathological changes in the brain, and therefore delay onset of the dementia syndrome.136 Alternatively, educational achievement may be a surrogate or an indicator of intelligent quotient, early life living environments, and occupational toxic exposure experienced over adulthood.133
Social network and social engagement
Evidence from longitudinal observational studies suggests that a poor social network or social disengagement is associated with cognitive decline and dementia.132,137
The risk for dementia and AD was also increased in older people with increasing social isolation and less frequent and unsatisfactory contacts with relatives and friends. Furthermore, a recent study suggested that low neuroticism in combination with high extraversion was the personality trait associated with the lowest dementia risk, and among socially isolated individuals even low neuroticism alone seemed to decrease the risk of dementia.138 Finally, low social engagement in late life and a decline in social engagement from middle age to late life were associated with a doubly increased risk of developing dementia and AD in late life.139,140 Rich social networks and high social engagement imply better social support, leading to better access to resources and material goods. Rich and large social networks also provide affective and intellectual stimulation that could influence cognitive function and different health outcomes through behavioral, psychological, and physiological pathways.132,141
Physical activity
Regular physical exercise was reported to be associated with a delay in onset of dementia and AD among cognitively healthy elderly.142 In the Kungsholmen Project, the component of physical activity presenting in various leisure activities, rather than sports and any specific physical exercise, was related to a decreased dementia risk.143 In addition, low-intensity activity such as walking may reduce the risk of dementia and cognitive decline.144 A strong protective effect of regular physical activity in middle age against the development of dementia and AD in late life was reported, especially for persons with the APOE ε4 allele.145 As it may take years to achieve high levels of physical fitness, brief periods of exercise training may not have substantial benefits on cognitive processes, but could still be detectable in the subsets of cognitive domains that are more sensitive to the agerelated decrements. Physical activity is important not only in promoting general and vascular health, but also in promoting brain plasticity, and it may also affect several gene transcripts and neurotrophic factors that are relevant for the maintenance of cognitive functions.
Mental activity
Various types of mentally demanding activities have been examined in relation to dementia and AD, including knitting, gardening, dancing, playing board games and musical instruments, reading, social and cultural activities, and watching specific television programs, which often showed a protective effect.147,114 Due to the cultural and individual differences in choosing specific activities, some researchers summarize mentally stimulating activities into a composite, score, which showed that a cognitive activity score involving participation in seven common activities with information processing as a central component was associated with a reduced risk of AD, even after controlling for APOE ε4 allele, medical conditions, and depressive symptoms.148,149 The Swedish Twin Studyshowed that greater complexity of work, and particularly complex work with people, may reduce the risk of AD.150 The Canadian Study of Health and Aging found that high complexity of work appeared to be associated with a reduced risk of dementia, but mostly for vascular dementia.151 In supporting of these findings, the recent neuroimaging study suggested that a high level of complex mental activity across the lifespan was correlated with a reduced rate of hippocampal atrophy.152
Other etiologic hypotheses (inflammation, toxic exposure, and other factors)
Inflammation
A higher level of serum C-reactive protein (CRP) in midlife was linked to an increased risk of both Alzheimer type and vascular dementias, suggesting that inflammatory markers may reflect both peripheral disease and cerebral mechanisms related to dementia, and that these processes are measurable for a long time before dementia is manifested.153 Follow-up studies of older adults also showed an association of serum inflammatory markers (eg, CRP and interleukin-6) measured at older ages with an increased incidence of dementia and AD154,155 As additional evidence supporting the inflammatory involvement in AD and dementia, recent follow-up studies and the systematic review of observational studies concludes that long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, >2 years) may have beneficial effect against AD and dementia.156,157 In addition, experimental research found that neuritic plaques in the brain are associated with inflammatory proteins. Therefore, it seems plausible to hypothesize that inflammatory mechanisms may play a role in the processes leading to neurodegeneration. However, neuropathological studies found no evidence for an association between use of NSAIDs and reduced burden of AD pathological changes.158 Furthermore, the results from the clinical trial of anti-inflammatory drugs (celecoxib or naproxen) in AD prevention, which was suspended due to increased cardiovascular risks associated with celecoxib, failed to show any beneficial effect of using these drugs against AD, instead, an increased risk for AD related to drug therapy were observed (see section on Intervention trials toward primary prevention).159-161
Toxic exposures
Manual work as the lifetime principal occupation has been related to AD and dementia in some studies,162 suggesting a possible implication of occupational toxic exposures in the development of the dementia disorders. Occupational exposures to heavy metals such as aluminum and mercury have been suggested as a risk factor for AD, and even high consumption of aluminum from drinking water may be a risk factor for AD.163 However, this has not been confirmed.164 In addition, occupational exposure to extremely-low-frequency electromagnetic fields (ELF-EMF) has been related to an increased risk of dementia and AD in a number of follow-up studies.165,166 The meta-analysis of epidemiological evidence suggests an association between occupational exposure to ELF-EMF and AD.167 The biological plausibility linking ELF-EMF to AD has been previously described.168
Other factors
First, traumatic brain injury has been extensively investigated as a possible, risk factor for AD. The meta-analysis of case-control studies supported an association between a history of previous head injury and the risk of developing AD.169 In contrast, some longitudinal studies found that AD risk was not associated with head trauma or associated with only severe head injury.170,171 Second, an association between hormone replacement therapy and a reduced risk of dementia and AD among postmenopausal women had been frequently reported in numerous observational studies until 2004 when, instead of a protective effect, a significantly increased risk of dementia associated with estrogen therapy was found in the Women's Health Study (see section on intervention trials toward primary prevention). Finally, several studies have reported an association between depression and an elevated risk of later development of dementia and AD, but it remains arguable as to whether depression is a preclinical symptom or a pure risk factor for dementia and AD.172-174
Interventions toward Alzheimer's disease
Current evidence tends to support the notion that dementia onset may be postponed by implementing interventions toward the potential etiologic factors (both risk and protective factors) (ie, primary prevention) and by early detection (ie, secondary prevention), whereas appropriate care and pharmacotherapy for patients with AD and dementia (ie, tertiary prevention) may help stabilize cognitive functions, reduce agitation, control neuropsychiatrie symptoms, and improve the quality of life.
