Abstract
For nearly two decades now, transcranial magnetic stimulation (TMS) has been available as a noninvasive clinical tool to treat patients suffering from major depression. In this period, a bulk of animal and human studies examined TMS parameters to improve clinical outcome. However, the neurobiological mechanisms underlying mood changes remain an important focus of research. In addition to having an effect on neuroendocrinological processes, neurotransmitter systems, and neurotrophic factors, TMS may not only affect the stimulated cortical regions, but also those connected to them. Therefore, we will review current human data on possible neurobiological mechanisms of repetitive (r) TMS implicated in the deregulated neurocircuitry present in unipolar depression. Furthermore, as the rTMS application can be considered as a “top-down” neuronal intervention, we will focus on the neuronal pathways linked with the stimulated area and we will present an integrative model of action.
Keywords: rTMS, depression, unipolar, neurobiological mechanism, treatment, neurocircuitry
Abstract
Desde hace casi dos décadas se dispone de la estimulación magnética transcraneal (EMT) como una herramienta clínica no invasora para tratar a los pacientes con depresión mayor. En este períod o se han examinado numerosos estudios clínicos y en animales acerca de los parámetros de la EMT para mejorar sus resultados clínicos. Sin embargo, los mecanismos neurobiológicos a la base de los cambios del ánimo siguen constituyendo un importante foco de investigación. Además de tener un efecto sobre los procesos neuroendocrinos, los sistemas de neurotransmisión y los factores neurotróficos, la EMT no solo puede afectar las regiones corticales estimuladas, sino también las que están conectadas con ellas. Por lo tanto, se revisarán los datos actuales en humanos acerca de los posibles mecanismos neurobiológicos de la EMT repetitiva que participan en los neurocircuitos disregulados de la depresión unipolar. Además, ya que la aplicación de la EMT repetitiva se puede considerar como una intervención neuronal “desde arriba hacia abajo”, la revisión se centrará en las vías neuronales relacionadas con el área estimulada y se presentará un modelo de acción integrador.
Abstract
Depuis presque 20 ans, la stimulation magnétique transcrânienne (TMS) constitue un outil clinique non invasif pour traiter les patients souffrant de dépression majeure. Pendant ce temps, un grand nombre d'études humaines et animales ont examiné les paramètres de la TMS afin d'en améliorer les résultats cliniques. Cependant, les mécanismes neurobiologiques sous-tendant les modifications de l'humeur restent un important sujet de recherche. Outre l'effet sur les processus neuroendocriniens, sur les systèmes de neurotransmission et les facteurs neurotrophiques, la TMS toucherait non seulement les régions corticales stimulées mais aussi celles qui lui sont connectées. Nous allons donc analyser les données humaines actuelles sur les mécanismes neurobiologiques possibles de la rTMS impliqués dans le dysfonctionnement des circuits neuronaux dans la dépression unipolaire. De plus, la rTMS pouvant être considérée comme une technique neuronale « descendante », nous nous consacrerons aux voies neuronales liées aux aires stimulées et nous présenterons un modèle d'action intégratif.
Unipolar major depression is one of the most common mental diseases worldwide.1,2 Unfortunately, not all patients respond to the available pharmacological treatment algorithms and refractory depression is not uncommon.3 Furthermore, the underlying pathophysiological mechanisms of this affective disorder are still under debate.4 In spite of these neurobiological uncertainties, we are in need of alternative treatment options.5 Repetitive transcranial magnetic stimulation (rTMS; a type of TMS that occurs in a rhythmic and repetitive form) has been put forward as a new technique to treat this debilitating illness.6 Current evidence suggests that rTMS applied to the dorsolateral prefrontal cortex (DLPFC) is a promising treatment strategy for depression, but not all patients show a positive