Interventions toward primary prevention
Primary intervention strategies
Theoretically, even if the mechanisms of vascular and psychosocial factors being involved in the pathogenesis and clinical expression of AD are still not fully understood, primary prevention seems possible as most vascular factors and disorders, psychosocial factors, and lifestyle factors are modifiable or amenable to management.38,175 One intervention strategy is to target vascular pathways, including management of midlife, high blood pressure and obesity, high blood glucose level, and diabetes. In addition, preventing recurrent cerebrovascular disease and maintaining sufficient cerebral blood perfusion by adequately managing heart failure and avoiding very low blood pressure may help postpone clinical expression of the dementia syndrome, especially among very old people. The second strategy is to maintain the more active and socially integrated lifestyles by establishing extensive social networks and frequently participating in social, physical, and intellectually stimulating activities, which may reduce the risk or delay the onset of AD.38,132 Taken together, the most effective strategy may be to encourage, people implementing multiple preventive measures throughout the life course, including high educational attainment in childhood and early adulthood, active control of vascular factors and disorders over adulthood, and maintenance of mentally, physically, and socially active lifestyles during middle age and later in life.
Intervention trials toward primary prevention
The main clinical and intervention trials toward primary prevention by targeting the possible risk and protective factors for AD and dementia are summarized in Table II. 160,161,176-182 Antihypertensive treatments in reducing the risk of dementia and AD have been tested in a few clinical trials. The pooling analysis of the 2007 Cochrane review, based essentially on three clinical trials (SHEP,183 Syst-Eur,184 and SCOPE185), found no convincing evidence that blood pressure-lowering therapy among elderly individuals with hypertension could prevent dementia. However, in this review the SCOPE trial, which did not show any effect of blood pressure-lowering treatment by candesartan on the risk of dementia, was actually not a placebo-controlled trial due to ethical considerations. By contrast, the placebo-controlled PROGRESS trial among individuals with cerebrovascular disease, (transient ischemic attacks and stroke), which did find a beneficial effect of antihypertensive therapy on cognitive function related to recurrent stroke, was not included.186 The cognition substudy of the double-blind placebo-controlled Hypertension in the Very Elderly Trial (HYVET-COG) among people 80+ years found a nonsignificant reduction in the risk of dementia related to antihypertensive treatment. Encouragingly, when data from this clinical trial were pooled together with those from three other doubleblind placebo-controlled trials (SHEP, Syst-Eur, and PROGRESS), antihypertensive treatment could reduce the risk of dementia by 13 % (hazard ratio, 0.87; 95 % CI, 0.76-1.00; P=0.045).177 It is worthwhile to notice that dementia or AD was the secondary end point in all major clinical trials of antihypertensive therapy, with a relatively short period of follow-up because the trials had to be terminated when benefits of the treatments were clearly shown in terms of primary end points (usually cardiac and cerebrovascular disease). In this context, if the beneficial effect of lowering blood pressure on dementia is not as strong as for cardiovascular disease, a longer period of observation is needed to demonstrate, any significant beneficial effect.
Table II. Major clinical trials of primary prevention against dementia and Alzheimer's disease. AD, Alzheimer's disease; ADAPT, Alzheimer's Disease Antiinflammatory Prevention Trial; CI, confidence inten/al; HR, hazard ratio; HYVET-COG, Hypertension in the Very Elderly Trial-Cognitive Function Assessment; MCI, mild cognitive impairment; SCOPE, Study on Cognition and Prognosis in the Elderly; PROGRESS, Perindopril Protection Against Recurrent Stroke Study; SHEP, Systolic Hypertension in the Elderly Program; Syst-Eur, Systolic Hypertension in Europe.
| Authors, years | Participants | Interventions | Outcomes | Main findings |
| McGuinnes et al, 176 2008 | n=15295, age ≥ 60 years, from SHEP, Syst-Eur, and SCOPE Trials | Blood pressure-lowering therapy | Dementia, AD | Dementia: HR 0.89 (95 % CI 0.69 - 1.16) |
| AD: HR 0.85 (95 % CI 0.63 - 1.15) | ||||
| Peters et al, 177 2008 | HYVET-COG: n=33365, age ≥ 80 years | Blood pressure-lowering therapy | Dementia | HYVET-COG: HR 0.86 (95 % CI 0.67 - 1.00) P=0.045 |
| Meta-analysis (SHEP, Syst-Eur, PROGRESS, and HYVET-COG): n=16595, age ≥ 60 years | Meat-analysis: HR 0.87 (95 % CI 0.76 - 1.00), P=0.045 | |||
| Shumaker et al, 178 2004; Espeland et al, 179 2004 | n=7479, age ≥ 65 years, community-dwelling women | Estrogen alone or estrogen plus progestin | Dementia and MCI, cognitive function | Estrogen: dementia: HR 1.76 (95 % CI 1.19 - 2.60); |
| dementia or MCI: 1.38 (1.01 - 1.89) | ||||
| Estrogen plus progestin: no effect on cognition | ||||
| Heart Protection Study Collaborative Group, 180,181 2002 | n=20536, age 40 - 80 years, had vascular disease, and total cholesterol level ≥ 3.5 mmol/L |
|
Dementia and cognitive impairment | Incidence rates: Treatment vs placebo groups: |
| For dementia: 0.3% vs 0.3 % | ||||
| For cognitive impairment: 23.7 % vs 24.2 % | ||||
| ADAPT Research Groups,160,161 2007, 2008 | n=2528, age ≥ 70 years, had a family history of AD | Celecoxib or naproxen | AD, cognitive function | AD celecoxib HR 2.0 (95 % CI 0.8 - 5.0, P=0.14); naproxen HR 2.4 (95 % CI 0.95 - 5.8, P=0.06) |
| Cognition cognitive function was not improved | ||||
| DeKosky et al,182 2008 | n=3069, age ≥ 75 years, with normal cognition or MCI | Extract of Ginkgo biloba | Dementia, AD | Dementia: HR 1.12 (95 % CI 0.94 - 1.33) |
| AD: HR 1.16 (95 % CI 0.97 - 1.39) |
Estrogen therapy among postmenopausal women has been linked to a considerably lower risk of AD and dementia in numerous observational studies, but the large-scale, clinical trial of the Women's Health Initiative Memory Study (WHI-MS) showed that estrogen therapy alone or in combination with progestin did not reduce incidence of probable dementia and mild cognitive impairment (MCI); instead, the active treatments with estrogen and estrogen plus progestin were found to be associated with a twofold increased risk for both dementia and MCI.178,179 It has been argued that in the WHI-MS hormone replacement therapy was given 10 to 15 years after the menopause when the “window of critical time” for putative beneficial effects of estrogen therapy mayhave been missed.187
Other major clinical trials of primary prevention against dementia, such as those with NSAIDs and vitamin E supplementation, have so far failed to confirm any efficacy against dementia and AD (Table II), while more clinical trials (eg, use of cholesterol-lowering drugs and blood glucose lowering drugs) are ongoing.188,189 The lack of success in most primary prevention trials underscores the need of new strategies in future intervention studies.