outcome.7,8 Current clinical outcome studies report rather modest superiority compared with placebo (sham).9-11 To date, it remains unclear which TMS parameters, such as stimulation duration and intensity, can produce the most benefits.6,8,9,12 Moreover, there is no consensus of the exact brain localization for individual coil placement.13 To answer these important questions, it would be important to gain more insight in the underlying neurobiological working mechanisms of rTMS. To date, no clear theoretical framework has yet emerged as to why rTMS treatment could result in a “normalization” of mood in depressed patients.14,15
rTMS, the dorsolateral prefrontal cortex, and unipolar depression
The majority of rTMS treatment studies target the DLPFC15,17 The (dorsolateral) prefrontal cortex is implicated in regulating affective states, providing cognitive control over stress and emotion responsiveness.18 A variety of studies has shown that a series of daily sessions of high frequency ( HF)-rTMS delivered to the left DLPFC or low frequency (LF)-rTMS applied to the right DLPFC are effective in reducing symptoms in clinically depressed populations.10,11,15 rTMS can either activate or suppress motor, sensory, or cognitive functions, depending on the brain location and parameters of its delivery: LF-rTMS (<1Hz) is considered to “inhibit” cortical regional activity, while HF-rTMS (>1Hz) “activates” cortical areas.19,20 The stimulation effects not only affect neuronal activities in the stimulated regions primarily but also those connected to them secondarily21,22 The vast majority of rTMS studies in major depression target the left DLPFC with HF stimulation.10
The rationale to use the DLPFC as the rTMS target area originates from brain imaging research, where patients with unipolar depression show prefrontal abnormalities (predominantly on the left).23,24 Decreased neuronal activities in the (dorsolateral) prefrontal regions, as well as in the rostral anterior cingulate cortex (ACC) areas, closely connected to the DLPFC, are often reported.23 These frontal hypoactivities result in apathy, psychomotor slowness, and impaired executive functioning. Besides dysfunctional “frontocingulate networks,” other neuronal pathways between the orbital and medial prefrontal cortex, the amygdala, and hippocampus are implicated in the pathophysiology of mood disorders.24,25
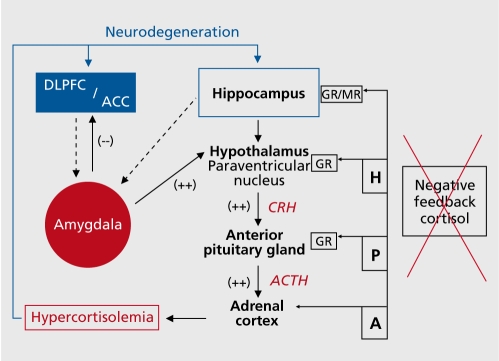
Endocrinological disturbances and hypothalamic-pituitary-adrenal ( HPA) system deregulations are commonly found.26,27 The most consistently described biological abnormality in chronic major depression is a failing negative feedback system resulting in hypercortisolism28 (Figure 1).
Figure 1. Theoretical framework of deregulated cortico-thalamiclimbic pathways in unipolar major depression. In major depression there is a pronounced shift in the homeostasis with diminished activity in the prefrontal cortex (DLPFC and dorsal ACC - blue), enhanced activity in the amygdala (red) and activation of the core stress system. Hyperactivity in limbic areas results in higher neural activities at the hypothalamic level, evoking higher corticotrophin-releasing hormone (CRH) secretions, resulting in elevations of cortisol levels. Hippocampal dysfunction may also result in reduction of the inhibitory regulation of the HPA axis, which could then lead to hypercortisolemia.18 The failing negative feedback system results in chronic hypercortisolemia. In long-term depressive episodes, chronically elevated levels of cortisol contribute to hippocampal and cortical atrophy27 and reducing hippocampal ability to inhibit amygdala hyperactivity.76 Abnormal modulation of cortical-hippocampal-amygdala pathways may contribute to chronically hypersensitive stress responses, mediating features of anxiety, anhedonia, and affective dyscontrol.76 Additionally, the dysfunctional ACC fails to serve its inhibitory role in emotional regulation on the amygdala, resulting in further motivational and affective disruption.77 DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; ACTH, adenocorticotropic hormone; HPA, hypothalamic-pituitaryadrena.
Brain imaging studies and rTMS
Brain imaging results suggest that antidepressant response to rTMS might vary as a function of stimulation frequency29,30 and may depend on pretreatment prefrontal brain metabolism.31,32 For instance, the stimulation of prefrontal regions with lower metabolic activity with HF-rTMS may significantly improve clinical outcome.29 However, opposite results, where higher baseline metabolic activities in the DLPFC bilaterally were associated with better clinical outcome, have been reported as well.32-35 Additionally, higher baseline activities in the ACC are not only predictive for treatment outcome in pharmacological antidepressant trials, electrophysiological imaging studies, and sleep deprivation studies,23,36,37 but high pretreatment ACC activities were also a positive clinical predictor in HF-rTMS treatment protocols.32,38
Concerning the neurobiological effects, rTMS seems to influence metabolic activity of the ACC.39 Whereas right-sided LF-rTMS showed metabolic ACC decreases,40 left-sided HF-rTMS treatment resulted in higher ACC metabolic activity,41,42 especially in those subdivisions of the ACC which are strongly interconnected with DLPFC areas.17,32 However, in some reports successful HF-rTMS treatment did not result in significant ACC metabolic increases.43 Furthermore, Luborzewski et al44 failed to demonstrate neurochemical ACC alterations post HF-rTMS, and Loo et al45 demonstrated that one session of LF-rTMS seemed rather to deactivate the ACC than to activate it. Altogether, the majority of successful rTMS treatment studies correspond with pathophysiological models of major depression that are based on dysfunctions within fronto-cingulate networks,23-25 although the direction of the changes is not consistent over studies.
Neuroendocrinology and rTMS
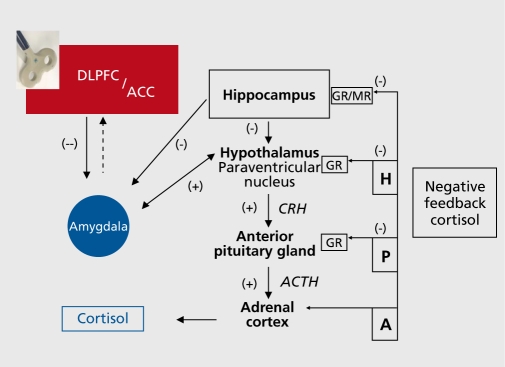
An important aspect of the physiology of rTMS could be related to the endocrinological response of the HPA axis.46,47 Keck48 proposed that rTMS influences occur at the hypothalamic level, suggesting that the (dorsolateral) prefrontal cortex participates in the rTMS-induced blunted response of HPA activity. HF-rTMS would inhibit cortisol-releasing hormone synthesis and release (Figure 2).. Some studies have examined this hypothesis in depressed patients.47 For instance, in a sample of severely depressed patients, salivary cortisol concentrations decreased immediately after one active left DLPFC HF-rTMS session and not after sham rTMS.48 Pridmore50 observed normalization of the dexamethasone suppression test in a small sample of medicated depressed subjects after multiple sessions of HF-rTMS.
Figure2. Visualization of a theoretical working mechanism of HF-rTMS applied to the DLPFC on the HPA-system in unipolar major depression. In the left hand corner a figure-of-eight shaped repetitive transcranial magnetic stimulation (rTMS) coil is depicted. rTMS treatment results in increased neuronal activity in the (dorsolateral) prefrontal cortex, which through cortico-subcortical trans-synaptic connections (presumably through frontocingulate networks)32 suppresses hypothalamic and/or indirectly amygdala overactivity, resulting in CRH decreases and ultimately in decreased salivary cortisol concentrations. In line with successful pharmacological interventions, successful rTMS treatment results in normalization of the negative feedback system.46 The areas in red represent an elevated neuronal activity. The blue areas represent the reverse, a diminished level of neuronal activity. ACTH, adenocorticotropic hormone DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; GR, glucocorticoid receptors; MR, mineral-corticoid receptor; CRH, corticotrophin-releasing hormone; ACTH: adenocorticotrope hormone; H, Hypothalamus; P, Pituitary gland; A, Adrenal cortex; (-), inhibitory; (+) ,excitatory.