New strategies for primary prevention: a life-course approach and multidomain interventions
The life-course approach considers biological, environmental, and psychosocial factors acting during early childhood, middle age, and late life as relevant for the development of dementia and AD. This approach seeks to identify the “time windows” when exposures exert the greatest effect on the development of disease and to determine whether concurrent exposures could have interactive effects over the lifespan.190 In this context, the risk of late-life dementia and AD is likely to be determined by accumulative effects or complex interactions of genetic, biological, psychosocial, and environmental exposures experienced over the life course. For instance, the brain structural and functional reserve related to education and other psychosocial factors can be conceived as the sum of their lifetime input of education and related activities.191 In addition, the life-course approach model introduces the concept of “time windows” at exposure, which might be highly relevant for chronic disorders with a long time latent period such as AD.38 A certain factor might increase the risk of the disease if a subject is exposed during a specific time period, whereas the same factor may show a different effect on the disease in another life period, due to, for example, differential interactions with other factors or selective survival. For instance, high blood pressure and high BMI in midlife are risk factors for late-life dementia, whereas low blood pressure and low BMI among older people are associated with an increased risk of dementia and AD.61,65,74
Furthermore, intervention studies integrating several different domains of intervention have not yet been implemented so far. The disappointing results of previous intervention trials focusing on a single intervention agent or component in older adults or in already cognitively impaired individuals point out that a few key issues need to be taken into account in future trials: (i) time window of interventions - interventions starting earlier in life may be more effective; (if) target group - a healthy, relative young population will require relatively long follow-up periods, large sample sizes, and considerable financial resources; and (Hi) outcome measures - cognitive impairment may be better than “conversion” to clinical dementia. Several multidomain intervention trials are being planned or ongoing such as the Dose-Response to Exercise Training (DR's EXTRA), the Cognitive Substudy of the Finnish Diabetes Prevention Study, and the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). In the FINGER study, individuals at an increased risk for developing dementia being identified according to the CAIDE Dementia Risk Score are targeted for intervention.192 The 2-year multidomain interventions include four main components: (i) nutritional guidance; (ii) physical activity; (iii) cognitive training and social activity; and (iv) intensive monitoring and management of metabolic and vascular risk factors. The FINGER study will be the first carefully-designed randomized intervention trial to clarify to what extent a multidomain intervention will delay the onset of cognitive impairment and dementia among persons with an increased risk of the disease. These data are urgently needed for health education and for planning community health service.
Secondary prevention
Alzheimer's disease is characterized by a preclinical phase, possibly lasting years, during which progressive neurodegeneration in the brain is occurring before typical clinical symptoms (eg, cognitive deficits and subtle cognitive disturbances) become detectable.193 Theoretically, detection of AD at early stage may provide an opportunity for implementing therapeutic intervention to more effectively delay its progression to clinical dementia. However, there remains a challenge as to how to identify individuals during the preclinical phase of the disease, although some clinical markers, neuroimaging biomarkers, and biochemical markers have been investigated.194 First, numerous studies have suggested that deficits in specific cognitive domains such as episodic memory and verbal ability are conceivable up to 10 years before the dementia syndrome can be clinically diagnosed, with a more evident decline occurring over the final few years. The term “mild cognitive impairment” has been used in clinical settings to identify individuals with isolated memory loss (ie, “amnestic” type MCI), which is more likely to represent the preclinical phase of Alzheimer dementia. However, population-based followup studies have frequently shown that individuals with MCI represent a very heterogeneous group in terms of prognosis195,196; although elderly persons with MCI had increased risk of progressing to dementia, a substantial proportion remained stable or even reverted to normal during the next few years. Second, biochemical markers in serum and cerebrospinal fluid such as (β-amyloid and τ-protein have been proposed for early detection of AD, but these markers are not sufficiently reliable in making diagnosis of AD in the presymptomatic phase.39,194 Finally, during the last decade neuroimaging has emerged as a useful tool to define AD at both preclinical and earlyclinical phases of the disease. For example, the amyloid positron emission tomography imaging tracer ligands offer opportunity for measuring β-amyloid in the brain in vivo, which provides the possibility for early diagnosis and for monitoring the course of antiamyloid therapy in AD.197,198 Furthermore, the medial-temporal lobe atrophy seen on volumetric MRI has been used in the identification of MCI and early AD as well as in the assessment of progression of MCI and early AD.199,200 Successful secondary prevention relies on both reliable detection of the disease at an early stage and availability of efficacious interventions for slowing down progression of the disease. However, while efforts are being made to find ways to effectively counteract the course of AD, some methodological issues facing research on disease-modifying therapies and interventions remain a challenge.201,202
Tertiary prevention
The tertiary prevention aims to avoid functional disability, and if possible, to improve quality of life for patients with AD. Cognitive training may help maintain cognitive function, slow down cognitive decline, and improve wellbeing for people with mild dementia. Current medications widely used for AD and dementia, including cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and the N-methyl-D-aspartate-receptor antagonist (memantine), are designed to target clinical symptoms of the disease such as cognitive and neuropsychiatrie disturbances.201 The efficacy of antioxidant treatments in AD has not been proven. For example, the Cochrane review found no evidence of efficacy of vitamin E in the treatment of AD and in the prevention of progression of MCI to AD,203 although the randomized clinical trials of the Physicians' Health Study II suggested that a long-term (β-carotene supplementation might provide cognitive benefits among men.204
Furthermore, high-dose B vitamin supplements did not slow down cognitive decline among individuals with mild to moderate AD.205 Intensive research is ongoing in an attempt to develop disease-modifying drugs by targeting the key neuropathological processes in AD such as β-amyloid protein.206
Summary
Alzheimer's disease represents an increasing challenge to public health and the health care system, and has had tremendous impact at both the individual and the societal levels. Epidemiologic research has provided sufficient evidence that vascular risk factors in middle-aged and older adults play a significant role in the development and progression of dementia and AD, whereas extensive social network and active engagement in mental, social, and physical activities may postpone the onset of the dementing disorder. Multidomain community intervention trials are warranted to determine to what extent preventive strategies toward optimal control of multiple vascular factors and disorders, as well as the maintenance of an active lifestyle, are effective against dementia and AD.
Acknowledgments
This work was supported in part by grants from the Swedish Research Council in Medicine, the Swedish Council for Working Life and Social Research (FAS), the Future Leader of Aging Research in Europe (FLARE)-FAS Program (CQ), the Alzheimer Foundation Sweden, and the Gamla Tjànarinnor Foundation.
Selected abbreviations and acronyms
- AD
Alzheimer's disease
- APOE
apolipotrotein E
- BMI
body mass index
- ELF-EMF
extremely-low-frequency electromagnetic fields
- HYVET-COG
Hypertension in the Very Elderly Trial-Cognitive Function Assessment
- MCI
mild cognitive impairment
- PROGRESS
Perindopril Protection Against Recurrent Stroke Study
- SCOPE
Study on Cognition and Prognosis in the Elderly
- SHEP
Systolic Hypertension in the Elderly Program
- Syst-Eur
Systolic Hypertension in Europe Trial
- WHI-MS
Women's Health Initiative-Memory Study
Contributor Information
Chengxuan Qiu, Aging Research Center, Karolinska Institutet-Stockholm University and Stockholm Gerontology Research Center, Stockholm, Sweden.
Miia Kivipelto, Aging Research Center, Karolinska Institutet-Stockholm University and Stockholm Gerontology Research Center, Stockholm, Sweden.
Eva von Strauss, Aging Research Center, Karolinska Institutet-Stockholm University and Stockholm Gerontology Research Center, Stockholm, Sweden.
REFERENCES
- 1.United Nations Organization. World population ageing: 1950-2050. New York: U. N. P. o. Ageing, United Nations; 2001. www.un.org/esa/population/publications/worldageing19502050/. Accessed March 22, 2009 [Google Scholar]
- 2.The Centers for Disease Control and Prevention. Public health and aging: trends in aging - United States and worldwide. JAMA. 2003;289:1371–1373. [PubMed] [Google Scholar]
- 3.Lobo A., Launer LJ., Fratiglioni L., et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology. 2000;54:S4–S9. [PubMed] [Google Scholar]
- 4.Plassman BL., Langa KM., Fisher GG., et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferri CP., Prince M., Brayne C., et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wimo A., Winblad B., Agüero-Torres H., von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord. 2003;17:63–67. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 8.Dong MJ., Peng B., Lin XT., Zhao J., Zhou YR., Wang RH. The prevalence of dementia in the People's Republic of China: a systematic analysis of 1980-2004 studies. Age Ageing. 2007;36:619–624. doi: 10.1093/ageing/afm128. [DOI] [PubMed] [Google Scholar]
- 9.Scazufca M., Menezes PR., Vallada HP., et al. High prevalence of dementia among older adults from poor socioeconomic backgrounds in São Paulo, Brazil. Int Psychogeriatr. 2008;20:394–405. doi: 10.1017/S1041610207005625. [DOI] [PubMed] [Google Scholar]
- 10.von Strauss E., Viitanen M., De Ronchi D., Winblad B., Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort. with a large sample of nonagenarians. Arch Neurol. 1999;56:587–592. doi: 10.1001/archneur.56.5.587. [DOI] [PubMed] [Google Scholar]
- 11.Corrada MM., Brookmeyer R., Berlau D., Paganini-Hill A., Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 12.Kalaria RN., Maestre GE., Arizaga R., et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llibre Rodriguez JJ., Ferri CP., Acosta D., et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZX., Zahner GE., Roman GC., et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol. 2005;62:447–453. doi: 10.1001/archneur.62.3.447. [DOI] [PubMed] [Google Scholar]
- 15.Llibre Rodríguez J., Valhuerdi A., Sanchez II., et al. The prevalence, correlates and impact of dementia in Cuba: a 10/66 group population-based survey. Neuroepidemiology. 2008;31:243–251. doi: 10.1159/000165362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fratiglioni L., Launer LJ., Andersen K., et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurology. 2000;54:S10–S15. [PubMed] [Google Scholar]
- 17.Kawas C., Gray S., Brookmeyer R., Fozard J., Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 18.Kukull WA., Higdon R., Bowen JD., et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 19.Jorm AF., Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 20.The Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology. 2000;55:66–73. [PubMed] [Google Scholar]
- 21.Miech RA., Breitner JC., Zandi PP., Khachaturian AS., Anthony JC., Mayer L. Incidence of AD may decline in the early 90s for men, later for women: the Cache County study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 22.Matthews F., Brayne C. The incidence of dementia in England and Wales: findings from the five identical sites of the MRC CFA Study. PLoS Med. 2005;2:e193. doi: 10.1371/journal.pmed.0020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravaglia G., Forti P., Maioli F., et al. Incidence and etiology of dementia in a large elderly Italian population. Neurology. 2005;64:1525–1530. doi: 10.1212/01.WNL.0000160107.02316.BF. [DOI] [PubMed] [Google Scholar]
- 24.Chandra V., Pandav R., Dodge HH., et al. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 25.Mathers C., Leonardi M. Global burden of dementia in the year 2000. World Health Organization (WHO), 2003. www.who.int/entity/healthinfo/statistics/bod_dementia.pdf. Accessed March 22, 2009. [Google Scholar]
- 26.Agüero-Torres H., Fratiglioni L., Guo Z., Viitanen M., von Strauss E., Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88:1452–1456. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzman R., Hill LR., Yu ES., et al. The malignancy of dementia: predictors of mortality in clinically diagnosed dementia in a population survey of Shanghai, China. Arch Neurol. 1994;51:1220–1225. doi: 10.1001/archneur.1994.00540240064017. [DOI] [PubMed] [Google Scholar]
- 28.Helzner EP., Scarmeas N., Cosentino S., Tang MX., Schupf N., Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta KM., Yaffe K., Pérez-Stable EJ., et al. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology. 2008;70:1163–1170. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganguli M., Dodge HH., Shen C., Pandav RS., DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62:779–784. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 31.Larson EB., Shadlen MF., Wang L., et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 32.Helmer C., Joly P., Letenneur L., Commenges D., Dartigues JF. Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol. 2001;154:642–648. doi: 10.1093/aje/154.7.642. [DOI] [PubMed] [Google Scholar]