In addition, in a sham-controlled left prefrontal HFrTMS trial, Szuba et al51 found acute mood and serum thyroid-stimulating hormone elevations in drug-free depressed patients after each active stimulation session. Mood improvement was only observed after active HFrTMS. These observations could imply that the clinical effects of rTMS act in a similar way to pharmacological interventions: clinical improvement after antidepressant treatment has been associated with a normalization of HPA system function and different antidepressants may act in the same way in attenuating the HPA axis.52,53 However, it has to be noted that in depressed patients HPA system abnormalities are not consistently observed.47
Neurotransmitter systems and rTMS
Only a few studies have examined the rTMS effects on neurotransmitter systems in major depression. Because rTMS treatment resulted in psychomotor symptom improvement, such as a reduction in motor slowness in bodily movement and speech, increased voice volume, and facial inexpressivity, some authors suggested that a possible working mechanism of action could be by activating the dopaminergic system.54,55 Indeed, several brain imaging studies using dopaminergic ligands point to an rTMS-related release in endogenous dopamine when stimulating prefrontal cortical areas,56,57 although others found no impact on the dopaminergic system at all.58,59 In major depression, the serotonergic system has been extensively investigated, and serotonin (5-HT) is an important excitatory transmitter involved in HPAsystem regulation.60 In a severely depressed medication-resistant sample, successful clinical outcome after HF-rTMS treatment was associated with DLPFC 5HT2A receptor upregulation and hippocampal 5-HT2A receptor downregulation, measured with 123I-5-IR91150 SPECT61 Interestingly, this prefrontal increase of this type of postsynaptic receptor agrees with treatment response findings after treatment with selective serotonin uptake inhibitors (SSRIs) and electroconvulsive therapy (ECT).62,63 As clinical recovery is reported to be associated with increased brain-derived neurotrophic factor (BDNF) expression in the hippocampus,64 it was suggested that the observed 5-HT2A receptor downregulation in the hippocampus would be associated with BDNF increases in this area comparable to the effects of most pharmacological antidepressant agents.65 However, as rTMS responders seem to be resistant to acute mood changes after trypthophan depletion,66 it may be possible that the neurobiological influence of rTMS does not only depend on the central availability of serotonin to exert antidepressant effects. In short, whether the rTMS effects are attributed to the modulation of only the serotonergic system remains unclear. A beneficial treatment outcome has been related to glutaminergic increases under the stimulated area (left DLPFC) in depressed patients.44 From an electrophysiological point of view, stimulation of the DLPFC might influence 5-HT2A receptors in the hippocampus via (glutaminergic) pyramidal neurons.67 Furthermore, research on the chronic effects of TMS on hippocampal evoked potentials demonstrates that TMS is accompanied by changes in the local hippocampal inhibitory circuits (g-aminobutyric acid, GABA).68 The implication of glutaminergic/GABAergic deficits in major depression has been proposed, but to date the influence of rTMS on the glutaminergic/ GABA system has only been demonstrated in healthy individuals.69,70 A single active HF-rTMS session increased glutamate/glutamine levels in the prefrontal cortices, suggesting that this application may act via the stimulation of the glutaminergic prefrontal neurons.69 Concerning the inhibitory effects, active rTMS resulted in increases in cortical inhibition; however, in this study only the left motor cortex was stimulated.70
Neurotrophic factors and rTMS
Brain and endocrinological data indirectly suggest that a clinical beneficial rTMS outcome affects neurotrophic factors in the brain.71 Animal studies already demonstrated increases in the expression of BDNF in the rat hippocampus after the application of long-term HFrTMS similar to antidepressant drug treatment and ECT72 In a sample of drug-resistant depressed patients, Zanardini et al73 reported on a normalizing rTMS effect of initially decreased serum BDNF. Yukimisa et al74 demonstrated that changes in serum BDNF correlated positively (rs=0.34) with changes on the 17-item Hamilton Depression Rating Scale in all depressed patients treated with HF-rTMS. These trend-like correlations were found to be significant when comparing responders with nonresponders; an increase of BDNF levels was only observed in those patients who clinically responded to the HF-rTMS treatment.
Conclusions
In unipolar depressed patients, beneficial rTMS treatment has immediate and prolonged neurobiological effects. Neurobiological data support the choice of the left DLPFC as a valid rTMS target site to intervene with the neuronal pathways deregulated in major depression. The observed changes in a depressionrelated neurocircuitry seem to agree with other successful treatment modalities, such as pharmacological antidepressant treatment and ECT. Although further research is required, biological data indicate that depressed patients with some kind of “preserved” cortico- subcortical neurocircuitries could be susceptible to rTMS treatment. Displaying a metabolically more active fronto-cingulate network at baseline indicates a possible better clinical outcome. This observation is consistent with the hypothesis that the synchronized modulation of “dysfunctional fronto-cingulate pathways” is critical for illness remission.23 In short, successful rTMS treatment seems to result in a cascade of neurobiological changes in brain areas linked with the stimulated area, supporting the integrative model of action depicted in Figures 1 and 2. Whether the rTMS effects are modulated by NT systems or neurotrophic factors remains to be clarified.