- 33.Hebert LE., Scherr PA., Bienias JL., Bennett DA., Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 34.Alzheimer's Association. 2009 Alzheimer's Disease Facts and Figures. (www.alz.org/national/documents/report_alzfactsfigures2009.pdf. Accessed April 6, 2009. [Google Scholar]
- 35.Wimo A., Winblad B., Jönsson L. An estimate of total worldwide societal costs of dementia in 2005. Alzheimers Dement. 2007;3:81–91. doi: 10.1016/j.jalz.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Jönsson B. The economic impact of diabetes. Diabetes Care. 1998;21 Suppl3:C7–C10. doi: 10.2337/diacare.21.3.c7. [DOI] [PubMed] [Google Scholar]
- 37.Brookmeyer R., Gray S., Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fratiglioni L., von Strauss E., Qiu CX. Epidemiology of the dementias of old age. In Dening T, Jacoby R, Oppenheimer C, Thomas A, eds. The Oxford Textbook of Old Age Psychiatry. 4th ed. New York, NY: Oxford. University Press;2008:391–406. [Google Scholar]
- 39.Blennow K., de Leon MJ., Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 40.Green RC., Cupples LA., Go R., et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 41.Huang W., Qiu C., von Strauss E., Winblad B., Fratiglioni L. APOE genotype, family history of dementia, and Alzheimer disease risk: a 6-year follow-up study. Arch Neurol. 2004;61:1930–1934. doi: 10.1001/archneur.61.12.1930. [DOI] [PubMed] [Google Scholar]
- 42.Qiu C., Kivipelto M., Aguero-Torres H., Winblad B., Fratiglioni L. Risk and protective effects of the APOE gene towards Alzheimer's disease in the Kungsholmen project: variation by age and sex. J Neurol Neurosurg Psychiatry. 2004;75:828–833. doi: 10.1136/jnnp.2003.021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slooter AJ., Cruts M., Kalmijn S., et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol. 1998;55:964–968. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 44.Farrer LA., Sherbatich T., Keryanov SA., et al. Association between angiotensin-converting enzyme and Alzheimer disease. Arch Neurol. 2000;57:210–214. doi: 10.1001/archneur.57.2.210. [DOI] [PubMed] [Google Scholar]
- 45.Bertram L., McQueen MB., Mullin K., Blacker D., Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 46.Fratiglioni L., Wang HX. Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies. Behav Brain Res. 2000;113:117–120. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 47.Wang HX., Fratiglioni L., Frisoni GB., Viitanen M., Winblad B. Smoking and the occurrence of Alzheimer's disease: cross-sectional and longitudinal data in a population-based study. Am J Epidemiol. 1999;149:640–644. doi: 10.1093/oxfordjournals.aje.a009864. [DOI] [PubMed] [Google Scholar]
- 48.Hill G., Connelly J., Hebert R., Lindsay J., Millar W. Neyman's bias re-visited. J Clin Epidemiol. 2003;56:293–296. doi: 10.1016/s0895-4356(02)00571-1. [DOI] [PubMed] [Google Scholar]
- 49.Tyas SL., White LR., Petrovitch H., et al. Mid-life smoking and late-life dementia: the Honolulu- Asia Aging Study. Neurobiol Aging. 2003;24:589–596. doi: 10.1016/s0197-4580(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 50.Ott A., Slooter AJ., Hofman A., et al. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 51.Merchant C., Tang MX., Albert S., Manly J., Stern Y., Mayeux R. The influence of smoking on the risk of Alzheimer's disease. Neurology. 1999;52:1408–1412. doi: 10.1212/wnl.52.7.1408. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal NT., Bienias JL., Bennett DA., et al. The relation of cigarette smoking to incident Alzheimer's disease in a biracial urban community population. Neuroepidemiology. 2006;26:140–146. doi: 10.1159/000091654. [DOI] [PubMed] [Google Scholar]
- 53.Anstey KJ., von Sanden C., Salim A., O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 54.Peters R., Poulter R., Warner J., Beckett N., Burch L., Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anttila T., Helkala EL., Viitanen M., et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang W., Qiu C., Winblad B., Fratiglioni L. Alcohol consumption and incidence of dementia in a community sample aged 75 years and older. J Clin Epidemiol. 2002;55:959–964. doi: 10.1016/s0895-4356(02)00462-6. [DOI] [PubMed] [Google Scholar]
- 57.Ruitenberg A., van Swieten JC., Witteman JC., et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 58.Mukamal KJ., Longstreth WT. Jr., Mittleman MA., Crum RM., Siscovick DS. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke. 2001;32:1939–1946. doi: 10.1161/hs0901.095723. [DOI] [PubMed] [Google Scholar]
- 59.Ding J., Eigenbrodt ML., Mosley TH. Jr., et al. Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2004;35:16–21. doi: 10.1161/01.STR.0000105929.88691.8E. [DOI] [PubMed] [Google Scholar]
- 60.Paul CA., Au R., Fredman L., et al. Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. 2008;65:1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5:713–720. doi: 10.1016/S1474-4422(06)70526-9. [DOI] [PubMed] [Google Scholar]
- 62.Kivipelto M., Ngandu T., Fratiglioni L., et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 63.Rosengren A., Skoog I., Gustafson D., Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 64.Whitmer RA., Gunderson EP., Barrett-Connor E., Quesenberry CP. Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitzpatrick AL., Kuller LH., Lopez OL., et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart R., Masaki K., Xue QL., et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 67.Buchman AS., Wilson RS., Bienias JL., Shah RC., Evans DA., Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 68.Johnson DK., Wilkins CH., Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 69.Dahl AK., Löppönen M., Isoaho R., Berg S., Kivelä SL. Overweight and obesity in old age are not associated with greater dementia risk. J Am Geriatr Soc. 2008;56:2261–2266. doi: 10.1111/j.1532-5415.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 70.Nourhashemi F., Deschamps V., Larrieu S., Letenneur L., Dartigues JF., Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 71.Atti AR., Palmer K., Volpato S., Winblad B., De Ronchi D., Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008;56:111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 72.Launer LJ., Ross GW., Petrovitch H., et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 73.Kivipelto M., Helkala EL., Laakso MP., et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 75.Skoog I., Lernfelt B., Landahl S., et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 76.Petitti DB., Crooks VC., Buckwalter JG., Chiu V. Blood pressure levels before dementia. Arch Neurol. 2005;62:112–116. doi: 10.1001/archneur.62.1.112. [DOI] [PubMed] [Google Scholar]