Selected abbreviations and acronyms
- ACC
anterior cingulate cortex
- BDNF
brain-derived neurotrophic factor
- DLPFC
dorsolateral prefrontal cortex
- HF
high-frequency
- HPA
hypothalamic-pituitary-adrenal
- LF
low-frequency
- rTMS
repetitive transcranial magnetic stimulation
Contributor Information
Chris Baeken, Department of Psychiatry and Center for Neurosciences, Vrije Universiteit Brussel (VUB), Brussels, Belgium.
Rudi De Raedt, Ghent University, Department of Experimental Clinical and Health Psychology, Ghent, Belgium.
REFERENCES
- 1.Belmaker RH., Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB. The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41:189–206. doi: 10.1016/j.jpsychires.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. . Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 4.Wong ML., Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 5.George MS., Nahas Z., Li X., et al. Novel treatments of mood disorders based on brain circuitry (ECT, MST, TMS, VNS, DBS). . Semin Clin Neuropsychiatry. 2002;7:293–304. doi: 10.1053/scnp.2002.35229. [DOI] [PubMed] [Google Scholar]
- 6.Gross M., Nakamura L., Pascual-Leone A., Fregni F. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta-analysis comparing the recent vs. the earlier rTMS studies. . Acta Psychiatr Scand. 2007;116:165–173. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Reardon JP., Solvason HB., Janicak PG., et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. . Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Brakemeier EL., Luborzewski A., Danker-Hopfe H., Kathmann N., Bajbouj M. Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS). J Psychiatr Res. 2007;41:606–615. doi: 10.1016/j.jpsychires.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Martin JL., Barbanoj MJ., Schlaepfer TE., Thompson E., Perez V., Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–491. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- 10.Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39:65–75. doi: 10.1017/S0033291708003462. [DOI] [PubMed] [Google Scholar]
- 11.Schutter DJ. Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychol Med. 2010;40:1789–1795. doi: 10.1017/S003329171000005X. [DOI] [PubMed] [Google Scholar]
- 12.Epstein CM. TMS stimulation coils. In: Wasserman EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, eds. The Oxford Handbook of Transcranial Stimulation. Oxford, UK; New York, NY Oxford University Press; 2008:25–32. [Google Scholar]
- 13.Fitzgerald PB., Oxley TJ., Laird AR., Kulkarni J., Egan GF., Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Toro M., Montes JM., Talavera JA. Functional cerebral asymmetry in affective disorders: new facts contributed by transcranial magnetic stimulation. J Affect Disord. 2001;66:103–109. doi: 10.1016/s0165-0327(00)00276-7. [DOI] [PubMed] [Google Scholar]
- 15.Padberg F., George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. . Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Buck R. The biological affects: a typology. . Psychol Rev. 1999;106:301–336. doi: 10.1037/0033-295x.106.2.301. [DOI] [PubMed] [Google Scholar]
- 17.Paus T., Barrett J. Transcranial magnetic stimulation (TMS) of the human frontal cortex: implications for repetitive TMS treatment of depression. J Psychiatry Neurosci. 2004;29:268–279. [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson RJ., Lewis DA., Alloy LB., et al. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- 19.Chen R., Gerloff C., Classen J., Wassermann EM., Hallett M., Cohen LG. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol. 1997;105:415–421 . doi: 10.1016/s0924-980x(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 20.Maeda F., Keenan JP., Tormos JM., Topka H., Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 21.Speer AM., Kimbrell TA., Wassermann EM., et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- 22.Knoch D., Treyer V., Regard M., Muri RM., Buck A., Weber B. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage. 2006;3:641–648. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 24.Drevets WC., Price JL., Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. . Brain Structure Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. . Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501 . doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 27.Erickson K., Drevets W., Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Gold PW., Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 29.Kimbrell TA., Little JT., Dunn RT., et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. . Biol Psychiatry. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 30.Speer AM., Kimbrell TA., Wassermann EM., et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133–1141 . doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- 31.Speer AM., Benson BE., Kimbrell TK., et al. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. . J Affect Disord. 2009;115:386–394. doi: 10.1016/j.jad.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baeken C., De Raedt R., Van Hove C., Clerinx P., De Mey J., Bossuyt A. HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. . CNS Spectr. 2009;14:439–448. doi: 10.1017/s1092852900020411. [DOI] [PubMed] [Google Scholar]
- 33.Herwig U., Lampe Y., Juengling FD., et al. Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res. 2003;37:267–275. doi: 10.1016/s0022-3956(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 34.Paillère Martinot ML., Galinowski A., Ringuenet D., et al. Influence of prefrontal target region on the efficacy of repetitive transcranial magnetic stimulation in patients with medication-resistant depression: a [(18)F]-fluorodeoxyglucose PET and MRI study. Int J Neuropsychopharmacol. 2010;13:45–59. doi: 10.1017/S146114570900008X. [DOI] [PubMed] [Google Scholar]
- 35.Li CT., Wang SJ., Hirvonen J., et al. Antidepressant mechanism of addon repetitive transcranial magnetic stimulation in medication-resistant depression using cerebral glucose metabolism. J Affect Disord. 2010;127:219–229. doi: 10.1016/j.jad.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Wu J., Buchsbaum MS., Gillin JC., et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. . Am J Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 37.Pizzagalli D., Pascual-Marqui RD., Nitschke JB., et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. . Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 38.Langguth B., Wiegand R., Kharraz A., et al. Pre-treatment anterior cingulate activity as a predictor of antidepressant response to repetitive transcranial magnetic stimulation (rTMS). . Neuroendocrinology Letters. 2007;28:633–638. [PubMed] [Google Scholar]
- 39.Paus T., Castro-Alamancos MA., Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. . Eur J Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 40.Kito S., Fujita K., Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58:29–36. doi: 10.1159/000154477. [DOI] [PubMed] [Google Scholar]
- 41.Kito S., Fujita K., Koga Y. Changes in regional cerebral blood flow after repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in treatment-resistant depression. . J Neuropsychiatry Clin Neurosci. 2008;20:74–80. doi: 10.1176/jnp.2008.20.1.74. [DOI] [PubMed] [Google Scholar]
- 42.Shajahan PM., Glabus MF., Steele JD., et al. Left dorso-lateral repetitive transcranial magnetic stimulation affects cortical excitability and functional connectivity, but does not impair cognition in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:945–954. doi: 10.1016/s0278-5846(02)00210-5. [DOI] [PubMed] [Google Scholar]
- 43.Nahas Z., Teneback CC., Kozel A., et al. Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. . J Neuropsychiatry Clin Neurosci. 2001;13:459–470. doi: 10.1176/jnp.13.4.459. [DOI] [PubMed] [Google Scholar]
- 44.Luborzewski A., Schubert F., Seifert F., et al. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. J Psychiatr Res. 2007;41:606–615. doi: 10.1016/j.jpsychires.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Loo CK., Sachdev PS., Haindl W., et al. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psych Med. 2003;33:997–1006. doi: 10.1017/s0033291703007955. [DOI] [PubMed] [Google Scholar]
- 46.Post A., Keck ME. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res. 2001;35:193–215. doi: 10.1016/s0022-3956(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 47.Schutter DJ., van Honk J. An endocrine perspective on the role of steroid hormones in the antidepressant treatment efficacy of transcranial magnetic stimulation. Psychoneuroendocrinology. 2010;35:171–178. doi: 10.1016/j.psyneuen.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Keck ME. rTMS as treatment strategy in psychiatric disordersneurobiological concepts. Suppl Clin Neurophysiol. 2003;56:100–116. doi: 10.1016/s1567-424x(09)70213-2. [DOI] [PubMed] [Google Scholar]
- 49.Baeken C., De Raedt R., Leyman L., et al. The impact of one HF-rTMS session on mood and salivary cortisol in treatment resistant unipolar melancholic depressed patients. J Affect Disord. 2009;113:100–108. doi: 10.1016/j.jad.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Pridmore S. Rapid transcranial magnetic stimulation and normalization of the dexamethasone suppression test. Psychiatry Clin Neurosci. 1999;53:33–37. doi: 10.1046/j.1440-1819.1999.00467.x. [DOI] [PubMed] [Google Scholar]
- 51.Szuba MP., O'Reardon JP., Ral AS., et al. Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry. 2001;50:22–27. doi: 10.1016/s0006-3223(00)01118-5. [DOI] [PubMed] [Google Scholar]
- 52.Keck ME., Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 53.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. . J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 54.Hoeppner J., Padberg F., Domes G., et al. Influence of repetitive transcranial magnetic stimulation on psychomotor symptoms in major depression. Eur Arch Psychiatry Clin Neurosci. 2010;260:197–202. doi: 10.1007/s00406-009-0039-8. [DOI] [PubMed] [Google Scholar]