- 77.Morris MC., Scherr PA., Hebert LE., Glynn RJ., Bennett DA., Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. 2001;58:1640–1646. doi: 10.1001/archneur.58.10.1640. [DOI] [PubMed] [Google Scholar]
- 78.Qiu C., von Strauss E., Fastbom J., Winblad B., Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60:223–228. doi: 10.1001/archneur.60.2.223. [DOI] [PubMed] [Google Scholar]
- 79.Verghese J., Lipton RB., Hall CB., Kuslansky G., Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:1667–1672. doi: 10.1212/01.wnl.0000098934.18300.be. [DOI] [PubMed] [Google Scholar]
- 80.Ruitenberg A., den Heijer T., Bakker SL., et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 81.Yasar S., Corrada M., Brookmeyer R., Kawas C. Calcium channel blockers and risk of AD: the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 2005;26:157–163. doi: 10.1016/j.neurobiolaging.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Khachaturian AS., Zandi PP., Lyketsos CG., et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- 83.Peila R., White LR., Masaki K., Petrovitch H., Launer LJ. Reducing the risk of dementia: efficacy of long-term treatment of hypertension. Stroke. 2006;37:1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- 84.Haag MD., Hofman A., Koudstaal PJ., Breteler MM., Strieker BH. Duration of antihypertensive drug use and risk of dementia. A prospective cohort study. . Neurology. 2009;72:1727–1734. doi: 10.1212/01.wnl.0000345062.86148.3f. [DOI] [PubMed] [Google Scholar]
- 85.Hoffman LB., Schmeidler J., Lesser GT., et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72:1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kivipelto M., Helkala EL., Laakso MP., et al. Apolipoprotein E Â4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 87.Whitmer RA., Sidney S., Selby J., Johnston SC., Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 88.Tan ZS., Seshadri S., Beiser A., et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 89.Reitz C., Tang MX., Luchsinger J., Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mielke MM., Zandi PP., Sjogren M., et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 91.Solomon A., Kåreholt I., Ngandu T., et al. Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology. 2007;68:751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 92.Stewart R., White LR., Xue QL., Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2007;64:103–107. doi: 10.1001/archneur.64.1.103. [DOI] [PubMed] [Google Scholar]
- 93.Björkhem I., Heverin M., Leoni V., Meaney S., Diczfalusy U. Oxysterols and Alzheimer's disease. Acta Neurol Scand Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 94.Jick H., Zornberg GL., Jick SS., Seshadri S., Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 95.Rockwood K., Kirkland S., Hogan DB., et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 96.Haag MD., Hofman A., Koudstaal PJ., Strieker BH., Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity: the Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 97.Rea TD., Breitner JC., Psaty BM., et aI. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 98.Zandi PP., Sparks DL., Khachaturian AS., et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 99.Li G., Larson EB., Sonnen JA., et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 100.Arvanitakis Z., Schneider JA., Wilson RS., et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70 (19 Pt 2):1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 101.Zandi PP., Anthony JC., Khachaturian AS., et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 102.Barberger-Gateau P., Raffaitin C., Letenneur L., et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 103.Gray SL., Anderson ML., Crane PK., et al. Antioxidant vitamin supplement use and risk of dementia or Alzheimer's disease in older adults. J Am Geriatr Soc. 2008;56:291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 104.Scarmeas N., Stern Y., Mayeux R., Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scarmeas N., Stern Y., Tang MX., Mayeux R., Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luchsinger JA., Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 107.Malouf M., Grimley EJ., Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev. 2003;CD004514 doi: 10.1002/14651858.CD004514. [DOI] [PubMed] [Google Scholar]
- 108.Laitinen MH., Ngandu T., Rovio S., et al. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dement Geriatr Cogn Disord. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 109.Huang TL., Zandi PP., Tucker KL., et al. Benefits of fatty fish on dementia risk are stronger for those without APOE e4. Neurology. 2005;65:1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 110.Schaefer EJ., Bongard V., Beiser AS., et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 111.Arvanitakis Z., Wilson RS., Bienias JL., Evans DA., Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 112.Akomolafe A., Beiser A., Meigs JB., et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 113.Irie F., Fitzpatrick AL., Lopez OL., et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE e4: the Cardiovascular Health Study Cognition Study. . Arch Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Biessels GJ., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 115.Roberts RO., Geda YE., Knopman DS., et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–1073. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu W., Qiu C., Gatz M., Pedersen NL., Johansson B., Fratiglioni L. Midand late-life diabetes in relation to the risk of dementia: a populationbased twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu WL., Qiu CX., Winblad B., Fratiglioni L. The effect of borderline diabetes mellitus on the risk of dementia and Alzheimer disease. Diabetes. 2007;56:211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 118.Korf ES., White LR., Scheltens P., Launer LJ. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care. 2006;29:2268–2274. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- 119.Rönnemaa E., Zethelius B., Sundelöf J., et al Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71:1065–1071. doi: 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- 120.Xu WL., von Strauss E., Qiu CX., Winblad B., Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer's disease: a population-based cohort study. Diabetologia. 2009;52:1031–1039. doi: 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- 121.Vanhanen M., Koivisto K., Moilanen L., et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67:843–847. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 122.Muller M., Tang MX., Schupf N., Manly JJ., Mayeux R., Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24:185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vermeer SE., Prins ND., den Heijer T., Hofman A., Koudstaal PJ., Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 124.Honig LS., Tang MX., Albert S., et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 125.Newman AB., Fitzpatrick AL., Lopez O., et al. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 126.Beeri MS., Rapp M., Silverman JM., et al. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology. 2006;66:1399–1404. doi: 10.1212/01.wnl.0000210447.19748.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiu C., Winblad B., Marengoni A., Klarin I., Fastbom J., Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a populationbased cohort study. Arch intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 128.Laurin D., Masaki KH., White LR., Launer LJ. Ankle-to-brachial index and dementia: the Honolulu-Asia Aging Study. Circulation. 2007;116:2269–2274. doi: 10.1161/CIRCULATIONAHA.106.686477. [DOI] [PubMed] [Google Scholar]
- 129.van Oijen M., de Jong FJ., Witteman JC., Hofman A., Koudstaal PJ., Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 130.Snowdon DA., Greiner LH., Mortimer JA., Riley KP., Greiner PA., Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 131.Esiri MM., Nagy Z., Smith MZ., Barnetson L., Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 132.Fratiglioni L., Paillard-Borg S., Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 133.Qiu C., Bäckman L., Winblad B., Agüero-Torres H., Fratiglioni L. The influence of education on clinically diagnosed dementia: incidence and mortality data from the Kungsholmen Project. Arch Neurol. 2001;58:2034–2039. doi: 10.1001/archneur.58.12.2034. [DOI] [PubMed] [Google Scholar]
- 134.Ngandu T., von Strauss E., Helkala EL., et al. Education and dementia: what lies behind the association? Neurology. 2007;69:1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- 135.Karp A., Kåreholt I., Qiu C., Bellander T., Winblad B., Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer's disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- 136.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 137.Fratiglioni L., Wang HX., Ericsson K., Maytan M., Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 138.Wang HX., Karp A., Herlitz A., Crowe M., Kåreholt I., Winblad B., Fratiglioni L. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72:253–259. doi: 10.1212/01.wnl.0000339485.39246.87. [DOI] [PubMed] [Google Scholar]
- 139.Wang HX., Karp A., Winblad B., Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 140.Saczynski JS., Pfeifer LA., Masaki K., et al. The effect of social engagement on incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol. 2006;163:433–440. doi: 10.1093/aje/kwj061. [DOI] [PubMed] [Google Scholar]
- 141.Bennett DA., Schneider JA., Tang Y., Arnold SE., Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 142.Larson EB., Wang L., Bowen JD., et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 143.Karp A., Paillard-Borg S., Wang HX., Silverstein M., Winblad B., Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 144.Abbott RD., White LR., Ross GW., Masaki KH., Curb JD., Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 145.Rovio S., Kåreholt I., Helkala EL., et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 146.Verghese J., Lipton RB., Katz MJ., et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 147.Crowe M., Andel R., Pedersen NL., Johansson B., Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer's disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2003;58:P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- 148.Wilson RS., Bennett DA., Bienias JL., Aggarwal NT., et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 149.Wilson RS., Mendes De Leon CF., Barnes LL., et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 150.Andel R., Crowe M., Pedersen NL., et al. Complexity of work and risk of Alzheimer's disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2005;60:P251–P258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- 151.Kroger E., Andel R., Lindsay J., Benounissa Z., Verreault R., Laurin D. Is complexity of work associated with risk of dementia? The Canadian Study of Health and Aging. Am J Epidemiol. 2008;167:820–30. doi: 10.1093/aje/kwm382. [DOI] [PubMed] [Google Scholar]
- 152.Valenzuela MJ., Sachdev P., Wen W., Chen X., Brodaty H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS ONE. 2008;3:e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmidt R., Schmidt H., Curb JD., Masaki K., White LR., Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 154.Engelhart MJ., Geerlings Ml., Meijer J., et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 155.Tan ZS., Beiser AS., Vasan RS., et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 156.Etminan M., Gill S., Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szekely CA., Breitner JC., Fitzpatrick AL., et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arvanitakis Z., Grodstein F., Bienias JL., et al., Bennett DA. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008;70:2219–2225. doi: 10.1212/01.wnl.0000313813.48505.86. [DOI] [PubMed] [Google Scholar]
- 159.ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin Trials. 2006;1:e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lyketsos CG., Breitner JC., Green RC., et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 161.Martin BK., Szekely C., Brandt J., et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qiu C., Karp A., von Strauss E., Winblad B., Fratiglioni L., Bellander T. Lifetime principal occupation and risk of Alzheimer's disease in the Kungsholmen project. Am J Ind Med. 2003;43:204–211. doi: 10.1002/ajim.10159. [DOI] [PubMed] [Google Scholar]
- 163.Rondeau V., Jacqmin-Gadda H., Commenges D., Helmer C., Dartigues JF. Aluminum and silica in drinking water and the risk of Alzheimer's disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am J Epidemiol. 2009;169:489–496. doi: 10.1093/aje/kwn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Perl DP., Moalem S. Aluminum and Alzheimer's disease, a personal perspective after 25 years. J Alzheimers Dis. 2006;9 (3 suppl):291–300. doi: 10.3233/jad-2006-9s332. [DOI] [PubMed] [Google Scholar]
- 165.Feychting M., Jonsson F., Pedersen NL., Ahlbom A. Occupational magnetic field exposure and neurodegenerative disease. Epidemiology. 2003;14:413–419. doi: 10.1097/01.EDE.0000071409.23291.7b. [DOI] [PubMed] [Google Scholar]
- 166.Qiu C., Fratiglioni L., Karp A., Winblad B., Bellander T. Occupational exposure to electromagnetic fields and risk of Alzheimer's disease. Epidemiology. 2004;15:687–694. doi: 10.1097/01.ede.0000142147.49297.9d. [DOI] [PubMed] [Google Scholar]
- 167.Garcia AM., Sisternas A., Hoyos SP. Occupational exposure to extremely low frequency electric and magnetic fields and Alzheimer disease: a meta-analysis. Int J Epidemiol . 2008;37:329–340. doi: 10.1093/ije/dym295. [DOI] [PubMed] [Google Scholar]
- 168.Sobel E., Dunn M., Davanipour Z., Qian Z., Chui HC. Elevated risk of Alzheimer's disease among workers with likely electromagnetic field exposure. Neurology. 1996;47:1477–1481. doi: 10.1212/wnl.47.6.1477. [DOI] [PubMed] [Google Scholar]
- 169.Fleminger S., Oliver DL., Lovestone S., Rabe-Hesketh S., Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mehta KM., Ott A., Kalmijn S., et al. Head trauma and risk of dementia and Alzheimer's disease: the Rotterdam Study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 171.Himanen L., Portin R., Isoniemi H., Helenius H., Kurki T., Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year followup study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 172.Dal Forno G., Palermo MT., Donohue JE., Karagiozis H., Zonderman AB., Kawas CH. Depressive symptoms, sex, and risk for Alzheimer's disease. Ann Neurol. 2005;57:381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 173.Andersen K., Lolk A., Kragh-Sorensen P., Petersen NE., Green A. Depression and the risk of Alzheimer disease. Epidemiology. 2005;16:233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- 174.Amieva H., Le Goff M., Millet X., et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 175.Qiu CX., De Ronchi D., Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- 176.McGuinness B., Todd S., Passmore AP., Bullock R. Systematic review: Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. J Neurol Neurosurg Psychiatry. 2008;79:4–5. doi: 10.1136/jnnp.2007.118505. [DOI] [PubMed] [Google Scholar]
- 177.Peters R., Beckett N., Forette F., et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 178.Shumaker SA., Legault C., Kuller L., et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 179.Espeland MA., Rapp SR., Shumaker SA., et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 180.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 181.Heart Protection Study Collaborative Group. MROBHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 182.DeKosky ST., Williamson JD., Fitzpatrick AL., et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 184.Forette F., Seux ML., Staessen JA., et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 185.Lithell H., Hansson L., Skoog I., et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized doubleblind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 186.Tzourio C., Anderson C., Chapman N., et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 187.Gibbs RB., Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 188.Jones RW., Kivipelto M., Feldman H., et al. The Atorvastatin/Donepezil in Alzheimer's Disease Study (LEADe): design and baseline characteristics. Alzheimers Dement. 2008;4:145–153. doi: 10.1016/j.jalz.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 189.Cukierman-Yaffe T., Gerstein HC., Williamson JD., et al. Relationship between baseline glycemie control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32:221–226. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Whalley LJ., Dick FD., McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 191.Richards M., Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- 192.Kivipelto M., Ngandu T., Laatikainen T., Winblad B., Soininen H., Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 193.Bäckman L., Small BJ., Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. . Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- 194.DeKosky ST., Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 195.Palmer K., Wang HX., Backman L., Winblad B., Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 196.Ganguli M., Dodge HH., Shen C., DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 197.Nordberg A. Amyloid imaging in Alzheimer's disease. Curr Opin Neurol. 2007;20:398–402. doi: 10.1097/WCO.0b013e3281a47744. [DOI] [PubMed] [Google Scholar]
- 198.Forsberg A., Engler H., Almkvist O., et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 199.Dubois B., Feldman HH., Jacova C., et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 200.Ridha BH., Barnes J., van de Pol LA., et al. Application of automated medial temporal lobe atrophy scale to Alzheimer disease. Arch Neurol. 2007;64:849–854. doi: 10.1001/archneur.64.6.849. [DOI] [PubMed] [Google Scholar]
- 201.Jelic V., Kivipelto M., Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol Neurosurg Psychiatry. 2006;77:429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cummings JL., Doody R., Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology. 2007;69:1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- 203.Isaac MG., Quinn R., Tabet N. Vitamin E for Alzheimer's disease and mild cognitive impairment. Cochrane Database Syst Rev. 2008;3:CD002854. doi: 10.1002/14651858.CD002854.pub2. [DOI] [PubMed] [Google Scholar]
- 204.Grodstein F., Kang JH., Glynn RJ., Cook NR., Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II. Arch intern Med. 2007;167:2184–2190. doi: 10.1001/archinte.167.20.2184. [DOI] [PubMed] [Google Scholar]
- 205.Aisen PS., Schneider LS., Sano M., et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rafii MS., Aisen PS. Recent developments in Alzheimer's disease thera- doi: 10.1186/1741-7015-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]