- 55.Baeken C., De Raedt R., Santermans L., et al. HF-rTMS treatment decreases psychomotor retardation in medication-resistant melancholic depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:684–687. doi: 10.1016/j.pnpbp.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 56.Pogarell O., Koch W., Pöpperl G., et al. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res. 2006;40:307–314. doi: 10.1016/j.jpsychires.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Pogarell O., Koch W., Pöpperl G., et al. Acute prefrontal rTMS increases striatal dopamine to a similar degree as D-amphetamine. . Psychiatry Res. 2007;156:251–55. doi: 10.1016/j.pscychresns.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Kuroda Y., Motohashi N., Ito H., Ito S., Takano A., Nishikawa T., Suhara T. Effects of repetitive transcranial magnetic stimulation on [11C]raclopride binding and cognitive function in patients with depression. J Affect Disord. 2006;95:35–42. doi: 10.1016/j.jad.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 59.Kuroda Y., Motohashi N., Ito H., et al. Chronic repetitive transcranial magnetic stimulation failed to change dopamine synthesis rate: preliminary L-[b-11C]DOPA positron emission tomography study in patients with depression. . Psychiatry Clin Neurosci. 2010;64:659–662. doi: 10.1111/j.1440-1819.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 60.Neumeister A., Charney DS. Monoaminergic transmitter systems. In: D'haenen, HAH, den Boer, JA and Willner, P, eds. . Biological Psychiatry. Chichester, UK: John Wiley & Sons Ltd; 2002:727–738. [Google Scholar]
- 61.Baeken C., De Raedt R., Bossuyt A., et al. The impact of HF-rTMS treatment on serotonin 2A receptors in unipolar melancholic depression. Brain Stimulation. In press. doi: 10.1016/j.brs.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Burnet PW., Sharp T., LeCorre SM., Harrison PJ. Expression of 5-HT receptors and the 5-HT transporter in rat brain after electroconvulsive shock. Neurosci Lett. 1999;277:79–82. doi: 10.1016/s0304-3940(99)00857-5. [DOI] [PubMed] [Google Scholar]
- 63.Zanardi R., Artigas F., Moresco R., et al. Increased 5-hydroxytryptamine2 receptor binding in the frontal cortex of depressed patients responding to paroxetine treatment: a positron emission tomography scan study. J Clin Psychopharmacol. 2001;21:53–58. doi: 10.1097/00004714-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Martinowich K., Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 65.Vaidya VA., Terwilliger RM., Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 66.O'Reardon JP., Cristancho P., Pilania P., Bapatla KB., Chul S., Peshek AD. Patients with a major depressive episode responding to treatment with repetitive transcranial magnetic stimulation (rTMS) are resistant to the effects of rapid tryptophan depletion. Depress Anxiety. 2007;24:537–544. doi: 10.1002/da.20261. [DOI] [PubMed] [Google Scholar]
- 67.Celada P., Puig M., Amargos-Bosch M., Adell A., Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- 68.Levkovitz Y., Grisaru N., Segal M. Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology. 2001;24:608–616. doi: 10.1016/S0893-133X(00)00244-X. [DOI] [PubMed] [Google Scholar]
- 69.Michael N., Gösling M., Reutemann M., et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17:2462–2468. doi: 10.1046/j.1460-9568.2003.02683.x. [DOI] [PubMed] [Google Scholar]
- 70.Daskalakis ZJ., Möller B., Christensen BK., Fitzgerald PB., Gunraj C., Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 71.Brunoni AR., Boggio PS., Fregni F. Can the 'yin and yang' BDNF hypothesis be used to predict the effects of rTMS treatment in neuropsychiatry? . Med Hypotheses. 2008;71:279–282. doi: 10.1016/j.mehy.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Müller MB., Toschi N., Kresse AE., Post A., Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology. 2000;23:205–215. doi: 10.1016/S0893-133X(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 73.Zanardini R., Gazzoli A., Ventriglia M., et al. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. . J Affect Disord. 2006;91:83–86. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Yukimasa T., Yoshimura R., Tamagawa A., et al. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- 75.Sullivan RM., Gratton A. Prefrontal cortical regulation of hypothalamicpituitary-adrenal function in the rat and implications for psychopathology: side matters. . Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 76.Ressler KJ., Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 77.Maletic V., Robinson M., Oakes T., Iyengar S., Ball SG., Russell J. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract. 2007;61:2030–2040. doi: 10.1111/j.1742-1241.2007.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]